Published online Jun 20, 2024. doi: 10.5493/wjem.v14.i2.92157
Revised: February 6, 2024
Accepted: March 18, 2024
Published online: June 20, 2024
Processing time: 150 Days and 12.1 Hours
Traditional descriptions of liver anatomy refer to a smooth, convex surface contacting the diaphragm. Surface depressions are recognized anatomic variants. There are many theories to explain the cause of the depressions. We discuss the theory that these are caused by hypertrophic muscular bands in the diaphragm.
Core Tip: Surface depressions of the liver are a recognized anatomic variant Transillumination is a method that is useful to evaluate the association with hypertrophic muscular bands in the diaphragm. Using this technique, we determined that hyper
- Citation: Cawich SO, Gardner MT, Shetty R, Louboutin JP, Dabichan Z, Johnson S. Liver surface depressions in the presence of diaphragmatic muscular bands on trans-illumination. World J Exp Med 2024; 14(2): 92157
- URL: https://www.wjgnet.com/2220-315x/full/v14/i2/92157.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i2.92157
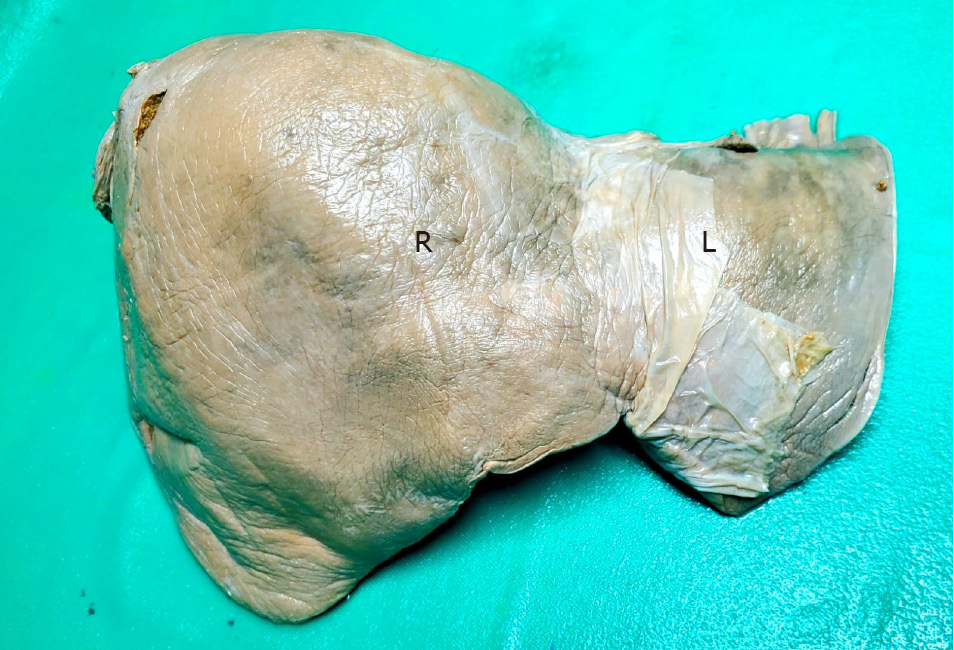
Traditional descriptions of liver anatomy refer to a smooth, rounded, convex surface contacting the diaphragm, as demonstrated in Figure 1[1]. The presence of surface depressions is a recognized anatomic variant[2-4].
While the cause for these surface depressions has not been definitively elucidated, several theories have been put forward, including: compression by ribs[5,6], pulmonary emphysema[7], congenital parenchymal weakness zones[8-10], hepatic trauma[11], tumour necrosis leading to desmoplasia and subsequent capsular retraction[10,11,12], regression of liver metastases after chemotherapy[11,13], adjacent inflammatory foci (gallbladder empyema, liver abscesses or cirrhosis) leading to parenchymal scarring and capsular retraction[14], localized iatrogenic injury after trans-arterial chemo-embolization (TACE)[15], fibrous bands and diaphragmatic scars[16]. Although none have been definitively proven as the cause for surface depressions, diaphragmatic muscular bands have gained little attention as an aetiologic factor. These are well-defined fascicles that connect the central diaphragmatic tendon to the rib cage. We discuss our observations during cadaveric dissections regarding the association of diaphragmatic muscular bands and surface depressions on the liver.
