Published online Jun 20, 2024. doi: 10.5493/wjem.v14.i2.91519
Revised: February 7, 2024
Accepted: April 11, 2024
Published online: June 20, 2024
Processing time: 172 Days and 14.1 Hours
Mitochondrial dysfunction is a key driver of cardiovascular disease (CVD) in metabolic syndrome and diabetes. This dysfunction promotes the production of reactive oxygen species (ROS), which cause oxidative stress and inflammation. Angiotensin II, the main mediator of the renin-angiotensin-aldosterone system, also contributes to CVD by promoting ROS production. Reduced activity of sir
Core Tip: Sodium-glucose cotransporter-2 inhibitors, diabetes drugs, unlock tissue protection via diverse pathways. They boost mitochondrial efficiency, curb oxidative stress and inflammation, and enhance autophagy. Clinical trials show cardiovascular benefits, suggesting immense potential beyond diabetes and even towards anti-aging therapy.
- Citation: Sanz RL, García Menéndez S, Inserra F, Ferder L, Manucha W. Sodium-glucose cotransporter-2 inhibitors protect tissues via cellular and mitochondrial pathways: Experimental and clinical evidence. World J Exp Med 2024; 14(2): 91519
- URL: https://www.wjgnet.com/2220-315x/full/v14/i2/91519.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i2.91519
Despite ongoing research and preventive efforts, cardiovascular disease (CVD) remains the leading cause of global mortality, a sobering statistic highlighted by the American Heart Association in its 2021 update[1]. In individuals with metabolic syndrome and diabetes, this grim reality is heavily influenced by mitochondrial dysfunction, which acts as a crucial driver of disease progression and persistence, ultimately contributing to adverse cardiac remodeling and events. The renin-angiotensin system plays another pivotal role, with elevated angiotensin II (Ang II) activity fueling oxidative stress and localized inflammatory responses. Furthermore, a decline in sirtuins (SIRTs) appears to be a key player in this complex interplay. These proteins exert intricate control over cellular responses to environmental cues, impacting oxidative stress levels and interacting with Ang II in various pathways linked to fibrosis, apoptosis, inflammation, and cardiac and vascular remodeling[2-6]. Recent research has revealed a fascinating interplay between sodium-glucose cotransporter-2 inhibitors (SLGT2i) and the sirtuin-renin-angiotensin-aldosterone system (RAAS) crosstalk, which has profound implications for mitochondrial function.
Beyond their established role as deacetylases[7], SIRTs wield another hidden power: modulating oxidative stress through intricate regulation of cellular adaptive responses[8]. This means that SIRTs not only remove acetyl groups from proteins but also adjust how cells respond to oxidative stress, akin to a team of tiny biochemists fine-tuning the cellular environment. Specifically, Ang II, a key player in the RAAS, acts as a kind of conductor, stimulating SIRTs via their type 1 receptor and the production of reactive oxygen species (ROS)[9]. Think of it as Ang II turning up the volume on SIRT activity, leading to increased ROS production. However, it is not one-sided. Ang II also throws a wrench in the works, down-regulating SIRT1 and 2 in the heart, like a dimmer switch lowering their brightness[10]. In contrast, SIRT3 adopts a different approach, inducing forkhead box O3 (FOXO3) to move into the cell nucleus, which ultimately leads to reduced catalase levels and increased ROS[10]. Imagine SIRT3 as a mischievous elf sneaking FOXO3 into the nucleus, causing some oxidative stress mischief.
Diving deeper into the mitochondrial realm, we find SIRT3, 4, and 5 holding court. Among them, SIRT3 reigns supreme, partnering with cyclophilin D to unlock the powerhouses’ hidden doors (the transition pores), influencing how freely things flow and ultimately impacting cell function. However, lurking in the shadows, Ang II orchestrates a nefarious play, wielding specific tools to adorn FOXO3a with an acetyl group[11-13]. This glamorous attire, sadly, mutes the antioxidant heroes like superoxide dismutase and catalase, allowing ROS to run rampant and culminate in a magnified heart (cardiac hypertrophy). Yet, SIRT3 stands as a beacon of hope, meticulously fine-tuning complex I, the conductor of the mitochondrial orchestra, ensuring smooth energy production[14]. This highlights the intricate waltz of SIRTs, each playing a unique melody within the symphony of cellular health. However, the plot thickens with SIRT4, a rogue in the story of cardiac remodeling. Unlike its benevolent brethren, it throws its lot in with Ang II, unleashing a torrent of destructive effects[15]. This alliance silences manganese-dependent superoxide dismutase, the valiant shield against oxidative stress, leading to a cascade of damage and, ultimately, a hypertrophied heart.
