INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoreactive activity of the immune system, the production of a variety of autoantibodies, abundant deposition of immune complexes, and damage to multiple tissues and organs. The ovaries, as female gonads, are the organs from which estrogen is secreted. Follicles mature and ovulate, playing an important role in endocrine and reproductive functions. SLE commonly occurs in women between 20 and 40 years of age. If immune complexes are deposited in ovaries, they can cause ovarian damage, manifesting as low ovarian reserve[1]. Currently, drugs such as tacrolimus, mycophenolate mofetil, cyclophosphamide, and prednisolone are the main treatment options for patients with SLE. However, some of these drugs, such as cyclophosphamide, have ovarian toxicity. They may cause less menstruation, amenorrhea, infertility, low ovarian reserve, premature ovarian failure and other clinical manifestations in women with SLE. Therefore, this review briefly summarizes the abnormal changes in ovarian function indicators, the ovarian damage caused by clinical medications, and the measures to improve ovarian function in female patients with SLE in recent years, hoping to provide insights into the ovarian reserve function of patients with SLE and the clinical treatment for patients with SLE with reproductive needs.
ABNORMAL OVARIAN FUNCTION IN PATIENTS WITH SLE
Ovarian reserve refers to the quantity and quality of oocytes in the ovary and is closely related to female fertility[2]. Clinically, the most commonly used indicators to evaluate ovarian reserve include the menstrual cycle, antral follicle count (AFC), ovarian volume (OV), follicle stimulating hormone (FSH), corpus luteum luteinizing hormone (LH) and anti-Müllerian hormone (AMH).
Menstrual cycle
A woman’s menstrual cycle can reflect her ovarian function and reproductive capacity. The length of the menstrual cycle, regular or disordered cycle, the amount of menstrual flow, and amenorrhea can all reflect ovarian function and hormone secretion levels. Female patients with SLE have been suggested to be more likely to suffer from menstrual disorders and even persistent amenorrhea. In a recent study of 40 SLE patients, 45% of the patients (18 patients) were reported to have irregular menstruation, including 11 patients with oligomenorrhea, 1 patient with irregular menstruation, 4 patients with oligomenorrhea and irregular menstruation, and 1 patient with amenorrhea, and the rate of menstrual abnormalities was significantly greater in these patients than in healthy control individuals[3]. This is consistent with the studies carried out by Shabanova[4] and Girbash[5]. In addition, the systemic lupus erythematosus disease activity index (SLEDAI) score of SLE patients with irregular menstrual cycles was notably greater than that of SLE patients with regular menstrual cycles.
Ovarian morphology
The ovarian morphology can indicate its reserve function. The number of primordial follicles in the ovary represents a woman’s ovarian reserve, and the AFC is generally used to quantify ovarian function[6]. A decrease in the number of AFCs often indicates that the responsiveness and reserve function of the ovary are reduced[6]. In addition, a smaller OV will cause lower sex hormone levels and ovarian responsiveness. Therefore, the OV is also an important indicator of ovarian reserve[7]. Girbash et al[5] compared 50 SLE patients with 50 healthy control individuals of similar age and found that the AFCs and OVs of SLE patients were markedly reduced, and both were related to age.
Sex hormones
Follicles are mainly composed of granulosa cells and eggs. Granulosa cells play a vital role in egg development, differentiation and maturation. Estradiol (E2) is secreted by follicular granulosa cells and can be used as an indicator of ovarian reserve. However, this indicator is susceptible to ovarian diseases and other indicators, and thus, it cannot be simply evaluated alone. Since the ovaries are regulated by the hypothalamic-pituitary-gonadal (HPG) axis, when the ovarian reserve is reduced, negative feedback occurs to promote FSH and LH secretion. Therefore, in clinical practice, FSH, FSH/LH, and E2 are often jointly tested as biochemical indicators for evaluating ovarian function[7]. If a patient’s ovarian reserve decreases, her E2 levels decrease, and her FSH and FSH/LH values increase accordingly. This phenomenon corresponds to the results after ovarian damage in SLE patients. In a prospective study, researchers measured FSH, E2, and LH on the third day of the menstrual cycle. They found that E2 levels were reduced in SLE patients, and FSH and LH levels were significantly greater than those in healthy control individuals[8].