The prevalence of surface depressions is difficult to discern, because there is no standardized nomenclature for this variant. A review of the literature revealed that many names have been ascribed to this variant, such as: hepatic surface grooves[5,7,11,16], diaphragmatic grooves[3], accessory sulci[6,8,16], hepatic fissures[3,8], portal fissures[8], accessory fissures[4], and capsular retraction[10,12]. Although there is heterogeneity in nomenclature, a detailed review of the descriptions appearing in the anatomic literature suggest that surface depressions are encountered in 5%[17] to 51%[18] of unselected persons across the globe.
In a prior publication on cadavers with liver surface depressions, we reported that the diaphragm appeared normal in all cases, without evidence of scars, fibrotic slips, ligamentous thickening or thickened muscular bands[16]. In fact, we wrote in our conclusion that our findings “did not support diaphragmatic pathology as a plausible explanation” for surface depressions[16]. Subsequently, however, we had the opportunity to inspect the diaphragm in living persons undergoing surgery, and observed thickened muscular bands in vivo while performing open surgery[19] as well as laparoscopic operations[20]. These observations directly challenged our prior statements and we were forced to re-visit this issue.
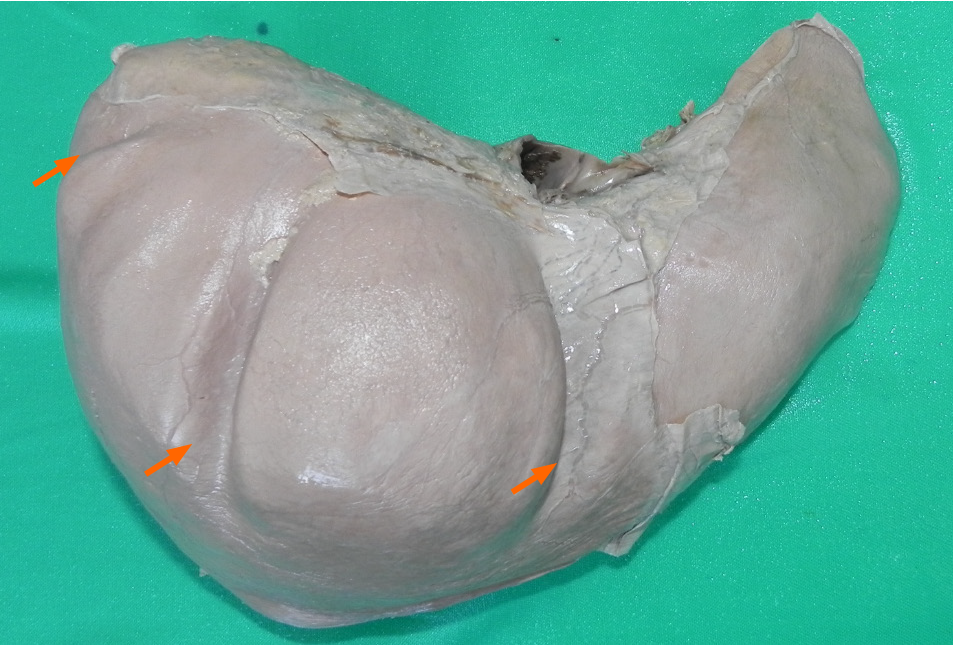
We postulated that the reason we had not observed diaphragmatic muscular bands in cadaveric studies was because they were not ligamentous thickening, scars or fibrotic slips. Instead, these areas appeared to be composed of hypertrophic muscular bands. Therefore, when muscle tone was absent at the post-mortem examinations, these bands would not be easily visible. However, if the bands were hypertrophic muscle, they should still be discernable by transillumination (Figure 2).