Concerning vascular reactivity, potassium and calcium channels have long been recognized as playing central roles. However, it has recently been reported that dapagliflozin promotes vasodilation by activating the protein kinase G pathway without altering the activity or expression of calcium or potassium channels[16]. Conversely, in adipose tissue, SGLT2i exert multiple actions that promote a healthy phenotype with reduced secretion of inflammatory adipokines such as leptin and increased secretion of adiponectin[17]. Furthermore, both adiponectin and the induction of macrophage polarization toward the macrophage 2 phenotype lead to adipocyte browning and increased brown adipose tissue activity, which promotes greater utilization of lipid substrates in a context in which inflammatory and lipotoxic effects are attenuated[17,18]. In addition, SGLT2i activate the adenosine monophosphate-activated protein kinase (AMPK)/SIRT1/peroxisome proliferator-activated receptor γ co-activator 1 α (PGC1α) pathway in adipose tissue, associated with changes in mitochondrial morphology and function[19]. Beyond their established glucose-lowering prowess, SGLT2i can shrink epicardial adipose tissue (EAT)[20]. This targeted attack on the fatty culprit dampens the flames of myocardial inflammation, offering dual protection against heart disease.
SGLT2i act like metabolic magicians in the liver, increasing the production of fibroblast growth factor 21[18-21], which boosts lipid oxidation and prevents the activation of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome. This leads to pyroptosis, a form of programmed cell death linked to insulin resistance and obesity. By preventing pyroptosis, SGLT2i improve insulin sensitivity and promote adipocyte browning. At the pancreatic level, SGLT2i protect from damage by inhibiting the activation of the NLRP3 inflammasome. Its direct effect on pancreatic α cells is controversial, as it leads to increased glucagon secretion and a consequent increase in hepatic gluconeogenesis[22,23].
There is considerable evidence about the positive cellular and mitochondrial effects of SGLT2i on the renal system. Specifically, less fibrosis, organ damage, and inflammatory damage have been demonstrated through the modulation of the SIRT1/AMPK/PGC1α pathway[24,25]. Inhibition of the mammalian target of rapamycin complex 1 -possibly secondary to elevated ketone bodies- was interestingly linked to autophagy, lower stress, and prevention of podocyte and endothelial injury[24]. Furthermore, SGLT2i can mitigate renal fibrosis by modulating transforming growth factor beta, autophagy, and peroxisome proliferator-activated receptor alpha through fatty acid oxidation[26,27]. Another beneficial effect at the tubular level is the reduction of serum uric acid levels, possibly attributed to the alteration of uric acid transport activity induced by glycosuria[28].
In addition to their classic pharmacological effects, SGLT2i have manifested pleiotropic actions that involve signaling pathways with SIRTs, especially SIRT3 and SIRT1, reducing oxidative stress, inflammation, and fibrosis[29,30]. SGLT2i constitutes a multifaceted defense against heart failure, striking on two fronts[31]. They wield a molecular scalpel, inhibiting the Na+/H+ exchanger to calm the storm of sodium and calcium overload within heart cells. Simultaneously, they ignite the metabolic spark, promoting a fasting-like state that fuels the engine of ketone production, providing an optimal energy source for the struggling heart. This mechanism led to the postulation that SGLT2i could activate SIRTs linked to the autophagy pathway and innate immunity. SGLT2i choreograph a wonderful cellular ballet (beclin 1, toll-like receptor 9, SIRT3, and mitochondria), weaving together threads of autophagy, oxidative stress, and mitochondrial health[32]. With SIRT3 as the key piece, they stimulate mitochondrial respiration, quiet the whispers of oxidative stress, silence the shouts of apoptosis and inflammasome signaling, and build a defensive wall against cardiac injury. In this regard, it is worth noting that SIRT3 deficiency -both in mice and in patients- caused the loss of the cardiac protective effects of SGLT2i.