Anti-Müllerian hormone
AMH is also secreted by follicular granulosa cells and remains relatively stable throughout the menstrual cycle. It is more sensitive and specific than other biochemical indicators and is therefore widely used as a direct indicator of ovarian reserve. Lawrenz et al[9] measured the AMH values of 33 premenopausal SLE patients and compared them with the AMH values of 33 age-matched healthy people. The authors found that the AMH levels in the SLE group were notably lower than those in the healthy group. Furthermore, they found that there was no correlation between the AMH value and the SLEDAI score. Conversely, in another case-control study of 40 SLE patients of childbearing age, the AMH level was significantly lower in patients with SLE than in healthy control individuals and was negatively correlated with SLEDAI[3]. Thus, the correlation between AMH levels and disease activity in SLE patients still needs further large-scale testing to draw an accurate conclusion.
EFFECTS OF SLE MEDICATION ON OVARIAN FUNCTION
Glucocorticoids
Glucocorticoids (GCs) are the preferred medication for treating SLE patients and can quickly suppress immune responses and reduce the damage of inflammatory reactions to the body, thereby rapidly alleviating the condition of SLE. However, GCs (especially high-dose GCs) can decrease levels of ovarian-related sex hormones and AMH secretion, decrease AFC and OV, and cause amenorrhea, and thus, GCs can impair ovarian function in patients with SLE. All these changes may be related to the inhibition of the HPG axis function by GC[10].
Whirledge et al[11] assumed that GCs affect ovarian function through the following three mechanisms. GCs influence ovarian function indirectly through altering the levels of circulating gonadotropins by acting on the hypothalamus and pituitary. Excessive GC levels inhibit gonadotropin-releasing hormone secretion, leading to hypogonadotropic hypogonadism. The second mechanism of glucocorticoid regulation is also indirect and affects the levels of metabolic hormones and growth factors, such as insulin-like growth factor-1. Finally, GCs regulate ovarian function by binding to GC receptors on ovarian cells, affecting LH activity and steroid biosynthesis. It can be concluded that GCs can extensively act on all links of the HPO axis to cause damage to ovarian function.
Cyclophosphamide
Cyclophosphamide (CTX), a commonly used immunosuppressant, unfortunately has strong gonadal toxicity and can lead to amenorrhea and infertility[12]. Boumpas et al[13] reported that 28% of SLE patients had persistent amenorrhea after CTX treatment, and 8% of the patients had temporary amenorrhea. SLE patients receiving high-dose CTX treatment were more likely to have persistent amenorrhea than were those receiving low-dose CTX treatment (39% vs 12%, respectively). In addition, amenorrhea occurs in 12% of patients younger than 25 years and in 62% of patients older than 31 years. The above studies have shown that the ovarian damage caused by CTX is closely related to the age of SLE patients at the start of CTX treatment and the cumulative dose over treatment time, which has been corroborated by the results of others[14,15]. Therefore, CTX should be used with caution in SLE patients of childbearing age who are willing to have children.
CTX can activate the PI3K-AKT-mTOR signaling pathway and increase the phosphorylation of AKT and other related proteins. Thus, it can directly affect the oocytes and granulosa cells of primordial follicles and accelerate the activation of primordial follicles[16]. Moreover, CTX-induced ovarian toxicity may be mediated by an inflammatory response, which can be caused by increasing the levels of proinflammatory cytokines [interleukin (IL)-6, IL-8, TNFα, etc.] and decreasing the levels of anti-inflammatory factors such as IL-10[17]. In addition, CTX changes the expression of pro- and antiapoptotic genes, leading to increased apoptosis in the ovary[18]. In conclusion, CTX induces oocyte damage and apoptosis in granulosa cells and follicles, resulting in premature depletion of follicular reserves.
In addition, other drugs used to treat SLE, such as hydroxychloroquine, tripterygium glycosides, mycophenolate mofetil and tacrolimus, are rarely reported to have effects on the ovary and need to be further studied.