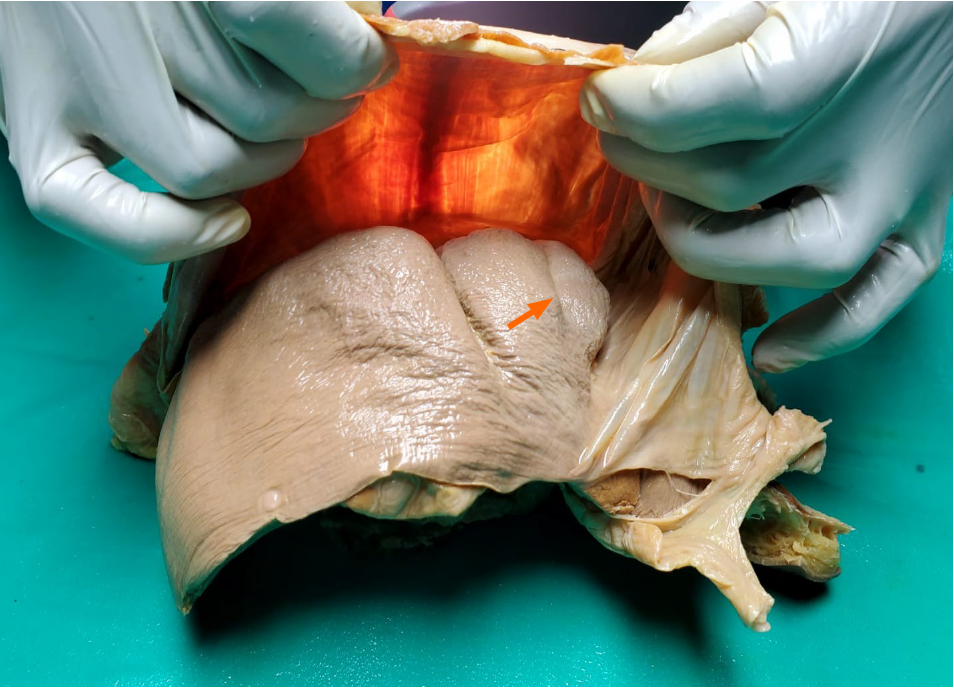
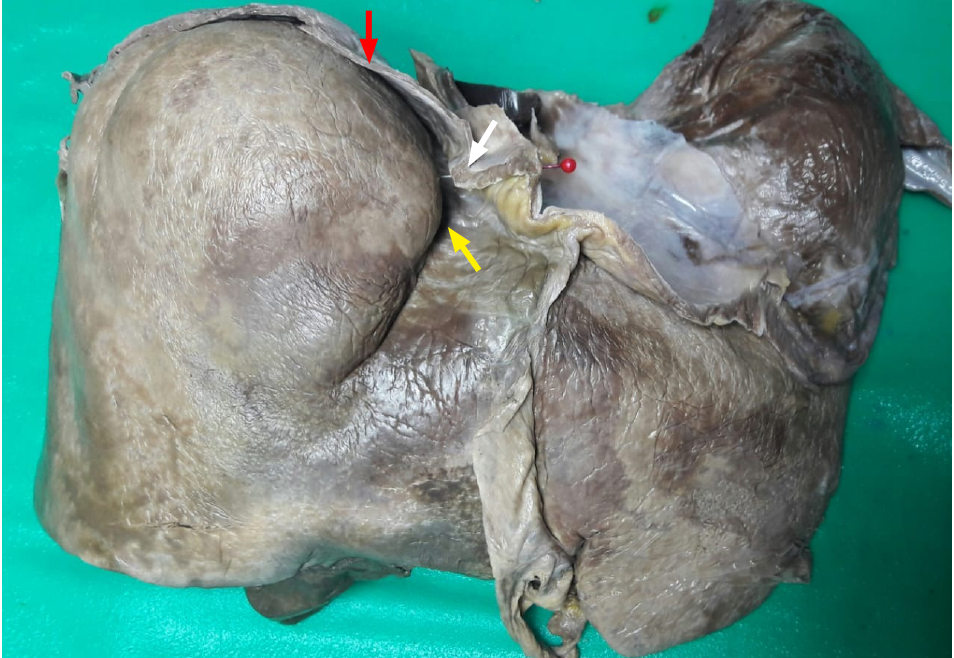
After approval was granted from the local institutional review board (CREC_SA.1034/06/2021), we observed cadaveric dissections for academic medical curricula at two university campuses in the Caribbean. From a total of 120 cadaveric dissections observed, 18 (15%) cadavers had surface depressions on the liver. During academic teachings, the liver and diaphragm were excised en-bloc and studied on the dissecting bench. We took this opportunity to apply a light source against the thoracic surface of the diaphragm and observations were made from the abdominal side (Figure 3). The presence of hypertrophic muscular bands, when present, and their correlation with surface depressions were recorded.
