Published online Jul 20, 2022. doi: 10.5493/wjem.v12.i4.68
Peer-review started: December 18, 2021
First decision: April 13, 2022
Revised: April 26, 2022
Accepted: June 23, 2022
Article in press: June 23, 2022
Published online: July 20, 2022
Processing time: 212 Days and 14.7 Hours
Collagen membrane and platelet-rich fibrin (PRF) have emerged as vital biomaterials in the field of periodontal regeneration. Minimally invasive techniques are being preferred by most periodontists, as it is patient compliant with fewer post-surgical complications as compared to conventional surgical techniques. Thus, in this study we have evaluated the effect of injectable PRF (i-PRF) with collagen membrane compared with collagen membrane alone using vestibular incision subperiosteal tunnel access (VISTA) technique for gingival recession coverage.
To compare the efficacy of VISTA using collagen membrane with collagen membrane soaked in injectable PRF for gingival recession coverage.
A split mouth randomized controlled clinical trial was designed;13 subjects having at least 2 teeth indicated for recession coverage were enrolled in this study. The sites were randomly assigned to control group (VISTA using collagen membrane alone) and the test group (VISTA using collagen membrane with i-PRF). The clinical parameters assessed were pocket depth, recession depth (RD), recession width (RW), relative attachment level, keratinised tissue width (KTW), keratinised tissue thickness (KTT), and percentage root coverage.
RD showed a statistically significant difference between the test group at 3 mo (0.5 ± 0.513) and 6 mo (0.9 ± 0.641) and the control group at 3 mo (0.95 ± 0.51) and 6 mo (1.5 ± 0.571), with P values of 0.008 and 0.04, respectively. RW also showed a statistically significant difference between the test group at 3 mo (1 ± 1.026) and 6 mo (1.65 ± 1.04) and the control group at 3 mo (1.85 ± 0.875) and 6 mo (2.25 ± 0.759), with P values of 0.008 and 0.001, respectively. Results for KTW showed statistically significant results between the test group at 1 mo (2.85 ± 0.489), 3 mo (3.5 ± 0.513), and 6 mo (3.4 ± 0.598) and the control group at 1 mo (2.45 ± 0.605), 3 mo (2.9 ± 0.447), and 6 mo (2.75 ± 0.444), with P values of 0.04, 0.004, and 0.003, respectively. Results for KTT also showed statistically significant results between test group at 1 mo (2.69 ± 0.233), 3 mo (2.53 ± 0.212), and 6 mo (2.46 ± 0.252) and the control group at 1 mo (2.12 ± 0.193), 3 mo (2.02 ± 0.18), and 6 mo (1.91 ± 0.166), with P values of 0.001, 0.001, and 0.001, respectively. The test group showed 91.6%, 81.6%, and 67% root coverage at 1 mo, 3 mo, and 6 mo, while the control group showed 82.3%, 66.4%, and 53.95% of root coverage at 1 mo, 3 mo, and 6 mo, respectively.
The use of minimally invasive VISTA technique along with collagen membrane and injectable form of platelet-rich fibrin can be successfully used as a treatment method for multiple or isolated gingival recessions of Miller’s class-I and class-II defects.
Core Tip: The use of minimally invasive vestibular incision subperiosteal tunnel access technique, along with collagen membrane acting as scaffold and chemoattractant with added benefit of injectable form of platelet-rich fibrin has the capacity of releasing more growth factors and regenerative cells responsible for tissue regeneration, can be successfully used as a treatment method for multiple or isolated gingival recessions of Miller’s class-I and class-II defects.
- Citation: Patra L, Raj SC, Katti N, Mohanty D, Pradhan SS, Tabassum S, Mishra AK, Patnaik K, Mahapatra A. Comparative evaluation of effect of injectable platelet-rich fibrin with collagen membrane compared with collagen membrane alone for gingival recession coverage. World J Exp Med 2022; 12(4): 68-91
- URL: https://www.wjgnet.com/2220-315x/full/v12/i4/68.htm
- DOI: https://dx.doi.org/10.5493/wjem.v12.i4.68
Gingival recession is a common feature affecting large populations leading to functional and aesthetic problems. While inflammation is the main etiologic factor for gingival recession, other anatomical factors, like thin biotype, abnormal tooth position (positioned too far buccally or lingually, direct trauma associated with malocclusion, aberrant frenal attachment, class-II division 2 malocclusion), and iatrogenic factors, like mechanical trauma (impaction of foreign bodies, faulty tooth brushing, poorly designed partial dentures) can also cause gingival recession. Subgingival restoration margins, the presence of calculus, periodontal disease, and smoking also plays role in the etiology of gingival recession[1-3].
Gingival recession is being treated using various therapeutic approaches with varying degrees of success depending on the etiology and treatment approach. Various periodontal surgical techniques for root coverage, like free gingival graft (FGG), subepithelial connective tissue graft (SCTG), semilunar flap, coronally advanced flap (CAF), and guided tissue regeneration (GTR), are available. Among them, CAF with connective tissue graft (CTG) is considered the gold standard for soft tissue augmentation and periodontal root coverage. It has some disadvantages, including harvesting from a donor site, limited tissue availability, and increased potential for post-harvesting morbidity[4].
With the introduction of various minimally invasive tunnelling techniques for gingival augmentation, similar results could be obtained. It tries to preserve the interdental papillae, unhampered blood supply, and faster wound healing. However, these procedures are quite technique sensitive and may cause tissue trauma to the sulcular epithelium leading to unfavorable healing outcomes[5].
To avoid these complications, a new minimally invasive approach for treating multiple gingival recession defects within the maxillary and mandibular aesthetic zone was introduced by Zadeh[4] called the vestibular incision subperiosteal tunnel access (VISTA) technique. Complete root coverage was observed for all VISTA treated sites along with a 1-2 mm gain in keratinised gingiva at the end of 12th mo follow-up period. These improvements were sustained at the 20th mo observation period[4]. Mansouri et al[6] compared the VISTA technique with the gold standard coronally advanced flap (CAF) technique using CTG for the treatment of gingival recession defects, which showed higher frequency of root coverage with the VISTA technique as compared to CAF. Mohamed et al[7] compared the efficacy of the VISTA technique with the tunnel technique (TUN) using acellular dermal matrix (ADM) allograft for gingival recession coverage. The 6-mo follow up results showed a statistically significant difference in favor of the VISTA + ADM technique than the TUN + ADM technique. This minimally invasive procedure promises adequate blood supply to the surgical site as it requires a small opening leading to the undermining of the periosteum, completely free from the area of root coverage, which further enhances the coronal positioning of the flap passively onto the exposed root surface[3].
Along with various techniques for root coverage procedure, several grafts, such as CTG, ADM allograft, Amniotic membrane, and bioactive glass, can be advocated for root coverage[3]. Adjunctive agents, like recombinant human growth factor, platelet rich plasma (PRP), and platelet-rich fibrin (PRF) have been used to accelerate healing and enhance clinical outcomes[3,8].
Collagen membrane is one of the materials used for gingival recession coverage, It is semipermeable, which allows nutrient passage and gas exchange and supports cell proliferation via its lattice-like structure and cell binding ability. It increases tissue volume as it is naturally absorbed and is replaced by host tissue. The chemotactic function encourages host cell migration and attachment, thus facilitating primary wound closure and reducing the likelihood of membrane exposure or potential wound/ membrane contamination[9].
Another agent that is commonly used for recession coverage is PRF, which is a leukocyte and platelet-rich fibrin biomaterial with a specific composition and 3D architecture that plays an important role in the release of growth factors, immune regulation, anti-infectious activities, and matrix remodeling during wound healing, and further serves as a scaffold for tissue regeneration by acting as a barrier membrane in guided bone regeneration (GBR) and guided tissue regeneration (GTR) procedures[10-14]. PRF has been utilized for the treatment of extraction sockets, gingival recessions, palatal wound closure, regeneration of periodontal defects, and hyperplastic gingival tissues[15].
Initially, PRF formulations were lacking a liquid concentrate of proteins, as standardized PRF had the majority of its growth factor encapsulated within its fibrin matrix. Recent advances in the field aim at developing a liquid formulation of PRF with no anticoagulants or fibrin matrix to allow the development of an injectable formulation of PRF, termed injectable PRF (i-PRF), which is a platelet concentrate in a liquid formulation that can be either utilized alone or combined easily with various biomaterials. It has a higher presence of regenerative cells with higher concentrations of growth factors and higher fibroblast migration, and has a higher expression of platelet-derived growth factor (PDGF), transforming growth factor (TGF-β), and type-1 collagen when compared to other formulations of PRF[16,17].
The purpose of the study was to compare the efficacy of the VISTA technique incorporating collagen membrane alone with the VISTA technique with collagen membrane soaked in injectable platelet-rich fibrin for gingival recession coverage.
The study was recommended by the Institutional Ethics Committee, under IEC/SCBDCH/049/20189 dated September 17, 2019 before its commencement and was conducted in accordance with the declaration of Helsinki of 1975 as revised in 2000. This was an interventional, parallel design, double blinded, randomized controlled trial performed from March 2020 to March 2021 in our department. A written informed consent was obtained from all participants after they received a written and oral explanation of study objectives, risks, and benefits. The study was prospectively registered with clinical trials registry (CTRI/2020/06/026141).
Sample size was calculated using G power 3.1.9.2 software (SPSS software India by Norman H Nie in 2015 G Power 3.1.9.2) considering 80% power, a 95%CI level with an effect size of 0.55 and a mean probing depth of 2.27mm before the treatment and 2.08mm after the treatment with a standard deviation of 0.34 mm respectively.
Inclusion criteria: (1) Both males and females of age ≥ 18 years with dentinal hypersensitivity or impaired aesthetics or difficulty in oral hygiene maintenance associated with gingival recession; (2) Subjects having Miller class I or II bilateral buccal gingival recession defects measuring ≥ 2 mm on the anterior teeth or premolars, on either arch; (3) Subjects who are not on any medication known to interfere with periodontal tissue health or healing within 6 mo of the study; and (4) Subjects having identifiable cementoenamel junction (CEJ) at recession sites.
Exclusion criteria: (1) History of systemic diseases (i.e. diabetes, autoimmune dysfunction, prolonged cortisone therapy, or chemotherapy) that would contraindicate periodontal surgical treatment; (2) Patients with deleterious habits like the use of tobacco chewing or smoking; (3) History of previous periodontal surgical treatment of the involved sites; (4) Presence of malocclusion and pathologic movement of teeth in involved sites; and (5) Presence of active carious lesions, restorations, or crowns at the CEJ, as well as non-vital teeth with radicular grooves and irregularities.
A simple random sampling technique by coin toss was done by an author (SCR) unaware of the clinical parameters, to decide which side/arch to act as test site and which as to control site of each patient. In sites included in the test group, recession sites were placed with collagen membrane soaked with injectable platelet-rich fibrin and in the control site only collagen membrane was placed.
After enrollment, all the participants underwent an initial non-surgical therapy including full mouth supra and subgingival scaling and root planning using ultrasonic scalers and hand instruments to ensure a healthy periodontium before the onset of surgical phase. Each of them was then given a standardized set of oral hygiene instructions both verbally and in a written format. Alginate impressions were taken 4 wk after signal recognition particle and study casts were poured. An acrylic template was fabricated on the study cast extending one tooth mesial and distal to the tooth indicated for extraction. This template was used as reference for the vertical measurements during the course of the study.
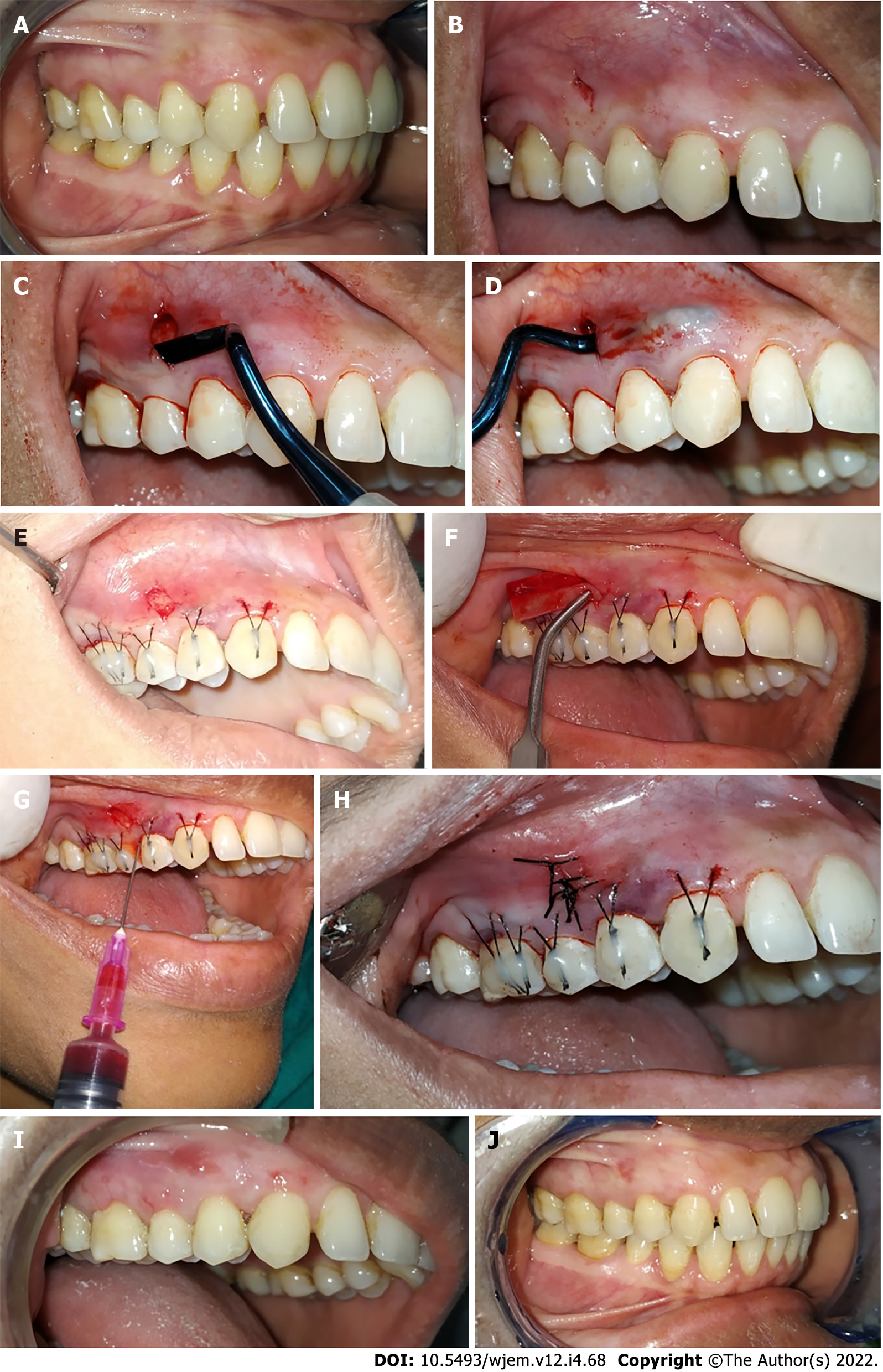
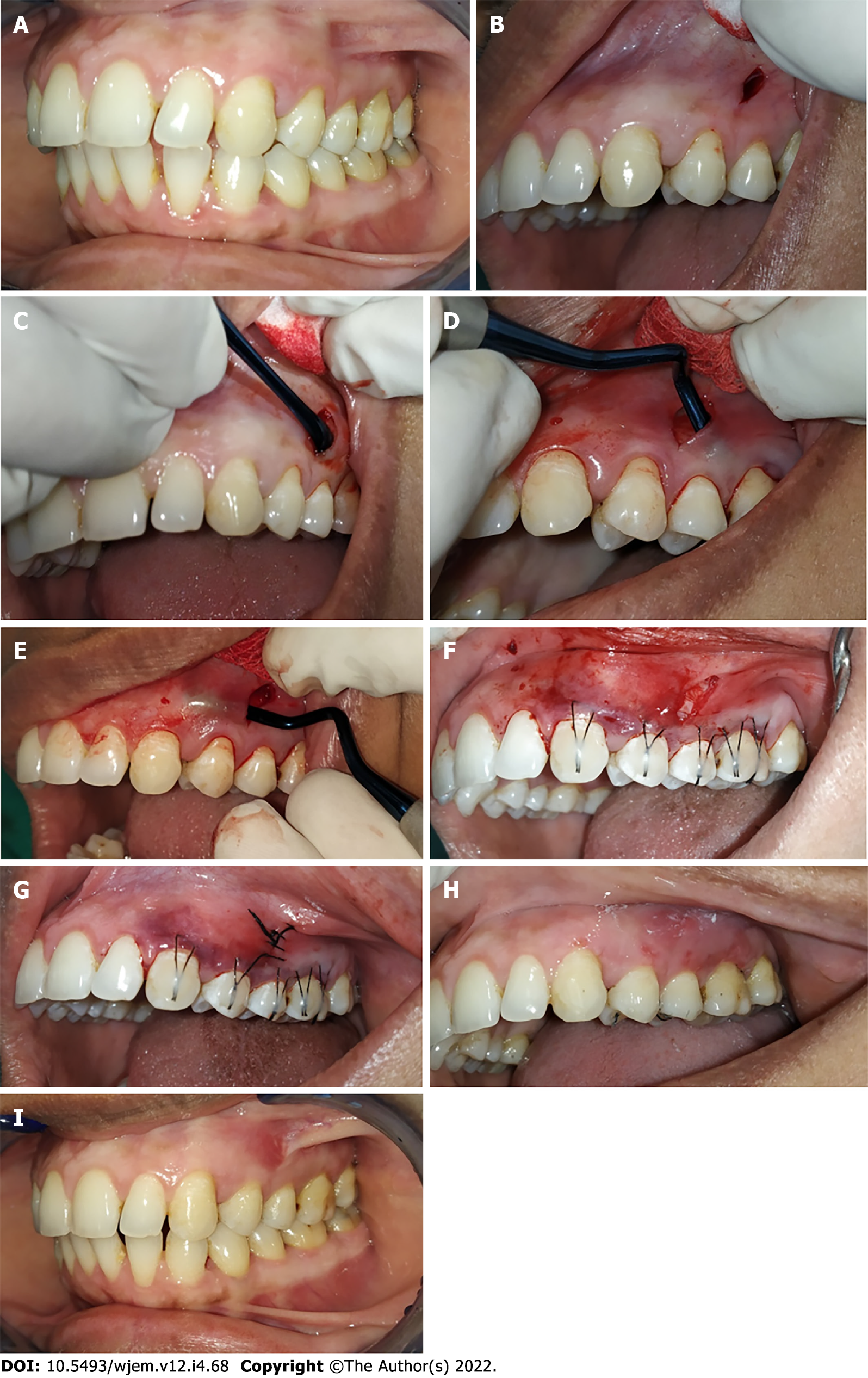
Clinical parameters were recorded at baseline (immediately before surgery) (Figure 1A and Figure 2A), as well as at 1 mo, 3 mo, and 6 mo follow-up appointments for control and test site groups. The clinical parameters recorded were as follows:
Plaque index (PI) as outlined by Silness P and Loe H (1964).
Gingival index (GI) as outlined by Loe H and Silness P (1963).
Probing depth (PD) measured with a UNC-15 periodontal probe as the distance from the gingival margin to the bottom of the pocket.
Recession depth (RD) was measured as the distance from the cementoenamel junction (CEJ) to the gingival margin at the mid-buccal surface using the UNC-15 probe.
Recession width (RW) was measured with a UNC-15 periodontal probe oriented horizontally and located at the most apical convexity of the CEJ, and horizontal distance between the mesial and distal gingival margin.
Relative attachment level (RAL) was measured mid-buccally with the reference point located at the apical end of the groove in the stent to the bottom of the periodontal pocket.
Keratinized tissue width (KTW) was measured from the most coronal extension of gingival margin to the mucogingival line.
Thickness of keratinized tissue (KTT) was measured by using an endodontic K-file (number-20, color code – yellow) with a silicon stop, perpendicular to the tissue surface and 2 mm apical to the gingival margin. After reaching the hard surface, the silicon stop was slid and placed in contact with the soft tissue. After removing the file, the distance between the tip of the file and the silicon stop was measured with a digital caliper accurate to the nearest 0.1 mm.
Percentage of root coverage was calculated according to the formula: % Root coverage = (Preopera
After extraoral scrubbing with 5% povidone-iodine solution, the patient was asked to rinse with 10 mL of 0.2% chlorhexidine digluconate solution for 1 min. Root debridement was done with an ultrasonic instrument followed by odontoplasty, carried out where necessary using a rotary finishing bur. The surgical site was anesthetized by local infiltration (2% lidocaine HCL with adrenaline 1:100000). The roots are then conditioned for 2 min with 24% buffered ethylenediaminetetraacetic acid gel to eliminate the smear layer.
For the test site, the vestibular incision subperiosteal tunnel access (VISTA) approach began with a vestibular access incision at an optimal position to gain access to the recession defects. The location of the access incision depends on the sites being treated, e.g., in cases where both premolars are indicated for recession coverage, the vertical access incision was given in between both the premolars. The incision was made through the periosteum using a No. 15 surgical blade (Bard-Parker) exposing the facial osseous plate (Figure 1B). A special set of patented periosteal elevators (VISTA 1-4) was used to elevate the periosteum and create the subperiosteal tunnel. The attached gingiva adjacent to the incision was elevated using VISTA 1, and the areas that are distant from the incision are elevated with VISTA 2, and interproximal areas were elevated with VISTA 3 and 4 instruments. With a VISTA 2 elevator, the tunnel was extended to at least one tooth beyond the teeth requiring root coverage, also beyond the mucogingival junction, and into the gingival sulcus of the teeth in the involved area, to aid in the mobilization of the mucoperiosteal flap (Figure 1C and D).
The mucogingival complex was coronally positioned using an anchored horizontal mattress suture. An anchored horizontal mattress suture was placed at a distance of 2-3 mm from gingival margin using 5-0 black braided suture with 3/8 reverse cutting needle. These anchored sutures were coronally positioned. The knot of the anchored sutures was moved on the facial enamel surfaces of the involved teeth to check the final position of the coronally advanced mucogingival complex. After that, the facial enamel surfaces of each tooth were briefly etched for 15 s, irrigated for 15 s, and dried with air. Thereafter, a bonding agent was applied over the prepared enamel surface and light cured. Then knots of anchored sutures were secured to the prepared facial aspect of each tooth by placing a small amount of flowable composite resin over the knot and was light cured (Figure 1E). This procedure effectively prevents apical relapse of the gingival margin during the initial stages of healing.
For the i-PRF preparation, first a tourniquet was tied around the arm of the patient, the skin over the antecubital vein was disinfected with Surgical spirit. Two tubes of 10 mL whole blood were collected by venipuncture of the antecubital vein. The collected blood was centrifuged at 700 rpm for 3 min (60 × g) (according to Miron RJ) at room temperature without any additives in restriction enzyme-mediated integration laboratory centrifuge machine. The i-PRF formed at the top layer, which was immediately collected into a 2 mL syringe with a 25-gauge needle. Then, commercially available collagen membrane (HEALIGUIDE Bio resorbable membrane, Advanced Biotech Products, INDIA) was trimmed according to the size of recession in the experimental site, and the trimmed collagen membrane was soaked with i-PRF for 10 min in a steel bowl and inserted into the experimental site with the help of tissue forceps (Figure 1F). Along with this, i-PRF was also injected at the mesial and distal aspects into the periodontal ligament and the facial aspect of the gingiva (Figure 1G). Finally, the vertical access incision was approximated and sutured with 5-0 black braided silk sutures, achieving primary wound closure (Figure 1H).
For the control site, a similar surgical technique was used to prepare the tunnel on the control site (Figure 2B-E). After that, collagen membrane was trimmed according to the size of the recession at the control site and soaked with normal saline for 10 min before being inserted into the tunnel. Similar to the test site, 5-0 black braided silk sutures was used to close the vertical access incision for achieving primary closure (Figure 2F and G).
All the patients were prescribed antibiotics and analgesics. Post-operative instructions were given to all patients and kept on a strict oral hygiene maintenance program. The vertical incision suture was removed after 1 wk and anchored sutures were removed after 3 wk post-surgery. The residual composite resin was removed using 16-flute tungsten carbide burs.
The follow-up was done every month for all the patients. During follow-up, oral prophylaxis was done and oral hygiene instructions were reinstituted. The measurements of clinical parameters were taken at 1, 3, and 6 mo postoperatively (Figure 1I and J, Figure 2H and I).
The data was analyzed using SPSS Ver 22 for windows, (IBM Corp, Armonik, United States). Descriptive statistics were expressed as a mean with standard deviations and proportions. Normally distributed data were analyzed using paired t-test for intragroup comparison and unpaired t-test for intergroup comparison. Skewed data were analyzed using the Wilcoxon signed rank test for intragroup and Mann-Whitney U test for intergroup comparison. The level of significance was set at P < 0.05.
The study consists of 13 subjects (7 males, 6 females) with the mean age of 36.7 ± 12.44 years (Table 1). All recession sites were divided into two groups: Group-I: Test sites (20 sites in which i-PRF with collagen membrane was used for recession coverage) and Group-II: Control sites (20 sites in which collagen membrane alone was used for recession coverage) (see flow diagram in Figure 3). Sample size was calculated using G power 3.1.9.2 software (SPSS software India by Norman H Nie in 2015 G Power 3.1.9.2).
| Demographic characteristics | Test, mean ± SD | Control, mean ± SD | |
| Sex | |||
| Male | 7 | ||
| Female | 6 | ||
| Age | 36.7 ± 12.44 | ||
| PI | 0.625 ± 0.151 | 0.625 ± 0.154 | |
| GI | 0.625 ± 0.164 | 0.625 ± 0.65 | |
| PD | 1.75 ± 0.444 | 2.05 ± 0.6 | |
| RD | 2.7 ± 0.86 | 2.9 ± 0.71 | |
| RW | 3.5 ± 0.6 | 3.7 ± 0.73 | |
| RAL | 7.3 ± 0.8 | 7.05 ± 0.82 | |
| KTW | 1.6 ± 0.5 | 1.35 ± 0.48 | |
| KTT | 1.64 ± 0.237 | 1.61 ± 0.201 |
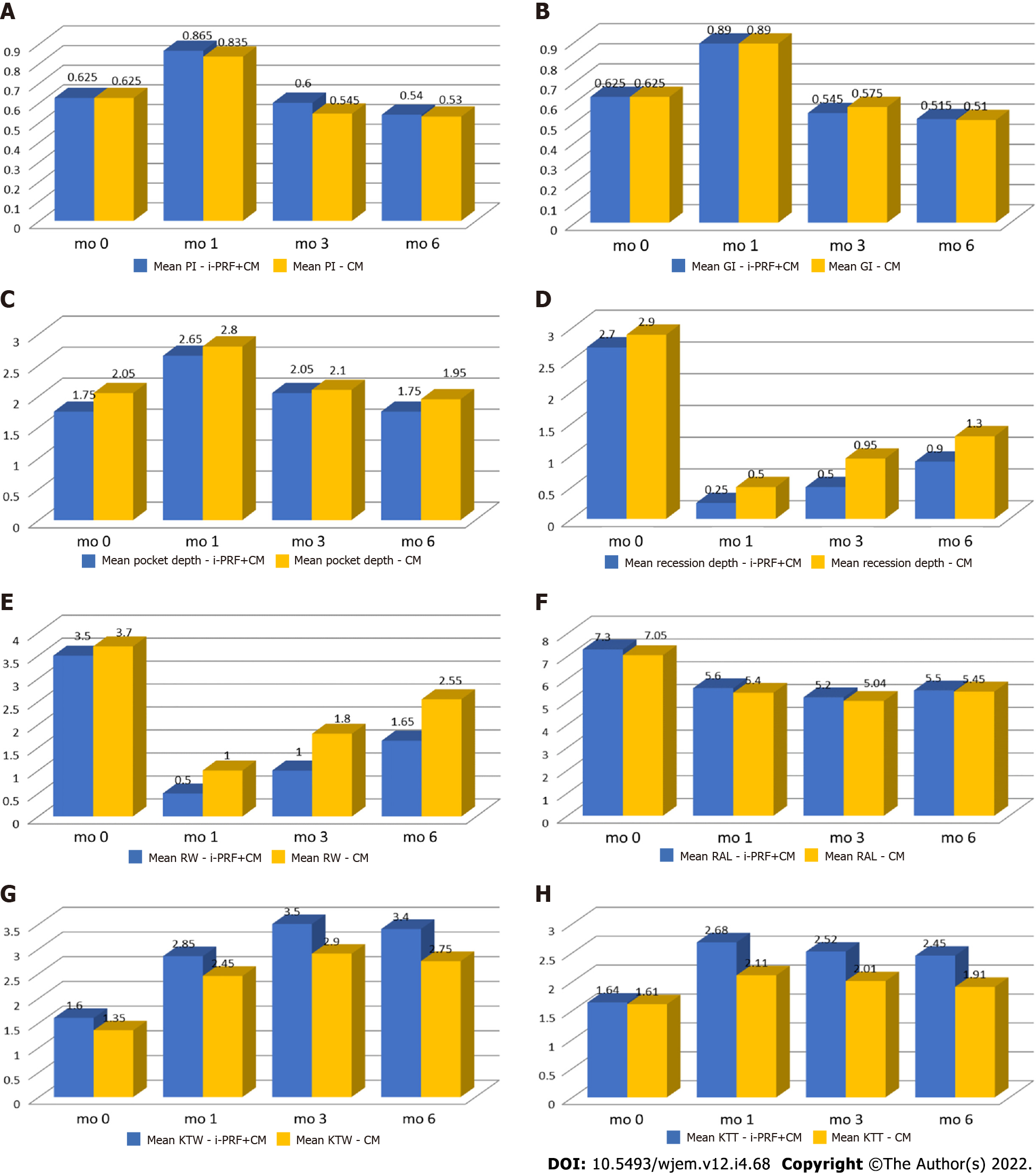
Mean plaque index scores of the test group were 0.625 ± 0.151, 0.865 ± 0.134, 0.6 ± 0.133, and 0.54 ± 0.127 and of the control group were 0.625 ± 0.154, 0.835 ± 0.172, 0.545 ± 0.139, and 0.56 ± 0.134 at baseline, post-operative 1 mo, 3 mo, and 6 mo, respectively. The plaque scores are statistically not significant at different time intervals in the intergroup comparison (Table 2, Figure 4A). However, there was a statistically significant difference between mean scores in the intragroup comparison between each time interval for individual groups (P < 0.01). No statistically significant difference found between baseline and postoperative 3 mo for the test group (P = 0.204) and between postoperative 3 mo and 6 mo for control group (P = 0.379) (Table 3).
| Time (mo) | Group | n | mean ± SD | P value | |
| 0 | i-PRF + CM | 20 | 0.625 | 0.15174 | 1.000 |
| CM | 20 | 0.625 | 0.15174 | NS | |
| 1 | i-PRF + CM | 20 | 0.865 | 0.13485 | 0.544 |
| CM | 20 | 0.835 | 0.17252 | NS | |
| 3 | i-PRF + CM | 20 | 0.6 | 0.13377 | 0.211 |
| CM | 20 | 0.545 | 0.13945 | NS | |
| 6 | i-PRF + CM | 20 | 0.54 | 0.12732 | 0.810 |
| CM | 20 | 0.53 | 0.13416 | NS | |
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 0.625 ± 0.151 | 0.001a | 0 | 0.625 ± 0.154 | 0.001a |
| 1 | 0.865 ± 0.134 | 1 | 0.835 ± 0.172 | ||
| 0 | 0.625 ± 0.151 | 0.204 | 0 | 0.625 ± 0.154 | 0.001a |
| 3 | 0.6 ± 0.133 | 3 | 0.545 ± 0.139 | ||
| 0 | 0.625 ± 0.151 | 0.001a | 0 | 0.625 ± 0.154 | 0.001a |
| 6 | 0.54 ± 0.127 | 6 | 0.53 ± 0.134 | ||
| 1 | 0.865 ± 0.134 | 0.001a | 1 | 0.835 ± 0.172 | 0.001a |
| 3 | 0.6 ± 0.133 | 3 | 0.545 ± 0.139 | ||
| 1 | 0.865 ± 0.134 | 0.001a | 1 | 0.835 ± 0.172 | 0.001a |
| 6 | 0.54 ± 0.127 | 6 | 0.56 ± 0.134 | ||
| 3 | 0.6 ± 0.133 | 0.004a | 3 | 0.545 ± 0.139 | 0.379 |
| 6 | 0.54 ± 0.127 | 6 | 0.56 ± 0.134 |
Mean gingival index scores of the test group were 0.625 ± 0.164, 0.89 ± 0.141, 0.545 ± 0.119, and 0.51 ± 0.149 and of the control group were 0.625 ± 0.65, 0.89 ± 0.18, 0.575 ± 0.155, and 0.51 ± 0.137 at baseline, post-operative 1 mo, 3 mo, and 6 mo, respectively. Intergroup comparison of gingival index scores revealed no statistically significant difference between mean scores at different time intervals (Table 4 and Figure 4B). However, there was a statistically significant increase in the gingival index scores between baseline and postoperative 1 mo for both groups (P < 0.01). There was a decrease in gingival index scores at subsequent time intervals for both the groups except between postoperative 3 mo and 6 mo (P = 0.137) (Table 5).
| mo | Group | n | mean ± SD | P value |
| 0 | i-PRF + CM | 20 | 0.625 ± 0.16504 | 1.0 |
| CM | 20 | 0.625 ± 0.16504 | NS | |
| 1 | i-PRF + CM | 20 | 0.89 ± 0.14105 | 1.0 |
| CM | 20 | 0.89 ± 0.18035 | NS | |
| 3 | i-PRF + CM | 20 | 0.545 ± 0.1191 | 0.497 |
| CM | 20 | 0.575 ± 0.15517 | NS | |
| 6 | i-PRF + CM | 20 | 0.515 ± 0.14965 | 0.913 |
| CM | 20 | 0.51 ± 0.13727 | NS |
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 0.625 ± 0.164 | 0.001a | 0 | 0.625 ± 0.165 | 0.001a |
| 1 | 0.89 ± 0.141 | 1 | 0.89 ± 0.18 | ||
| 0 | 0.625 ± 0.164 | 0.001a | 0 | 0.625 ± 0.165 | 0.001a |
| 3 | 0.545 ± 0.119 | 3 | 0.575 ± 0.155 | ||
| 0 | 0.625 ± 0.164 | 0.001a | 0 | 0.625 ± 0.165 | 0.001a |
| 6 | 0.51 ± 0.149 | 6 | 0.51 ± 0.137 | ||
| 1 | 0.89 ± 0.141 | 0.001a | 1 | 0.89 ± 0.18 | 0.001a |
| 3 | 0.545 ± 0.119 | 3 | 0.575 ± 0.155 | ||
| 1 | 0.89 ± 0.141 | 0.001a | 1 | 0.89 ± 0.18 | 0.001a |
| 6 | 0.51 ± 0.149 | 6 | 0.51 ± 0.137 | ||
| 3 | 0.545 ± 0.119 | 0.137 | 3 | 0.545 ± 0.155 | 0.137 |
| 6 | 0.51 ± 0.149 | 6 | 0.51 ± 0.137 |
Mean probing depth scores of the test group were 1.75 ± 0.444 mm, 2.65 ± 0.489 mm, 2.05 ± 0.489 mm, and 1.75 ± 0.444 mm and of the control group were 2.05 ± 0.6 mm, 2.8 ± 0.83 mm, 2.1 ± 0.3 mm, and 1.95 ± 0.223 mm at baseline, postoperative 1 mo, 3 mo, and 6 mo, respectively. In the intergroup comparison between the test group and control group, there was no statistically significant difference between mean scores at the different time interval between two groups (Table 6 and Figure 4C). However, there was a significant increase in probing depth between baseline and 1 mo and subsequent decrease in probing depth at postoperative 1 mo, 3 mo, and 6 mo, respectively for test group. Similarly, in the control group, there was an increase in probing depth between baseline and 1 mo. Although there was a decrease in probing depth between postoperative 1 mo and 3 mo which was not statistically significant, but there was a significant decrease between postoperative 3 mo and 6 mo. There was no significant difference between baseline and postoperative 6 mo, and postoperative 1 mo and 6 mo respectively (Table 7).
| Time (mo) | Group | n | mean ± SD | P value |
| 0 | i-PRF + CM | 20 | 1.75 ± 0.44426 | 0.082 |
| CM | 20 | 2.05 ± 0.60481 | NS | |
| 1 | i-PRF + CM | 20 | 2.65 ± 0.48936 | 0.492 |
| CM | 20 | 2.8 ± 0.83351 | NS | |
| 3 | i-PRF + CM | 20 | 2.05 ± 0.22361 | 0.560 |
| CM | 20 | 2.1 ± 0.30779 | NS | |
| 6 | i-PRF + CM | 20 | 1.75 ± 0.44426 | 0.080 |
| CM | 20 | 1.95 ± 0.22361 | NS |
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 1.75 ± 0.444 | 0.001a | 0 | 2.05 ± 0.6 | 0.001a |
| 1 | 2.65 ± 0.489 | 1 | 2.8 ± 0.83 | ||
| 0 | 1.775 ± 0.444 | 0.01a | 0 | 2.05 ± 0.6 | 0.002a |
| 3 | 2.05 ± 0.223 | 3 | 2.1 ± 0.3 | ||
| 0 | 1.75 ± 0.444 | 1.0 | 0 | 2.05 ± 0.6 | 0.08 |
| 6 | 1.75 ± 0.444 | 6 | 1.95 ± 0.223 | ||
| 1 | 2.65 ± 0.489 | 0.001a | 1 | 2.8 ± 0.83 | 0.748 |
| 3 | 2.05 ± 0.223 | 3 | 2.1 ± 0.6 | ||
| 1 | 2.65 ± 0.489 | 0.001a | 1 | 2.8 ± 0.83 | 0.428 |
| 6 | 1.75 ± 0.444 | 6 | 1.95 ± 0.223 | ||
| 3 | 2.45 ± 0.223 | 0.01a | 3 | 2.1 ± 0.3 | 0.001a |
| 6 | 1.75 ± 0.444 | 6 | 1.95 ± 0.223 |
Mean recession depth scores of the test group were 2.7 ± 0.86 mm, 0.25 ± 0.4 mm, 0.5 ± 0.5 mm, and 0.9 ± 0.64 mm and of the control group were 2.9 ± 0.71 mm, 0.5 ± 0.51 mm, 0.95 ± 0.51 mm, and 1.3 ± 0.57 mm at baseline, 1 mo, 3 mo, and 6 mo, respectively. In the intergroup analysis, there was no statistically significant difference between mean scores at baseline and 1 mo; however, there was a statistically significant difference in mean recession depth at 3 mo (P < 0.01) and 6 mo (P < 0.05) between both the test and control groups (Table 8 and Figure 4D). Within the group analysis, there was a statistically significant decrease in mean recession depth in both the groups between baseline and 1 mo (P = 0.001); 1 mo and 3 mo (P = 0.021; P = 0.001), and 3 mo and 6 mo (P = 0.002; P = 0.005), respectively (Table 9).
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 2.7 ± 0.86 | 0.001a | 0 | 2.9 ± 0.71 | 0.001a |
| 1 | 0.25 ± 0.4 | 1 | 0.5 ± 0.51 | ||
| 0 | 2.7 ± 0.86 | 0.001a | 0 | 2.9 ± 0.71 | 0.001a |
| 3 | 0.5 ± 0.5 | 3 | 0.95 ± 0.51 | ||
| 0 | 2.7 ± 0.86 | 0.001a | 0 | 2.9 ± 0.71 | 0.001a |
| 6 | 0.9 ± 0.64 | 6 | 1.3 ± 0.57 | ||
| 1 | 0.25 ± 0.4 | 0.021b | 1 | 0.5 ± 0.51 | 0.001a |
| 3 | 0.5 ± 0.5 | 3 | 0.95 ± 0.51 | ||
| 1 | 0.025 ± 0.4 | 0.001a | 1 | 0.5 ± 0.51 | 0.001a |
| 6 | 0.9 ± 0.64 | 6 | 1.3 ± 0.57 | ||
| 3 | 0.5 ± 0.5 | 0.002a | 3 | 0.95 ± 0.51 | 0.005a |
| 6 | 0.9 ± 0.64 | 6 | 1.3 ± 0.57 |
Mean recession width scores of the test group were 3.5 ± 0.6 mm, 0.5 ± 0.8 mm, 1 ± 1.02 mm, and 1.65 ± 1.03 mm and for the control group were 3.7 ± 0.73 mm, 1 ± 1.02 mm, 1.85 ± 0.85 mm, and 2.55 ± 0.75 mm at baseline, 1 mo, 3 mo and 6 mo, respectively. In the intergroup analysis, there was a statistically significant difference between the two groups at 3 mo (P < 0.01) and 6 mo (P < 0.01), respectively. There was no statistically significant difference at baseline and 1 mo (Table 10 and Figure 4E). In intragroup analysis, there was a statistically significant decrease in the recession width between baseline and 1 mo (P = 0.001), and an increase in the width of recession between 1 mo and 3 mo (P = 0.025; P = 0.004) in both groups, respectively. Similarly, there was an increase in the recession width between 3 mo and 6 mo (P = 0.009; P = 0.001) (Table 11).
| Time (mo) | Group | n | mean ± SD | P value | |
| 0 | i-PRF + CM | 20 | 3.5 | 0.607 | 0.400 |
| CM | 20 | 3.7 | 0.733 | NS | |
| 1 | i-PRF + CM | 20 | 0.5 | 0.889 | 0.107 |
| CM | 20 | 1 | 1.026 | NS | |
| 3 | i-PRF + CM | 20 | 1 | 1.026 | 0.080 |
| CM | 20 | 1.85 | 0.875 | ||
| 6 | i-PRF + CM | 20 | 1.65 | 1.04 | 0.001a |
| CM | 20 | 2.55 | 0.759 | ||
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 3.5 ± 0.6 | 0.001a | 0 | 3.7 ± 0.73 | 0.001a |
| 1 | 0.5 ± 0.8 | 1 | 1 ± 1.02 | ||
| 0 | 3.5 ± 0.6 | 0.001a | 0 | 3.7 ± 0.73 | 0.001a |
| 3 | 1 ± 1.02 | 3 | 1.85 ± 0.85 | ||
| 0 | 3.5 ± 0.6 | 0.001a | 0 | 3.7 ± 0.73 | 0.001a |
| 6 | 1.65 ± 1.03 | 6 | 2.55 ± 0.75 | ||
| 1 | 0.5 ± 0.8 | 0.025b | 1 | 1 ± 1.02 | 0.004a |
| 3 | 1 ± 1.02 | 3 | 1.85 ± 0.85 | ||
| 1 | 0.5 ± 0.8 | 0.001a | 1 | 1 ± 1.02 | 0.001a |
| 6 | 1.65 ± 1.03 | 6 | 2.55 ± 0.75 | ||
| 3 | 1 ± 1.02 | 0.009a | 3 | 1.85 ± 0.85 | 0.001a |
| 6 | 1.65 ± 1.03 | 6 | 2.55 ± 0.75 |
Mean relative attachment scores for the test group were 7.3 ± 0.8 mm, 5.6 ± 1.3 mm, 5.25 ± 1.29 mm, and 5.55 ± 1.09 mm and for the control group were 7.05 ± 0.82 mm, 5.4 ± 0.94 mm, 5.05 ± 0.94 mm, and 5.45 ± 0.82 mm at baseline, 1 mo, 3 mo, and 6 mo, respectively. In intergroup analysis, there was a statistically significant difference between the two groups at 3 mo (P < 0.01) and 6 mo (P < 0.01), respectively (Table 12 and Figure 4F). There was no statistically significant difference at baseline and 1 mo. There was a statistically significant decrease in the attachment level in both the groups between baseline and 1 mo (P = 0.001). There was a further decrease in the test group between 1 mo and 3 mo (P = 0.021) and between 3 mo and 6 mo for the control group (P = 0.021) (Table 13).
| Time (mo) | Group | n | mean ± SD | P value |
| 0 | i-PRF + CM | 20 | 7.3 ± 0.801 | 0.429 |
| CM | 20 | 7.05 ± 0.826 | NS | |
| 1 | i-PRF + CM | 20 | 5.65 ± 1.348 | 0.620 |
| CM | 20 | 5.4 ± 0.94 | NS | |
| 3 | i-PRF + CM | 20 | 5.25 ± 1.293 | 0.779 |
| CM | 20 | 5.05 ± 0.945 | NS | |
| 6 | i-PRF + CM | 20 | 5.55 ± 1.099 | 0.779 |
| CM | 20 | 5.45 ± 0.826 | NS |
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 7.3 ± 0.8 | 0.001a | 0 | 7.05 ± 0.82 | 0.001a |
| 1 | 5.6 ± 1.3 | 1 | 5.4 ± 0.94 | ||
| 0 | 7.3 ± 0.8 | 0.001a | 0 | 7.05 ± 0.82 | 0.001a |
| 3 | 5.25 ± 1.29 | 3 | 5.05 ± 0.94 | ||
| 0 | 7.3 ± 0.8 | 0.001a | 0 | 7.05 ± 0.82 | 0.001a |
| 6 | 5.55 ± 1.09 | 6 | 5.45 ± 0.82 | ||
| 1 | 5.6 ± 1.3 | 0.021b | 1 | 5.4 ± 0.94 | 0.124 |
| 3 | 5.25 ± 1.29 | 3 | 5.05 ± 0.94 | ||
| 1 | 5.6 ± 1.3 | 0.589 | 1 | 5.4 ± 0.94 | 0.868 |
| 6 | 5.55 ± 1.06 | 6 | 5.45 ± 0.82 | ||
| 3 | 5.52 ± 1.29 | 0.06 | 3 | 5.05 ± 0.94 | 0.021a |
| 6 | 5.55 ± 1.06 | 6 | 5.45 ± 0.82 |
The mean width of keratinized tissue scores for the test group were 1.6 ± 0.5 mm, 2.85 ± 0.48 mm, 3.5 ± 0.51 mm, and 3.4 ± 0.59 mm and for the control group were 1.35 ± 0.48 mm, 2.45 ± 0.6 mm, 2.9 ± 0.44 mm, and 2.75 ± 0.44 mm at baseline, 1 mo, 3 mo, and 6 mo, respectively. Intergroup comparison revealed statistically insignificant difference between the two groups at baseline, but there was a statistically significant difference at 1 mo, 3 mo, and 6 mo, respectively (Table 14 and Figure 4G). In intragroup comparison, there was a statistically significant increase in the width of keratinized tissue in both the groups (P = 0.001) between baseline and 1 mo and between 1 mo and 3 mo respectively (P = 0.001; P = 0.013) (Table 15).
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 1.6 ± 0.5 | 0.001a | 0 | 1.35 ± 0.48 | 0.001a |
| 1 | 2.85 ± 0.48 | 1 | 2.45 ± 0.6 | ||
| 0 | 1.6 ± 0.5 | 0.001a | 0 | 1.35 ± 0.48 | 0.001a |
| 3 | 3.5 ± 0.51 | 3 | 2.9 ± 0.44 | ||
| 0 | 1.6 ± 0.5 | 0.001a | 0 | 1.35 ± 0.48 | 0.001a |
| 6 | 3.4 ± 0.59 | 6 | 2.75 ± 0.44 | ||
| 1 | 2.85 ± 0.48 | 0.001a | 1 | 2.45 ± 0.6 | 0.001a |
| 3 | 3.5 ± 0.51 | 3 | 2.9 ± 0.44 | ||
| 1 | 2.85 ± 0.48 | 0.001a | 1 | 2.45 ± 0.6 | 0.001a |
| 6 | 3.4 ± 0.59 | 6 | 2.75 ± 0.44 | ||
| 3 | 3.5 ± 0.51 | 0.001a | 3 | 2.9 ± 0.44 | 0.001a |
| 6 | 3.4 ± 0.59 | 6 | 2.75 ± 0.44 |
The mean thickness of keratinized tissue observed for the test group were 1.64 ± 0.237 mm, 2.68 ± 0.233 mm, 2.52 ± 0.211 mm, and 2.45 ± 0.252 mm and for the control group were 1.61 ± 0.201 mm, 2.11 ± 0.193 mm, 2.01 ± 0.179 mm, and 1.91 ± 0.166 mm at baseline, 1 mo, 3 mo, and 6 mo, respectively. In intergroup analysis, there was no statistically significant difference found between the mean thickness of keratinized tissue between the two groups at baseline (P > 0.05). However, there was a statistically significant difference at 1 mo, 3 mo, and 6 mo, respectively (Table 16 and Figure 4H). In intragroup analysis, there was a statistically significant increase in the thickness of keratinized tissue in both the groups at all time intervals (P = 0.001) (Table 17).
| i-PRF + CM in mo | mean ± SD | P value | CM in mo | mean ± SD | P value |
| 0 | 1.64 ± 0.237 | 0.001a | 0 | 1.61 ± 0.201 | 0.001a |
| 1 | 2.68 ± 0.233 | 1 | 2.11 ± 0.193 | ||
| 0 | 1.64 ± 0.237 | 0.001a | 0 | 1.61 ± 0.201 | 0.001a |
| 3 | 2.52 ± 0.211 | 3 | 2.01 ± 0.179 | ||
| 0 | 1.64 ± 0.237 | 0.001a | 0 | 1.61 ± 0.201 | 0.001a |
| 6 | 2.45 ± 0.252 | 6 | 1.91 ± 0.166 | ||
| 1 | 2.68 ± 0.233 | 0.001a | 1 | 2.11 ± 0.193 | 0.001a |
| 3 | 2.52 ± 0.211 | 3 | 2.01 ± 0.179 | ||
| 1 | 2.68 ± 0.233 | 0.001a | 1 | 2.11 ± 0.193 | 0.001a |
| 6 | 2.45 ± 0.252 | 6 | 1.91 ± 0.166 | ||
| 3 | 2.52 ± 0.211 | 0.001a | 3 | 2.01 ± 0.179 | 0.001a |
| 6 | 2.45 ± 0.252 | 6 | 1.91 ± 0.166 |
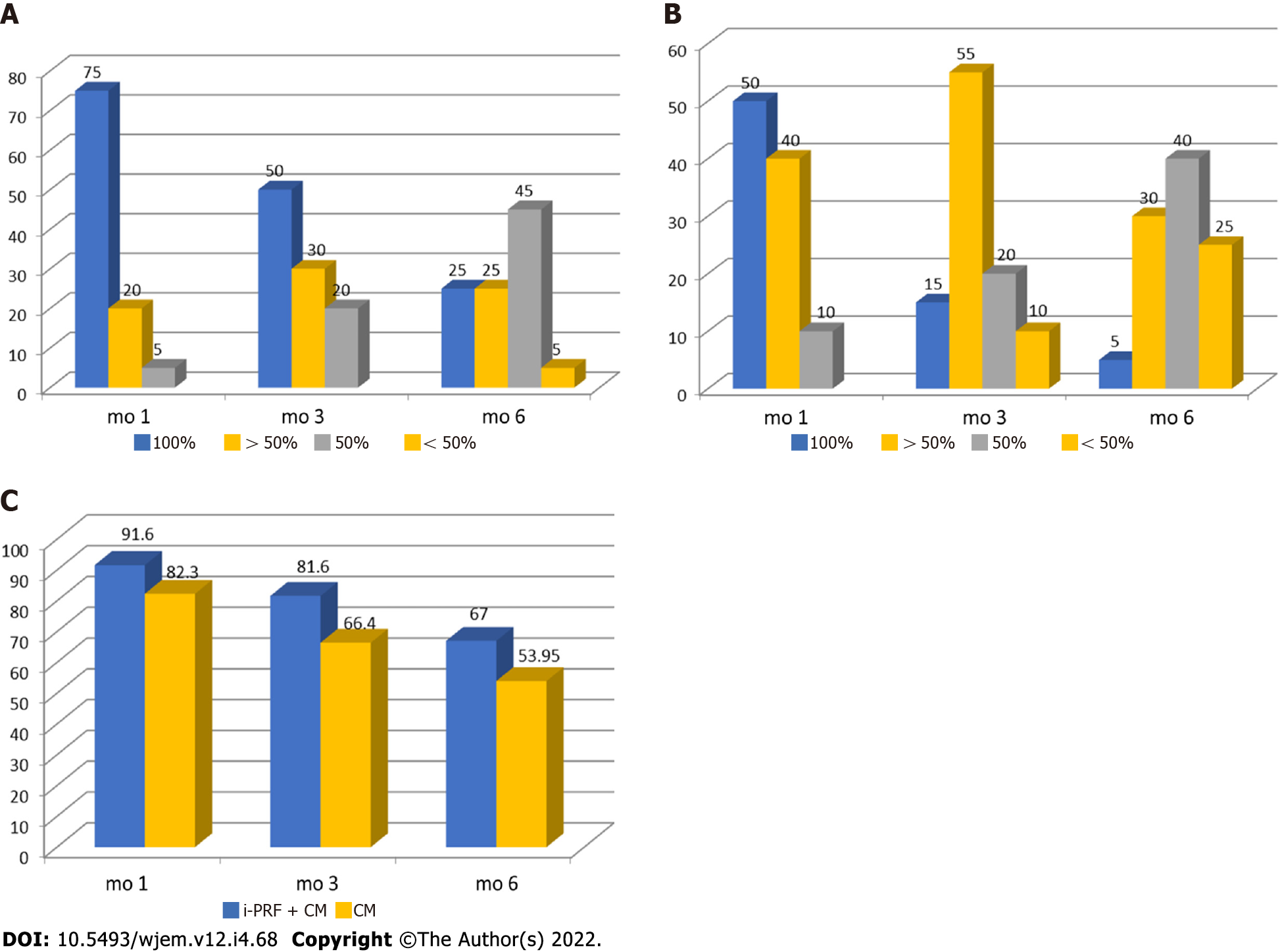
In the analysis of the percentage of root coverage for the test sites in which i-PRF with collagen membrane was used for recession coverage, it was found that at 1st postoperative month, about 75% of sites had 100% root coverage, 20% had of sites > 50% root coverage, and only 5% of sites had 50% root coverage. In 3rd postoperative month, 50% of sites had 100% root coverage, 30% had > 50% root coverage, and 20% of sites had 50% root coverage. In the 6th postoperative month, only 25% of sites remained at 100% root coverage, 25% of sites had > 50% root coverage, 45% of sites had 50% root coverage, and 5% of sites had < 50% root coverage (Figure 5A). While in the analysis of the percentage of root coverage for the control sites in which only collagen membrane was used for recession coverage, it was found that at 1st postoperative month about 50% of sites had 100% root coverage, 40% of sites had > 50% root coverage, and 10% of sites had 50% root coverage. In the 3rd postoperative month, only 15% of sites had 100% root coverage, 55% had > 50% root coverage, 20% of sites had 50% root coverage, and 10% of sites had < 50% of root coverage. In the 6th postoperative month, only 5% of sites remained at 100% root coverage, 30% of sites had > 50% root coverage, 40% of sites had 50% of root coverage, and 25% of sites had < 50% root coverage (Figure 5B).
In the overall percentage of root coverage, it was found that in the test group 91.6%, 81.6%, and 67% root coverage was found at 1 mo, 3 mo, and 6 mo, respectively, while in the control group it was found 82.3%, 66.4%, and 53.95% of root coverage at 1 mo, 3 mo and 6 mo, respectively (Table 18 and Figure 5C).
| Group | Month 1 | Month 3 | Month 6 |
| i-PRF + CM | 91.6 | 81.6 | 67 |
| CM | 82.3 | 66.4 | 53.95 |
Gingival recession defects present clinicians with significant therapeutic challenges, including restoration of protective anatomy of the mucogingival complex, reestablishment of the aesthetic balance between soft tissues and adjacent tooth structures, and, ideally, regeneration of the lost cementum, periodontal ligament and supporting alveolar bone[18]. Although a wide range of therapeutic alternative exist for treatment of isolated or multiple gingival recessions, according to the available systematic reviews, coronally advanced flap with subepithelial connective tissue graft is the most predictable approach and is considered as the gold standard for root coverage procedures[19-22].
The large avascular area, which usually leads to difficulty in restoring blood supply to the grafted tissue and which is vital for healing, the need for large amount of donor tissue, and the presence of non-carious cervical lesions, which are often associated with multiple gingival recessions, compound the problem[3]. Also, muscle pull during healing often leads to incomplete root coverage or relapse of the recession[4]. Taking all these factors into consideration, the VISTA technique, which is minimally invasive, does not compromise the blood supply, and yet results in improvement of all the clinical parameters, can be considered an accepted approach[3,4].
The advantage of the VISTA technique over other tunneling approaches and classical techniques of gingival augmentation is the degree of coronal advancement of the gingival margin advocated during the procedure[3]. Placement of the initial vertical access incision and the subperiosteal tunnel entrance far from the gingival margin reduces the risk of trauma to the gingiva, while at the same time maintains the integrity of the interdental papilla by avoiding papillary reflection and marginal tissue loss on the teeth being treated[3,5,23]. It also provides a wider access to the surgical region, improves visualization through the single incision with no visible scarring, maximizing the aesthetic outcome[3,6]. The positioning of the gingival margin to the most coronal level of the adjacent interproximal papilla rather than to the cementoenamel junction, with the help of coronally anchored suturing technique on the facial surface of each tooth, effectively minimizes micromotion of the regenerative site and prevents apical relapse of the gingival margin during the initial stages of healing by compensating for some degree of apical migration during the healing period[3].
Dandu et al[24] conducted a split mouth randomized controlled trial in 15 patients having bilateral Miller class I and II recession defects. Results revealed mean percentage root coverage of 87.37% + 17.78% with statistically significant gains in the width of keratinized gingiva and a clinical attachment level obtained at 9 mo. Reddy et al[8] conducted a case series study to evaluate clinical efficacy of the VISTA technique in combination with PRF and CTG in the treatment of gingival recession defects. Results obtained showed complete root coverage in all the cases at 6 mo and concluded that the VISTA technique overcomes the shortcoming of other treatment options and gives better results for multiple gingival recession defects. Garg et al[10] evaluated the efficacy of VISTA with or without PRF membrane in the treatment of multiple Millers class I and class II gingival recession defects. One hundred percent coverage was obtained in class I sites treated with VISTA approach with or without PRF-membrane. Millers class II recession defects showed 100% coverage with 80%-85% of CAL gain at site treated with VISTA + PRF membrane as compared to sites treated with VISTA technique, which only displayed 50% coverage. They concluded that the VISTA technique alone is a successful approach for the treatment of class-I and II multiple recession defects. Moreover, along with PRF-membrane, the VISTA technique has proven efficiency for treatment of class - III recession defects. Mansouri et al[5] compared the clinical efficacy of the VISTA technique with CTG vs CAF with CTG for the treatment of multiple gingival recession defects. Results revealed a significant decrease in recession depth, recession width, and clinical attachment level, and an increase in keratinized tissue width in both the groups. It was concluded that VISTA, as a minimally invasive approach, was able to treat gingival recession defects and reduce their height and width, yielding results similar to those obtained by the use of CAF, which is the gold standard procedure for root coverage. Mohamed et al[6] compared the Tunnel technique with the VISTA technique for the treatment of multiple gingival recessions with ADM. The percentage of root coverage between VISTA + ADM sites and tunnel + ADM sites was statistically significant in favor of VISTA + ADM. They concluded that an ADM allograft can be recommended as an alternative to connective tissue graft, but its combination with the VISTA technique is found to be more efficient than tunnel + ADM in treatment of Miller class I and II multiple gingival recessions and lead to more favorable root coverage.
Guided tissue regeneration is a reliable method for periodontal regeneration and the introduction of resorbable collagen membranes allowed clinicians to achieve a predictable, new connective tissue attachment over the exposed root surface[25-29]. Collagen membrane, acting as a barrier, mechanically prevents the epithelial cell migration during the initial stages of healing, allowing the regeneration of the treated root surface by connective tissue cells, eventually leading to the development of a new connective tissue attachment. The cross-linked structure slows the degradation rate, allowing the membrane to remain in the site for a sufficient period of time which prevents the apical migration of epithelial cells in late stages of healing, thus discouraging the formation of long junctional epithelial attachment and favoring the development of a connective tissue attachment[30,31].
Since the introduction of PRF[32], it has been used in various types of periodontal defects with good results. PRF is autologous, easy to prepare in a short period of time, and has little biochemical handling, giving it an advantage over other techniques. It has a matrix of fibrin, which has trapped platelets, leukocytes, and cytokines. It acts as a source of growth factors, which are released slowly over a period of 7 d and play an important role in recession coverage[33]. One drawback that limits the applications of PRF is that PRF is currently available only in a gel form, which is not conducive to being injected[34-36].
i-PRF also has similar properties as PRF; however, it is available in injectable form. It contains all components of PRF, including platelets, white blood cells, and all the clotting factors comprising fibrinogen in an uncoagulated form[37]. The major advantages of i-PRF over other platelet concentrates is that it contains a greater number of regenerative cells with higher concentrations of growth factors and leukocytes due to the “slow speed concept” of blood centrifugation[38,39]. Leukocytes have been known to play an important role in wound healing and tissue regeneration. With the increased number of these cells available, this possibly increases the release of growth factors like platelet-derived growth factor (PDGF), epidermal growth factor, transforming growth factor-β (TGF-β), and insulin-like growth factor-1[40,41].
According to Miron et al[16] when i-PRF was compared with PRP in terms of cell proliferation, PRP was more effective than injectable PRF. However, injectable PRF demonstrated significantly better results than PRP did, including cell migration and messenger ribonucleic acid expression of TGF-β, PDGF, and collagen type 1a2 at both 3- and 7-d intervals. Also, whereas PRP had completely dissolved over a period of 10 d, injectable PRF formed a small clot as a dynamic gel and maintained release of growth factor for over 10 d. Varela et al[42] observed that i-PRF induces higher cell migration and expression of TGF-β, PDGF, and type I collagen, which stimulates the differentiation of osteoblasts and deposits a mineral matrix. İzol et al[43] investigated the outcome of i-PRF on root coverage of free gingival graft surgery. The result showed a positive effect on the coverage of the root surface. Ucak Turer et al[44] investigated the combined effect of SCTG with i-PRF and SCTG alone in a coronally positioned flap procedure for the treatment of root coverage and observed that the combined effect of SCTG and i-PRF achieved a greater keratinized tissue width and showed predictable results in reduced gingival recession. Ozsagir et al[45] evaluated the efficacy of i-PRF alone and in combination with microneedling on gingival thickness and KTW in patients with a thin biotype. They stated that microneedling has a beneficial result on the augmentation of gingival thickness. Al-Maawi et al[46] analyzed the combination of an autologous i-PRF matrix as a drug delivery system, with five different xenogeneic collagen-based biomaterials (Mucograft®, Bio-Gide®, Mucoderm®, Collprotect® and BEGO®) histologically. They found that i-PRF could be used as a drug delivery system to support GTR/GBR and enhance their biomaterial bioactivity. Chai et al[47] conducted a comparative analysis study to compare the cellular regenerative activity of human dental pulp cells (hDPCs) when cultured with either i-PRF or traditional PRP. The findings from the study suggested that i-PRF promoted higher regeneration potential of hDPCs when compared with traditional PRP. Furthermore, i-PRF also reduced the inflammatory condition created by lipopolsacharrides and maintained a supportive regenerative ability for stimulation of odontoblastic differentiation and reparative dentin in hDPCs. Bennardo et al[48] conducted a split mouth randomized controlled trial to compare the efficacy of i-PRF and triamcinolone acetonide (TA) injective therapies in patients with symptomatic oral lichen planus (OLP). The results obtained with i-PRF are similar to those obtained with TA. It was concluded that although i-PRF injections do not represent a standard treatment option, they have proved to be equally effective in reducing symptoms and dimensions of OLP lesions.
The VISTA technique has been applied for gingival recession coverage using different regenerative materials like CTG[5,49,50], PRF[50,51], titanium PRF[51], ADM[6], GEM 21S[24], recombinant human platelet derived growth factor[4], and collagen membrane[52]; however, there was no study using i-PRF in combination with collagen membrane using VISTA technique for recession coverage.
In this split mouth randomized clinical trial, the full mouth plaque and gingival index scores remained low throughout the study period. It was observed that plaque and gingival index were increased in 1 mo, which could be due to the coronally advanced suture held in the facial enamel surface for 3 wk postoperatively leading to a difficulty in maintaining oral hygiene in the operated region. There was a reduction in the plaque and gingival indexes at the 3rd and 6th postoperative month, which is due to better patient compliance and regular oral hygiene instructions given to the patients, thereby enabling improved plaque control efficiency.
The change in mean probing depth in i-PRF with collagen membrane group was statistically insignificant between both groups, which is in accordance with observation by Geeti et al[53] Similarly, another study done by Mohamed et al[6] where they used acellular dermal matrix (ADM) for recession coverage showed reduction in probing depth score. The intergroup comparison in the present study was statistically insignificant at each time intervals which is in accordance with the study done by Subbareddy et al[3].
Recession depth in the present study revealed a significant reduction of the test and control groups at the end of 6 mo postoperatively. This is similar with the case series done by Raja Rajeswari et al[54] There was a significant difference in the intergroup comparison at 3 mo and 6 mo, which is in line with the split mouth study done by Subbareddy et al[3] in which VISTA + PRF was compared with VISTA + SCTG.
Reduction in recession width was statistically significant in each postoperative visit in comparison to baseline for both the groups. This is in agreement with the study done by Mansouri et al[5] in which VISTA was compared with coronally advanced flap procedure using connective tissue graft. The present study shows i-PRF with collagen membrane is equally effective in reducing the width of a recession when compared to VISTA with CTG.
There was a significant increase in attachment gain level for both i-PRF + CM and CM groups at 6 mo, which is in accordance with Mansouri et al[5]. Improvement in clinical attachment may be because of recession coverage that results from coronal shift of attachment apparatus during coronally advance flap procedures.
In the present study, the width of keratinized gingiva in the subjects of both groups showed significant increase in 1 mo and 3 mo, and it was sustained at least until 6 mo. These results are in accordance with study done by Mohamed et al[6], though the study used VISTA + PRF for recession coverage. Similarly, a study done by Dandu et al[24] showed gain in width of keratinized gingiva in which VISTA with collagen membrane enhanced with GEM 21S was used for recession coverage.
There was a significant gain in the mean thickness of keratinized gingiva in both the test and control groups, which is similar to the results of study using VISTA with PRF done by Geeti et al[53] and Raja Rajeswari et al[54].
In the overall percentage of root coverage of the present study, it was observed that at 1 mo there was 91% and 82% of root coverage, which reduced to 67% and 53% of root coverage at 6 mo for test and control groups, respectively. It was also observed that at the end of 6 mo, 25% (5) of the sites had complete root coverage for test group while only 5% (1) of the sites had complete root coverage for control group. Similarly, in the study by Mansouri et al[5], mean root coverage achieved was 70.69%, with 50% of cases having complete root coverage in the VISTA with CTG group. A study done by Subbareddy et al[3] showed that in the test group involving VISTA with PRF, 30.33% of sites obtained complete root coverage, whereas the remaining sites constituting 69.67% partial root coverage.
In the overall assessment of the results of the study, it was observed that probing depth, recession depth, recession width, and the relative attachment level, both the test and control sites had similar results. Width of keratinized tissue, thickness of keratinized tissue, and the percentage of root coverage in i-PRF with collagen membrane had better results than sites where only collagen membrane was used for recession coverage. This can be attributed to the VISTA technique, as it was a minimally invasive surgery which not only reduces the trauma to the operating site, but also preserves the major blood vessels of the flap and blood supply to the area, resulting in better nourishment of the collagen membrane.
The use of i-PRF is not only helpful for enrichment of the collagen membrane with various growth factors responsible for tissue regeneration, but also injecting it into the mesial and distal aspects into periodontal ligament and into the facial aspects of gingiva is an added benefit for stimulation of wound healing[55].
This study must be interpreted with consideration of relatively small sample size (13 subjects) and the shorter study duration (6 mo).
Based on the results of the study it can be concluded that the use of the minimally invasive VISTA technique, along with a collagen membrane acting as scaffold and chemoattractant with the added benefit of an injectable form of PRF with the capacity of releasing more growth factors and regenerative cells responsible for tissue regeneration, can be successfully used as a treatment method for multiple or isolated gingival recessions of Miller’s class-I and class-II defects though further multicentric longitudinal studies are needed to be carried out to validate to the results of the present study.
Gingival recession is being treated using various therapeutic approaches with varying degrees of success depending on the etiology and treatment approach. Among them, coronally advanced flap technique with a connective tissue graft is considered the gold standard for soft tissue augmentation and periodontal root coverage. However, this technique has some disadvantages, including harvesting from a donor site, limited tissue availability, and increased potential for post-harvesting morbidity. With the introduction of the minimally invasive vestibular incision subperiosteal tunnel access (VISTA) technique, similar results could be obtained. It tries to preserve the interdental papillae and unhampered blood supply while maintaining the marginal integrity and minimizing the micromotion of flap for faster wound healing with no visible scarring to maximize the aesthetic outcome. This study is an attempt to find the efficacy of the VISTA technique using collagen membrane soaked in autologous injectable formulation of platelet-rich fibrin, termed as injectable platelet-rich fibrin (i-PRF) for the treatment of multiple gingival recession coverage.
The main topic is to compare the efficacy of minimally invasive VISTA technique for the treatment of multiple gingival recession coverage using a collagen membrane or a collagen membrane soaked in i-PRF. Placement of the initial vertical access incision and the subperiosteal tunnel entrance being far from the gingival margin reduces the risk of trauma to the gingiva, while at the same time maintaining the integrity of the interdental papilla by avoiding papillary reflection and marginal tissue loss of the teeth being treated. It also provides wider access to the surgical region and improves visualization through a single incision with no visible scarring, maximizing the aesthetic outcome. The positioning of the gingival margin to the most coronal level of the adjacent interproximal papilla rather than to the cementoenamel junction, with the help of the coronally anchored suturing technique on the facial surface of each tooth, effectively minimizes micromotion of the regenerative site and prevents apical relapse of the gingival margin during the initial stages of healing. The use of i-PRF also has similar properties as PRF, but has the added benefit of being available in an injectable form. It contains all components of PRF, including platelets, white blood cells, and all the clotting factors comprising fibrinogen in an uncoagulated form, making them readily available. The major advantage of i-PRF over other platelet concentrates is that it contains a greater number of regenerative cells with higher concentrations of growth factors and leukocytes. With the increased number of cells, there is possibly an increased release of growth factors like platelet-derived growth factor, epidermal growth factor, transforming growth factor and insulin-like growth factor-1.
The main objective is to compare the efficacy of the VISTA technique incorporating collagen membrane alone with the VISTA technique with collagen membrane soaked in injectable platelet-rich fibrin for gingival recession coverage in terms of clinical parameters like pocket depth, recession width, recession depth, width of keratinized gingiva, thickness of keratinized tissue, and the percentage of root coverage. In the overall assessment of the result of the study, it was observed that probing depth, recession depth, recession width, and relative attachment level are similar between the test and control sites. However, the width of keratinized tissue, the thickness of keratinized tissue, and the percentage of root coverage had better results for sites treated with i-PRF than sites where only collagen membrane was used for recession coverage. This can be attributed to the VISTA technique as it was a minimally invasive surgery, which not only reduces the trauma to the operating site, but also preserves the major blood vessels of the flap and blood supply to the area, resulting in better nourishment of the collagen membrane. The use of i-PRF is not only helpful for the enrichment of collagen membrane with various growth factors responsible for tissue regeneration, but also injecting it into the mesial and distal aspects of periodontal ligament and into the facial aspects of gingiva is an added benefit for stimulation of wound healing.
The data was analyzed using SPSS Ver 22 for windows, (IBM Corp, Armonik, United States). Descriptive statistics were expressed as a mean with standard deviations and proportions. Normally distributed data were analyzed using a paired t-test for intragroup comparison and an unpaired t-test for intergroup comparison. Skewed data were analyzed using the Wilcoxon signed rank test for intragroup and Mann-Whitney U test for intergroup comparison. The level of significance was set at P < 0.05.
The result of the study observed that probing depth, recession depth, recession width, and relative attachment level are similar in test sites compared with control sites. However, the width of keratinized tissue, the thickness of keratinized tissue, and the percentage of root coverage had better results in sites treated with i-PRF with collagen membrane than sites where only collagen membrane was used for recession coverage. This can be attributed to the VISTA technique, as it is a minimally invasive surgery which not only reduces the trauma to the operating site, but also preserves the major blood vessels of the flap and blood supply to the area, resulting in better nourishment of the collagen membrane. The use of i-PRF is not only helpful for the enrichment of collagen membrane with various growth factors responsible for tissue regeneration, but also injecting it into the mesial and distal aspects of periodontal ligament and into the facial aspects of gingiva is an added benefit for stimulation of wound healing.
The VISTA technique has been applied for gingival recession coverage using different regenerative materials like connective tissue graft, PRF, titanium PRF, acellular dermal matrix, GEM 21S, recombinant human platelet derived growth factor, and collagen membrane; however, there was no study using i-PRF in combination with collagen membrane using VISTA technique for gingival recession coverage. The results of the study proposed that the use of minimally invasive VISTA technique, along with collagen membrane with the added benefit of the injectable form of platelet-rich fibrin have the capacity of releasing more growth factors and regenerative cells responsible for tissue regeneration, can be successfully used as a treatment method for multiple or isolated gingival recessions of Miller’s class-I and class-II defects.
This study must be interpreted with consideration of the relatively small sample size (13 subjects) and shorter study duration (6 mo). A long term follow-up study with larger sample size is required.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bennardo F, Italy S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Li X
| 1. | Tugnait A, Clerehugh V. Gingival recession-its significance and management. J Dent. 2001;29:381-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Niswade G. Paripex- Gingival recession-a stigma to the tooth. Indian J Res. 2017;3:72-75. |
| 3. | Subbareddy BV, Gautami PS, Dwarakanath CD, Devi PK, Bhavana P, Radharani K. Vestibular Incision Subperiosteal Tunnel Access Technique with Platelet-Rich Fibrin Compared to Subepithelial Connective Tissue Graft for the Treatment of Multiple Gingival Recessions: A Randomized Controlled Clinical Trial. Contemp Clin Dent. 2020;11:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Zadeh HH. Minimally invasive treatment of maxillary anterior gingival recession defects by vestibular incision subperiosteal tunnel access and platelet-derived growth factor BB. Int J Periodontics Restorative Dent. 2011;31:653-660. [PubMed] |
| 5. | Dutta S, Nasim F, Pal D, Singh MK, Chakrabarty H. A novel approach of treating gingival recession by Vestibular Incision Subperiosteal Tunnel Access along with palatal connective tissue graft. IP Int J Periodontol Implantol. 2020;5:37-40. [DOI] [Full Text] |
| 6. | Mansouri SS, Moghaddas O, Torabi N, Katayoun Ghafari. Vestibular incisional subperiosteal tunnel access vs coronally advanced flap with connective tissue graft for root coverage of Miller’s class I and II gingival recession: A randomized clinical trial. J Adv Periodontal Implant Dent. 2019;11:12-20. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Mohamed A, Marssafy L. Comparative clinical study between Tunnel and VISTA approaches for the treatment of multiple gingival recessions with acellular dermal matrix allograft. Egypt Dent J. 2020;66:247-259. [DOI] [Full Text] |
| 8. | Reddy S, Prasad MGS, Bhowmik N, Singh S, Pandit HR, Vimal SK. Vestibular incision subperiosteal tunnel access (VISTA) with platelet-rich fibrin (PRF) and connective tissue graft (CTG) in the management of multiple gingival recession- A case series. Int J Appl Dent Sci. 2016;2:34-37. |
| 9. | Kimble KM, Eber RM, Soehren S, Shyr Y, Wang HL. Treatment of gingival recession using a collagen membrane with or without the use of demineralized freeze-dried bone allograft for space maintenance. J Periodontol. 2004;75:210-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Garg S, Arora SA, Chhina S, Singh P. Multiple Gingival Recession Coverage Treated with Vestibular Incision Subperiosteal Tunnel Access Approach with or without Platelet-Rich Fibrin - A Case Series. Contemp Clin Dent. 2017;8:464-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1016] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 12. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 13. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 481] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 14. | Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 689] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 15. | Jain V, Triveni MG, Kumar AB, Mehta DS. Role of platelet-rich-fibrin in enhancing palatal wound healing after free graft. Contemp Clin Dent. 2012;3:S240-S243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig. 2017;21:2619-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 17. | Agrawal DR, Jaiswal PG. Injectable Platelet Rich Fibrin (i-PRF): A Gem in Dentistry. Int J Cur Res Rev. 2020;12:25-30. [DOI] [Full Text] |
| 18. | Rutuja PK, Lisa C, Rakhewar PS. The vestibular incision subperiosteal tunnel access (VISTA) for treatment of maxillary anterior gingival recession defects- a case report. Int J Health Sci Res. 2017;7:360-365. |
| 19. | Buti J, Baccini M, Nieri M, La Marca M, Pini-Prato GP. Bayesian network meta-analysis of root coverage procedures: ranking efficacy and identification of best treatment. J Clin Periodontol. 2013;40:372-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Cairo F, Nieri M, Pagliaro U. Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. J Clin Periodontol. 2014;41 Suppl 15:S44-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 21. | Moghaddas H, Esfahanian V, Moghaddas O. Efficacy of subepithelial connective tissue grafts in the treatment of Miller’s Class I and II gingival recessions. J Isfahan Dent Sch. 2011;7:337-353. |
| 22. | Roccuzzo M, Bunino M, Needleman I, Sanz M. Periodontal plastic surgery for treatment of localized gingival recessions: a systematic review. J Clin Periodontol. 2002;29 Suppl 3:178-94; discussion 195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Chatterjee A, Sharma E, Gundanavar G, Subbaiah SK. Treatment of multiple gingival recessions with vista technique: A case series. J Indian Soc Periodontol. 2015;19:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Dandu SR, Murthy KR. Multiple Gingival Recession Defects Treated with Coronally Advanced Flap and Either the VISTA Technique Enhanced with GEM 21S or Periosteal Pedicle Graft: A 9-Month Clinical Study. Int J Periodontics Restorative Dent. 2016;36:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Cortellini P, Clauser C, Prato GP. Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol. 1993;64:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Paolantonio M. Treatment of gingival recessions by combined periodontal regenerative technique, guided tissue regeneration, and subpedicle connective tissue graft. A comparative clinical study. J Periodontol. 2002;73:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Al-Hamdan K, Eber R, Sarment D, Kowalski C, Wang HL. Guided tissue regeneration-based root coverage: meta-analysis. J Periodontol. 2003;74:1520-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Kapare K, Gopalakrishnan D, Kathariya R, Tyagi T, Bagwe S. Evaluation of efficacy of a novel resorbable collagen membrane for root coverage of Miller's Class I and Class II recession in the maxillary anteriors and premolars. J Indian Soc Periodontol. 2016;20:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Yuan KH, Kun LH. Systematic Review of the Clinical Performance of Connective Tissue Graft and Guided Tissue Regeneration in the Treatment of Gingival Recessions of Miller's Classification Grades I and II. J Exp Clin Med. 2010;2:63-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Wang HL, Al-Shammari KF. Guided tissue regeneration-based root coverage utilizing collagen membranes: technique and case reports. Quintessence Int. 2002;33:715-721. [PubMed] |
| 31. | Wang HL, O'Neal RB, Thomas CL, Shyr Y, MacNeil RL. Evaluation of an absorbable collagen membrane in treating Class II furcation defects. J Periodontol. 1994;65:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Choukroun J, Adda F, Schoeffer C, Vervelle A. The opportunity in perio-implantology: The PRF. Implantodontie. 2000;42:55-62. |
| 33. | Chandran P, Sivadas A. Platelet-rich fibrin: Its role in periodontal regeneration. Saudi J Dent Res. 2013;5:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Saini K, Chopra P, Sheokand V. Journey of Platelet Concentrates: A Review. Biomed Pharmacol J. 2020;13:185-191. [DOI] [Full Text] |
| 35. | Prathamesh F, Vijaysinh M. Platelet concentrates - preparation protocols and recent advances. Int J Sci Res. 2010;9:1-3. [DOI] [Full Text] |
| 36. | Gil A, Bakhshalian N, Min S, Zadeh HH. Treatment of multiple recession defects with vestibular incision subperiosteal tunnel access (VISTA): A retrospective pilot study utilizing digital analysis. J Esthet Restor Dent. 2018;30:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Shah R, Gowda TM, Thomas R, Kumar T, Mehta DS. Biological activation of bone grafts using injectable platelet-rich fibrin. J Prosthet Dent. 2019;121:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44:87-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 39. | Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 40. | Mourão CF, Valiense H, Melo ER, Mourão NB, Maia MD. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev Col Bras Cir. 2015;42:421-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 41. | Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 42. | Varela HA, Souza JCM, Nascimento RM, Araújo RF Jr, Vasconcelos RC, Cavalcante RS, Guedes PM, Araújo AA. Injectable platelet rich fibrin: cell content, morphological, and protein characterization. Clin Oral Investig. 2019;23:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | İzol BS, Üner DD. A New Approach for Root Surface Biomodification Using Injectable Platelet-Rich Fibrin (I-PRF). Med Sci Monit. 2019;25:4744-4750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Ucak Turer O, Ozcan M, Alkaya B, Surmeli S, Seydaoglu G, Haytac MC. Clinical evaluation of injectable platelet-rich fibrin with connective tissue graft for the treatment of deep gingival recession defects: A controlled randomized clinical trial. J Clin Periodontol. 2020;47:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Ozsagir ZB, Saglam E, Sen Yilmaz B, Choukroun J, Tunali M. Injectable platelet-rich fibrin and microneedling for gingival augmentation in thin periodontal phenotype: A randomized controlled clinical trial. J Clin Periodontol. 2020;47:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Al-Maawi S, Herrera-Vizcaíno C, Orlowska A, Willershausen I, Sader R, Miron RJ, Choukroun J, Ghanaati S. Biologization of Collagen-Based Biomaterials Using Liquid-Platelet-Rich Fibrin: New Insights into Clinically Applicable Tissue Engineering. Materials (Basel). 2019;12:3993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Chai J, Jin R, Yuan G, Kanter V, Miron RJ, Zhang Y. Effect of Liquid Platelet-rich Fibrin and Platelet-rich Plasma on the Regenerative Potential of Dental Pulp Cells Cultured under Inflammatory Conditions: A Comparative Analysis. J Endod. 2019;45:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Bennardo F, Liborio F, Barone S, Antonelli A, Buffone C, Fortunato L, Giudice A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Kriti V, Triveni G, Kumar AB, Mehta DS. Minimally Invasive Treatment of Mandibular Anterior Lingual Defects by Vestibular Incision Subperiosteal Tunnel Access (VISTA Technique) and Connective Tissue Graft: A Case Report. Clin Adv Periodontics. 2017;7:1-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 50. | Reddy S, Prasad MGS, Bhowmik N, Singh S, Pandit HR, Vimal SK. Vestibular incision subperiosteal tunnel access (VISTA) with platelet rich fibrin (PRF) and connective tissue graft (CTG) in the management of multiple gingival recession- A case series. Int J Appl Dent Sci. 2016;2:34-37. |
| 51. | Agarwal MC, Rathore P, Gummaluri SS, Agarwal P, Kumari S. Vestibular Incision Subperiosteal Tunnel Access with Titanium-Prepared Platelet-Rich Fibrin - A Golden Approach for Treating Multiple Recession Defects in Esthetic Zone. Contemp Clin Dent. 2019;10:682-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Pawar B, Bhuse K, Shetty A, Shetty D. A Fantastic Approach for Multiple Recession Coverage: Vestibular Incision Subperiosteal Tunnel Access Technique (Vista)-A Case Report. IOSR J Dent Med Sci. 2016;15:52-56. |
| 53. | Geeti G, Komal P, Mansi B, Manish K, Ashish K. Platelet Rich Fibrin (PRF) Reinforced Vestibular Incision Subperiosteal Tunnel Access (VISTA) Technique for Recession Coverage. Clin Adv Periodontics. 2014;5:248-253. [DOI] [Full Text] |
| 54. | S RR, Kumar TA, Gowda TM, Mehta DS, Kumar A. Management of Multiple Gingival Recessions with the VISTA Technique: An 18-Month Clinical Case Series. Int J Periodontics Restorative Dent. 2018;38:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Tuttle D, Kurtzman G, Bernotti AL. Gum Drop Technique: Minimally Invasive Soft-Tissue Platelet-Rich Plasma Grafting for Marginal Soft-Tissue Recession. Compend Contin Educ Dent. 2018;39:e9-e12. [PubMed] |