Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.89320
Peer-review started: October 27, 2023
First decision: November 30, 2023
Revised: December 11, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: March 20, 2024
Processing time: 143 Days and 21.4 Hours
Gestational diabetes is typically diagnosed in the late second or third trimester of pregnancy. It is one of the most common metabolic disorders among expectant mothers, with potential serious short- and long-term complications for both maternal and offspring health. C-peptide is secreted from pancreatic beta-cells into circulation in equimolar amounts with insulin. It is a useful biomarker to estimate the beta-cell function because it undergoes negligible hepatic clearance and consequently it has a longer half-life compared to insulin. Pregnancy induces increased insulin resistance due to physiological changes in hormonal and metabolic homeostasis. Inadequate compensation by islet beta-cells results in hyperglycemia. The standard oral glucose tolerance test at 24-28 wk of gestation sets the diagnosis. Accumulated evidence from prospective studies indicates a link between early pregnancy C-peptide levels and the risk of subsequent gestational diabetes. Elevated C-peptide levels and surrogate glycemic indices at the beginning of pregnancy could prompt appropriate strategies for secondary prevention.
Core Tip: Understanding the diagnostic role of C-peptide measurements in predicting hyperglycemia during pregnancy can facilitate timely interventions in clinical practice, potentially preventing gestational diabetes in a subset of predisposed women. Further research is necessary to confirm the utility of C-peptide testing in pregnancy and define the appropriate diagnostic thresholds.
- Citation: Milionis C, Ilias I, Lekkou A, Venaki E, Koukkou E. Future clinical prospects of C-peptide testing in the early diagnosis of gestational diabetes. World J Exp Med 2024; 14(1): 89320
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/89320.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.89320
Gestational diabetes (GD) presents a mounting global public health challenge, with its prevalence varying from 7.1% to 27.6% across different International Diabetes Federation regions[1]. Its epidemiological pattern parallels that of type 2 diabetes mellitus (T2DM) in the same ethnic groups[2]. Typically emerging in the late second or third trimester, GD heightens the risk of various adverse maternal and fetal effects. Moreover, women with a history of GD face an elevated susceptibility to developing T2DM later in life. However, this relative risk is considerably variable and hinges on factors like the amount of time after delivery, ethnicity, age, body mass index, family history of diabetes, and previous affected pregnancies[3].
Maintaining optimal blood sugar levels is crucial to managing pregnant women with GD. Identifying early those at risk of dysglycemia during pregnancy is challenging because it can enable timelier interventions and, in turn, foster more effective glycemic control. Early diagnosis and treatment of GD can lead to improved maternal metabolic profiles and the creation of favorable intrauterine conditions for fetal development. Managing dysglycemia before the 20th week of pregnancy may contribute to a decreased incidence of a composite of adverse neonatal outcomes[4].
GD can be regarded as a pre-gravid subclinical glycemic dysregulation unmasked in late gestation[5]. Presently, blood glucose, insulin, and occasionally triglycerides are widely used in clinical practice to determine insulin resistance through the calculation of various indices[6]. However, these methods suffer from significant variability in defining appropriate threshold levels, making it challenging to estimate optimal cut-off points[7]. Given the specific physiological conditions of pregnancy, diagnosing glucose intolerance in pregnant women typically relies on blood glucose measurements in the fasting state and after glucose load.
Accurate identification of GD is of paramount significance. The standard approach involves an oral glucose tolerance test (OGTT) in the late second or early third trimester. Still, various physiological factors, including previous physical activity, preparatory diet, fasting duration, rate of intestinal glucose absorption, hydration status, and stress, can influence the glycemic response to oral glucose load. Moreover, technical inadequacies such as errors in sample handling and a lack of analytical standardization can compromise the reliability of this test[8]. It is also worth noting that postprandial blood sugar measurements in pregnant women only detect a manifested disturbance of glucose metabolism. Consequently, exploring the use of other biochemical markers that are more accurate and timelier in revealing dysglycemia during pregnancy is essential.
In contrast, C-peptide measurement has rarely been employed for diagnostic purposes in assessing insulin resistance. Using C-peptide as a biochemical marker is promising. Yet, its current role is limited to differentiating between type 1 diabetes mellitus (T1DM) and T2DM in cases of ambiguous hyperglycemia, as well as in the differential diagnosis of hypoglycemia. However, C-peptide testing could be instrumental in several other facets of diabetes care, including estimating the progression of insulin resistance (and deficiency), predicting the onset of T2DM, and evaluating the risk of diabetic complications. This review particularly focuses on the potential utilization of C-peptide testing in early pregnancy to assess the risk of GD. It also analyzes the rationale for the practical integration of C-peptide testing in the clinical setting for this purpose.