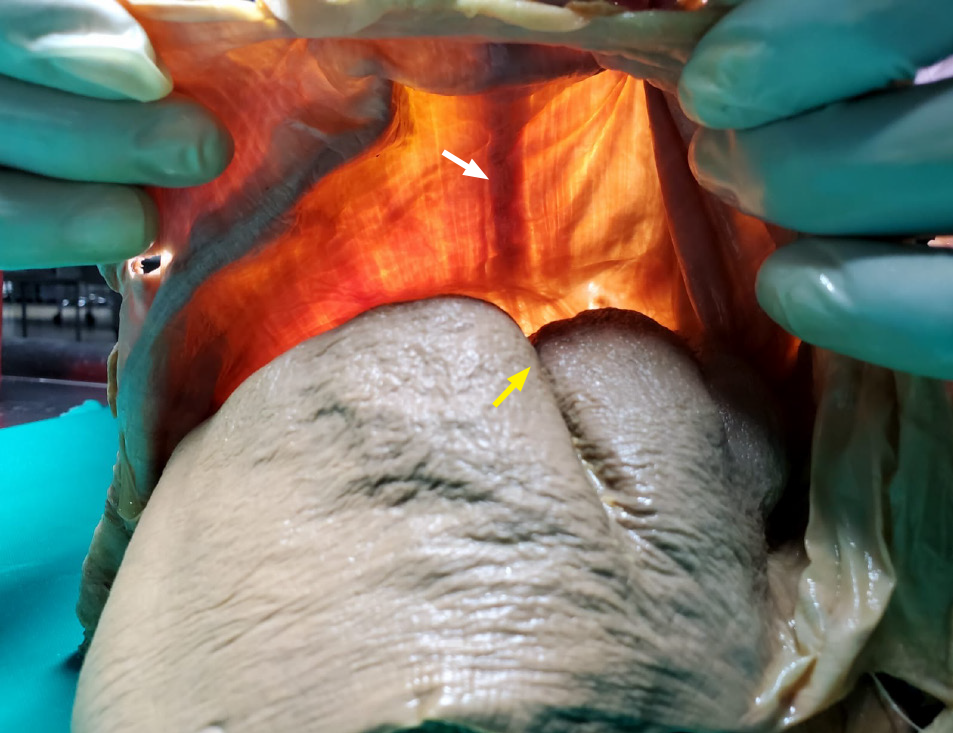
There were hypertrophic muscular bands visible on trans-illumination in 12 (67%) of the cadavers with surface depressions (Figure 4). Apart from hypertrophic muscular bands, there were no other causes of compression in these 12 cases.
These bands were not readily visible upon gross inspection. Admittedly, we did not appreciate differences in the diaphragm in our previous publications[16]. Upon close inspection after transillumination, however, we noted that the diaphragmatic thickness was greater adjacent to surface depressions (Figure 5).
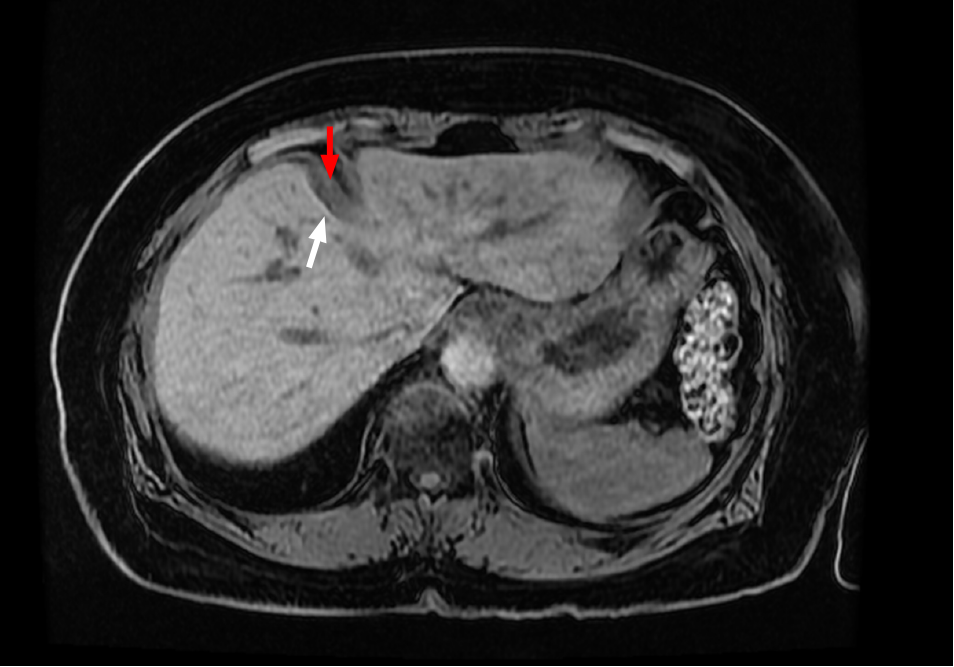
In addition, we noticed the presence of muscular bands occurring adjacent to surface depressions in all of seven living patients undergoing abdominal imaging for varied indications (Figure 6). These observations support our theory that hypertrophic muscular bands are intimately related to the corresponding surface depressions.
At the turn of the century, animal studies demonstrated that beta-catenin was an important factor responsible for stimulating hepatocyte growth, and its regulation had the potential to alter the size and shape of the liver[21,22]. Suksaweang et al[22] also theorized that there were specific areas in the liver, known as growth zones, that are susceptible to changes in beta-catenin activity.
Beta-catenin has since been shown to be up-regulated in humans with venous congestion secondary to cardiac failure[23]. It is also well known that there are areas between the inter-segmental and inter-sectional planes of the liver where the parenchyma is relatively less-well vascularized[11,24]. These “watershed areas” would be less responsive to the effect of beta-catenin up-regulation, and hence not proliferate as readily as surrounding parenchyma[11]. We suggest that hypertrophic muscular bands in proximity to these “watershed areas” could be responsible for the surface depressions. This could also explain the frequent location of surface depressions closely related to the inter-sectional planes[8,25].
The presence of surface depressions does have clinical significance, especially in light of the popularity of medical imaging in modern medicine. As more patients are subjected to medical imaging and these variants will be detected increasingly. When detected, these variants can be misinterpreted for metastatic liver secondaries in patients with known malignancies[1,2,4]. In those who have sustained blunt abdominal trauma, the depressions can be mistaken for liver lacerations[2,16]. There are also prior reports of these variants being mistaken for Chilaiditi’s syndrome on imaging[13]. In all of these cases, mis-interpretations can negatively affect clinical care decisions[16].
On the other hand, we have found that surface depressions can sometimes be advantageous. For example, in patients who require liver resections, we have modified our practice to routinely attempt to transect the parenchyma where surface depressions are present. This significantly reduces the thickness of the parenchymal transection line, reduces bleeding and so has the potential to improve patient outcomes[16].
Surface depressions of the liver are a recognized anatomic variant, with clinical implications when present. We suggest that it is time to adopt standardized nomenclature. We also theorize that diaphragmatic muscular bands play a prominent role in the formation of surface depressions.
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Srimani P, Saha A. Liver morphology: anatomical study about the outer aspects. Surg Radiol Anat. 2020;42:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Ragavan S, Muraleedharan A, Bage NN, Devi R. A comprehensive study and extensive review of morphological variations of liver with new insights. Surg Radiol Anat. 2022;44:455-466. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Chaudhari HJ, Ravat MK, Vaniya VH, Bhedi AN. Morphological Study of Human Liver and Its Surgical Importance. J Clin Diagn Res. 2017;11:AC09-AC12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sambhav K, Krishna H, Dixit SG, Ghatak S. Morphological Study of Variations of the Human Cadaveric Liver and Its Clinical Implications. Cureus. 2023;15:e35507. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Newell RLM, Morgan-Jones R. Grooves in the superior surface of the liver. Clin Anat. 1993;6:333-336. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Muktyaz H, Nema U, Suniti MR, Mahboobul H. Anatomical study of Accessory Sulci of Liver and its Clinical Significance in North Indian Population. Int J Med Health Sci. 2013;2:222-229. |
| 8. | Macchi V, Feltrin G, Parenti A, De Caro R. Diaphragmatic sulci and portal fissures. J Anat. 2003;202:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Joshi SD, Joshi SS, Athavale SA. Some interesting observations on the surface features of the liver and their clinical implications. Singapore Med J. 2009;50:715-719. [PubMed] |
| 10. | Yang DM, Kim HS, Cho SW, Kim HS. Pictorial review: various causes of hepatic capsular retraction: CT and MR findings. Br J Radiol. 2002;75:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Cawich SO, Ali RRA, Gardner MT, Charles J, Sandy S, Pearce NW, Naraynsingh V. Hepatic surface grooves in Trinidad and Tobago. Surg Radiol Anat. 2020;42:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Sans N, Fajadet P, Galy-Fourcade D, Trocart J, Jarlaud T, Chiavassa H, Giron J, Railhac JJ. Is capsular retraction a specific CT sign of malignant liver tumor? Eur Radiol. 1999;9:1543-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Young ST, Paulson EK, Washington K, Gulliver DJ, Vredenburgh JJ, Baker ME. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol. 1994;163:1385-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ohtomo K, Baron RL, Dodd GD 3rd, Federle MP, Ohtomo Y, Confer SR. Confluent hepatic fibrosis in advanced cirrhosis: evaluation with MR imaging. Radiology. 1993;189:871-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kostov S, Selçuk I, Watrowski R, Dineva S, Kornovski Y, Slavchev S, Ivanova Y, Dzhenkov D, Yordanov A. Surgical Anatomy of the Liver-Significance in Ovarian Cancer Surgery. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Gardner MT, Cawich SO, Shetty R, Pearce NW, Naraynsingh V. Hepatic surface grooves in an Afro-Caribbean population: a cadaver study. Ital J Anat Embryol. 2015;120:117-126. [PubMed] |
| 17. | Othman FB, Latiff AA, Suhaimi FH, Das S. Accessory sulci of the liver. An anatomical study with clinical implications. Saudi Med J. 2008;29:1247-1249. [PubMed] |
| 18. | Singh HR, Rabi S. Study of Morphoogical Variations of Liver in Humans. Transl Res Anat. 2019;14:1-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Cawich SO, Gardner MT, Pearce NW, Shetty R. Narayansingh V. Association between hepatic surface grooves and diaphragmatic slips. Italian J Anat Embryol. 2017;122:64-66. [DOI] [Full Text] |
| 20. | Cawich SO, Spence R, Mohammed F, Gardner MT, Sinanan A, Naraynsingh V. The liver and Chilaiditi's syndrome: Significance of hepatic surface grooves. SAGE Open Med Case Rep. 2017;5:2050313X17744979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Dev Biol. 2004;266:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 2006;20:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Sureka B, Sharma N, Khera PS, Garg PK, Yadav T. Hepatic vein variations in 500 patients: surgical and radiological significance. Br J Radiol. 2019;92:20190487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Ono ML, Murakami G, Sato TJ, Sawada K. Hepatic grooves and portal segmentation. Kaibogaku Zasshi. 2000;75:517-523. [PubMed] |