Specifically, at least three central processes are presently known by which SGLT2i can activate SITRs: One of them is that SGLT2i stimulate the fasting process cellularly by promoting gluconeogenesis through activating the sensitive element binding protein cyclic adenosine monophosphate. Thus, the SIRT1 promoter regulates its transcription[33]. SGLT2i engages in a clever cellular game, manipulating nicotinamide adenine dinucleotide (NAD+) levels to turn on SIRTs, the molecular maestros[34].
Furthermore, the AMPK and SIRT1 signaling pathways are reciprocally activated. Their complementary functions demonstrate that AMPK promotes mitochondrial biogenesis and mitochondrial DNA replication and can activate SIRT1 by increasing intracellular NAD+[35]. SIRT1 and AMPK, the cellular power couple, engage in mutual crosslink activation. Fueled by caloric restriction and SGLT2i, they synergistically boost each other’s power, modulating a symphony of metabolic efficiency and stress resilience[19].
In the realm of clinical information, the signaling pathways involving SGLT2i and SIRT1, 3, and 6 take center stage. In this regard, several clinical studies are of particular interest. The EMPEROR-Preserved trial marks a seismic shift in the landscape of heart failure management, demonstrating that SGLT2i’s protective umbrella extends beyond diabetes[36]. This landmark study opens doors for a wider population struggling with preserved ejection fraction heart failure, offering them a powerful weapon against CVD. In this context, Packer elucidates heart failure mechanisms, focusing on hyperinsulinemia-driven EAT expansion and its pro-inflammatory consequences, and also highlights the beneficial effects of SGLT2i on EAT, reducing inflammation and improving cardiac health[20].
Emerging evidence suggests that SGLT2i may hold promise as an anti-aging agent. These drugs appear to target key pathways involved in aging, including inflammation, cellular energy regulation, and the harmful effects of senescent cells. Similar to the established anti-aging drug metformin, SGLT2i may offer benefits through mechanisms such as reducing free radical production, activating