Published online Sep 20, 2021. doi: 10.5493/wjem.v11.i4.44
Peer-review started: January 12, 2021
First decision: July 8, 2021
Revised: July 26, 2021
Accepted: September 1, 2021
Article in press: September 1, 2021
Published online: September 20, 2021
Processing time: 247 Days and 0.8 Hours
Although the detection of viral particles by reverse transcription polymerase chain reaction (RT-PCR) is the gold standard diagnostic test for coronavirus disease 2019 (COVID-19), the false-negative results constitute a big challenge.
To examine a group of patients diagnosed and treated as possible COVID-19 pneumonia whose multiple nasopharyngeal swab samples were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by RT-PCR but then serological immunoglobulin M/immunoglobulin G (IgM/IgG) antibody against SARS-CoV-2 were detected by rapid antibody test.
Eighty possible COVID-19 patients who had at least two negative consecutive COVID-19 RT-PCR test and were subjected to serological rapid antibody test were evaluated in this study.
The specific serological total IgM/IgG antibody against SARS-CoV-2 was detected in twenty-two patients. The mean age of this patient group was 63.2± 13.1-years-old with a male/female ratio of 11/11. Cough was the most common symptom (90.9%). The most common presenting chest computed tomography findings were bilateral ground glass opacities (77.2%) and alveolar consolidations (50.1%). The mean duration of time from appearance of first symptoms to hospital admission, to hospital admission, to treatment duration and to serological positivity were 8.6 d, 11.2 d, 7.9 d, and 24 d, respectively. Compared with reference laboratory values, serologically positive patients have shown increased levels of acute phase reactants, such as C-reactive protein, ferritin, and procalcitonin and higher inflammatory markers, such as erythrocyte sedimentation rate, lactate dehydrogenase enzyme, and fibrin end-products, such as D-dimer. A left shift on white blood cell differential was observed with increased neutrophil counts and decreased lymphocytes.
Our study demonstrated the feasibility of a COVID-19 diagnosis based on rapid antibody test in the cases of patients whose RT-PCR samples were negative. Detection of antibodies against SARS-CoV-2 with rapid antibody test should be included in the diagnostic algorithm in patients with possible COVID-19 pneumonia.
Core Tip: This is the first clinical retrospective study in Turkey that reports the features of the patients that were diagnosed and treated as possible coronavirus disease 2019 (COVID-19) cases whose multiple nasopharyngeal swab samples were negative by reverse transcription polymerase chain reaction (RT-PCR) but serological immunoglobulin M/immunoglobulin G antibody against severe acute respiratory syndrome coronavirus 2 was detected by a rapid antibody test. Our study demonstrated the feasibility of COVID-19 diagnosis based on rapid antibody tests in the cases of patients whose RT-PCR samples were negative. An effective diagnosis for COVID-19 is likely to require a hybrid strategy of PCR and serologic testing with the radiological demonstration.
- Citation: Yıldırım F, Gulhan PY, Diken ÖE, Capraz A, Simsek M, Yildirim BB, Taysi MR, Ozturk SY, Demirtas N, Ergil J, Dirican A, Uzar T, Karaman I, Ozkaya S. Role of serological rapid antibody test in the management of possible COVID-19 cases. World J Exp Med 2021; 11(4): 44-54
- URL: https://www.wjgnet.com/2220-315x/full/v11/i4/44.htm
- DOI: https://dx.doi.org/10.5493/wjem.v11.i4.44
The coronavirus disease-2019 (COVID-19) is a unique pneumonia caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that typically causes various degrees of respiratory disease[1]. Currently, the entire world is battling COVID-19 pneumonia, which can be lethal in high-risk patient groups. Although a COVID-19 diagnosis is generally based on clinical, laboratory, and radiological features of the patients, the gold standard test for diagnosis is the real time reverse transcription polymerase chain reaction (RT-PCR) assay from respiratory samples[2,3]. However, several studies have indicated the concerns regarding the sensitivity of RT-PCR tests[4,5]. False negative results are thought to originate from several technical issues, including the high variability of RT-PCR tests, low nasopharyngeal viral load, manual mistakes performing the test, inappropriate collection and transportation of samples, and timing of specimen in relation to onset of symptoms, whereas false positive results are rarely seen[4].
Rapid antibody card tests can produce results in as short as fifteen minutes by detecting immunoglobulin M (IgM) and immunoglobulin G(IgG) antibodies produced against SARS-CoV-2, and they have been approved in Europe, as well as in China. Although the specificity of these tests is lower than with PCR, in some cases they can aid in the diagnosis of possible COVID-19 patients. In this retrospective study; we aimed to investigate whether these rapid antibody tests would be useful in the diagnostic challenge faced in suspected, possible COVID-19 pneumonia patients whose PCR tests were negative but has radiologically and clinically features that are consistent with COVID-19 pneumonia.
We retrospectively evaluated the clinical characteristics, laboratory results, and radiological features of 80 possible COVID-19 patients with multiple negative RT-PCR tests and reported the characteristics of 22 serologically positive COVID-19 patients.
In Turkey, rapid antibody test kits for COVID-19 were become commercially available at the beginning of April 2020. Symptomatic RT-PCR-negative patients who were suspected to be infected with SARS-CoV-2 based on epidemiological history, laboratory results, and positive radiological findings were included in the study. Until September 2020, we were able to test 80 suspected RT-PCR negative possible COVID-19 patients; 22 serologically positive cases were detected. All patients had a contact history and most patients had a history of a family member who tested positive with RT-PCR for COVID-19 disease. All COVID-19 antibody test positive cases had fever and at least one respiratory system symptom such as cough, dyspnea, or sputum. Herein, we introduced features of 22 serologically positive COVID-19 cases. High resolution computed tomography (HRCT) was used for the radiological assessment. In patients with possible COVID-19 pneumonia, ground-glass formation and/or consolidative opacities distributed usually bilateral, peripheral, and mostly basal, were considered as positive HRCT findings. The patients with negative RT-PCR tests were tested for specific antibodies against SARS-CoV-2 following the COVID-19 treatment, which was in average 24 d after the initiation of symptoms.
Samples were taken from the patients with oro-nasopharyngeal and nasal swabs and analyzed by RT-PCR. The humoral responses against SARS-CoV-2 were tested with rapid card test with blood samples of patients. The blood taken from the patient was dropped on this rapid card test and the total antibody response (either IgM or IgG) was analyzed. The clinical samples were anonymized and used in accordance with local ethical guidelines. Total antibody levels against SARS-CoV-2 were noted. We used the Colloidal Gold SARS-CoV-2 IgG/IgM Rapid Test (Beijing Hotgen Biotech Co., Ltd), which is a lateral flow chromatographic immunoassay detecting total antibodies produced against the SARS-CoV-2. The anti-SARS-CoV-2 virus IgM, if present in the specimen, will bind to the SARS-CoV-2 conjugates. The immunocomplex is then captured by the anti-human IgM line, forming a burgundy colored M Line, indicating a SARS-CoV-2 virus IgM positive test result.
Mean and standard deviations were given for normally distributed metric variables. Frequencies and percentages were given for non-metric variables.
The demographic and clinical characteristics of 22 serologically positive RT-PCR negative COVID-19 patients were shown in Table 1. Each of these patients had at least two consecutive negative PCR tests, taken at a minimum of 2 d apart. The mean age was 63.2 ± 13.1-years-old and male to female ratio was 11/11. The mean duration of time from appearance of first symptoms to hospital admission, to hospital admission, to treatment duration and to serological positivity were 8.6 ± 7.2, 11.2 ± 5.4, 7.9 ± 3.2 and 24 ± 17 d, respectively.
| n (%) | |
| Agein yr | |
| mean ± SD | 63.2 ± 13.1 |
| Gender | |
| Male/Female | 11/11 |
| Symptoms, n (%) | |
| Cough | 20 (90.9) |
| Dyspnea | 14 (63.6) |
| Fever | 10 (45.4) |
| Chest pain | 8 (36.3) |
| Duration in d, mean ± SD | |
| From first symptom to admission | 8.6 ± 7.2 |
| Hospital stay | 11.2 ± 5.4 |
| From symptoms to antibody test | 24 ± 17 |
| Drug treatment | 7.9 ± 3.2 |
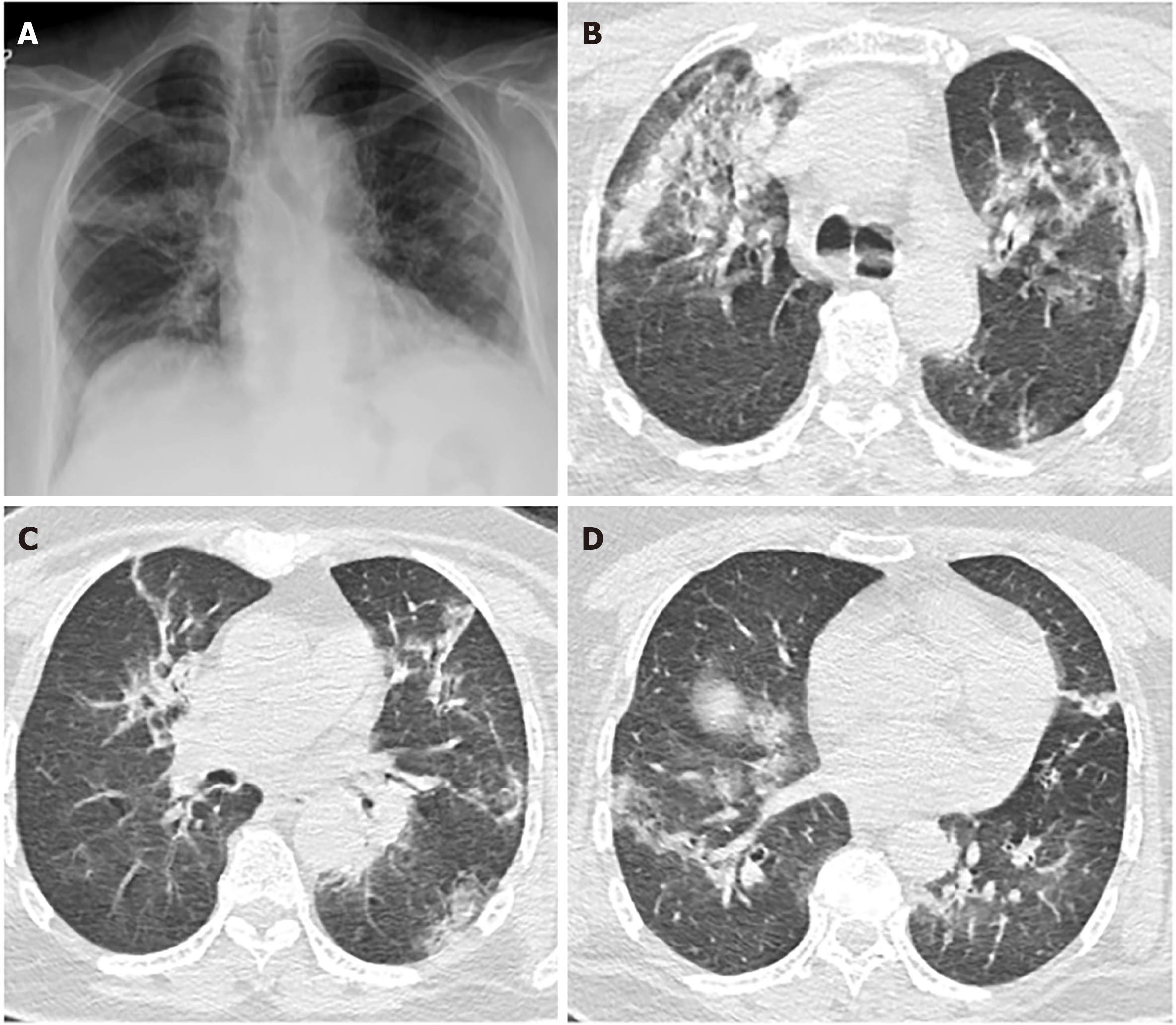
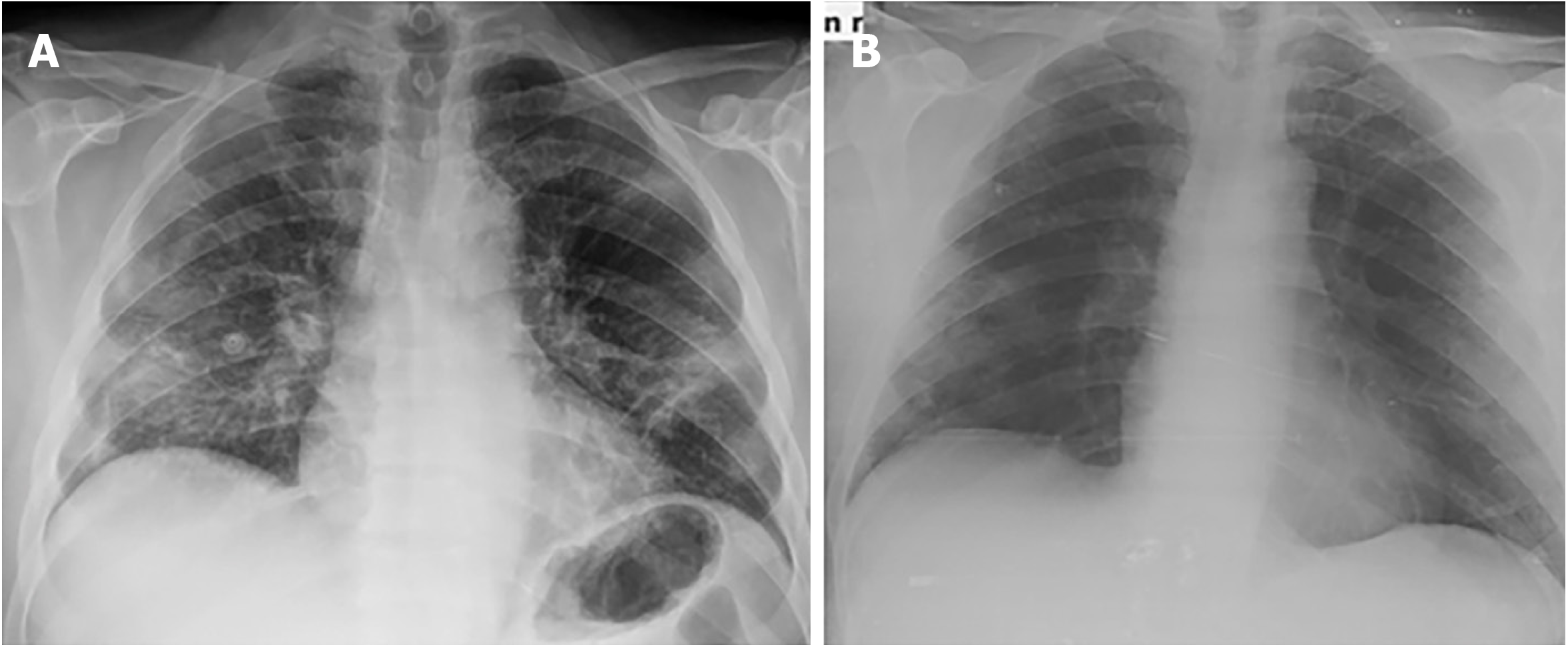
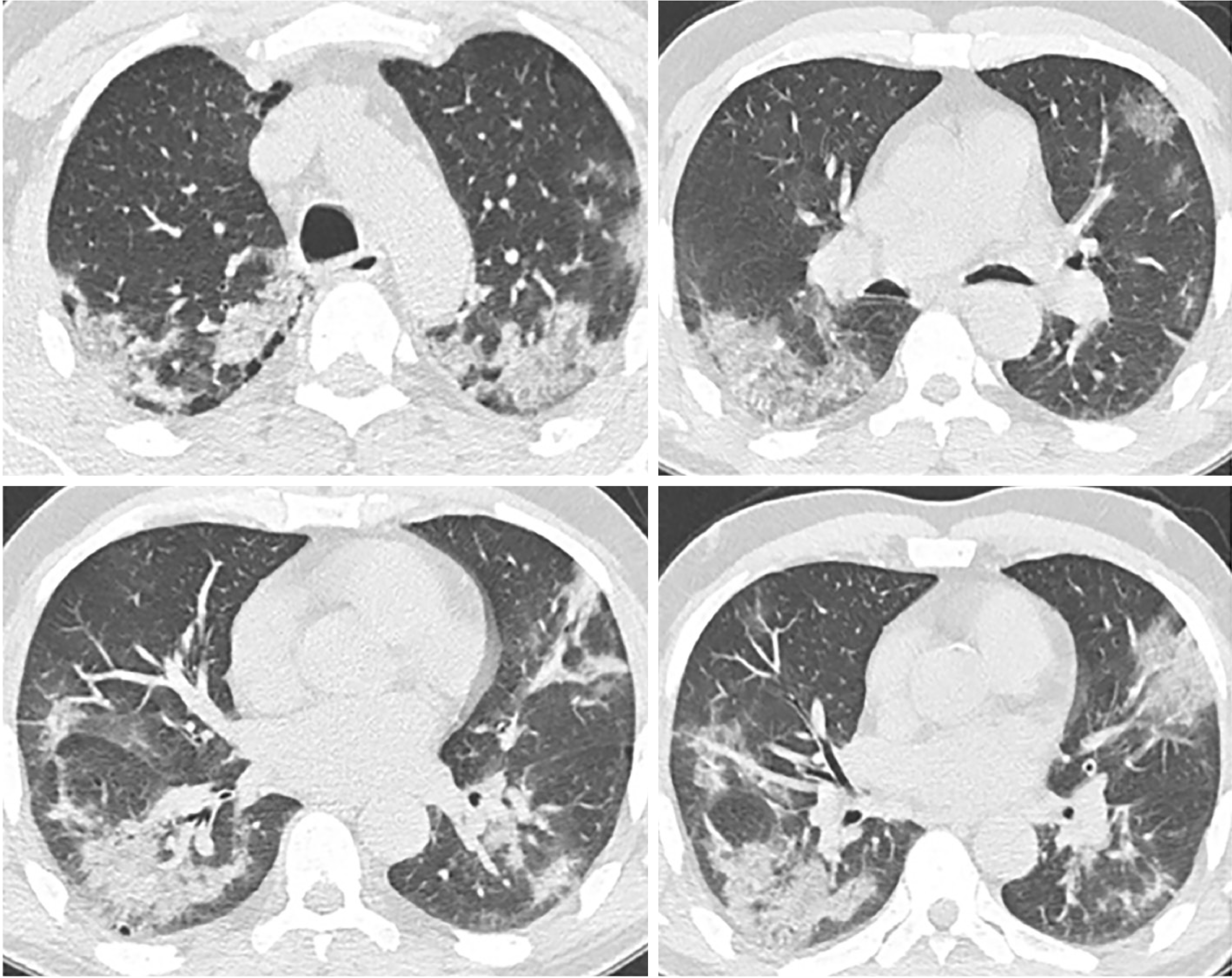
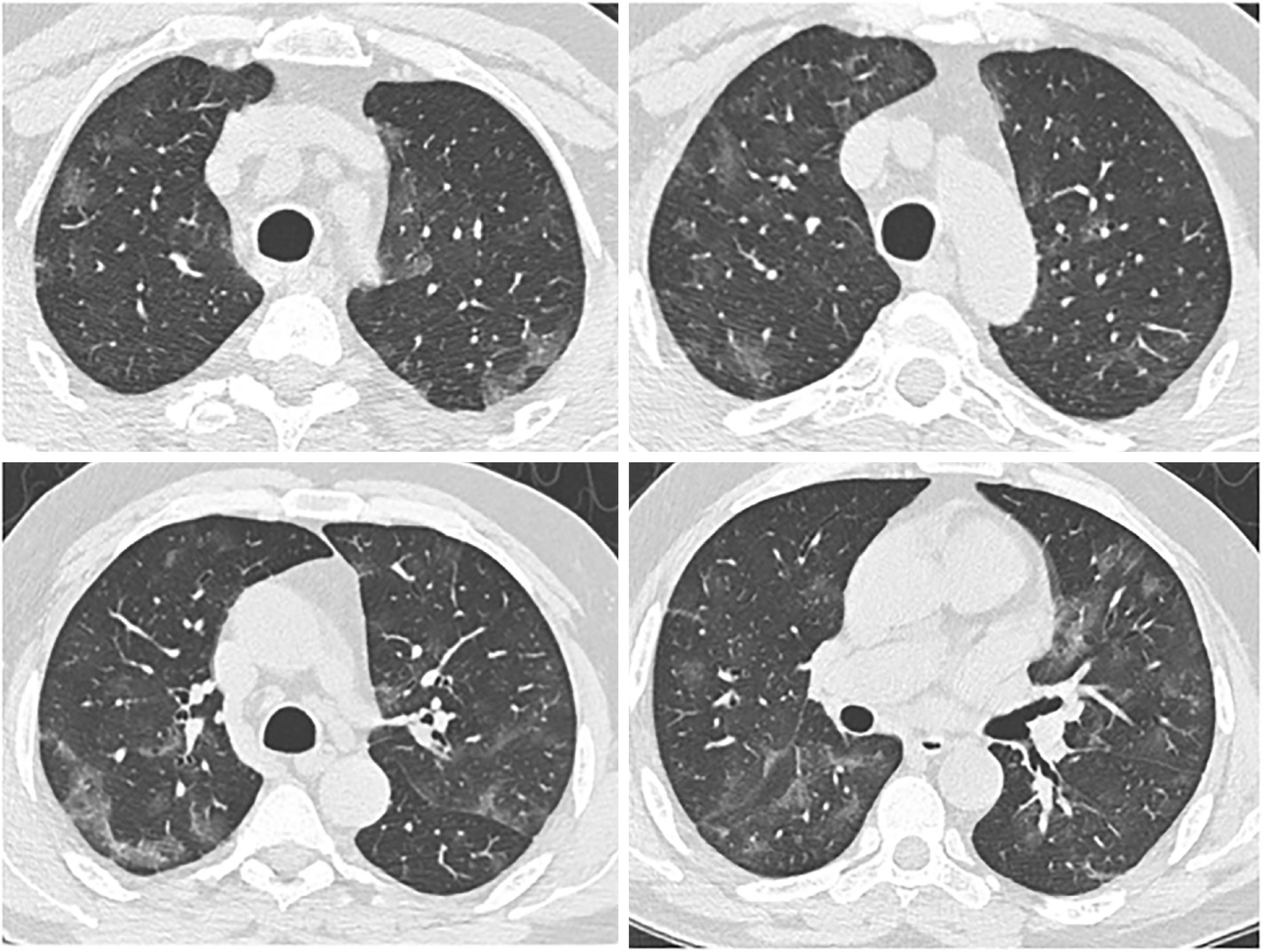
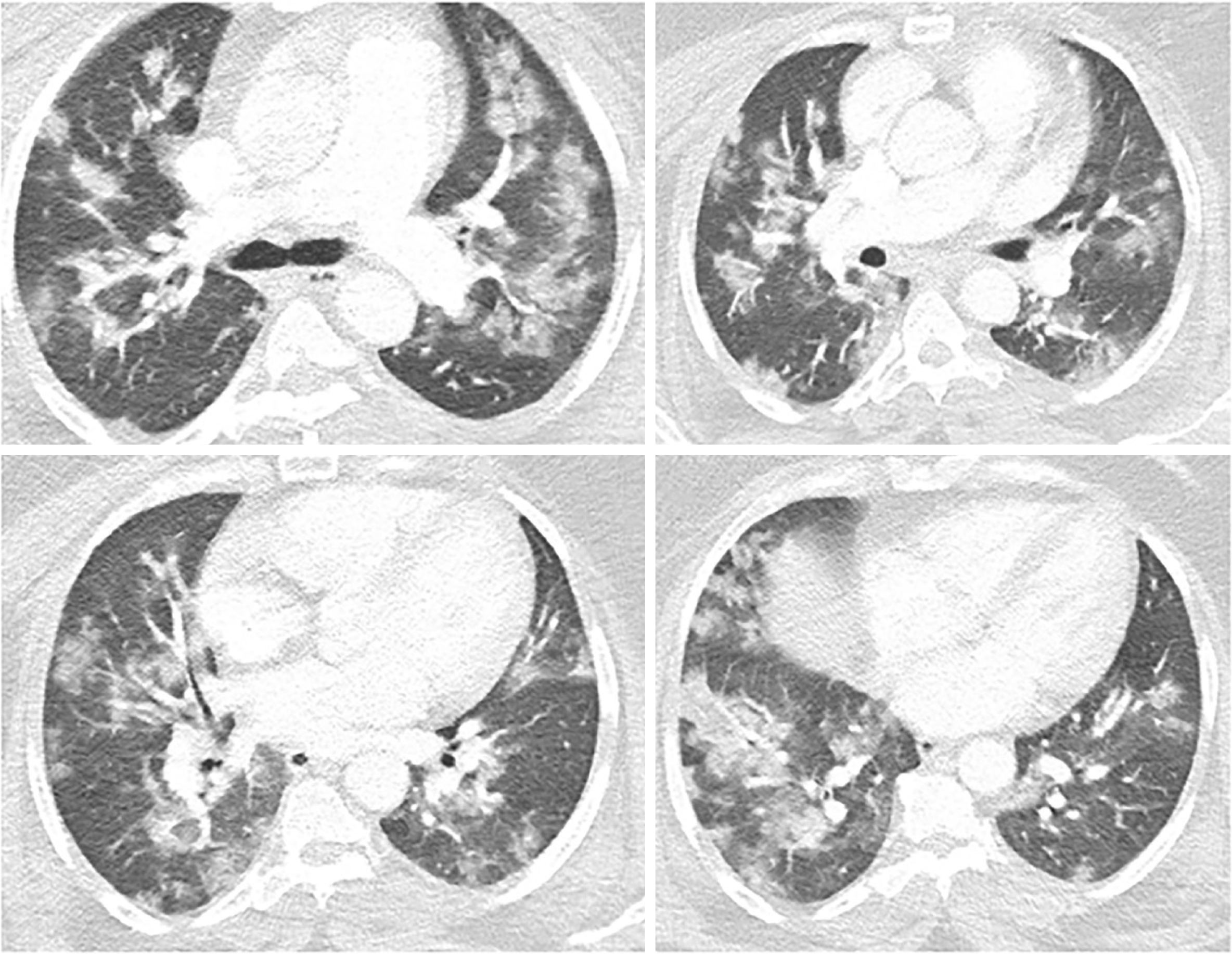
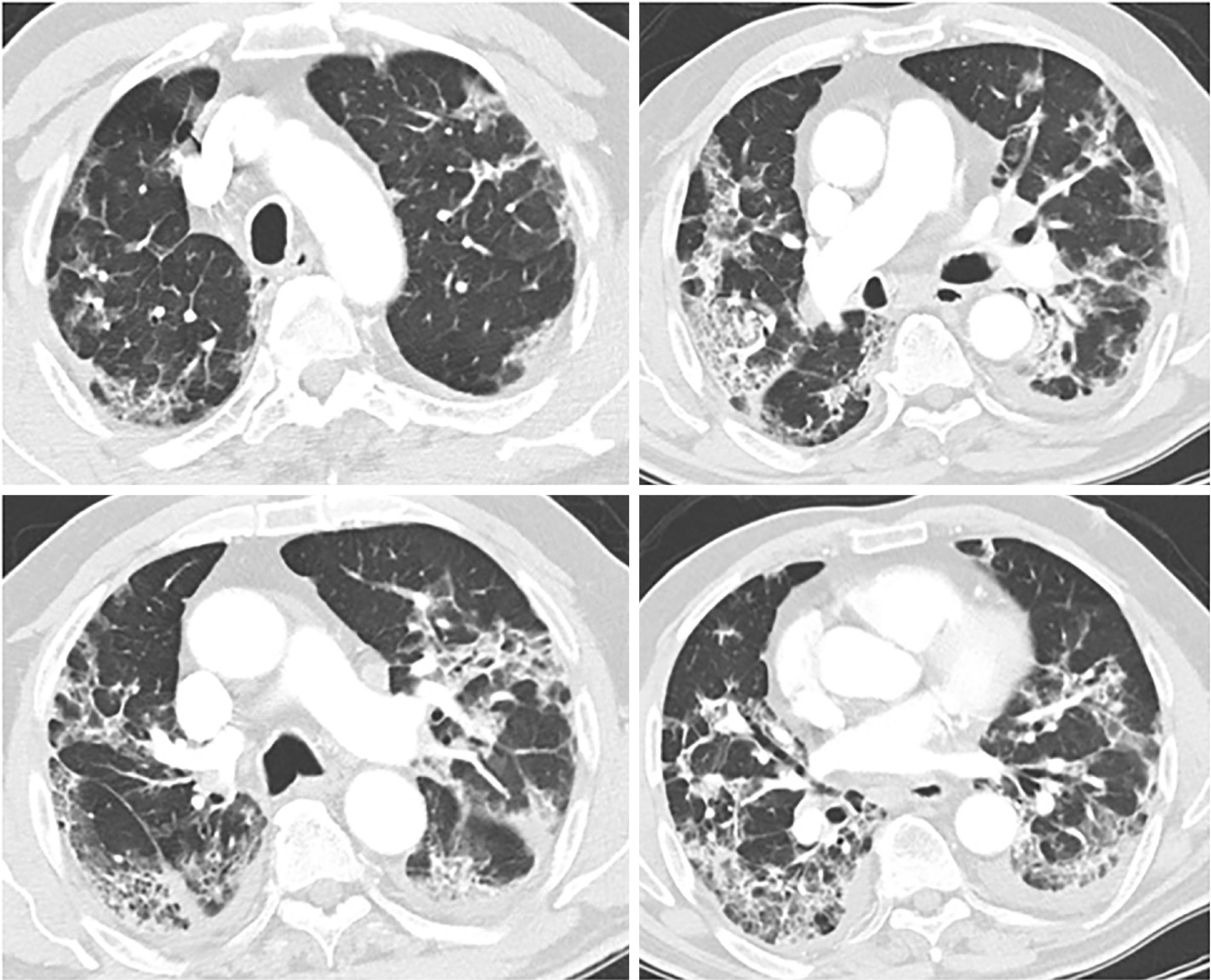
The radiological findings and drug regimens were shown in Table 2. The radiological findings, such as bilateral reticular and ground-glass opacities were demonstrated in Figures 1-5. Also, dense consolidations were noted in Figures 3 and 5. The bilateral fibroreticular infiltrates with crazy-paving patterns are shown in Figure 6. Hydroxychloroquine and/or azithromycine and/or favipiravir therapy was initiated by the consensus of infectious disease specialists and pulmonologists according to the clinical, laboratory, and radiological findings of the patients. The selection of the drug regimen was made based on the clinical evaluation of each patient.
| n (%) | |
| Radiology | |
| GGO | 17 (77.2) |
| Consolidation | 11 (50) |
| Nodular infiltrates | 4 (18.1) |
| Fibroreticular infiltrates | 3 (5.9) |
| Drug regimens | |
| HCQ+Azithromycine + Favipravir | 11 (50) |
| HCQ+Azithromycine | 7 (31.8) |
| Favipravir | 4 (18.1) |
The laboratory results of the patients were given in Table 3. Compared with reference laboratory values, serologically positive patients have shown increased levels of acute phase reactants such as C-reactive protein, ferritin, and procalcitonin, higher inflammatory markers, such as erythrocyte sedimentation rate, lactate dehydrogenase enzyme, and fibrin end-products, such as D-dimer. A left shift on white blood cell differential observed with increased neutrophil counts and decreased lymphocytes.
| Value | mean ± SD |
| ESR in mm/h | 68.5 ± 41.7 |
| LDH in U/L | 362 ± 152 |
| CRP in mg/L | 95 ± 101 |
| Ferritinin μg/L | 778 ± 684 |
| WBCs | 8621 ± 3549 |
| Lymphocytes, n (%) | 1430 ± 530 |
| Lymphocytes, n (%) | 22 ± 10.8 |
| Neutrophils, n (%) | 5390 ± 2450 |
| Neutrophils as % | 70.5 ± 12.3 |
| D-Dimer in mg/L | 1875 ± 2757 |
| Procalcitonin in mg/L | 0.15 ± 0.03 |
In patients with possible COVID-19 pneumonia, rapid identification, isolation, and treatment of infected individuals will be a key step to prevent onward community transmission. Currently, COVID-19 diagnosis is made by the direct detection of SARS-CoV-2, supported by clinical, laboratory and radiological features of the suspected patients. According to the first COVID-19 case series by Bai et al[4]; the sensitivity of CT was estimated to be 97% compared to PCR tests, which had 71% sensitivity[4]. Ai et al[5] also reported as the sensitivity of RT-PCR assays to be in the range of 60% to 70%[5]. Here, our results supported that chest CT results were more sensitive than RT-PCR results to suspect from a possible COVID-19 diagnosis.
It was suggested that PCR-negative cases with positive CT findings and high clinical suspicion may benefit from repeated RT-PCR testing[6]. Shi et al[7] reported that COVID-19 pneumonia might manifest with chest CT imaging abnormalities, even in asymptomatic patients, with rapid evolution from focal unilateral to diffuse bilateral ground glass opacities that progressed to, or coexisted with, consolidations within 1–3 wk[7]. Another study with 1099 patients from China revealed that 56% of patients had ground-glass opacities, but no radiological findings were reported in 18% of COVID-19 cases. Although bilateral and peripheral ground-glass opacities constitute the most typical CT findings, they were not specific for the COVID-19 disease[8,9]. Since radiological evaluation of the thorax is often the key diagnostic element in patients with possible COVID-19 pneumonia, like in our present study, the patients with positive CT findings but negative RT-PCR results should be isolated and re-evaluated[9,10]. Combined assessment of imaging features with clinical and laboratory findings is key to facilitate an early diagnosis of COVID-19. Therefore, we suggest that in RT-PCR-negative cases, radiological diagnosis should be supported with specific antibody detection. Our study demonstrated that the diagnosis of COVID-19 should be confirmed by rapid antibody test at least 5 d after the treatment of RT-PCR negative patients with typical CT findings.
SARS-CoV-2 can be detected in different tissues and body fluids. In our study, the nasopharyngeal and nasal swabs samples taken from the patients were utilized and assessed by RT-PCR test. In a study on 1070 specimens collected from 205 patients with COVID-19, bronchoalveolar lavage fluid specimens showed the highest positive rates (14 of 15; 93%), followed by sputum (72 of 104; 72%), nasal swabs (5 of 8; 63%), fibro-bronchoscopy brush biopsy (6 of 13; 46%), pharyngeal swabs (126 of 398; 32%), feces (44 of 153; 29%), and blood (3 of 307; 1%). None of the 72 urine specimens tested positive[9]. That study by Ding et al[9] supported that sensitivity of nasal and nasopharyngeal swabs for PCR tests remained questionable.
The first comprehensive study on the host humoral response against SARS-CoV-2 has shown that serological response can aid in the diagnosis of COVID-19, including those subclinical cases. In that study, IgA, IgM, and IgG response using an ELISA-based assay on the recombinant viral nucleocapsid protein was analyzed in 208 plasma samples from 82 confirmed and 58 probable cases[11,12]. The median duration of IgM and IgA antibody detection were 5 d (IQR 3-6), while IgG was detected on day 14 (IQR 10-18) after symptom onset, with a positive rate of 85.4%, 92.7% and 77.9% respectively. It was shown that detection efficiency by IgM ELISA was higher than that of PCR after 5.5 d of onset of symptoms. In another study of 173 patients, the seroconversion rates (median time) for IgM and IgG were 82.7% (12 d) and 64.7% (14 d), respectively. Our study also reported the mean duration of time from appearance of first symptoms to hospital admission, to hospital admission, to treatment duration and to serological positivity were 8.6 ± 7.2, 11.2 ± 5.4, 7.9 ± 3.2 and 24 ± 17 d, respectively. It was also reported that a higher titter of antibody was independently associated with severe course of diseases[13]. Since our study included only RT-PCR-negative serologically positive COVID-19 patients who were diagnosed and treated based on radiological and clinical findings, we were unable to compare the severity of RT-PCR-positive and RT-PCR-negative COVID-19 patients.
To date, several population-based studies demonstrated false-negative RT-PCR is a particular concern in the diagnosis of COVID-19. Baron et al[14] reported that among COVID-19 patients, the ratio of false-negative RT-PCR results was 18% compared to a negative serology ratio of 4%[14]. West et al[15] clearly stated that the variety in the test performance and diagnostic validity of different methods have not been well investigated, which raises concern for generating a false sense of security[15]. As Benoit[16] suggested, a multi-step strategy to limit the likelihood of COVID-19 patients to be labeled incorrectly as negative should be applied, which includes RT-PCR tests, serological testing, and clinical and radiological findings of the patients[16]. It should be noted that RT-PCR tests alone to define COVID-19 negative cohorts are not valid and likely to produce biased results based on many concerns regarding the sensitivity of RT-PCR assays.
Our study has several limitations, including low sample size and follow-up for serology results due to its retrospective nature; however, ideal research conditions are often difficult to be establish during a pandemic situation. Also, the comparison of laboratory and radiological findings between patients who demonstrated a seroconversion and those who did not could better reveal the differences and may give information about the severity of the disease course. In addition, this study did not differentiate the serological results in terms of specific IgM and IgG against SARS-CoV-2.
In conclusion, our study remarks the feasibility of total antibody testing by a rapid card test in the diagnosis of suspected PCR-negative COVID-19 patients who are likely to have false negative results or viral clearance of the upper respiratory tract. Even though there is no specific treatment for COVID-19, it is highly important to confirm the diagnosis of highly suspected cases to prevent further transmission and to prevent long-term complications. We suggest that detection of antibodies against SARS-CoV-2 with rapid-card test should be included in the diagnostic algorithm in PCR-negative patients with COVID-19 pneumonia. An effective diagnosis is likely to require a hybrid strategy of PCR and serologic testing with radiological demonstration.
Novel coronavirus disease (COVID-19) is unique pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that typically causes various degrees of respiratory disease. Currently, the entire world is battling COVID-19 pneumonia, which can be lethal in high-risk patient groups. Although COVID-19 diagnosis is generally made based on clinical, laboratory, and radiological features of the patients, the most common standard of care for diagnosis is the reverse transcription polymerase chain reaction (RT-PCR) assay.
Several studies have indicated concerns regarding the sensitivity of RT-PCR tests, and an alternative rapid test is required to confirm the diagnosis by RT-PCR test.
In this study; we aimed to investigate whether rapid antibody tests would be useful in the diagnostic challenge faced in suspected COVID-19 patients whose PCR tests were negative but has radiologically and clinically consistent features with COVID-19.
Eighty suspected COVID-19 patients who had at least two negative consecutive COVID-19 PCR tests and were subjected to serological rapid antibody tests were evaluated. The clinical and laboratory characteristics of serologically positive RT-PCR negative COVID-19 patients were presented in this study.
The specific serological total immunoglobulin M/immunoglobulin G antibody against SARS-CoV-2 was detected in 22 patients. The most common presenting chest computed tomography findings were bilateral ground glass opacities (77.2%) and alveolar consolidations (50.09%). The mean duration of time from appearance of first symptoms to hospital admission, to hospital admission, to treatment duration and to serological positivity were 8.6, 11.2, 7.9, and 24 d, respectively. Compared with reference laboratory values, serologically positive patients have shown increased levels of acute phase reactants such as C-reactive protein, ferritin, and procalcitonin, higher inflammatory markers, such as erythrocyte sedimentation rate, lactate dehydrogenase enzyme, and fibrin end-products, such as D-dimer. A left shift on white blood cell differential was observed with increased neutrophil counts and decreased lymphocytes.
Rapid serological card tests can be a feasible alternative in the diagnosis and treatment algorithm of suspected COVID-19 cases.
An effective diagnosis for COVID-19 is likely to require a hybrid strategy of PCR and serologic testing with radiological demonstration.
We thank our colleague Ali Ayata, MD, Assoc. Prof., who provided insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations/conclusions of this paper.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bhardwaj R, Gupta MK S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
| 1. | World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report–38. 27 February 2020. [cited 28 February 2020]. Available from: www.who.int/docs/default-source/coronaviruse/situation-reports/20200227-sitrep-38-covid-19.pdf?sfvrsn=9f98940c_2. |
| 2. | World Health Organization. 2020. Novel coronavirus (2019-nCoV) technical guidance: laboratory testing for 2019-nCoV in humans. World Health Organization, Geneva, Switzerland. [cited 28 February 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technicalguidance/laboratory-guidance. |
| 3. | Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5481] [Cited by in RCA: 4918] [Article Influence: 983.6] [Reference Citation Analysis (1)] |
| 4. | Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3006] [Cited by in RCA: 2687] [Article Influence: 537.4] [Reference Citation Analysis (0)] |
| 5. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3285] [Article Influence: 657.0] [Reference Citation Analysis (0)] |
| 6. | Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020;296:E41-E45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1213] [Cited by in RCA: 1211] [Article Influence: 242.2] [Reference Citation Analysis (1)] |
| 7. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 8. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 9. | Ding X, Xu J, Zhou J, Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol. 2020;127:109009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 10. | Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, Zeng B, Li Z, Li X, Li H. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 561] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 11. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 12. | Guo L, Ren L, Yang S, Xiao M, Chang, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang QW, Xu SY, Zhu HD, Xu YC, Jin Q, Sharma L, Wang L, Wang J. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020;71:778-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1043] [Cited by in RCA: 1085] [Article Influence: 217.0] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 1840] [Article Influence: 368.0] [Reference Citation Analysis (0)] |
| 14. | Baron RC, Risch L, Weber M, Thiel S, Grossmann K, Wohlwend N, Lung T, Hillmann D, Ritzler M, Bigler S, Egli K, Ferrara F, Bodmer T, Imperiali M, Heer S, Renz H, Flatz L, Kohler P, Vernazza P, Kahlert CR, Paprotny M, Risch M. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: a population-based study. Clin Chem Lab Med. 2020;58:2131-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | West CP, Montori VM, Sampathkumar P. COVID-19 Testing: The Threat of False-Negative Results. Mayo Clin Proc. 2020;95:1127-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 16. | Benoit JC. [Psychotherapy and medical relaxation. An interview with Dr. J-Cl. Benoit]. Soins Psychiatr. 1985;3-4. [PubMed] |