MEASURES TO IMPROVE OVARIAN FUNCTION IN PATIENTS WITH SLE
The recurrent attacks of SLE can significantly exacerbate the damage to affected tissues and organs. In addition, the ovarian damage resulting from the long-term application of glucocorticoids and immunosuppressants cannot be ignored. For patients with SLE of childbearing age, it is essential to protect their ovarian function and maintain fertility, thereby necessitating the urgent development of a treatment strategy to improve ovarian damage.
The clinical application of CTX enhances the recruitment of follicles, eventually leading to ovarian dysfunction. Therefore, inhibiting ovulation reduces the ovarian toxicity of CTX during treatment. Gonadotropin releasing hormone (GnRH) is secreted from the hypothalamus to the pituitary portal circulation, where it stimulates the pituitary to secrete FSH and LH and maintain the normal menstrual cycle. GnRH analog (GnRH-a) is a synthetic agonist of GnRH that can competitively bind to the gonadotropin-releasing hormone receptor on the pituitary gland and inhibit the secretion of FSH and LH, thus inhibiting ovulation[19]. In addition, clinical studies have reported that GnRH-a combined with CTX in the treatment of SLE reduces ovarian exposure to alkylation reagents, resulting in a reduction in ovarian damage[20,21].
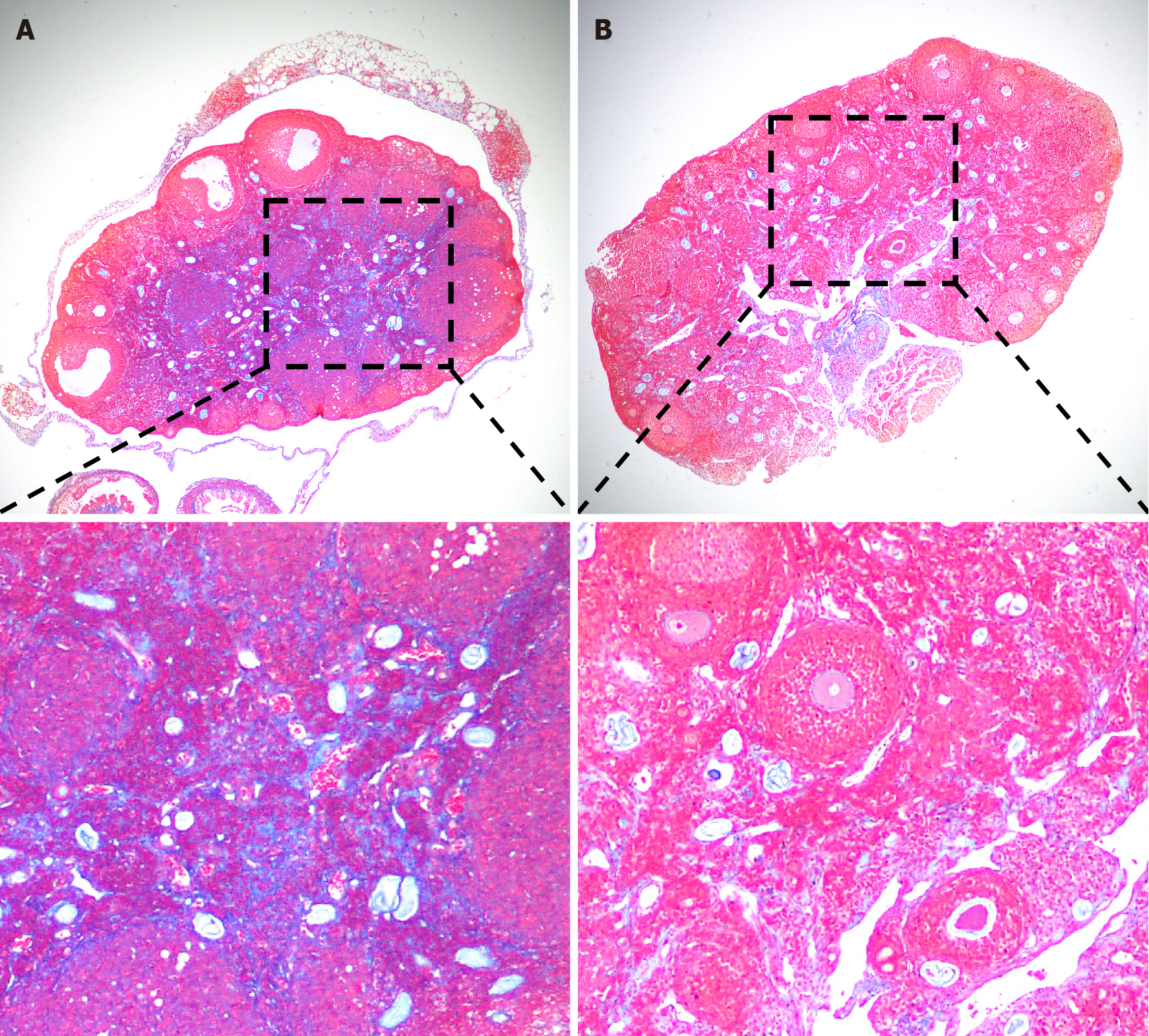
Severe ovarian damage profoundly impacts the entire reproductive system and can even result in infertility in severe cases. Currently, the cryopreservation of embryos and oocytes is considered a promising approach for preserving fertility. However, the social, ethical, and technical challenges associated with this strategy cannot be neglected. Mesenchymal stem cells (MSCs) are multipotent adult stem cells with low immunogenicity, self-renewal to maintain their stem cell properties, and multidirectional differentiation ability[22]. MSCs can migrate into the damaged ovary to increase the production of bioactive factors[23,24], such as vascular endothelial growth factor, hepatocyte growth factor, insulin-like growth factor, and other growth factors that promote immunomodulation, anti-inflammation, angiogenesis, anti-apoptosis, and anti-fibrosis. In recent years, MSC therapy has gradually been applied to treat ovarian insufficiency[25-27], and its efficacy