With the aim of providing an overview of the use of C-peptide determination in the early diagnosis of GD, a search in the database of PubMed was performed between October and November 2023. It used the terms ‘C-peptide’, ‘gestational diabetes’, ‘early diagnosis’, and ‘biomarkers’ in all possible combinations. Table 1 shows the relevant literature search. The selected articles included studies that explored novel clinical applications of C-peptide testing with a special focus on pregnancy. For a better understanding of the relevant physiology, review papers that explained the applicability of C-peptide measurements, analyzed aspects of insulin synthesis and secretion, or described the nosological entity of GD were also used after a targeted literature search.
| Query | Results |
| Search: ((gestational diabetes) AND (early diagnosis)) AND (biomarkers) | 344 |
| Search: ((C-peptide) AND (early diagnosis)) AND (biomarkers) | 96 |
| Search: ((C-peptide) AND (gestational diabetes)) AND (biomarkers) | 64 |
| Search: ((C-peptide) AND (gestational diabetes)) AND (early diagnosis) | 32 |
| Search: (((C-peptide) AND (gestational diabetes)) AND (early diagnosis)) AND (biomarkers) | 9 |
A narrative review can be defined as a thorough report of a body of literature that also includes interpretation and critique[9]. This method lacks cumbersome restrictions, which makes it suitable for the in-depth analysis of a particular topic, especially when existing data are incomplete[10]. The utility of C-peptide measurements in pregnancy has been researched sparingly. Hence, this article used a narrative approach to summarize the existing evidence on the underlying physiology of glucose homeostasis, elaborate on the importance of early diagnosis of dysglycemia, and speculate on possible preventive strategies. Although a narrative review may suffer from data selection bias, a purposeful search of the literature can mitigate this disadvantage and allow for a comprehensive investigation of the desired topic.
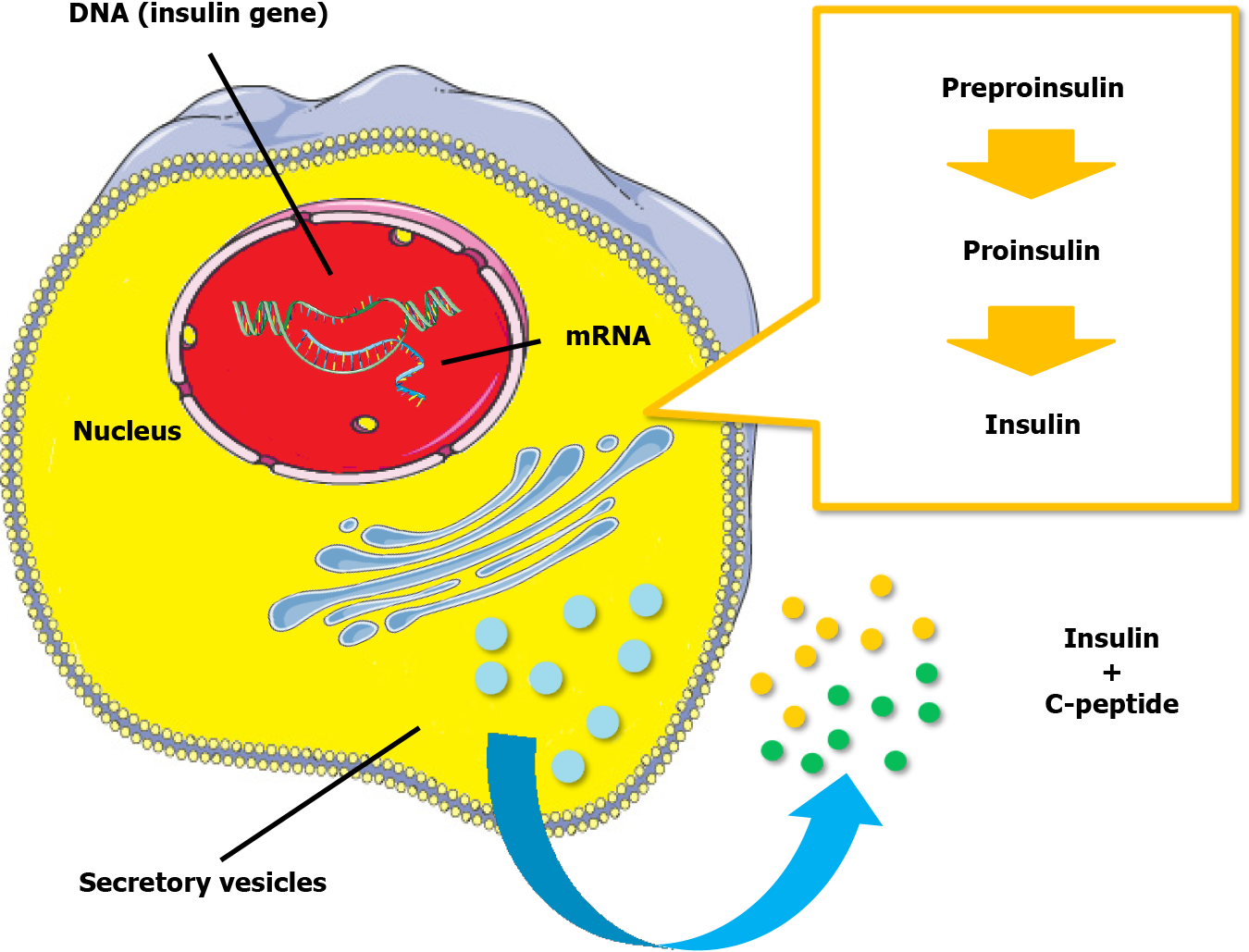
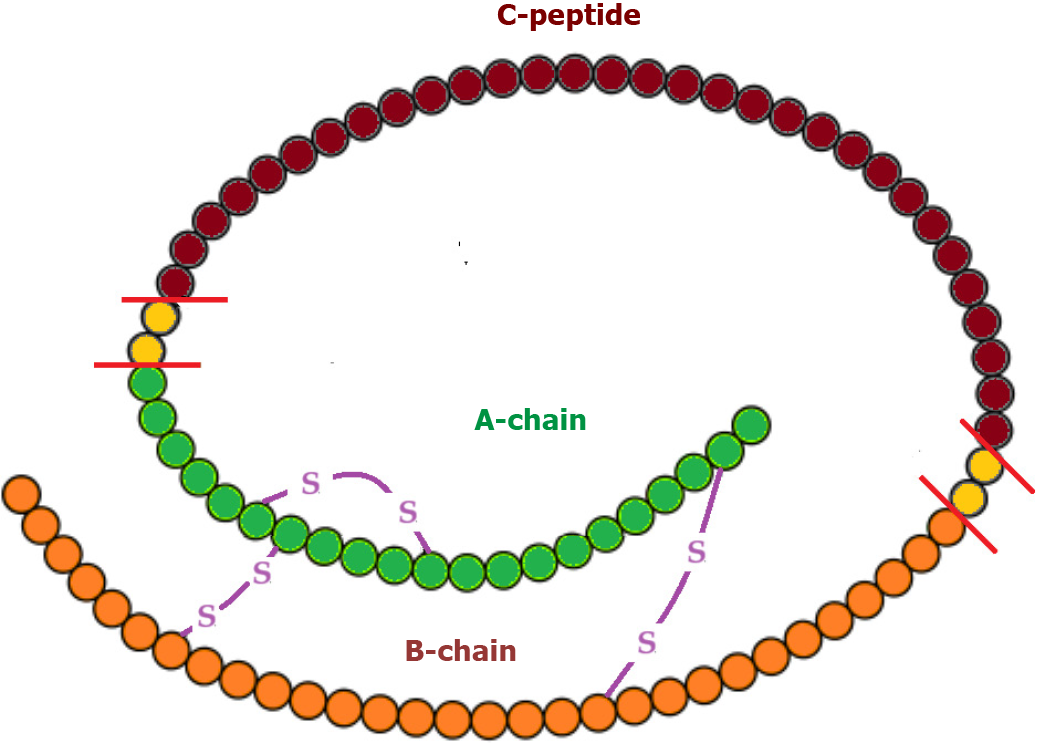
Insulin, a peptide hormone, is produced through the classical process of protein synthesis. The human insulin gene is located on the short arm of chromosome 11. It is transcribed into preproinsulin mRNA in the nucleus of pancreatic beta-cells in the islets of Langerhans. This mRNA is transferred to the cytoplasm, where it is translated into a 110-amino-acid precursor peptide (preproinsulin) in the rough endoplasmic reticulum. Preproinsulin is cleaved to proinsulin with the removal of the signal peptide almost immediately after synthesis. Proinsulin is a single 86-amino-acid chain containing the A and B chains of insulin (with 21 and 30 amino acids, respectively) and a 35-residue connecting segment (the C-peptide and four intervening amino acids). As proinsulin folds, the A and B chains form three disulfide bonds (two bridges between the A and B chains and an intra-chain bridge within the A chain). Proinsulin is then packaged into clathrin-coated secretory vesicles in the Golgi apparatus. Loss of the clathrin coating and conversion of proinsulin into insulin indicate maturation for exocytosis[11,12]. Figure 1 represents the process of insulin synthesis and secretion by the pancreatic beta-cell.
C-peptide, consisting of 31 amino acids, initially links the A and B chains of insulin within proinsulin, aiding correct protein folding and disulfide bond formation. In the Golgi apparatus, proinsulin is cleaved at two sites along the peptide chain through prohormone convertases 1 and 2, with carboxypeptidase E removing two pairs of amino acids from the protein’s ends. This process yields equimolar quantities of C-peptide and active insulin stored together in secretory vesicles in the cytoplasm of pancreatic beta-cells. Glucose and other secretagogues (leucine, arginine, acetylcholine, incretins, and vagal nerve stimulation) stimulate the co-release of both insulin and C-peptide into the bloodstream. Mature secretory vesicles might contain small amounts of proinsulin or partially split products that evade cleavage[11,12]. Figure 2 depicts the molecular structure of proinsulin.
Following secretion, insulin and C-peptide are routed through the portal circulation into the liver, where half of the pancreatic insulin undergoes first-pass hepatic clearance. Insulin is also metabolized in the kidney and the placenta. In contrast, C-peptide is only limitedly processed in the liver. It is primarily metabolized in the kidney, with a small fraction excreted unchanged in the urine (5%-10%). Consequently, the half-life of C-peptide is around 30 min, roughly ten times longer than that of endogenous insulin (approximately 3 min). Moreover, the molar ratio of insulin to C-peptide in peripheral circulation is less than 1. The stability of C-peptide in the bloodstream supports its clinical utility as a reliable marker of islet beta-cell function[13].
Besides its contribution to insulin structure, the exact biological actions of C-peptide in normal physiology remain only partially understood[14]. Available data indicate that C-peptide may bind to the cell membrane, possibly via a surface receptor coupled with a G-protein. Then, the latter triggers a signaling cascade that results in several effects, including elevation in the concentration of intracellular calcium, activation of the sodium-potassium adenosine triphosphatase (Na+-K+-ATPase), increased transcription of endothelial nitric oxide synthetase, stimulation of phosphoinositide 3-kinase, and activation of mitogen-activated protein kinases pathways, in multiple cell types from various tissues[15,16]. Non-receptor-mediated mechanisms of the C-peptide’s biological action have also been implied.
C-peptide likely exerts anti-inflammatory, anti-apoptotic, vasodilatory, and antioxidant effects in the vascular endothelium, either directly or through interactions with erythrocytes and immune cells. It may also participate in fine-tuning insulin signaling[17]. However, further research is necessary to confirm these presumptive functions, as well as their physiological role and clinical significance.
In pregnant women, hormonal and metabolic changes significantly influence carbohydrate metabolism, adapting to the nutritional demands of the growing fetus. The flow of glucose from maternal to fetal circulation relies on the gradient across the blood-placental barrier. Placental and other hormones (progesterone, estrogen, placental lactogen, leptin, cortisol, and growth hormone) induce insulin resistance, maintaining adequate maternal plasma glucose levels and ensuring unmarred glucose flow to the fetus. Heightened lipolysis in the adipose tissue of pregnant women favors fatty acid utilization by maternal tissues, increasing glucose availability for the fetus[18,19]. Changes in cytokine and adipokine production, circulating exosomes (both total and placenta-derived), and alterations in the gut microbiota might also contribute to increased insulin resistance during pregnancy[20]. Compensatory mechanisms involve expansion, proliferation, and possibly neogenesis of pancreatic beta-cells, leading to a two- to three-fold rise in insulin production[21,22]. Maternal insulin sensitivity returns to pre-pregnancy levels shortly after parturition.
GD manifests as pregnancy-related hyperglycemia resulting from insufficient pancreatic insulin secretion against heightened blood glucose levels due to the hormonal and metabolic homeostasis of pregnancy. It often arises from an underlying predisposition to beta-cell dysfunction in the presence of latent insulin resistance. Hormonal and metabolic changes during pregnancy exacerbate this state. Defects may occur at any stage of beta-cell function, including glucose sensing, proinsulin synthesis, post-translational modifications, and vesicle storage and exocytosis. Insulin resistance arises from disruptions in various phases of insulin signaling. As insulin resistance peaks in the third trimester, hyperglycemia typically becomes apparent around this time[18,19].
Early screening before the 15th week of gestation is crucial, especially for women with risk factors, to identify undiagnosed preexisting diabetes. Risk factors include advanced age, excessive weight, specific ethnicities (Indian, Southeast Asian, Arab, Mediterranean, Afro-Caribbean), family history of T2DM, previous macrosomic neonate delivery, and polycystic ovarian syndrome[23]. The gold-standard diagnostic approach is the OGTT at 24-28 wk, with different hyperglycemia thresholds indicating varying degrees of complication risk[24]. Adverse outcomes associated with GD include preeclampsia, preterm delivery, need for cesarean section, shoulder dystocia, neonatal hypoglycemia, neonatal jaundice, and increased birth weight[25].
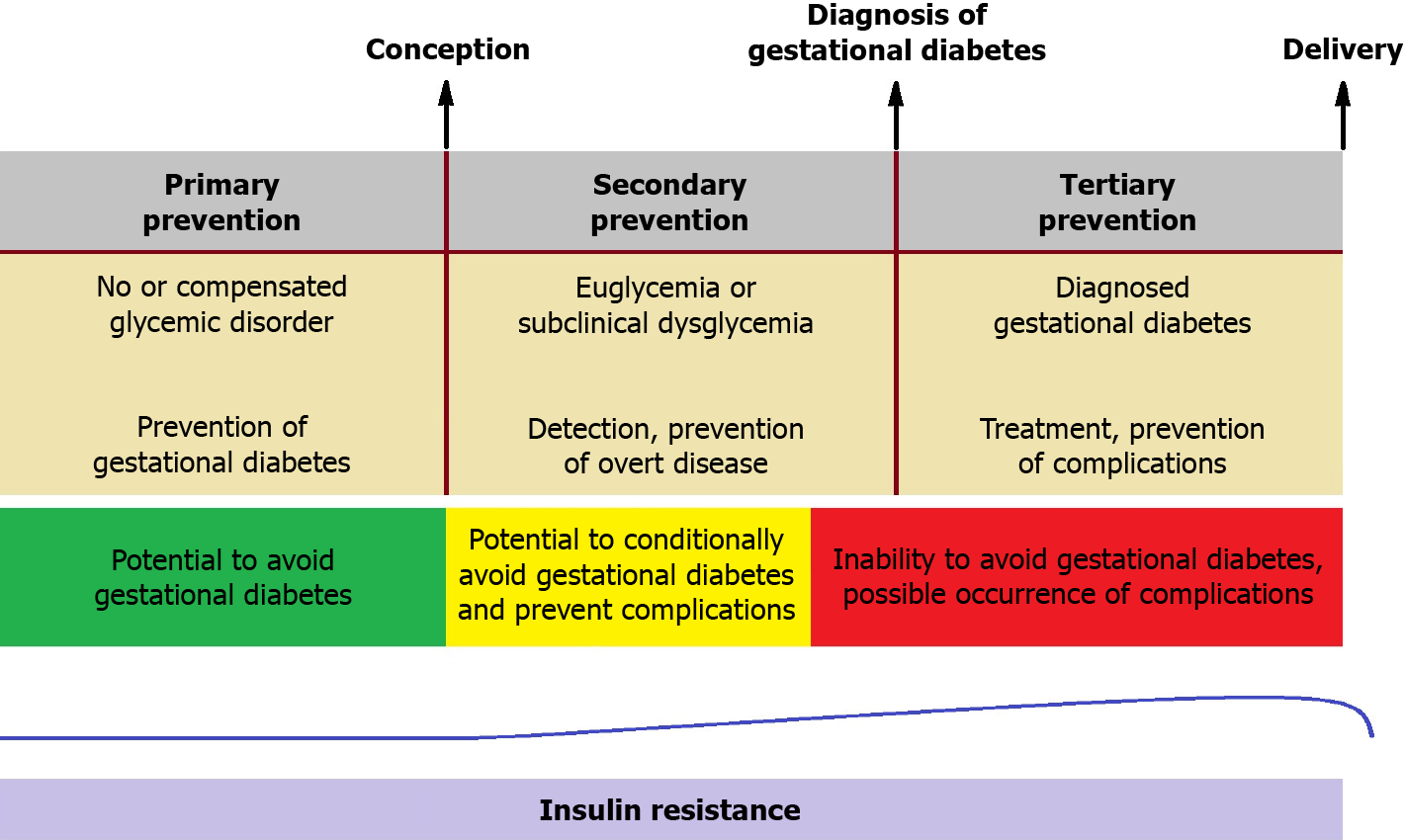
Pre-pregnancy lifestyle modifications (exercise and diet) may serve as primary prevention against GD[26]. Similar interventions in early gestation might prevent subsequent GD in high-risk individuals[27,28]. Nonetheless, no widely accepted strategy currently exists for secondary prevention (after conception) in the general pregnant population. An ideal diagnostic tool capable of detecting the condition prior to the onset of overt hyperglycemia, possibly in the first trimester, could enable timely interventions before adverse metabolic effects act on the fetus. Proper management of GD, including dietary plans, behavioral adjustments, and, if necessary, insulin therapy, can improve perinatal outcomes (tertiary prevention)[29]. Figure 3 outlines the timeline for potential diagnostic and therapeutic interventions in GD.
C-peptide testing serves as an important laboratory tool for evaluating pancreatic beta-cell function and finding application as a biochemical marker for insulin secretion assessment in clinical settings. It is preferred over insulin measurements in some instances owing to its slower degradation rate, which provides a broader time window to evaluate fluctuating beta-cell function. C-peptide is less affected by hemolysis, and its determination has a well-established standardization process[30]. Additionally, its measurement avoids the risk of cross-reactivity of immunoassays between endogenous and synthetic insulin[31].
Estimating C-peptide involves basal, random, and stimulated blood measurements. Fasting blood samples are obtained after an 8-10-h overnight fast, whereas random blood specimens can be drawn at any time during the day regardless of the last mealtime. Both of these approaches are simple and flexible and can be performed in an outpatient or inpatient setting. However, fasting C-peptide values might not capture subtle postprandial fluctuations. In addition, random C-peptide levels often require confirmation with stimulation methods. The latter include measurements at specific time intervals after glucose intake, glucagon administration, or a mixed meal[32]. Stimulation tests should be conducted primarily in diagnostically challenging cases, considering the potential inconveniences for both patients and personnel. C-peptide is prone to enzymatic proteolytic cleavage in serum gel or plain sample tubes but remains stable in whole blood specimens in ethylenediaminetetraacetic acid-prepared tubes for up to 24 h at room temperature[33].
Normal plasma C-peptide concentration in the fasting state ranges around 0.9-1.8 ng/mL, rising to 3-9 ng/mL postprandially in non-diabetic, non-obese individuals[34]. Elevated C-peptide blood levels may indicate insulin resistance, insulinoma, or kidney disease, whereas low concentrations are typical in T1DM or sometimes in T2DM.
Urinary C-peptide testing is a non-invasive procedure. It involves collecting samples in boric acid, which maintains C-peptide stability for up to 3 d at room temperature. In individuals with normal urinary function, the urinary C-peptide quantity represents 5%-10% of the total secretion by the pancreas. While 24-h urine collection provides a relatively accurate estimation of pancreatic secretion over an extended period, it can be time-consuming and inconvenient for the patient. The determination of spot urinary C-peptide concentration is less reliable because of the variability in the beta-cell secretory activity over the day. Nevertheless, the C-peptide to creatinine ratio in a spot sample correlates well with 24-h collection values, making it an attractive option for estimating islet beta-cell function. Gender differences should be considered due to variations in urinary creatinine levels between men and women[32].
C-peptide measurement is a key element in the differential diagnosis of hypoglycemia. When hypoglycemic episodes are confirmed, evaluating C-peptide blood concentration is essential. Low C-peptide levels alongside high insulin levels suggest factitious hypoglycemia due to excessive exogenous insulin administration. Elevated C-peptide levels in the presence of high insulin concentration should prompt consideration of stimulating insulin autoantibodies, insulinoma, or intoxication with insulin secretagogues (sulfonylureas or meglitinides)[35]. Furthermore, C-peptide is valuable for assessing insulin secretory reserve in patients with diabetes. In T1DM, C-peptide values decline rapidly during the initial 3-5 years since onset, with concentrations lower than 0.4 ng/mL indicative of pancreatic beta-cell failure and insulin deficiency[36].
There are some pitfalls associated with C-peptide testing. Its measurement in patients with chronic kidney failure is problematic due to the compromised renal metabolism. Cross-reactions with proinsulin and its partially processed forms, including C-peptide fragments, may lead to an overestimation of C-peptide concentration. High levels of antibodies binding to proinsulin or C-peptide can also yield falsely elevated readings with indirect immunoassays. While modern assays demonstrate high accuracy in quantifying blood C-peptide[37], awareness of the applied laboratory method remains crucial.
The escalating incidence of GD presents a growing global health challenge due to its potentially severe implications for both the mother and the offspring. Consequently, strategies for prevention, early identification, and optimal treatment are of utmost importance. As previously highlighted, the emergence of GD often results from an underlying deficit of islet beta-cells within a setting of pregnancy-induced insulin resistance. Thus, the early assessment of pancreatic beta-cell function, if feasible, holds significant promise for predicting the onset of this metabolic dysregulation. While the idea of employing C-peptide testing to evaluate insulin secretory capacity in pregnant women is appealing, it has not yet been extensively trialed in routine clinical practice. The theoretical advantage of C-peptide assessment, compared to the OGTT, lies in the potential for an earlier diagnosis (secondary prevention) of a pregnant woman’s predisposition to developing GD. This early assessment could allow for tailored interventions before the onset of glycemic dysregulation.
C-peptide can be readily measured in the serum of pregnant women, both in the fasting state and after stimulation. Given that glucose serves as the primary stimulus for endogenous insulin secretion, adjusting C-peptide levels based on blood sugar values using appropriate indices appears reasonable. Evaluating pancreatic beta-cell function through overnight fasting serum samples is readily comparable both within an individual over time and among different individuals, as basal insulin secretion remains relatively stable during fasting periods. However, post-stimulation examination reveals the maximum insulin secretory capacity of islet beta-cells, especially in the presence of insulin resistance. Hence, calculating the C-peptide (in ng/mL) to glucose (in mg/dL) ratio (multiplied by 100) in both fasting and post-stimulation serum samples could serve as a well-fitted marker for determining declining insulin secretion[38]. This approach holds promise for identifying pregnant women at high risk of developing GD early in their pregnancy.
Examining C-peptide levels in the urine represents a potentially attractive method for estimating islet beta-cell preserved function. While a substantial correlation exists between 24-h urinary excretion quantity and serum concentration, inter- and intra-personal differences in renal C-peptide clearance must be considered. Of course, collecting a 24-h urine sample can pose practical challenges. Correcting urinary C-peptide concentration for the respective creatinine levels facilitates the use of spot specimens in daily practice, albeit with presumably lower diagnostic accuracy[39]. Assessing C-peptide values in the urine during the initial weeks of gestation could serve as an alternative or complementary tool for evaluating the risk of subsequent hyperglycemia, with the necessary consideration of renal hemodynamic changes in pregnancy.
Recent efforts have concentrated on the early detection and management of glycemic dysregulation during pregnancy[40]. A large number of first-trimester biochemical markers have been tested for this purpose. These include glycemic, metabolic, inflammatory, hormonal, adipocyte-derived, and other molecules. Some of them may be useful for identifying women at high risk of developing GD. To apply the relevant findings in practice, further clinical trials as well as cost-effectiveness analyses of the use of these novel biomarkers are needed[41].
The potential integration of C-peptide testing for the early diagnosis of GD could prove to be a vital screening strategy. While current guidelines do not endorse its use for diagnosis during pregnancy, the future application of C-peptide measurement may hold promise for detecting abnormal glycemic homeostasis before the clinical manifestation of GD. This review offers valuable insights into the correlation between C-peptide levels and the progression of glucose intolerance in pregnancy. In this perspective, C-peptide testing could serve as a springboard for secondary prevention interventions during the period after conception but before the onset of frank hyperglycemia.
Pregnancy can be regarded as a stressful state that challenges a woman’s glycemic regulatory reserves. Physiological insulin resistance during pregnancy leads to adaptive changes in pancreatic beta-cell function, including increased insulin synthesis, heightened glucose-stimulated insulin secretion, elevated levels of glucokinase and glucose transporters (Glut-2 and Glut-5), increased glucose metabolism, and enhanced expression of soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins[42]. Failure of these cellular processes to adapt to the insulin demands of pregnancy results in disrupted glucose homeostasis. The OGTT conducted between 24 and 28 wk of pregnancy primarily serves tertiary prevention since the diagnosis of GD with this method is only established after the occurrence of hyperglycemia. Thus, the search for predictive biomarkers for secondary prevention that could potentially prevent hyperglycemia and subsequent complications at an earlier stage becomes imperative.
In the absence of pre-pregnancy hyperglycemia, current guidelines recommend diagnosing GD in the late second or early third trimester. Detecting and treating the disorder at this stage can have significant positive effects, albeit in a relatively small proportion of cases[25]. Therefore, it is significant to identify biomarkers that can assist in the prediction of GD at an earlier phase, primarily in the first trimester, triggering appropriate monitoring and treatment. Ideally, candidate laboratory tests for this purpose should be relatively affordable, readily available, automated, and minimally invasive[43].
GD is nowadays considered to be part of a progressive metabolic dysfunction that predates conception. A chronic beta-cell defect leads to elevated glycemia during pregnancy. Inadequate compensatory pancreatic secretion in the face of insulin resistance during the latter half of gestation results in hyperglycemia, which characterizes GD[42]. Assessing C-peptide concentration in the serum (or in the urine) and calculating relevant mathematical indices offers an optimal means of evaluating the amount of insulin secretion and sensitivity. Existing evidence suggests that elevated fasting C-peptide levels in early pregnancy are associated with a higher risk of subsequent GD, independently of factors such as maternal age, family history of diabetes, pre-pregnancy body weight, and parity[44-48]. Thus, the evaluation of C-peptide values, possibly in combination with other biochemical parameters, can potentially identify early glycemic disturbances from the initial weeks of pregnancy. Early diagnosis expands the time frame for targeted interventions that may prevent the onset of GD. However, further basic and clinical tests are required to validate these assumptions and determine the precise diagnostic cut-offs.
The pursuit of developing screening tests capable of detecting a disorder before the development of symptoms or at a stage when treatment is potentially more effective remains a longstanding goal in research and clinical practice. Managing GD is no exception to this objective. Following the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, which demonstrated a relationship between glucose levels after oral glucose load and adverse pregnancy outcomes[49], the OGTT at the late stages of pregnancy has become a cornerstone of diagnosis. However, advanced age, increased body weight, and family or personal history of GD may conditionate this approach. In addition, an earlier and more specific detection could improve glycemic progression and maternal and neonatal outcomes[50]. Novel indices that incorporate fasting and stimulated C-peptide values could be employed to assess glucose homeostasis and stratify the risk of hyperglycemia earlier in pregnancy[51]. Identifying appropriate biomarkers is an important milestone for designing effective prevention strategies and enhancing the health and well-being of both mothers and offspring. Prospective studies should be conducted to determine whether early interventions, such as lifestyle modifications, in the first trimester can impact the development of GD, the necessity for medical treatment, and perinatal outcomes.
GD is a common complication of pregnancy, with a rising incidence. Its consequences are far-reaching, associated with short-term adverse maternal and neonatal outcomes and long-term metabolic implications for both the mother and offspring. Biomarkers to identify women susceptible to the development of GD are useful to target prevention and prompt treatment. Unfortunately, the already well-known clinical risk factors lack specificity, while the OGTT offers a rather late diagnosis. The addition of novel biomarkers to predictive strategies for gestational diabetes may improve clinicians’ ability to identify pregnant women at risk of hyperglycemia prior to its development. This review concluded that the use of C-peptide as a biomarker is based on the comprehension of the pathophysiologic mechanisms of insulin resistance during gestation, and hence, it may provide further aid in the prediction of the relevant risk. However, the present article is a narrative review. As such, it has inherent flaws because of non-completely standardized methods of literature search, potential bias in the appraisal of retrieved data, and subjectivity in the interpretation of findings. Further research is certainly needed to validate C-peptide testing as a diagnostic tool in pregnancy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Gong F, China; Wu J, China S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 532] [Article Influence: 177.3] [Reference Citation Analysis (0)] |
| 2. | Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2004;21:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 554] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | Dennison RA, Chen ES, Green ME, Legard C, Kotecha D, Farmer G, Sharp SJ, Ward RJ, Usher-Smith JA, Griffin SJ. The absolute and relative risk of type 2 diabetes after gestational diabetes: A systematic review and meta-analysis of 129 studies. Diabetes Res Clin Pract. 2021;171:108625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Simmons D, Immanuel J, Hague WM, Teede H, Nolan CJ, Peek MJ, Flack JR, McLean M, Wong V, Hibbert E, Kautzky-Willer A, Harreiter J, Backman H, Gianatti E, Sweeting A, Mohan V, Enticott J, Cheung NW; TOBOGM Research Group. Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy. N Engl J Med. 2023;388:2132-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 150] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 5. | Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014;31:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 6. | Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19:160-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 7. | Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, Soewondo P. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16:102581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 255] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 8. | Bogdanet D, O'Shea P, Lyons C, Shafat A, Dunne F. The Oral Glucose Tolerance Test-Is It Time for a Change?-A Literature Review with an Emphasis on Pregnancy. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Greenhalgh T, Thorne S, Malterud K. Time to challenge the spurious hierarchy of systematic over narrative reviews? Eur J Clin Invest. 2018;48:e12931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 10. | Furley P, Goldschmied N. Systematic vs. Narrative Reviews in Sport and Exercise Psychology: Is Either Approach Superior to the Other? Front Psychol. 2021;12:685082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Maddaloni E, Bolli GB, Frier BM, Little RR, Leslie RD, Pozzilli P, Buzzetti R. C-peptide determination in the diagnosis of type of diabetes and its management: A clinical perspective. Diabetes Obes Metab. 2022;24:1912-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Novac C, Radulian G, Orzan A, Balgradean M. Short Update on C-Peptide and its Clinical Value. Maedica (Bucur). 2019;14:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Vejrazkova D, Vankova M, Lukasova P, Vcelak J, Bendlova B. Insights into the physiology of C-peptide. Physiol Res. 2020;69:S237-S243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 14. | Landreh M, Jörnvall H. Biological activity vs physiological function of proinsulin C-peptide. Cell Mol Life Sci. 2021;78:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wahren J, Larsson C. C-peptide: new findings and therapeutic possibilities. Diabetes Res Clin Pract. 2015;107:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Hills CE, Brunskill NJ. Intracellular signalling by C-peptide. Exp Diabetes Res. 2008;2008:635158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Yosten GL, Kolar GR. The Physiology of Proinsulin C-Peptide: Unanswered Questions and a Proposed Model. Physiology (Bethesda). 2015;30:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Sharma AK, Singh S, Singh H, Mahajan D, Kolli P, Mandadapu G, Kumar B, Kumar D, Kumar S, Jena MK. Deep Insight of the Pathophysiology of Gestational Diabetes Mellitus. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 1006] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 20. | Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J Diabetes Res. 2019;2019:5320156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 21. | Egan AM, Dow ML, Vella A. A Review of the Pathophysiology and Management of Diabetes in Pregnancy. Mayo Clin Proc. 2020;95:2734-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Moyce BL, Dolinsky VW. Maternal β-Cell Adaptations in Pregnancy and Placental Signalling: Implications for Gestational Diabetes. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Rodrigo N, Glastras SJ. The Emerging Role of Biomarkers in the Diagnosis of Gestational Diabetes Mellitus. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1278] [Article Influence: 639.0] [Reference Citation Analysis (70)] |
| 25. | Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 488] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 26. | Zhang C, Tobias DK, Chavarro JE, Bao W, Wang D, Ley SH, Hu FB. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: prospective cohort study. BMJ. 2014;349:g5450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 27. | Sadiya A, Jakapure V, Shaar G, Adnan R, Tesfa Y. Lifestyle intervention in early pregnancy can prevent gestational diabetes in high-risk pregnant women in the UAE: a randomized controlled trial. BMC Pregnancy Childbirth. 2022;22:668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 28. | Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, Kaaja RJ, Pöyhönen-Alho M, Tiitinen A, Huvinen E, Andersson S, Laivuori H, Valkama A, Meinilä J, Kautiainen H, Eriksson JG, Stach-Lempinen B. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL): A Randomized Controlled Trial. Diabetes Care. 2016;39:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 29. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Jeffrie Seley J, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S254-S266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 180] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 30. | Hörber S, Achenbach P, Schleicher E, Peter A. Harmonization of immunoassays for biomarkers in diabetes mellitus. Biotechnol Adv. 2020;39:107359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Ghazal K, Brabant S, Prie D, Piketty ML. Hormone Immunoassay Interference: A 2021 Update. Ann Lab Med. 2022;42:3-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 32. | Leighton E, Sainsbury CA, Jones GC. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017;8:475-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 33. | McDonald TJ, Perry MH, Peake RW, Pullan NJ, O'Connor J, Shields BM, Knight BA, Hattersley AT. EDTA improves stability of whole blood C-peptide and insulin to over 24 h at room temperature. PLoS One. 2012;7:e42084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Yosten GL, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am J Physiol Endocrinol Metab. 2014;307:E955-E968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Wong SL, Priestman A, Holmes DT. Recurrent hypoglycemia from insulin autoimmune syndrome. J Gen Intern Med. 2014;29:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Becht FS, Walther K, Martin E, Nauck MA. Fasting C-peptide and Related Parameters Characterizing Insulin Secretory Capacity for Correctly Classifying Diabetes Type and for Predicting Insulin Requirement in Patients with Type 2 Diabetes. Exp Clin Endocrinol Diabetes. 2016;124:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Oh J, Kim JH, Park HD. Clinical Utility and Cross-Reactivity of Insulin and C-Peptide Assays by the Lumipulse G1200 System. Ann Lab Med. 2018;38:530-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Saisho Y. Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 415] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 40. | Schaefer-Graf U, Napoli A, Nolan CJ; Diabetic Pregnancy Study Group. Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia. 2018;61:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Powe CE. Early Pregnancy Biochemical Predictors of Gestational Diabetes Mellitus. Curr Diab Rep. 2017;17:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Ernst S, Demirci C, Valle S, Velazquez-Garcia S, Garcia-Ocaña A. Mechanisms in the adaptation of maternal β-cells during pregnancy. Diabetes Manag (Lond). 2011;1:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Omazić J, Viljetić B, Ivić V, Kadivnik M, Zibar L, Müller A, Wagner J. Early markers of gestational diabetes mellitus: what we know and which way forward? Biochem Med (Zagreb). 2021;31:030502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Fu J, Retnakaran R. The life course perspective of gestational diabetes: An opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine. 2022;45:101294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Yang X, Ye Y, Wang Y, Wu P, Lu Q, Liu Y, Yuan J, Song X, Yan S, Qi X, Wang YX, Wen Y, Liu G, Lv C, Yang CX, Pan A, Zhang J, Pan XF. Association between early-pregnancy serum C-peptide and risk of gestational diabetes mellitus: a nested case-control study among Chinese women. Nutr Metab (Lond). 2022;19:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Chen X, Stein TP, Steer RA, Scholl TO. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res Care. 2019;7:e000632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Falcone V, Kotzaeridi G, Breil MH, Rosicky I, Stopp T, Yerlikaya-Schatten G, Feichtinger M, Eppel W, Husslein P, Tura A, Göbl CS. Early Assessment of the Risk for Gestational Diabetes Mellitus: Can Fasting Parameters of Glucose Metabolism Contribute to Risk Prediction? Diabetes Metab J. 2019;43:785-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Qiu C, Vadachkoria S, Meryman L, Frederick IO, Williams MA. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2005;193:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3783] [Cited by in RCA: 3687] [Article Influence: 216.9] [Reference Citation Analysis (0)] |
| 50. | Lorenzo-Almorós A, Hang T, Peiró C, Soriano-Guillén L, Egido J, Tuñón J, Lorenzo Ó. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc Diabetol. 2019;18:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 51. | Stopp T, Feichtinger M, Rosicky I, Yerlikaya-Schatten G, Ott J, Egarter HC, Schatten C, Eppel W, Husslein P, Mittlböck M, Tura A, Göbl CS. Novel Indices of Glucose Homeostasis Derived from Principal Component Analysis: Application for Metabolic Assessment in Pregnancy. J Diabetes Res. 2020;2020:4950584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |