INTRODUCTION
Our immune system can recognize some cancers with a high frequency of mutations as foreign and stimulate a tumor-specific immune response. Immune checkpoint inhibitors (ICIs) are antibodies that block inhibitory immune regulators such as cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1) and programmed death-ligand 1 (PD-L1). Checkpoint blockade by ICI enhance the body’s defense against cancer[1]. Since 2011, the Food and Drug Administration has approved several ICIs including CTLA-4 inhibitor (ipilimumab), PD-L1 inhibitors (atezolizumab, avelumab and durvalumab), and PD-1 inhibitors (nivolumab and pembrolizumab). These ICIs demonstrated tremendous efficacy for a broad range of cancers, including advanced-stage melanoma[2], renal cell carcinoma[3], and non-small-cell lung cancer[4].
Compared to chemotherapy, immune-related adverse events involving skin, liver and gastrointestinal (GI) tract are more common with ICIs[5]. Diarrhea/colitis represent the most common adverse effect[5,6] and is the most common reason for discontinuation of ICI therapy[2,7]. Its clinical presentations range from mild to severe colitis with some patients experiencing rapid progression to serious complications including bowel perforation and even death. The Common Terminology Criteria for Adverse Events version 5 is commonly used to access and grade disease severity. It is graded from 1 to 5 with grade 1 representing mild symptoms and grade 5 patient’s death related to ICI-induced colitis[8]. Prompt diagnosis and management of ICI-induced colitis is crucial for optimal outcome[9-11]. Unfortunately, its clinical, endoscopic and histopathologic presentations are non-specific and overlap with those of colitis caused by other etiologies, such as infection, other medications and graft-versus-host disease (GvHD). Thus, it may be challenging to make a timely diagnosis of ICI-induced colitis. For these reasons we believe it is important to raise awareness for this newer entity. In this review, we focus on the clinical features, differential diagnosis and management of ICI-induced colitis. Especially, we provide detailed histological differential diagnoses and supply ample microscopic images.
CLINICAL FEATURES
Incidence
The frequency of colitis complicating ICI therapy is variable depending on the ICI regimen and individual patient characteristics[5]. The highest incidence has been reported in patients treated with anti-CTLA-4 antibodies, ranging from 3.4%-15.5% for all grade colitis and 2.3%-8.3% for grade 3-4 colitis, followed by combination of anti-CTLA-4 and PD-1 with an incidence rate of 0.7%-12.8% for all grade colitis and 0.5%-8.3% for grade 3-4 colitis. The lowest incidence was in patients treated with anti-PD-1/L1 checkpoint inhibitors, ranging from 0.7%-2.6% for all grade colitis and 0.3%-1.0% for grade 3-4 colitis[12]. Patients with melanoma receiving anti-PD-1 agents seem to have a higher risk of developing ICI-induced colitis than those who receive anti PD-1 agents for non-small cell carcinoma [odds ratio (OR): 4.2; 95% confidence interval (CI): 1.3-14.0][7]. Although the mechanism is unclear, stage IV malignancies were associated with a lower incidence of diarrhea and colitis when compared to patients with stage III malignancies (35.3% vs 72.0%; P = 0.001)[13]. Caucasian patients have high risk of developing diarrhea/colitis (OR: 5.76; 95%CI: 2.03-16.36), while patients’ age and sex have no association with the incidence of diarrhea and colitis[13].
Interval from drug infusion to colitis
The median interval to onset of diarrhea is approximately 4-8 wk[5] after the first infusion. However, the range is broad with some patients experiencing symptoms as early as 1 wk after the exposure. Some patients developed symptoms months or even two years after discontinuation of the therapy. In these rare cases, the underlying mechanism was unclear[14,15].
Clinical presentations
Clinical presentations are usually non-specific and include diarrhea (92%), abdominal pain (82%), hematochezia (64%), fever (46%) and vomiting[16]. Disease severity is variable and can range from mild diarrhea to life-threatening colitis. In a meta-analysis, the overall mortality rate associated with ICI-induced colitis was 5% (225/3905). In this study, the correlation between the mortality and the grades of colitis was not analyzed. Sixty percent (135/225) of the fatality was from CTLA-4 inhibitor, 25.8% (58/225) from anti PD-1 or PD-L1 and 14.2% (32/225) from combined therapy[17]. Toxicities leading to fatal outcomes tend to occur early in the disease course and evolve rapidly, especially in patients receiving combination of agents. The median time to the onset of a fatal event is -14.5 d for ICI combination therapy, vs 40 d for ICI monotherapy (P < 0.001)[17]. ICI-induced colitis should be considered in the differential diagnosis in any patient treated with ICIs who presents with abdominal pain and or diarrhea.
ENDOSCOPIC FEATURES
Endoscopic presentation of ICI-related colonic inflammation varies from normal appearance, to edema, erythema, inflammatory exudate, erosions, aphthae, and ulcerations[18,19]. In the study published by Wang et al[18], ulceration was found in 40% (21/53), non-ulcerative inflammation in 42% (22/53) and no gross inflammation in 19% (10/53) of patients. Left-sided colitis was seen in 42% (18/43), left and right-sided colitis in 40% (17/43), ileocolonic disease in 14% (6/43) and 2% (1/43) had inflammation confined to the ileum. The distribution of the inflammation was diffuse (22/43, 51%), patchy (18/43, 42%) and less commonly, segmental (3/43, 7%)[18].
PATHOLOGICAL FINDINGS
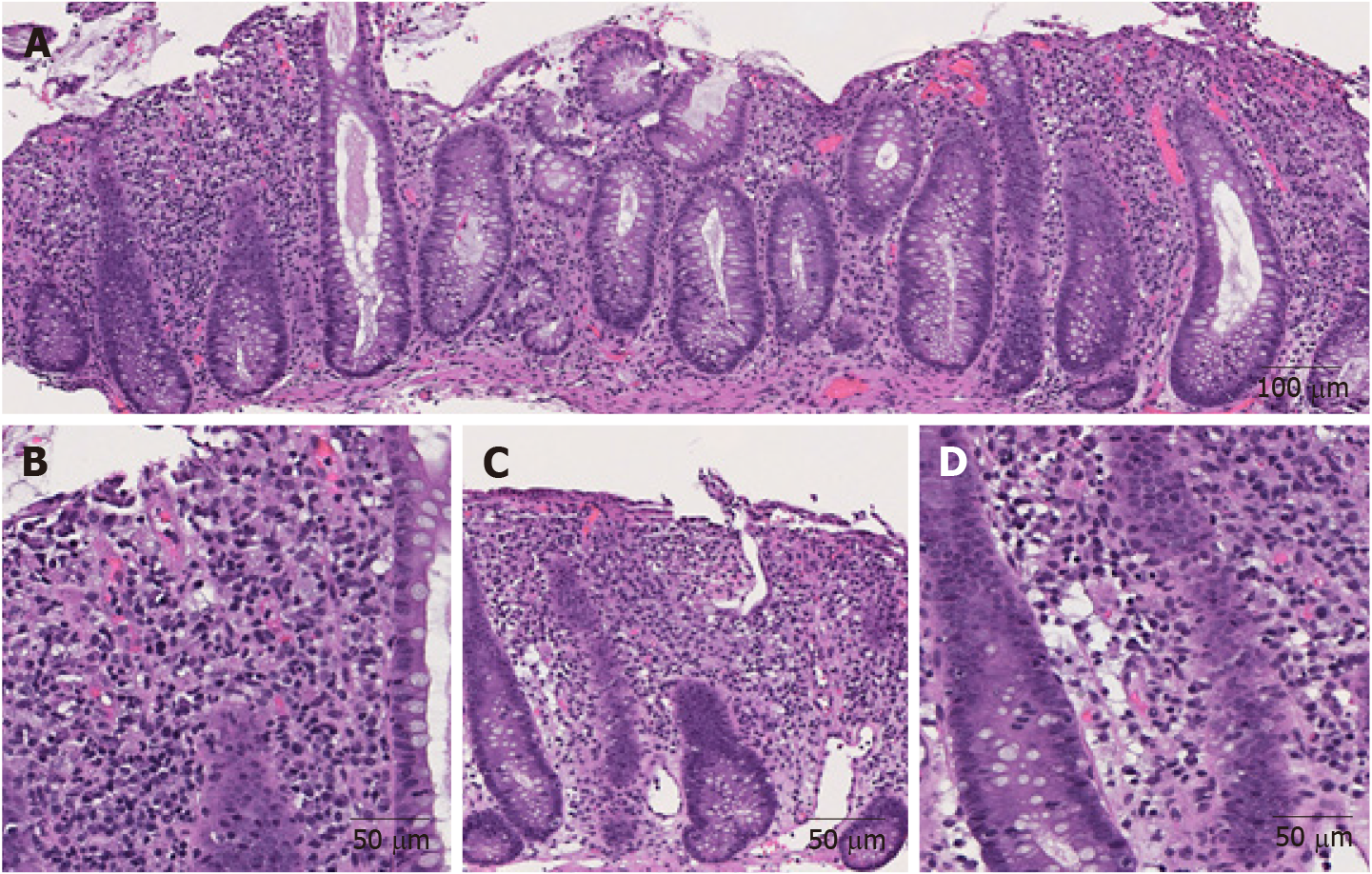
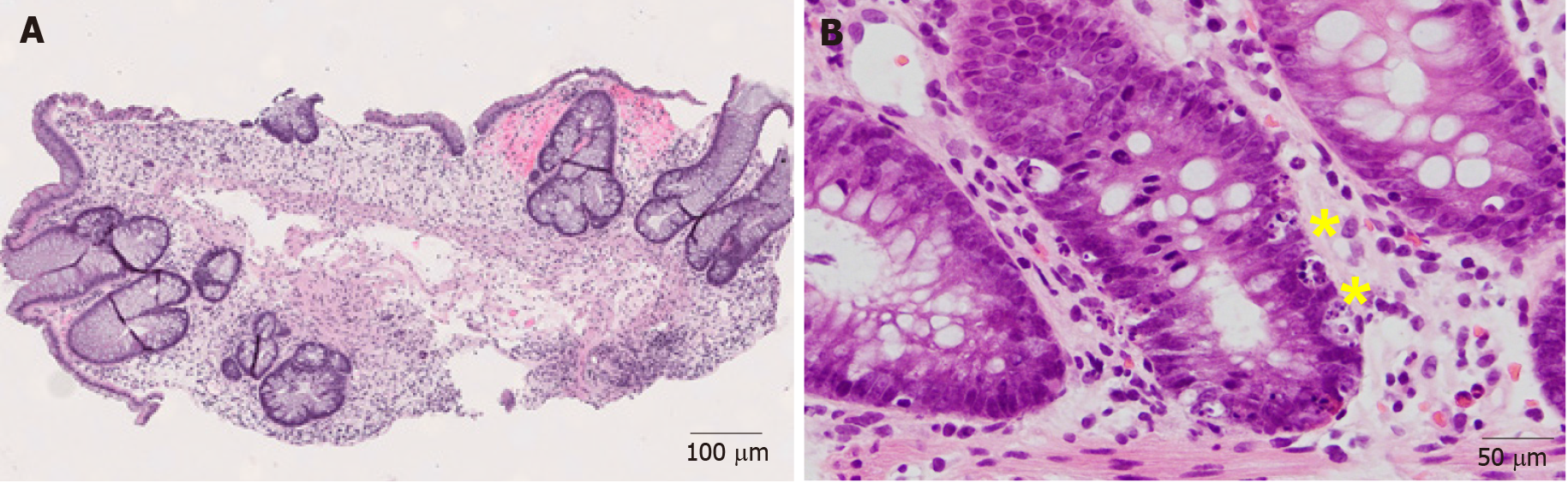
The morphological features of ICI-induced colitis are similar between patients receiving anti-CTLA-4, anti-PD-1 and anti-PD-L1 regimens. The spectrum of abnormalities ranges from minimal to severely active colitis. The histologic features may resemble those of infectious colitis characterized by increased inflammatory infiltrate in the lamina propria with lymphocytes, plasma cells, neutrophils, and intraepithelial neutrophils (Figures 1A and B)[18,19]. Cryptitis and crypt abscess are commonly seen (Figures 1C and D)[18,19]. Mild to prominent intraepithelial lymphocytosis (17%) and apoptotic cells (42%) may be evident[20]. Crypt architecture irregularities (36%) such as shortening of the crypts, loss of crypts, or slight irregularities in diameter and shape of the crypts (Figure 1C) may also be seen[19]. In addition, the presence of crypt irregularities might lead to a misdiagnosis of inflammatory bowel disease (IBD). Patterns of microscopic colitis (e.g., lymphocytic colitis and collagenous colitis) have been reported in about 10% of the cases[21,22].
Figure 1 Representative images of immune checkpoint inhibitor-induced colitis in a patient with metastatic melanoma treated with nivolumab and ipilimumab for 2 mo (Hematoxylin and eosin).
A: Active colitis characterized by mixed inflammatory cell infiltrates in the lamina propria and surface erosion (100 ×); B: High magnification of A. Note the neutrophils, lymphocytes, and plasma cells in the lamina propria (200 ×); C: Active colitis with mild crypt architectural irregularity (100 ×); D: Active colitis with neutrophilic cryptitis (200 ×).
PATHOPHYSIOLOGY
It is hypothesized that autoimmune related events are responsible for ICI-induced colitis. For example, the blockage of ICI leads to the release of T cells that had been previously suppressed. Likewise, immunosuppression therapy is usually effective for ICI-induced colitis[8,23,24]. In a study using endoscopic colon biopsies of ICI-induced colitis, marked activation and proliferation of cytotoxic effector CD8+ T cells was observed in the colonic tissue. T cell receptor sequence analysis showed that a substantial subset of these colitis-associated CD8+ T cells had originated from tissue-resident CD8+ T-cell populations. The authors speculated that following the activation of CD8+ T cells in the tissue, additional CD8+ and CD4+ T cells are recruited from the blood, leading to clinical progression of the colitis[25].
There is increasing evidence that gut microbiota plays an important role in the pathogenesis of ICI-induced colitis. For example, Chaput et al[26] reported that Bacteroidetes transplantation is associated with worse cancer outcome but lower incidence of ICI-induced colitis, whereas Faecalibacterium, in particular F. prausnitzii L2-6, butyrate-producing bacterium L2-21 and G. formicilis ATCC 27749, are associated with the development of ICI-induced colitis but favorable oncologic outcome. Currently the underlying mechanisms remain unclear. However, an animal study demonstrated that intestinal microbiome could mediate immune-related inflammation through host immune system[27].
DIFFERENTIAL DIAGNOSIS
A presumptive diagnosis of ICI-induced colitis can be considered in patients who develop diarrhea and abdominal pain while taking ICIs and have supportive endoscopic and/or histologic findings on GI biopsies. However, these clinical, endoscopic and histologic findings are non-specific; they can be seen in colitis caused by other etiologies. The diagnosis of ICI-induced colitis is one of exclusion and requires exclusion of other competing etiologies.
Infectious colitis
Colonic infection by bacteria, viruses, or parasites accounts for the majority of cases of patients presenting with acute diarrhea, fever, tenesmus and abdominal pain[24]. Common clinical presentation of infectious colitis is indistinguishable from that of ICI-induced colitis. Given that ICI-induced colitis patients are at increased risk for infectious colitis and ICI-induced colitis requires immunosuppressive therapy[8,23,24], microbiological studies and/or stool culture should be performed first to exclude the common infectious etiologies. The most common food borne pathogens in United States include Campylobacter, Salmonella, Escherichia coli O157.H7 and Norwalk virus. Common non-foodborne agents include Shigella, Yersinia, Coxsackie virus, rotavirus, enterovirus, and adenovirus[28].
For infectious colitis, endoscopic findings are usually non-specific, and show edema, erythema, erosion and ulceration. Microscopically, inflammatory infiltration of the lamina propria and neutrophil-mediated cryptitis and/or crypt abscess are often evident[30]. Unfortunately, endoscopic and microscopic findings are usually non-specific for distinguishing ICI-induced colitis from different infectious etiologies[28]. However, some specific histologic patterns may be helpful in identifying the infectious etiologies.
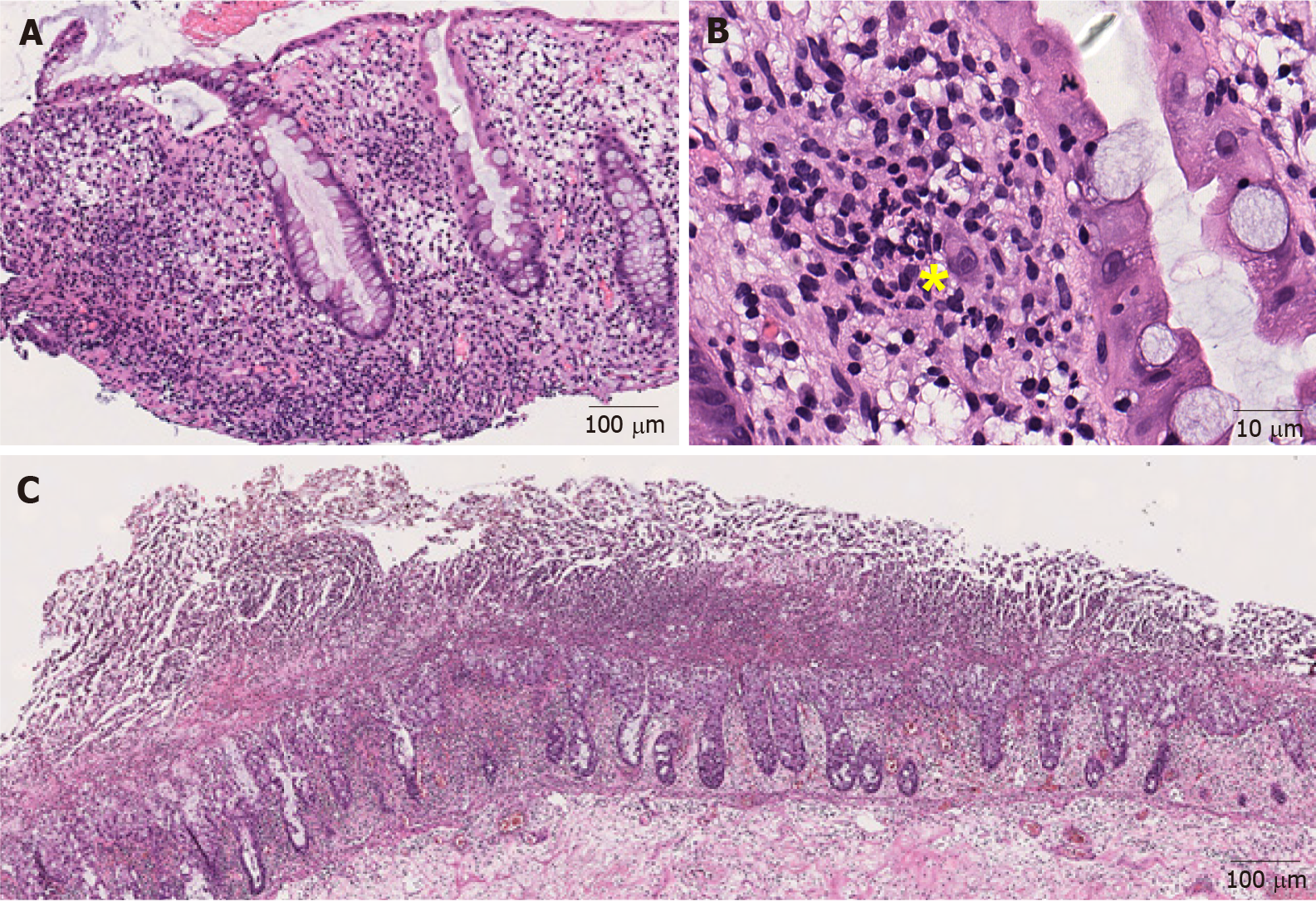
Cytomegalovirus: Cytomegalovirus (CMV) is an important opportunistic infectious agent in frankly immunosuppressed patients, immunocompetent patients undergoing chemotherapy, and the elderly[29]. CMV-associated colitis has been reported in patients with corticosteroid- refractory ICI-induced colitis[30-32]. It causes an active colitis injury pattern (Figure 2A). The diagnosis is made by identifying the typical large cells with basophilic cytoplasm and pathognomonic large, oval, eosinophilic intranuclear inclusions (owl-eye inclusions), usually seen at the base of the ulcer (Figure 2B). However, the sensitivity of detecting viral inclusions on histologic examination is low[33]. Immunohistochemical staining can be very helpful when the inclusions are poorly formed, rare, or obscured by inflammation[34].
Figure 2 Representative images of infectious colitis (Hematoxylin and eosin).
A: Low magnification view of cytomegaloviral (CMV) colitis. Notice the lymphocytes and neutrophils in the lamina propria (100 ×); B: Note an owl-eye inclusion characterized by enlarged nucleus with oval, eosinophilic intranuclear inclusion surrounded by clear halo, consistent with CMV inclusion (400 ×); C: Clostridium difficile colitis. Pseudomembranes composed of fibrin, neutrophils and necrotic epithelial cells are on the surface of the mucosal glands (40 ×). Yellow sign notes a viral inclusiona.
Clostridium difficile: Clostridium difficile (C. difficile) is the most common cause of hospital-acquired infectious diarrhea and is strongly associated with the use of clindamycin, fluoroquinolones, cephalosporins, monobactams, and carbapenems[35]. The clinical symptoms associated with C. difficile infection range from mild, self-limiting diarrhea to fulminant colitis and toxic megacolon, leading to bowel perforation, sepsis and/or multisystem organ failure[36]. C. difficile colitis causes pseudo-membranous colitis. Endoscopically, pseudomembranous colitis is characterized by elevated, discontinuous, yellow-white nodules or plaques. Microscopically, these nodules or plaques consist of mushroom-like laminated lesions on the surface of mucosal glands, composed of fibrin-rich exudates and mucus with embedded neutrophils and necrotic epithelial cells (Figure 2C)[37]. Pseudomembranous colitis can also be seen in other infections such as with E. coli O157, Shigella, and other Shiga toxin-producing organisms as well as acute ischemia, acute radiation injury, and in association with drugs, such as albendazole[38-40]. Superimposed[41] and concurrent[42] C. difficile infections have been documented in patients with ICI-induced colitis. Laboratory testing for either free toxins or toxigenic C. difficile in stool is required for confirmation of C. difficile colitis[43].
Yersinia: Yersinia enterocolitis is caused by Y. enterocolitica or Y. pseudotuberculosis, which are gram-negative coccobacilli. It is transmitted mostly by contaminated food and water[37]. Yersinia is an entero-invasive organism that primarily involves Peyer’s patches and the surrounding mucosa, forming aphthous and linear ulcers often mimicking Crohn’s disease (CD)[37]. Microscopically, Yersinia infection is characterized by epithelioid granulomas with associated prominent lymphoid tissue and mucosal ulceration[44]. Microbiologic cultures or molecular testing may be required to confirm the diagnosis.
Other medication-mediated colitis
A broad spectrum of drugs can cause GI toxicity. Symptoms are non-specific and include bloating, abdominal pain, cramping, diarrhea, weight loss, mucosal bleeding or anemia[45]. Due to the clinical and histological similarity with ICI-induced colitis, it should be considered in the differential diagnosis for ICI-induced colitis. Drugs that may lead to clinically significant colitis are listed herein:
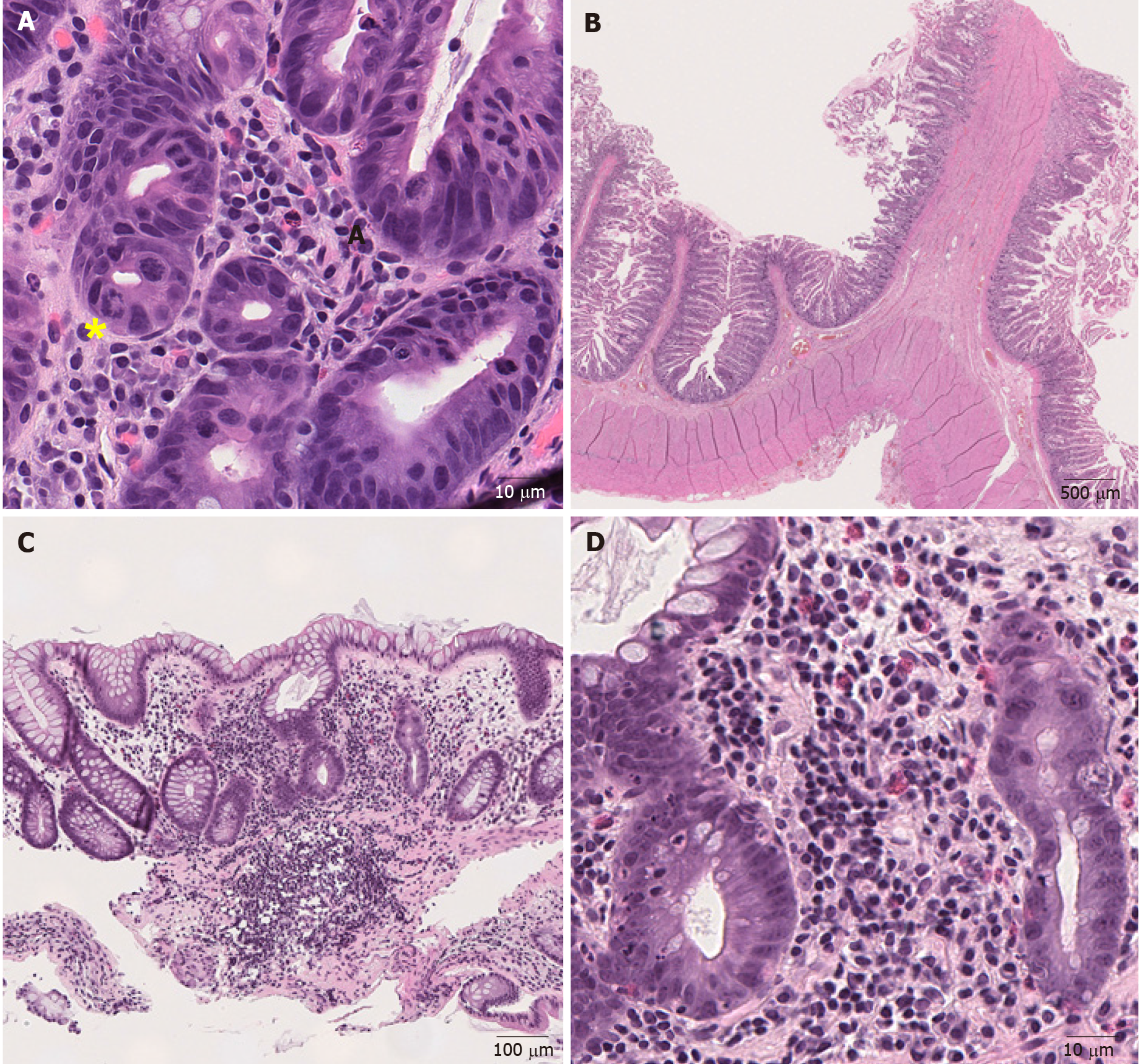
Chemotherapy: Chemotherapy-induced GI mucosal injury oftentimes manifests as diarrhea, odynophagia, nausea, emesis, anorexia, malabsorption, abdominal pain and cramping. Common endoscopic findings include mucosal erythema, erosions and ulcers. Microscopically, the crypts are attenuated and or dilated with minimal inflammation and the epithelium may undergo apoptosis and show some degree of atypia, such as hyperchromatic nuclei[46]. This finding is not specific and can be seen in other disorders, such as ischemic enterocolitis and GvHD. Some chemotherapeutic agents produce characteristic mucosal alterations. For example, taxanes prevent depolymerization[47], resulting in ring mitotic figures in the proliferative compartment of the mucosa throughout the GI tract (Figure 3A). Some patients are treated with traditional chemotherapy prior to ICIs, or receive ICIs combined with chemotherapy[48,49]. In these patients, chemotherapy-induced GI mucosal injury should be considered, though admittingly it may be very difficult to distinguish that with ICI-induced colitis. Medication history and clinical correlation are necessary to sort out the specific cause of colitis on an individual basis.
Figure 3 Representative images of medication-induced colitis (Hematoxylin and eosin).
A: Ring mitosis caused by docetaxel in duodenum (400 ×); B: Small bowel diaphragm. Fibrotic submucosa protrudes into the intestinal lumen and forms a diaphragm (8 ×); C: Low magnification view of mycophenolate mofetil-induced colitis. (100 ×); D: Higher magnification view of mycophenolate mofetil-induced colitis. There is an inflammatory cell infiltrate consisting of lymphocytes, plasma cells and eosinophils in the lamina propria with neutrophilic cryptitis (400 ×). Yellow sign notes a ring mitosis.
Nonsteroidal antiinflammatory drugs: Nonsteroidal antiinflammatory drugs (NSAIDs)-induced pathology can be seen throughout the GI tract. However, the only pathognomonic NSAIDs-associated lesion is diaphragm. Diaphragm is formed when the lumen of the small bowel is divided into short compartments by circular membranes of mucosa and sub-mucosa protruding into and obstructing the lumen (Figure 3B)[45]. Reactive gastropathy is highly suggestive of their usage but is not specific[45] and is seen in other conditions such as bile reflux[50]. Several forms of colitis associated with NSAIDs have been documented. The most common microscopic injury is epithelial erosion with mixed infiltration of lymphocytes and neutrophils[51,52]. NSAIDs-induced colitis may resemble lymphocytic colitis[51] and collagenous colitis[53], although the lymphocytosis and collagen deposition are usually patchier and less pronounced in NSAIDs-induced colitis.
All the histological presentations associated with NSAIDs use overlap with those of ICI-induced colitis. Given the widespread use of NSAIDs, NSAIDs-induced colitis should be always considered in the differential diagnosis for ICI-induced colitis. ICI induced adverse effect often involves multiple organs simultaneously[5], thus the history of NSAIDs use and the absence of other organ involvement would favor NSAIDs-induced colitis.
Mycophenolate mofetil: Mycophenolic acid is an immunosuppressive medication that is frequently used in solid organ transplant patients. One of the two forms, mycophenolate mofetil is well known to cause significant GI mucosal toxicity[54]. Nausea, vomiting, abdominal pain and watery diarrhea are frequent symptoms[54]. Endoscopic findings range from normal (47%), erythema (33%) to erosions/ulcers (19%)[55]. Histological findings include acute colitis pattern of injury with neutrophilic cryptitis or crypt abscesses (Figures 3C and D) (50%), crypt architecture distortion with lymphoplasmacyte-predominant lamina propria inflammation (36%), the presence of enterocyte apoptosis (Figure 3D) without lamina propria inflammation (8.3%), and mucin-depleted crypts with no or minimal lamina propria inflammation and crypt dropout (5.6%)[55]. All these features overlap with those of ICI-induced colitis. History of drug use is required to distinguish these two.
Many other drugs can damage the GI tract. FDA Adverse Event Reporting System Public Dashboard is a great platform to report and search for adverse events related to specific drugs and therapeutic biologic products (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard). Awareness of adverse effects related to drugs, careful review of the medication history and clinical correlation are essential to recognize and distinguish drug-induced GI injury from other causes.
IBD
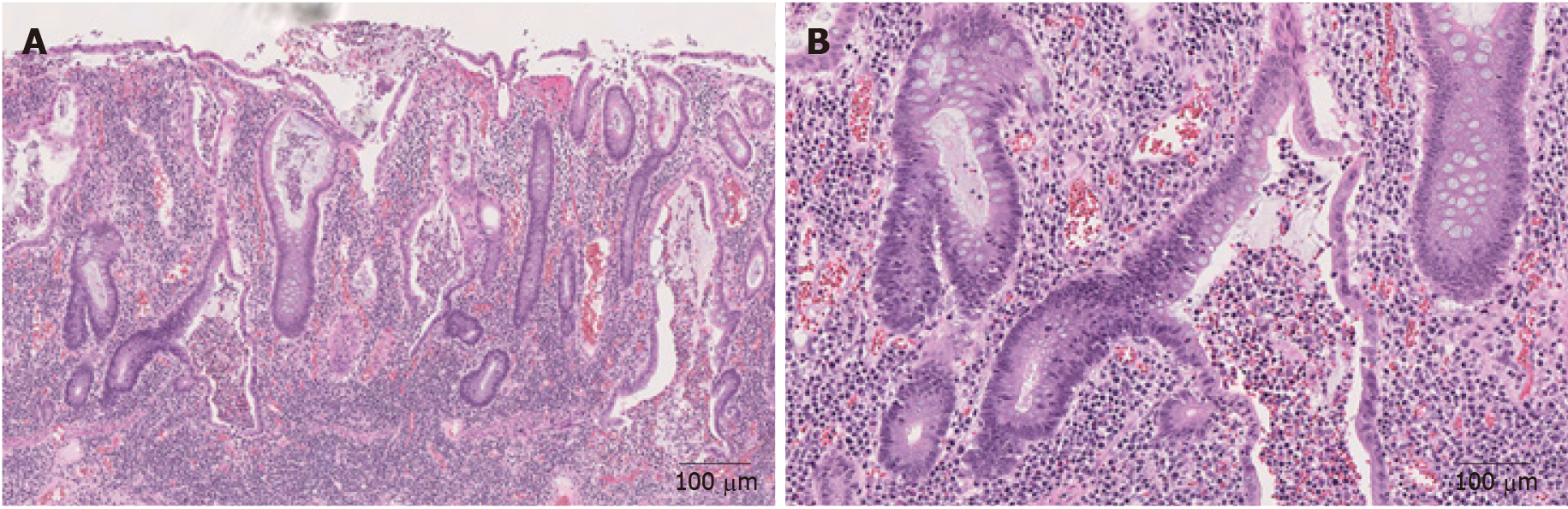
IBD is a form of chronic immunologically mediated intestinal disorders, consisting of CD and ulcerative colitis (UC)[56]. UC involves the rectum and continuously extends to involve proximal colonic mucosa. CD is distinguished from UC by skipped transmural inflammation of any part of the GI tract. Fissures/fistula, noncaseating sarcoidal granulomas, and transmural involvement are more characteristic of CD[37,57]. Some ICI-induced colitis resemble IBD with its patchy or segmental distribution[5], left colon involvement[5], and crypt architecture irregularities (Figures 4A and B)[19]. Usually, IBD has an insidious onset in contrast to ICI-induced colitis which often has a quick onset of symptoms after initiation of therapy[5]. Other features may aid in differentiating these two entities. Multiple organ involvement would favor ICI-induced colitis[5], fissures/fistula, noncaseating sarcoidal granulomas, and transmural involvement would favor CD and basal lymphoplasmacytosis would favor both UC and CD[37]. It may be challenging to distinguish superimposed ICI-induced colitis from IBD flare up only, as both show active colitis[18,37,55]. In this setting, the presence of crypt apoptosis would favor ICI-induced colitis as apoptosis is unusual in IBD[20].
Figure 4 Representative images of inflammatory bowel disease (Hematoxylin and eosin).
A: Mucosal inflammation with marked crypt distortion and neutrophilic cryptitis and abscesses in ulcerative colitis (40 ×); B: Higher magnification view shows crypt abscess (100 ×).
GvHD
GvHD refers to a phenomenon wherein the donor’s immune cells recognize the recipient’s cells as foreign and attack and damage them. It is a common and serious complication of allogeneic hematopoietic cell transplantation, occurring in 30% to 70% of patients[58]. GI tract is the second most common site affected by GvHD following skin. Although esophageal web, stricture or stenosis in the upper to mid third of the esophagus is sufficient to establish the diagnosis of chronic GvHD, neither clinical nor endoscopic presentations of colonic involvement is specific for GvHD[59]. In GvHD, crypt injury, loss and ulcer may be found in severe disease (Figure 5A). Apoptotic bodies are commonly found at the deeper portion of the crypts in small and large intestinal mucosa (Figure 5B)[59] closely resembling ICI-induced colitis, although lamina propria inflammation is usually sparse in GvHD (Figure 5A). Both ICI-induced colitis and GvHD involve variable organs other than GI tract, such as skin and liver[5,59], which make it even more challenging to distinguish them. Clinical history and medication history are necessary to sort out the specific cause of colitis on an individual basis.
Figure 5 Representative images of graft-versus-host disease (Hematoxylin and eosin).
A: Colonic graft-versus-host disease characterized by marked crypt architectural distortion and paucity of lamina propria inflammation (40 ×); B: On higher magnification, enterocyte apoptosis (yellow sign) are readily identified (200 ×).
TREATMENT
Comprehensive diagnostic protocol and management guidelines/recommendations regarding ICI-induced colitis were recently published[8,23,24]. Management varies according to the grade of colitis. In patients with only mild diarrhea (CTCAE grade 1), ICI therapy should be continued with close monitoring for dehydration. Once colitis reaches grade 2 or 3, ICI therapy should be suspended, but may restart anti PD-1 or PD-L1 agents if the patients recover to grade 1 or less following treatment. All ICI treatment should be permanently discontinued for patients with grade 4 colitis[24]. Although there is no definitive evidence to support their use, current guidelines universally recommend corticosteroids as initial management for ICI-induced colitis that is grade 2 or of higher grade[8,23,24]. Immunosuppressant maintenance therapy (< 10 mg prednisone equivalent dose) may be offered for initial treatment for grade 2 colitis if the infectious work-up in stool is negative. If diarrhea persists, 1 mg/kg/d prednisone or equivalent should be administered. Patients with grade 3 colitis generally start with high-dose corticosteroids (prednisone 1 to 2 mg/kg/d or methylprednisolone 1 to 2 mg/kg/d). If symptoms persist ≥ 3 to 5 d or recur after improvement, IV corticosteroid or stronger immunosuppressive agents are recommended, such as tumor necrosis factor-α blocker infliximab. Grade 4 colitis patients should start with IV corticosteroid and start infliximab 5 to 10 mg/kg if the symptoms are refractory to corticosteroid within 2 to 3 d[8,24]. Mucosal ulceration or extensive colitis is an indication for early escalation to infliximab[8].
Approximately two-thirds of patients respond to initial management with corticosteroids and do not require any further treatment[24,60,61]. Steroid tapers are typically performed over 4-6 wk, depending on the severity of the initial inflammation and the rapidity of the initial response[24,60,61]. A small fraction of patients fails to respond to corticosteroids as well as infliximab. In these patients, confirmation of ongoing inflammation and exclusion of opportunistic infections is essential. Based on patients’ risk factors, investigations should consider repeat stool cultures, C. difficile and CMV testing, and ova and parasite testing[23,32].
ALTERNATIVE STRATEGIES FOR TREATMENT-REFRACTORY ICI-INDUCED COLITIS
Currently there are case reports and small series reporting the use of alternative strategies for treatment-refractory ICI-induced colitis, including vedolizumab, fecal microbiota transplantation (FMT), and extracorporeal photopheresis (ECP). These will be briefly explored below.
Vedolizumab
Vedolizumab is a humanized monoclonal antibody that specifically recognizes the α4β7 heterodimer and modulates inflammation in the GI tract without inducing systemic immunosuppression[62]. Vedolizumab may benefit ICI-induced colitis patients who are refractory to infliximab and/or those with contraindication for its use. In a retrospective study of 28 patients with ICI-induced colitis who were refractory to corticosteroids, 32% of them did not respond to infliximab. Vedolizumab was administered using the same protocol for IBD. After 15 mo of follow up, 86% of the patients achieved and sustained clinical remission. Endoscopic remission was achieved in 54% (7/13) of the patients and 29% (5/17) achieved histologic remission[63]. Another small series also showed favorable outcome of vedolizumab use. Six out of seven steroid-dependent and/or partially refractory ICI-induced colitis patients experienced steroid-free remission of enterocolitis without related side effects[64]. It seems that a larger prospective study to evaluate the efficacy of vedolizumab is warranted.
FMT
FMT is the transfer of stool from a healthy donor into the colon of a patient whose disease is a result of an altered microbiome. The goal of FMT is to restore the normal microbiota. The most effective and well-studied indication for FMT is recurrent C. difficile infection[65]. Given the potential association between ICI-induced colitis and altered gut microbiota[26,27], FMT could be an effective treatment for treatment-refractory ICI-induced colitis. Wang et al[66] reported the first case series wherein ICI-induced colitis was successfully treated with FMT. Both patients achieved complete resolution of clinical symptoms and eventually returned to normal, daily solid bowel movements. Endoscopic evaluation demonstrated reduced inflammation and resolution of ulcerations. Additional clinical trials are needed to validate the utility of this approach.
ECP
ECP is an immunomodulatory therapy wherein white blood cells are isolated and are exposed to 8-methoxypsoralenand and ultraviolet A-irradiation ex vivo before being re-infused to the patient[67]. ECP has been used for the treatment of chronic GvHD and in clinical trial for acute GvHD[67]. Apostolova et al[68] reported a patient with ICI-induced colitis who had a complete response following ECP. A 29-year-old man developed symptoms of dermatitis, thyroiditis, hepatitis, and colitis after two doses of ipilimumab and nivolumab combination therapy. The dermatitis, thyroiditis, and hepatitis resolved after the discontinuation of ICI and the initiation of glucocorticoid treatment. However, the colitis did not show durable response with glucocorticoids for a total of 23 wk, infliximab (two single doses during a 4-wk period), and cyclosporine (during a 14-wk period). During the next 8 mo, he underwent two cycles of ECP on two consecutive days every 2 to 4 wk, which resulted in a complete resolution of his colitis. Immunosuppression was tapered without a rebound of the colitis symptoms[68]. This preliminary result is promising and warrants further studies.
CONCLUSION
Colitis constitutes the most common adverse effect of ICI therapy. Its clinical, endoscopic and histopathologic manifestations are not specific and resemble those of infectious colitis, other medication-mediated colitis, GvHD, and IBD. ICI-induced colitis can rapidly progress to cause ulceration, perforation and even death when there is a delay in diagnosis and appropriate treatment. The diagnosis of ICI-induced colitis is one of exclusion and requires exclusion of all other competing etiologies, including infectious colitis, medication-mediated colitis, GvHD and IBD.
Currently high dose corticosteroids are used as initial management followed by infliximab for steroid-refractory colitis. When patients do not respond to corticosteroids or infliximab, concurrent infectious colitis such as C. difficile and CMV colitis should be considered and excluded. Vedolizumab and FMT are promising treatment options for treatment-refractory ICI-induced colitis.