has been confirmed by clinical trials. Using a polycystic ovary syndrome (PCOS) mouse model, Chugh et al[27] confirmed that intraovarian injection of bone marrow mesenchymal cells significantly reduced steroid gene expression, thereby inhibiting the inflammatory response and restoring ovarian function. Transplantation of human umbilical cord MSCs (UC-MSCs) into patients with ovarian insufficiency can increase E2 levels and the number of follicles, improve follicular development, and result in successful clinical pregnancy[27]. Furthermore, our recent experiments demonstrated that UC-MSC transplantation improves ovarian function by inhibiting ovarian fibrosis in SLE model mice (Figure 1). Hence, MSCs are of great significance in the treatment of immune-related ovarian insufficiency, such as pregnancy complicated with SLE. Clinical data from trials carrying out MSC therapy to treat women with SLE are expected to validate this new approach in the future.
Figure 1 Umbilical cord mesenchymal stem cell (UC-MSC) transplantation reduced ovarian fibrosis in MRL/Lpr (MRL/Mpj-Faslpr/J, #000485, The Jackon Laboratories, United States) mice.
A: Representative masson staining of ovaries from MRL/Lpr mice in the PBS group; B: Representative masson staining of ovaries from MRL/Lpr mice in the umbilical cord mesenchymal stem cell transplantation group. Magnification: 40 × (top) and 100 × (bottom).
CONCLUSION
In summary, SLE damages ovarian and reproductive function in female patients of childbearing age. Disorders of the immune system can lead to autoimmune oophoritis, which injures the ovaries and reduces the ovarian reserve. Additionally, when treated with glucocorticoids, cyclophosphamide, triptolide polyglycosides, etc., gonadal toxicity will occur, resulting in HPO axis disorders and even adverse pregnancy outcomes. To better address ovarian dysfunction in SLE patients, the key unanswered question is how proinflammatory niches cause ovarian damage and loss of function. Therefore, it is important to seek safe and effective treatments for SLE and ovarian insufficiency. MSCs have been widely used to treat SLE and have great potential for treating ovarian insufficiency-related diseases. They are expected to become an ideal and reliable treatment for SLE combined with ovarian insufficiency in the near future.