autophagy, and modulating the inflammatory response. Remarkably, SGLT2i may also positively impact the gut microbiome, further contributing to their potential anti-aging effects. This multifaceted action against inflammaging, a chronic low-grade inflammation linked to accelerated aging and age-related diseases, makes SGLT2i particularly interesting candidates for therapeutic repurposing[37]. However, robust clinical studies are crucial to validate the anti-aging potential of SGLT2i beyond their established role in diabetes management. While preliminary results are promising, further research is needed to confirm their efficacy and safety in this context.
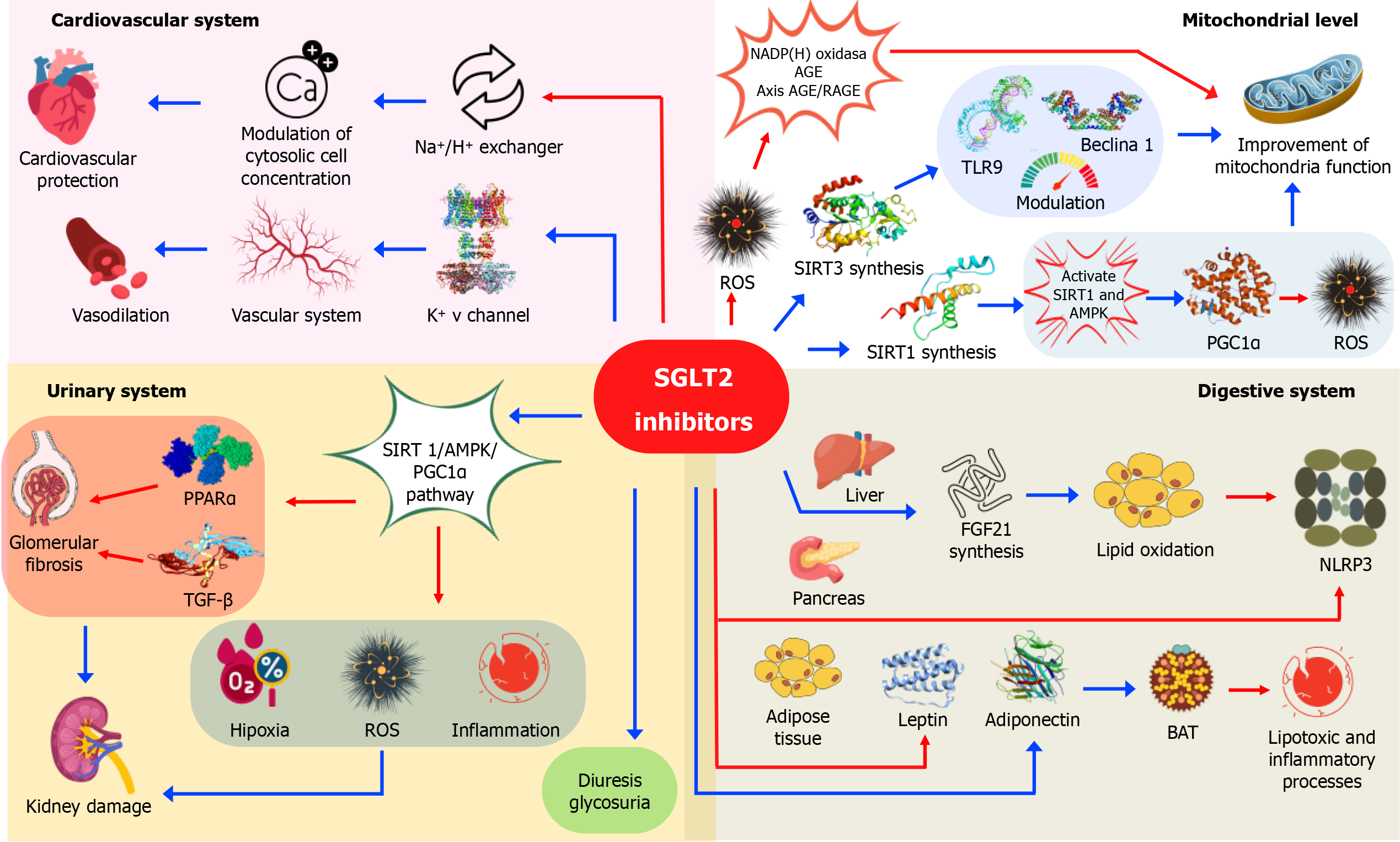
Finally, a lack of nutrients, as well as low energy production at the cellular level, can mitigate a large number of cardiometabolic disorders. In particular, the SIRT and AMPK pathways induce the hypoxia-inducible factor 1 beta and promote ketosis[38]. Consequently, autophagy/mitophagy are stimulated with beneficial effects on cardiac cells, reducing oxidation-inflammation[39]. Specifically, SGLT2i encourage no less than three critical SIRTs present in mitochondria. Furthermore, recent evidence demonstrates that SLGT2i mimic mitochondrial function, reducing inflammation and oxidative stress. Figure 1 provides a graphical overview of the beneficial effects of SGLT2i across various systems and at the mitochondrial level.
Added to the classic action of SGLT2i, which is linked to both inhibiting renal glucose reabsorption and triggering metabolic reprogramming through increased glycosuria and reduced glucotoxicity, a growing body of research demonstrates their pleiotropic effects across various cell types and organs, mediated by distinct signaling pathways that contribute to their beneficial outcomes. Table 1 summarizes the main studies showing tissue protection by SGLT2i. The pleiotropic effects of SGLT2i on diverse cellular and mitochondrial signaling pathways in multiple organs and tissues are well-documented by metabolomics studies. However, the possibility of accompanying epigenetic modifications requires further investigation[40,41].
| SGLT2i | Study design | Animal/human | Protected tissue | Effect | Ref. |
| Dapagliflozin | Animal study | Rabbits | Blood vessels | Activation of Kv channels and PKG | [16] |
| Empagliflozin | Animal study | Mice | Adipose tissue, and liver | Induction of anti-inflammatory macrophage 2 phenotype of macrophages | [18] |
| Canagliflozin | Animal study | Mice | Adipose tissue | Induction of AMPK-SIRT1-Pgc-1α signalling pathway | [19] |
| Canagliflozin | Animal study | Mice | Liver | Enhancing FGF21-ERK1/2 pathway activity | [21] |
| Empagliflozin | Animal study | Mice | Pancreas | Activation of the NLRP3/caspase-1/GSDMD pathway | [23] |
| Canagliflozin | Animal study | Mice | Kidney | Normalized Pin1 expression and AMPK activation | [25] |
| Canagliflozin | Animal study | Mice | Kidney | Autophagy modulation | [26] |
| Empagliflozin and canagliflozin | Cell culture | Renal cells | Kidney | Block basal and TGF-β1-induced expression | [27] |
| Luseogliflozin | Human study and animal study | Human and xenopus laevis oocytes | Kidney | Uric acid transport activity | [28] |
| Empagliflozin | Animal study | Mice | Heart | Improving mitochondrial homeostasis | [30] |
| Empagliflozin | Animal study | Mice | Heart | Modulation of the Beclin 1-TLR9-SIRT3 complexes in the mitochondria | [32] |
| Empagliflozin | Human study | Human | Heart | Reduced the combined risk of cardiovascular death or hospitalization for heart failure | [36] |
| Dapagliflozin | Animal study | Mice | Kidney, liver, and heart | Induction of the AMPK-mTORC1 signaling | [41] |
The introduction of SGLT2i holds the potential to transform the clinical prognosis of cardio-reno-metabolic diseases based on the aforementioned mechanisms. Therefore, the findings presented in this review, beyond encouraging results from large clinical trials, also raise significant expectations for future advancements. Conceptually, SGLT2i are understood to act in various target organs as a protective agent, regulating the delicate balance between oxygen consumption and energy production. Their effects at the mitochondrial level, particularly on oxidative stress and cell protection, are crucial determinants of their efficacy.
| 1. | Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254-e743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 3577] [Article Influence: 894.3] [Reference Citation Analysis (0)] |
| 2. | Ferder L, Inserra F, Martínez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep. 2006;8:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Cabandugama PK, Gardner MJ, Sowers JR. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med Clin North Am. 2017;101:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Verdejo HE, del Campo A, Troncoso R, Gutierrez T, Toro B, Quiroga C, Pedrozo Z, Munoz JP, Garcia L, Castro PF, Lavandero S. Mitochondria, myocardial remodeling, and cardiovascular disease. Curr Hypertens Rep. 2012;14:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am J Physiol Heart Circ Physiol. 2015;309:H15-H44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Maissan P, Mooij EJ, Barberis M. Sirtuins-Mediated System-Level Regulation of Mammalian Tissues at the Interface between Metabolism and Cell Cycle: A Systematic Review. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal. 2018;28:643-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 574] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 8. | Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY). 2013;5:144-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 1052] [Article Influence: 105.2] [Reference Citation Analysis (1)] |
| 10. | Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849-14854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 12. | Manucha W, Ritchie B, Ferder L. Hypertension and insulin resistance: implications of mitochondrial dysfunction. Curr Hypertens Rep. 2015;17:504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309:H1375-H1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 14. | Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447-14452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1030] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 15. | Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ, Liu DP. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2017;38:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Li H, Shin SE, Seo MS, An JR, Choi IW, Jung WK, Firth AL, Lee DS, Yim MJ, Choi G, Lee JM, Na SH, Park WS. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci. 2018;197:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Packer M. Mitigation of the Adverse Consequences of Nutrient Excess on the Kidney: A Unified Hypothesis to Explain the Renoprotective Effects of Sodium-Glucose Cotransporter 2 Inhibitors. Am J Nephrol. 2020;51:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, Mayoux E, Kaneko S, Ota T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine. 2017;20:137-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 19. | Yang X, Liu Q, Li Y, Tang Q, Wu T, Chen L, Pu S, Zhao Y, Zhang G, Huang C, Zhang J, Zhang Z, Huang Y, Zou M, Shi X, Jiang W, Wang R, He J. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte. 2020;9:484-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | Packer M. Differential Pathophysiological Mechanisms in Heart Failure With a Reduced or Preserved Ejection Fraction in Diabetes. JACC Heart Fail. 2021;9:535-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Huang S, Wu B, He Y, Qiu R, Yang T, Wang S, Lei Y, Li H, Zheng F. Canagliflozin ameliorates the development of NAFLD by preventing NLRP3-mediated pyroptosis through FGF21-ERK1/2 pathway. Hepatol Commun. 2023;7:e0045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Saponaro C, Pattou F, Bonner C. SGLT2 inhibition and glucagon secretion in humans. Diabetes Metab. 2018;44:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Liu P, Zhang Z, Wang J, Zhang X, Yu X, Li Y. Empagliflozin protects diabetic pancreatic tissue from damage by inhibiting the activation of the NLRP3/caspase-1/GSDMD pathway in pancreatic β cells: in vitro and in vivo studies. Bioengineered. 2021;12:9356-9366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Gao YM, Feng ST, Wen Y, Tang TT, Wang B, Liu BC. Cardiorenal protection of SGLT2 inhibitors-Perspectives from metabolic reprogramming. EBioMedicine. 2022;83:104215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 25. | Inoue MK, Matsunaga Y, Nakatsu Y, Yamamotoya T, Ueda K, Kushiyama A, Sakoda H, Fujishiro M, Ono H, Iwashita M, Sano T, Nishimura F, Morii K, Sasaki K, Masaki T, Asano T. Possible involvement of normalized Pin1 expression level and AMPK activation in the molecular mechanisms underlying renal protective effects of SGLT2 inhibitors in mice. Diabetol Metab Syndr. 2019;11:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Yang Y, Li Q, Ling Y, Leng L, Ma Y, Xue L, Lu G, Ding Y, Li J, Tao S. m6A eraser FTO modulates autophagy by targeting SQSTM1/P62 in the prevention of canagliflozin against renal fibrosis. Front Immunol. 2022;13:1094556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 27. | Pirklbauer M, Schupart R, Fuchs L, Staudinger P, Corazza U, Sallaberger S, Leierer J, Mayer G, Schramek H. Unraveling reno-protective effects of SGLT2 inhibition in human proximal tubular cells. Am J Physiol Renal Physiol. 2019;316:F449-F462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, Tamai I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35:391-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 29. | Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 549] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 30. | Zou R, Shi W, Qiu J, Zhou N, Du N, Zhou H, Chen X, Ma L. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol. 2022;21:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 31. | Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 32. | Wang CY, Chen CC, Lin MH, Su HT, Ho MY, Yeh JK, Tsai ML, Hsieh IC, Wen MS. TLR9 Binding to Beclin 1 and Mitochondrial SIRT3 by a Sodium-Glucose Co-Transporter 2 Inhibitor Protects the Heart from Doxorubicin Toxicity. Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Penke M, Larsen PS, Schuster S, Dall M, Jensen BA, Gorski T, Meusel A, Richter S, Vienberg SG, Treebak JT, Kiess W, Garten A. Hepatic NAD salvage pathway is enhanced in mice on a high-fat diet. Mol Cell Endocrinol. 2015;412:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2659] [Cited by in RCA: 2554] [Article Influence: 159.6] [Reference Citation Analysis (0)] |
| 36. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2727] [Article Influence: 681.8] [Reference Citation Analysis (0)] |
| 37. | Scisciola L, Olivieri F, Ambrosino C, Barbieri M, Rizzo MR, Paolisso G. On the wake of metformin: Do anti-diabetic SGLT2 inhibitors exert anti-aging effects? Ageing Res Rev. 2023;92:102131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 38. | Packer M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors: Potential Role in Mediating the Heart Failure Benefits of SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors. Circ Heart Fail. 2020;13:e007197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 39. | Peng K, Yang F, Qiu C, Yang Y, Lan C. Rosmarinic acid protects against lipopolysaccharide-induced cardiac dysfunction via activating Sirt1/PGC-1α pathway to alleviate mitochondrial impairment. Clin Exp Pharmacol Physiol. 2023;50:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Martinez-Moreno JM, Fontecha-Barriuso M, Martin-Sanchez D, Guerrero-Mauvecin J, Goma-Garces E, Fernandez-Fernandez B, Carriazo S, Sanchez-Niño MD, Ramos AM, Ruiz-Ortega M, Ortiz A, Sanz AB. Epigenetic Modifiers as Potential Therapeutic Targets in Diabetic Kidney Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Kogot-Levin A, Riahi Y, Abramovich I, Mosenzon O, Agranovich B, Kadosh L, Ben-Haroush Schyr R, Kleiman D, Hinden L, Cerasi E, Ben-Zvi D, Bernal-Mizrachi E, Tam J, Gottlieb E, Leibowitz G. Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition. JCI Insight. 2023;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |