Published online Jan 20, 2021. doi: 10.5493/wjem.v11.i1.1
Peer-review started: June 11, 2020
First decision: October 21, 2020
Revised: November 2, 2020
Accepted: November 11, 2020
Article in press: November 11, 2020
Published online: January 20, 2021
Processing time: 214 Days and 12.2 Hours
Inflammatory bowel diseases (IBDs) are closely linked to nutrition. The latest research indicates that diet and nutrition are significantly involved in the etiopathogenesis of the disease, although their specific role throughout its clinical course still remains unclear. This study reviewed how diet and nutrition are associated with IBD development and management. Even though specific diets have been shown to bring about positive outcomes, there is currently no scientific consensus regarding an appropriate diet that would benefit all IBD patients. We suggest that individualized dietary recommendations are of the greatest importance and that diets should be planned to provide individual IBD patients with specific nutrient requirements while keeping all the clinical aspects of the patients in mind. Further research is clearly necessary to investigate nutritional factors involved in IBD development and, especially, to evaluate the applications of the diets during the course of the disease.
Core Tip: Although inflammatory bowel disease (IBD) affects the gastrointestinal tract, the role of diet in the course of disease is often underestimated. Many studies have assessed the effect of diet in the risk of developing IBD, and the importance of nutrition in the etiopathogenesis of IBD was confirmed by the fast increase in its incidence and prevalence over the last two decades. We discuss the role of diet and nutrition in the etiology and management of IBD based on the data provided in the literature and set out an agenda for future research.
- Citation: de Castro MM, Pascoal LB, Steigleder KM, Siqueira BP, Corona LP, Ayrizono MLS, Milanski M, Leal RF. Role of diet and nutrition in inflammatory bowel disease. World J Exp Med 2021; 11(1): 1-16
- URL: https://www.wjgnet.com/2220-315x/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5493/wjem.v11.i1.1
Inflammatory bowel diseases (IBDs) are characterized by chronic and relapsing inflammation of different segments in the gastrointestinal tract. The etiology is not yet fully understood and the course of the disease is characterized by periods of exacerbation and remission. A multifactorial etiology has been confirmed. An interaction between environmental factors and gut microbiota in genetically susceptible individuals may cause a dysregulation of both the innate and adaptive immune responses[1-5]. The environmental factors include stress, pollution, breastfeeding, smoking, use of antibiotics, chemical products and diet[6]. Some of these risk factors are potentially reversible, such as smoking, use of antibiotics and diet[7].
Numerous studies point to the benefits of breastfeeding in the field of medical and public health practice, and in IBD development[8,9]. A meta-analysis performed on 17 relevant articles that examined whether breastfeeding may protect against ulcerative colitis (UC) and Crohn’s disease (CD) showed heterogeneous results that nonetheless support the hypothesis that breastfeeding is associated with lower risks of IBD development[10]. In another and more recent systematic review with meta-analysis, it was established that breastfeeding duration had a dose-dependent association, with the strongest decrease in risk for CD and UC occurring when breastfeeding lasted for at least 12 mo when compared to 3 or 6 mo, which confirmed the protective effect of breastfeeding on the development of IBD[11].
These protective effects are related to the antibodies (SIgA and SIgM), cytokines, immune cells, growth factors and high concentrations of oligosaccharides provided and released by breast milk and its components. These factors seem to provide defense and promote the production of bacteria that benefit neonatal intestinal microbiota, thus improving innate mucosal immunity development[12-14]. Therefore, breastfeeding, especially in families affected by IBD, should be encouraged for all the beneficial effects already mentioned in the literature[8-10].
Although IBD affects the gastrointestinal tract, the role of diet in the course of disease is often underestimated. Several studies have evaluated the effect of diet on the risk of developing IBD. The importance of nutrition in the etiopathogenesis of IBDs was confirmed by the fast increase in the incidence and prevalence of such diseases in the last two decades in populations with a previously low incidence[7].
There was also a steady increase in the incidence and prevalence of CD in the developed world, particularly in Australia and Europe. These changes can be partially explained by the recent higher awareness and better diagnostics for IBD. However, this increase may also be related to the higher degree of Westernization, since diet is one of the key factors in the initiation, duration and treatment of the disease[15,16].
Some studies point to the association of the incidence of IBD with dietary excess or even a deficit of several nutrients. Additionally, dietary components are involved in dysbiosis on the intestinal mucosa, which can become thinner and more permeable to pathogens and antigens, leading to a low-grade, but persistent inflammation[17]. IBD is associated with intestinal dysbiosis, which is characterized by a generalized alteration in the diversity and abundance of bacterial species[18-20].
Regarding nutritional status, malnutrition was historically present mainly during periods of exacerbation of the disease, and malnourished IBD patients must be treated properly, as they are more likely to have a worse prognosis, complication rates, mortality and quality of life[7]. However, several studies have indicated an increase in the prevalence of overweight and obesity - predominantly in remission patients[21]. We have demonstrated that in CD patients, 55% and 28% of those in remission and with active disease were overweight or obese[22], respectively, which suggests that these patients are currently receiving more effective treatments. This allows them to maintain the same behavior as the rest of the population, which typically follows a sedentary lifestyle and consumes a hypercaloric diet.
Obesity has also been associated with the risk of developing IBD. The relevant factors include epigenetic changes observed in both obesity and IBD patients, changes in the gut microbiome and high levels of intestinal inflammation[23,24].
Thus, further insight into the role of diet in the pathophysiology of IBD may help to identify preventive or therapeutic targets, and improve a patient's quality of life. Our aim is to review the literature on the role of diet and nutrition in the etiology and management of IBD and set the agenda for future research.
There is currently no consensus in the medical community regarding nutritional guidelines for adult IBD patients. The lack of randomized controlled trials testing the specific diets and dietary patterns make it impossible to make strong recommendations[25]. An exception is exclusive enteral nutrition (EEN), which is recommended as first-line therapy for children and adolescents with acute active CD in order to induce remission[7,26]. This lack of consensus is due to limited available research data. The fact that the exact action mechanism of these diets is not completely understood, in addition to the different effects caused by differences in the gastrointestinal physiology of each patient, make it impossible to formulate a guideline. The European Society for Clinical Nutrition and Metabolism (ESPEN) guideline recommends that no specific diet be followed during remission phases, as it does not seem to be effective in obtaining remission[7].
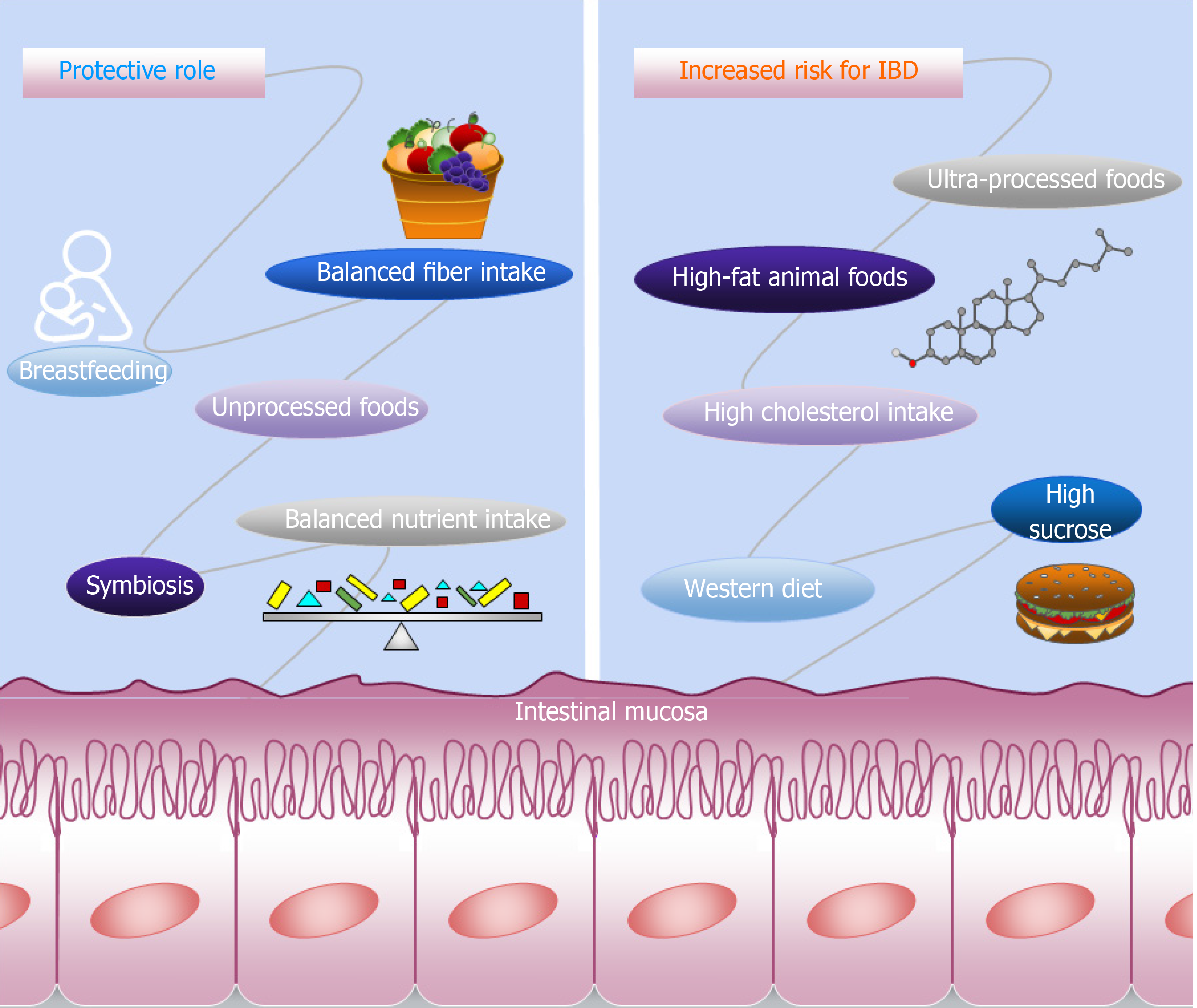
Several compounds in the diet that influence the development and maintenance of IBD have been identified, while others seem to play a protective role. In this review we will address the potential of these dietary compounds in IBD treatment based on recent literature, and will also describe the differences between UC and CD in relation to these compounds. Figure 1 shows a schematic representation of the dietetic components that influence IBD.
Recently, two dose-response meta-analyses found no evidence of an association between macronutrients and IBD risk[27,28]. However, a higher intake of fiber may play a protective role in CD development, while in sugar subtypes, high sucrose intake was linked to the risk of UC and CD[27,28]. Despite the inconclusive association between the macronutrients and IBD risk demonstrated by the two meta-analyses, studies have shown that high animal fat and cholesterol intake is associated with UC risk, and that a long-term intake of fast food, which is rich in fats and sugars, is a risk factor for CD[29,30]. The high intake of saturated fats and monosaccharides and a low intake of fiber are linked with increased risk of CD development[31,32]. The EpiCom cohort study demonstrated that daily fast food and high sugar consumption were associated with earlier onset of IBD, as well as an increased risk of disease severity and surgery in UC[30]. Although diets high in fructose are associated with metabolic diseases[33], a negative association was found between fructose intake and IBD risk[29].
Diet is an important factor influencing the composition of gut microbiota and changes in bacterial species[30]. Martinez-Medina and collaborators demonstrated in 2014 that a high-fat and high-sugar diet can lead to dysbiosis, with an increase in abundance of Bacteroides spp. and Ruminococcus torques in mice. Thus, new studies have pointed out that the westernization of the diet alters the microbiota to a composition that increases the risk of developing IBD[34,35].
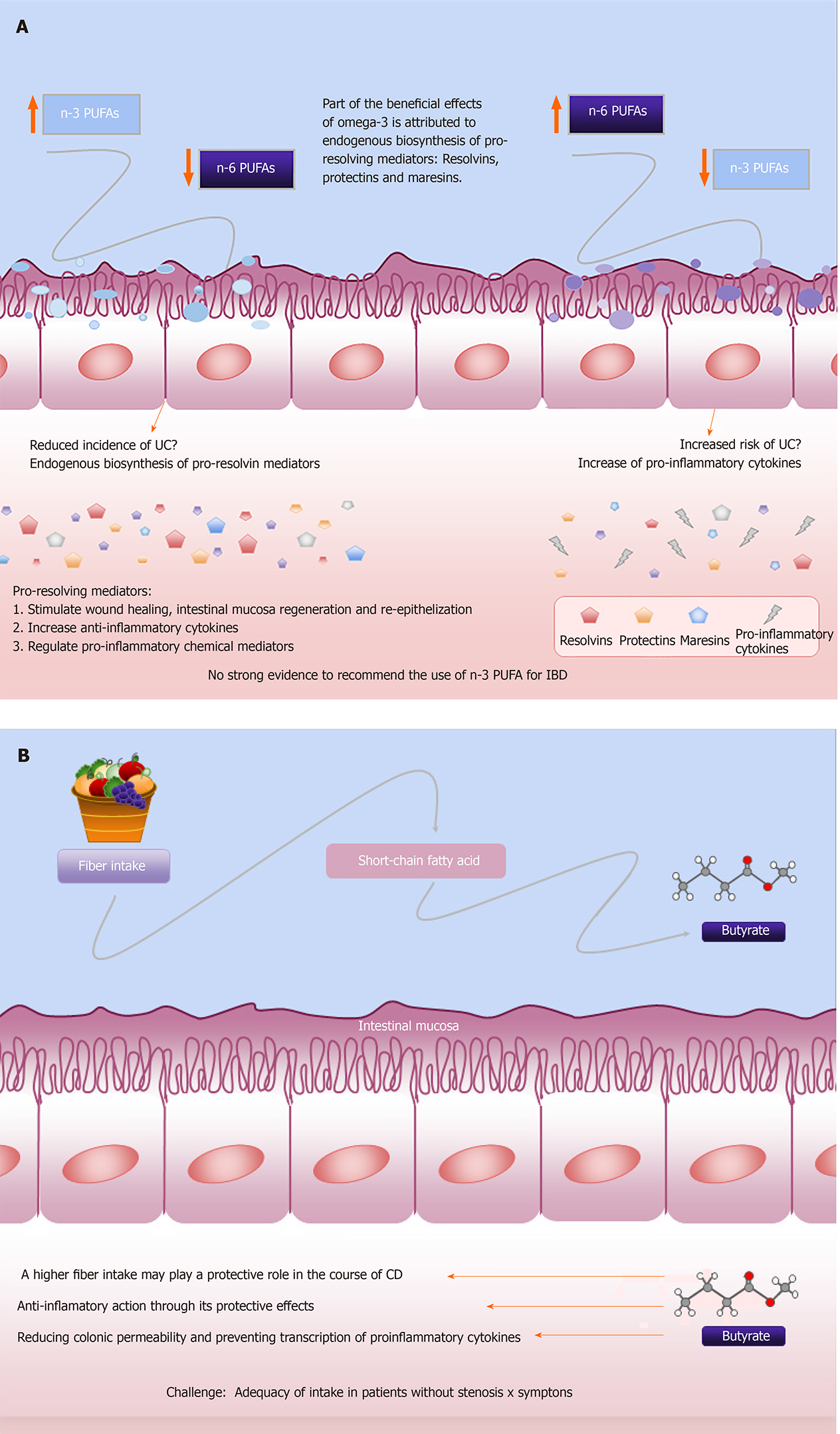
In addition, consumption of linoleic acid, a dietary n-6 polyunsaturated fatty acid (PUFA) has been associated with an increased risk of UC, while high intake of n-3 PUFAs is associated with a reduced incidence of UC[36,37]. Belluzzi et al[38] (1996) tested a new formulation of n-3 PUFAs, with the mixture of 45% eicosapentaenoic acid (EPA) and 20% docosahexaenoic acid (DHA) in enteric-coated capsules, in 78 CD patients in remission but with high risk of relapse. After 1 year of treatment, 59% of patients who were treated with the new formulation of n-3 PUFAs were still in remission, compared to only 26% in the placebo group and logistic regression analysis indicated that only n-3 PUFAs treatment reduced the likelihood of relapse. On the other hand, another study treated 700 CD patients with a similar preparation of n-3 PUFAs and this did not reduce the relapse rate in CD patients between groups[39]. This result can be explained by (1) the varied clinical inclusion criteria used to identify patients at high risk of relapse and (2) the inadequacy of the different therapies instituted for these patients[39,40]. A systematic review analyzing the effect of n-3 PUFAs in six studies in 1039 patients with CD did not rule out a possible beneficial effect of n-3 PUFAs in these patients[40].
Consequently, although we still have conflicting literature on whether or not to indicate omega-3 for patients with IBD, it seems that these divergent results are related to the different design of clinical trials and most likely due to the different n-6/n-3 ratio adopted in these studies. Indeed, the Western diet often results in the important disequilibrium in the n-6/n-3 PUFAs ratio, which can reach up to 20:1. Such an imbalance represents a pro-inflammatory stimulus that can affect the onset of many underlying conditions, including IBD[41-43]. The recommendation for the ratio of omega-3 to omega-6 is 5:1 according to the ESPEN guideline[44].
Furthermore, some components of n-3 PUFAs, EPA and DHA have been identified in the past decades and are related in processes of the anti-inflammatory effects of n-3 PUFAs, such as the inhibition of genes that lead to inflammatory processes and in the control of immunological and inflammatory responses. It is now known that part of the beneficial effects attributed to omega-3 in different diseases related to inflammatory disorders is attributed to endogenous biosynthesis of resolvins, protectins and maresins, as pro-resolving mediators[41,42].
These n-3 PUFAs-derived anti-inflammatory molecules can counteract and regulate pro-inflammatory chemical mediators; increase anti-inflammatory cytokines; and stimulate wound healing, intestinal mucosa regeneration and re-epithelialization. This may offer a fascinating new complementary approach to IBD treatment and its effects are currently being investigated by different research groups[40]. Despite its metabolic performance, the existing data are insufficient to justify the recommended use of n-3 PUFAs for UC and CD in clinical practice as well as for maintenance of remission in these patients. This was highlighted by the guideline for clinical nutrition in IBD[7,40,44], although it looks promising. Figure 2A describes the effects of PUFAs on IBD.
Taken together, these studies indicate that the primary factor to consider for IBD dietary recommendation is the quality of macronutrients (mainly for simple carbohydrates and animal fat) rather than the amount itself. According to the ESPEN guideline, the requirements for macronutrients in IBD patients are similar to those in the healthy population[7]. However, intake of protein in active adult IBD patients should be increased to provide 1.2-1.5 g/(kg·d), due to the proteolytic and catabolic response during active inflammation[7].
Nevertheless, future large-scale prospective designed studies are necessary to evaluate the relationship between quality macronutrients with IBD risk and management, and to make strong recommendations regarding the intake of macronutrients.
Although the literature is still unclear regarding the association between dietary components and the development of IBD, many articles demonstrate a positive effect of dietary fiber consumption[31,45,46], and its important role in the prevention of CD[7,45]. A prospective study found that patients who consumed a diet with a fiber content of 24.3 g/d resulted in a 40% decrease in the risk of CD development, although no association was observed for UC[31]. A meta-analysis of observational studies suggested that dietary fiber intake could decrease the risk of developing IBD, in addition to a reduction of 13% of CD risk for every 10 g/d increment in fiber. Among the mechanisms proposed in the literature are: (1) Anti-inflammatory action through the protective effects of butyrate; (2) Reduction in colonic permeability; and (3) Prevention of transcription of proinflammatory cytokines[47,48]. In addition, dietary fiber has been shown to have an effect on the microbiome, exerting a regulatory influence on the immunological homeostasis[46,49].
Recently, a European prospective multi-center cohort study investigated the association between fiber intake and the development of IBD, and contrary to other studies, no association between dietary fiber intake and the odds of developing CD and UC was found. The authors indicated that the low number of cases (104 with CD and 221 with UC) could be responsible for a weak association and a lack of statistical accuracy. Another limitation is related to information that was not collected in the study, including breastfeeding and the use of antibiotics, which could influence IBD risk and present unclear associations[50].
Furthermore, dietary fermentable carbohydrates, including fermentable oligo-saccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are a family of poorly absorbed short-chain carbohydrates that can induce gut symptoms in quiescent IBD[51]. A low FODMAP diet has been assessed for IBD patients and the results are discussed below. Patients with IBD usually report that high-fiber foods may worsen symptoms, and they also believe that foods may influence their disease course[52]. Foods rich in fiber may be hard to digest during active inflammation, so IBD patients tend to avoid fibers, despite the beneficial effect in maintaining remission that has been demonstrated by the intake of dietary fibers[47,53]. In addition, the latest dietary guidelines recommend a low insoluble fiber intake for those with intestinal stenosis. They emphasize that there is no strong evidence to support this approach, although it seems to be coherent[7,25]. Figure 2B describes the effects of fiber on IBD.
Therefore, future studies should investigate whether a protective effect by specific sources of fiber is associated with IBD development and the mechanisms involved.
Diet is an important nutritional factor to be taken into account when assessing the impact of nutrients, as well as the existence of dietary patterns involved in both the pathogenesis and the clinical progression of IBD. Recent dietary research has shown that whole dietary patterns are more representative and more important than isolated individual nutrient evaluations[54].
For example, westernized lifestyle has been associated with changes in dietary habits. The Western diet, which is high in fat and protein, mainly from animal sources, and low in fruits and vegetables, has been shown to predispose individuals to IBD. Devkota et al[55] (2012) confirmed that the consumption of a high milk-fat diet altered the environments of bacterial proliferation and promoted an increase of the sulfite-reducing pathobiont, Bilophila wadsworthia. Furthermore, the authors observed a bloom of Bilophila wadsworthia in mice who were supplemented with taurocholic acid - which is a bile acid byproduct resulting from the conjugation of taurine with cholic acid – which suggests an explanation of how western high saturated fat diets may increase the prevalence of IBD[55].
Our research group has identified three dietary patterns among patients with CD. These patterns are: (1) “Traditional + FODMAP”; (2) “fitness style”; and (3) “snacks and processed foods”. These patterns can be considered good indicators of patients’ eating habits, and facilitate the understanding of the relationship between diets and diseases[54]. Therefore, in general, results of dietary pattern studies should be considered when providing nutritional counseling to patients.
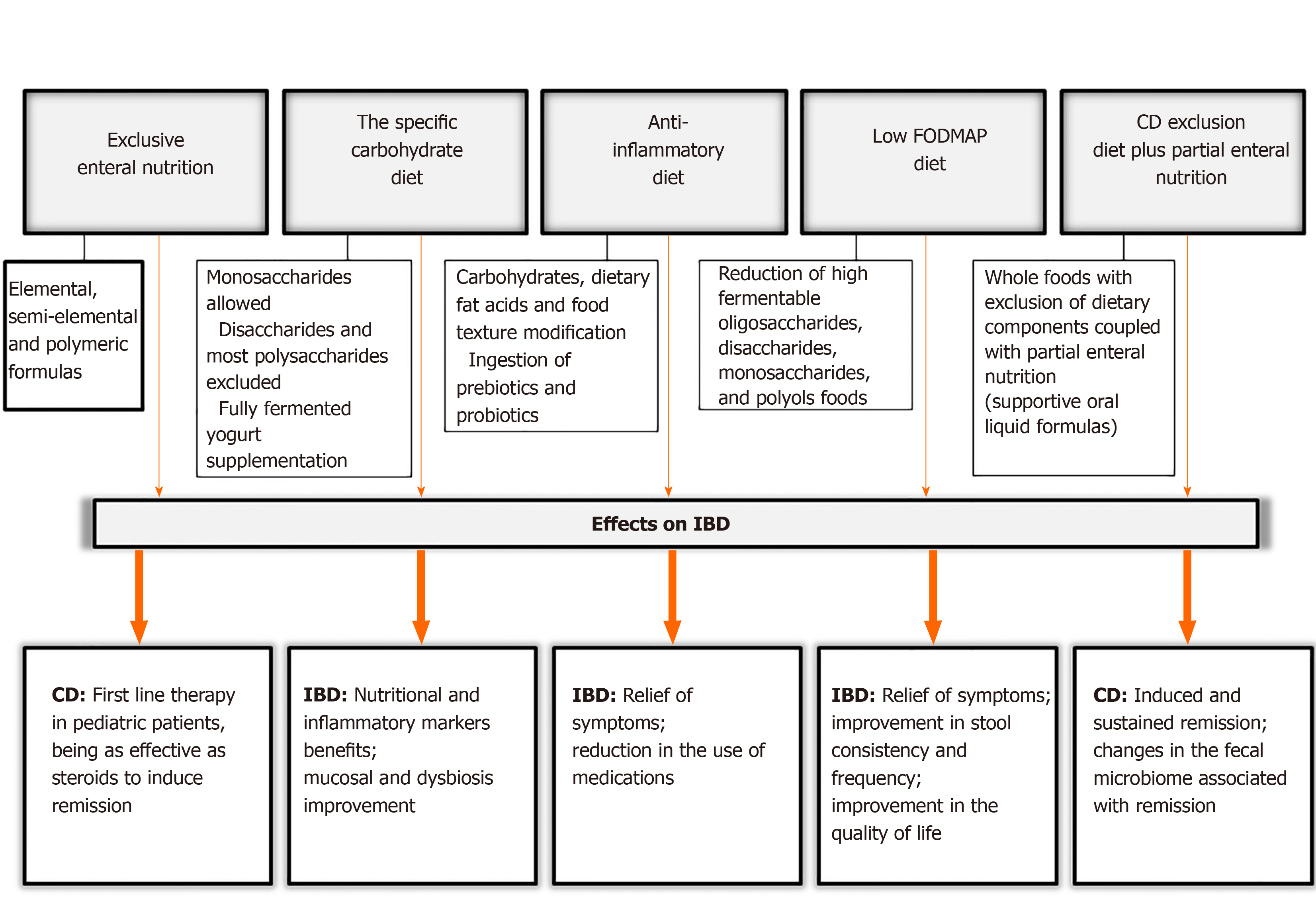
Considering the nutritional factors mentioned above and the risk of IBD development, there are specific diets which are proposed for IBD treatment in the scientific literature. We have noted some important aspects of these dietary treatments and evaluated their effects on IBD. These are summarized in Figure 3.
EEN is based on the administration of a liquid nutrition formula for 4-12 wk (with most requiring 6-8 wk) either orally or via a feeding tube[26]. EEN formulas can be classified as elemental, which contain individual amino acids; semi-elemental, with peptides of varying chain length; and polymeric formulas, which contain intact proteins[56]. Information about food reintroduction after this period is still scarce and inconclusive. However, most centers follow a gradual reintroduction of the normal diet over a period of 2-3 wk[57].
The study of Connors et al[58] (2017) included 111 newly diagnosed pediatric CD patients. They reported that EEN as an induction therapy resulted in a reduced need for steroid treatment, with no increased need for biological therapy or surgery[58]. However, this response to EEN is not observed in UC[59,60].
EEN has demonstrated many benefits such as mucosal healing, weight gain and linear growth, improved bone turnover and better quality of life[61-63]. The exact mode of action of EEN for CD treatment is uncertain, but may include: (1) Inhibiting the expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-1β; (2) increased release of vascular endothelial growth factor; and (3) transforming growth factor-β. In addition, a mucosal immunity activation with maintenance of intestinal homeostasis and the formation of the intestinal mucosal barrier by essential amino acids have been observed[64].
Some studies report changes in the gut microbiome induced by EEN[64-67], which are also important in the mechanisms’ understanding. The microbiome metabolic role is also relevant when considering the impact of EEN on its metabolic function[68]. Walton et al[68] (2016) demonstrated that there is a reduction in the concentrations of metabolites production during EEN treatment, which include potentially toxic compounds such as 1-propanol and 1-butanol, methyl and ethyl esters of short-chain fatty acids. These compounds could be involved in the immunological attack on the gut microbiota[68]. Interestingly, Mottawea et al[69] (2016) identified Atopobium parvulum as a microbe controlling the central hub of H2S-production, which is involved in H2S-detoxification. The decreased amount of the H2S-detoxification protein is a hallmark of CD activity. A systematic review reporting the effects of EEN on the microbiome concluded that, despite variable methods and the sample sizes used in the studies and the microbiota compositions in different people, a reduction in microbiota diversity and richness was reported. These EEN-induced metabolomic changes may play a role in achieving remission[65].
Current guidelines recommend EEN as the first line therapy for children with CD, especially at the time of diagnosis. The rates of clinical remission in EEN-treated children are up to 80%[7,26]. However, as this dietary therapy completely excludes food intake for several weeks, the adherence by children is difficult and uncertain. This approach could cause extreme emotional and social challenges for an entire family. For this reason, an adaptation of the EEN dietary intervention has recently been proposed and is discussed below.
Specific carbohydrate diet (SCD) is a popular diet for IBD treatment, mainly in the lay literature[70,71]. The features of this diet include a modified carbohydrate diet allowing monosaccharides, excluding disaccharides and most polysaccharides. The SCD is a very restrictive diet and allows fruits and vegetables containing more amylose rather than amylopectin, nuts, nut-derived flours, dry-curd cottage cheese, meats, eggs, butter as well as oils. It eliminates sucrose, maltose, isomaltose, lactose, all true and pseudo-grains and grain-derived flours, potatoes, okra, corn, fluid milk, soy, cheeses with high amounts of lactose and most food additives and preservatives. The diet is supplemented with fully fermented yogurt, to free it from lactose. Some recommended cultures are Lactobacillus bulgaricus, Lactobacillus acidophilus, and Streptococcus thermophilus[71].
This diet is based on the absorption of specific selected carbohydrates that require minimal digestive processes. Although also uncertain, it is hypothesized that the mechanism of action lies in the decrease in intestinal inflammation by changes in the fecal microbiome[71].
Although studies have reported a positive impact of the SCD in IBD patients, such as improvement in anemia and albumin[72], inflammatory markers[72,73], clinical and biochemical parameters, dysbiosis, and the number of patients achieving clinical remission[74,75] and mucosal healing[75], a careful scientific evaluation is necessary, as there are limitations regarding this diet. Only a few studies with small sample sizes have assessed this diet, which limit the accuracy of estimated effects; besides, the long-term effect must be evaluated.
Additionally, a very restrictive diet requires important lifestyle changes and the patients require follow-up to provide proper nutrition, taking into consideration that specific dietary deficiencies can occur as a consequence of the restricted foods, particularly dairy products that contain vitamin D and calcium; the limited intake of grains, fruits and vegetables can result in folate, thiamine, vitamins B6, C and A deficiencies. Another important concern is reported in the pilot study by Cohen et al[75] (2014) in which 33% of the patients lost weight following this restrictive diet.
The anti-inflammatory diet for IBD (IBD-AID) is derived from the SCD and was developed by a group at the University of Massachusetts Medical School. This diet was proposed for patients who are refractory to pharmacological therapy. The treatment was not as beneficial as required and its goal was to obtain and maintain remission with a decreased frequency and severity of flares[76].
The IBD-AID consists of five components: The first includes the modification of carbohydrates, such as lactose and refined or processed to complex carbohydrates; the second incorporates the ingestion of prebiotics and probiotics; the third modifies dietary fat acids; the fourth detects the overall dietary pattern and missing nutrients, and identifies intolerances; and the fifth component modifies the food texture according to 4 phases, beginning with soft or pureed foods if in active flare, or based on the symptoms reported. Patients often advanced to a more whole food diet according to the improvement of their symptoms[76].
In a case-series of 11 adult patients with IBD following the IBD-AID for 4 wk or more, all of the patients were able to interrupt at least one of their prior IBD medications, in addition to experiencing a reduction of their symptoms[76]. Again, it is a very restrictive diet with risk of nutritional deficiencies as a consequence of the restriction of foods, particularly micronutrients. Due to the limited number of studies with small sample sizes, prospective studies to evaluate the application of this diet on IBD are needed with rigorous analysis and a greater number of patients. Thus, its efficacy and mechanisms have not been elucidated, which makes its recommendation very difficult, especially as the long-term effects are not known.
FODMAPs are poorly absorbed molecules in the small intestine which are rapidly fermented in the colon by bacteria. This process can lead to abdominal pain, bloating, flatulence, and diarrhea[77,78].
Patients with IBD usually have functional symptoms similar to those seen in irritable bowel syndrome (IBS) patients, despite having quiescent disease[79]. Most studies on the low-FODMAP diet were performed in patients with IBS, and evidence shows that this diet can lead to significant improvement in the symptoms[80-85], stool consistency[84,85] and a decrease in functional gastrointestinal symptoms[85].
Patients with IBD presenting IBS-like symptoms also had a significant reduction in overall symptoms, as well as an increase in quality of life[86]. A meta-analysis showed that the adherence to a low-FODMAP diet compared to a normal Western diet results in the improvement of symptoms related to IBS and IBD, in addition to a significant reduction of symptom severity and improvement in the quality of life scores[87].
Additionally, patients with IBD are at risk of dysbiosis. The effects of the low-FODMAP diet on the microbiome and metabolites are important since the modification of the carbohydrate content of the diet potentially alters the gut microbiota. A study found that a variation in FODMAP intake was associated with important changes in fecal microbiota, which reflects a prebiotic effect of increasing FODMAPs in CD patients[88]. It was demonstrated in a trial of the low-FODMAP diet that IBD patients experienced relief in gut symptoms, higher health-related quality of life scores, and a reduction in the fecal abundance of microbes believed to participate in regulation of the immune response[51].
Although this diet shows positive effects for IBD patients, it should be followed with caution, as the restriction of dietary component intake may lead to nutritional deficiencies, especially in long-term use, which can have a negative impact in the course of the disease.
An alternative diet to EEN was developed based on partial enteral nutrition (PEN) that involves whole foods with exclusion of dietary components, for active CD children and young adults. The CD exclusion diet plus PEN (CDED + PEN) is a specialized diet coupled with supportive oral liquid formulas, and comprises two phases lasting 12 wk[25,89]. The first phase consists of a period of 6 wk with 50% PEN for calculated energy requirement that involves a more restrict diet: Gluten, dairy products, gluten-free baked goods and breads, animal fat, processed meats, products containing emulsifiers, canned goods, and all packaged products with an expiration date are not allowed, and a polymeric formula providing 50% of calories. The second 6-wk phase supplies 25% calories in a liquid formula, in addition to a fixed portion of whole grain bread, small amounts of nuts, fruits, legumes and vegetables are allowed. Up to 18-20 g of fiber per day is allowed.
In the Sigall-Boneh et al[89] (2014) study, by week 6, remission was obtained in 70.2% of patients, with similar rates in children and adults, 70.1% and 69.2%, respectively. At week 12 and after the reintroduction of some foods, 84% of patients in remission with follow-up remained in remission[89]. The same research group evaluated this diet expanding its use to anti-TNF biologics refractory patients, and showed that clinical remission was obtained in 62% of patients who were on CDED with or without PEN after 6 wk, suggesting that the effect of the decrease in inflammation may be due to the exclusion of specific dietary components rather than supplementation[90].
More recently, Levine et al[91] (2019) compared the efficacy of CDED + PEN with EEN in inducing and sustaining corticosteroid-free remission in children. Group 1 (CDED) received the protocol diet as documented before over 12 wk; group 2 received standard EEN for the first 6 wk and then 25% PEN + free diet during the next 6 wk with gradual reintroduction. At week 6, no statistically significant difference in inducing remission was observed between the groups[91]. However, more CDED + PEN patients achieved sustained remission (75.6%, P = 0.01) and were more likely to maintain corticosteroid-free remission compared to EEN patients at week 12 (87.5% vs 56%, P = 0.01). The authors also performed a microbiome analysis and demonstrated that changes within CDED + PEN patients indicated more differentiated communities with treatment than EEN patients, showing a rebound effect in which the re-exposure to food did not help to maintain the microbiological changes induced by EEN[91].
To date, of the diets proposed for CD patients, this one has some advantages, including it is not restrictive in terms of essential nutrients and it is more balanced, as it allows access to whole foods.
Diet is one of several important environmental factors associated with IBD etiopathology. The majority of studies address association but not causality. Therefore, our understanding is limited concerning how environmental factors may be involved and until now it is still unknown whether diet is a primary or secondary factor.
It has been reported that diet changes the microbiome, and thus contributes to modifying, for example, quality of life, lifestyle and clinical symptoms. For this reason, several non-lay and lay diets have been proposed and IBD patients are asking their physicians which would be the appropriate type of diet to choose.
The data point out the importance of nutrients in the etiopathogenesis and management of IBD. In particular, components such as breastfeeding and high intake of n-3 PUFAs might have a protective effect on UC, while high consumption of sucrose, animal fat and cholesterol, as well as linoleic acid are associated with increased risk of UC. Regarding CD, breastfeeding and high intake of fiber may protect against the disease, whilst high sucrose, saturated fat and monosaccharide intake, and long-term fast food consumption are considered risk factors.
Specific diets for IBD should be considered with caution and as an adjunct to IBD therapy. Currently there is no scientific consensus regarding an appropriate diet that would benefit all IBD patients. In particular, individualized dietary recommendations are of the greatest importance and diets should be planned by a multidisciplinary team which includes a dietitian in order to provide individual IBD patients with the specific nutrient requirements while keeping in mind all of the patients’ clinical aspects.
Among the diets described, CDED + PEN[91] is characterized by a reduction in the consumption of processed and industrialized foods. Interestingly, in Brazil we observe similar recommendations in ‘The Dietary Guidelines for the Brazilian Population’. These guidelines recommend consuming natural or minimally processed foods, and moderating the consumption of processed foods while avoiding ultra-processed foods. These important Brazilian guidelines aim to provide information on healthy eating habits in order to reduce and prevent nutritionally related diseases[92]. We suggest that IBD patients follow a diet based on the above Guideline recommendations, because the use of processed and ultra-processed foods can result in unbalanced nutritional compositions which can lead to chronic diseases such as obesity and can also increase the risk of nutritional deficiencies.
Considering the data, there are several important questions that remain to be answered: (1) How do dietary products influence gut microbiota? (2) How does diet predispose individuals to IBD? and (3) Is IBD-associated dysbiosis a cause or a consequence of clinical outcomes?
This review aims to discuss the scientific aspects involving diet and IBD. Additional research is required to determine if diets have a role in IBD etiology and how to determine the specific diet that can benefit patients with IBD. Specific diets can affect the inflammatory pathways and the mechanisms involved. Further long-term interventional studies should explore dietary interventions for IBD. Results will support the formulation of nutritional guidelines which will assist professionals in clinical practice. Dietary therapy may be more important than it is currently believed to be.
We thank Professor Torriani T for revising the English version of our manuscript.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Brazilian Study Group in Inflammatory Bowel Diseases (GEDIIB); European Crohn’s Colitis Organisation (ECCO); Pan American Crohn’s Colitis Organisation (PANCCO); International Society of University Colon and Rectal Surgeons (ISUCRS); and Coloproctology Brazilian Society (SBCP).
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsujikawa T S-Editor: Chen XF L-Editor: Webster JR P-Editor: Xing YX
| 1. | Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R; European Society of Pathology (ESP); European Crohn's and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 2. | de Souza HSP. Etiopathogenesis of inflammatory bowel disease: today and tomorrow. Curr Opin Gastroenterol. 2017;33:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:112-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Mokry M, Middendorp S, Wiegerinck CL, Witte M, Teunissen H, Meddens CA, Cuppen E, Clevers H, Nieuwenhuis EE. Many inflammatory bowel disease risk loci include regions that regulate gene expression in immune cells and the intestinal epithelium. Gastroenterology. 2014;146:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. 2012;3:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Tuvlin JA, Raza SS, Bracamonte S, Julian C, Hanauer SB, Nicolae DL, King AC, Cho JH. Smoking and inflammatory bowel disease: trends in familial and sporadic cohorts. Inflamm Bowel Dis. 2007;13:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Forbes A. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39:632-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 8. | Binns C, Lee M, Low WY. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac J Public Health. 2016;28:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 9. | Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Klement E, Cohen RV, Boxman J, Joseph A, Reif S. Breastfeeding and risk of inflammatory bowel disease: a systematic review with meta-analysis. Am J Clin Nutr. 2004;80:1342-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Xu L, Lochhead P, Ko Y, Claggett B, Leong RW, Ananthakrishnan AN. Systematic review with meta-analysis: breastfeeding and the risk of Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;46:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 12. | Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 13. | Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Lessons from mother: Long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes. 2014;5:663-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Rautava S. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis. 2016;7:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Uranga JA, López-Miranda V, Lombó F, Abalo R. Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol Rep. 2016;68:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 18. | Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1123] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 19. | Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 937] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 2063] [Article Influence: 158.7] [Reference Citation Analysis (0)] |
| 21. | Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 22. | de Castro MM, Corona LP, Pascoal LB, Rodrigues BL, de Lourdes Setsuko Ayrizono M, Rodrigues Coy CS, Leal RF, Milanski M. Impaired nutritional status in outpatients in remission or with active Crohn's disease - classified by objective endoscopic and imaging assessments. Clin Nutr ESPEN. 2019;33:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 23. | Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6397] [Cited by in RCA: 5666] [Article Influence: 354.1] [Reference Citation Analysis (1)] |
| 24. | Yi JM, Kim TO. Epigenetic alterations in inflammatory bowel disease and cancer. Intest Res. 2015;13:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Levine A, Rhodes JM, Lindsay JO, Abreu MT, Kamm MA, Gibson PR, Gasche C, Silverberg MS, Mahadevan U, Boneh RS, Wine E, Damas OM, Syme G, Trakman GL, Yao CK, Stockhamer S, Hammami MB, Garces LC, Rogler G, Koutroubakis IE, Ananthakrishnan AN, McKeever L, Lewis JD. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 26. | Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, Buderus S, Martín-de-Carpi J, De Ridder L, Fagerberg UL, Hugot JP, Kierkus J, Kolacek S, Koletzko S, Lionetti P, Miele E, Navas López VM, Paerregaard A, Russell RK, Serban DE, Shaoul R, Van Rheenen P, Veereman G, Weiss B, Wilson D, Dignass A, Eliakim A, Winter H, Turner D; European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology; Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 843] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 27. | Zeng L, Hu S, Chen P, Wei W, Tan Y. Macronutrient Intake and Risk of Crohn's Disease: Systematic Review and Dose-Response Meta-Analysis of Epidemiological Studies. Nutrients. 2017;9:500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Wang F, Feng J, Gao Q, Ma M, Lin X, Liu J, Li J, Zhao Q. Carbohydrate and protein intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Clin Nutr. 2017;36:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 218] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Burisch J, Pedersen N, Cukovic-Cavka S, Turk N, Kaimakliotis I, Duricova D, Bortlik M, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, Krabbe S, Andersen V, Dahlerup JF, Kjeldsen J, Salupere R, Olsen J, Nielsen KR, Manninen P, Collin P, Katsanos KH, Tsianos EV, Ladefoged K, Lakatos L, Ragnarsson G, Björnsson E, Bailey Y, O'Morain C, Schwartz D, Odes S, Giannotta M, Girardin G, Kiudelis G, Kupcinskas L, Turcan S, Barros L, Magro F, Lazar D, Goldis A, Nikulina I, Belousova E, Martinez-Ares D, Hernandez V, Almer S, Zhulina Y, Halfvarson J, Arebi N, Tsai HH, Sebastian S, Lakatos PL, Langholz E, Munkholm P; EpiCom-group. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe--an ECCO-EpiCom study. J Crohns Colitis. 2014;8:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 32. | Chiba M, Nakane K, Komatsu M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm J. 2019;23:18-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018;128:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 34. | Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 397] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 35. | Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 435] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 36. | IBD in EPIC Study Investigators, Tjonneland A , Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G, Lindgren S, Grip O, Palli D, Day NE, Khaw KT, Bingham S, Riboli E, Kennedy H, Hart A. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 37. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, Willett WC, Richter JM, Chan AT. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn's disease. Gut. 2014;63:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 38. | Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 469] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 39. | Scaioli E, Liverani E, Belluzzi A. The Imbalance between n-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int J Mol Sci. 2017;18:2619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1021] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 42. | Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2128] [Cited by in RCA: 2252] [Article Influence: 204.7] [Reference Citation Analysis (0)] |
| 43. | Chan SS, Luben R, Olsen A, Tjonneland A, Kaaks R, Lindgren S, Grip O, Bergmann MM, Boeing H, Hallmans G, Karling P, Overvad K, Venø SK, van Schaik F, Bueno-de-Mesquita B, Oldenburg B, Khaw KT, Riboli E, Hart AR. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn's disease. Aliment Pharmacol Ther. 2014;39:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Bischoff SC. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 428] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 45. | Amre DK, D'Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, Israel D, Mack D, Ghadirian P, Deslandres C, Chotard V, Budai B, Law L, Levy E, Seidman EG. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. Am J Gastroenterol. 2007;102:2016-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 46. | Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 865] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 47. | Liu X, Wu Y, Li F, Zhang D. Dietary fiber intake reduces risk of inflammatory bowel disease: result from a meta-analysis. Nutr Res. 2015;35:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Venkatraman A, Ramakrishna BS, Shaji RV, Kumar NS, Pulimood A, Patra S. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2003;285:G177-G184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Issa M, Saeian K. Diet in inflammatory bowel disease. Nutr Clin Pract. 2011;26:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Andersen V, Chan S, Luben R, Khaw KT, Olsen A, Tjonneland A, Kaaks R, Grip O, Bergmann MM, Boeing H, Hultdin J, Karling P, Overvad K, Oldenburg B, Opstelten J, Boutron-Ruault MC, Carbonnel F, Racine A, Key T, Masala G, Palli D, Tumino R, Trichopoulou A, Riboli E, Hart A. Fibre intake and the development of inflammatory bowel disease: A European prospective multi-centre cohort study (EPIC-IBD). J Crohns Colitis. 2018;12:129-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 51. | Cox SR, Prince AC, Myers CE, Irving PM, Lindsay JO, Lomer MC, Whelan K. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients With Inflammatory Bowel Disease: A Randomised, Double-blind, Placebo-controlled, Cross-over, Re-challenge Trial. J Crohns Colitis. 2017;11:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 52. | Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, Lewis JD. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 53. | Myklebust-Hansen T, Aamodt G, Haugen M, Brantsæter AL, Vatn MH, Bengtson MB. Dietary Patterns in women with Inflammatory Bowel Disease and Risk of Adverse Pregnancy Outcomes: Results from The Norwegian Mother and Child Cohort Study (MoBa). Inflamm Bowel Dis. 2017;24:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | de Castro MM, Corona LP, Pascoal LB, Miyamoto JÉ, Ignacio-Souza LM, de Lourdes Setsuko Ayrizono M, Torsoni MA, Torsoni AS, Leal RF, Milanski M. Dietary Patterns Associated to Clinical Aspects in Crohn's Disease Patients. Sci Rep. 2020;10:7033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1421] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 56. | Gatti S, Galeazzi T, Franceschini E, Annibali R, Albano V, Verma AK, De Angelis M, Lionetti ME, Catassi C. Effects of the Exclusive Enteral Nutrition on the Microbiota Profile of Patients with Crohn's Disease: A Systematic Review. Nutrients. 2017;9:832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Sood A, Ahuja V, Kedia S, Midha V, Mahajan R, Mehta V, Sudhakar R, Singh A, Kumar A, Puri AS, Tantry BV, Thapa BR, Goswami B, Behera BN, Ye BD, Bansal D, Desai D, Pai G, Yattoo GN, Makharia G, Wijewantha HS, Venkataraman J, Shenoy KT, Dwivedi M, Sahu MK, Bajaj M, Abdullah M, Singh N, Singh N, Abraham P, Khosla R, Tandon R, Misra SP, Nijhawan S, Sinha SK, Bopana S, Krishnaswamy S, Joshi S, Singh SP, Bhatia S, Gupta S, Bhatia S, Ghoshal UC. Diet and inflammatory bowel disease: The Asian Working Group guidelines. Indian J Gastroenterol. 2019;38:220-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Connors J, Basseri S, Grant A, Giffin N, Mahdi G, Noble A, Rashid M, Otley A, Van Limbergen J. Exclusive Enteral Nutrition Therapy in Paediatric Crohn's Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis. J Crohns Colitis. 2017;11:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Triantafillidis JK, Vagianos C, Papalois AE. The role of enteral nutrition in patients with inflammatory bowel disease: current aspects. Biomed Res Int. 2015;2015:197167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, Dias JA, Bronsky J, Braegger CP, Cucchiara S, de Ridder L, Fagerberg UL, Hussey S, Hugot JP, Kolacek S, Kolho KL, Lionetti P, Paerregaard A, Potapov A, Rintala R, Serban DE, Staiano A, Sweeny B, Veerman G, Veres G, Wilson DC, Ruemmele FM; European Crohn's and Colitis Organization; European Society for Paediatric Gastroenterology; Hepatology; and Nutrition. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 61. | Yamamoto T, Nakahigashi M, Saniabadi AR, Iwata T, Maruyama Y, Umegae S, Matsumoto K. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn's disease: a prospective study. Inflamm Bowel Dis. 2007;13:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Whitten KE, Leach ST, Bohane TD, Woodhead HJ, Day AS. Effect of exclusive enteral nutrition on bone turnover in children with Crohn's disease. J Gastroenterol. 2010;45:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Kuriyama M, Kato J, Morimoto N, Fujimoto T, Kono H, Okano N, Miyaike J, Morita T, Okada H, Suzuki S, Yoshioka T, Shiode J, Shiratori Y, Kazuhide Y; Japan West Crohn's Disease Study Group. Enteral nutrition improves health-related quality of life in Crohn's disease patients with long disease duration. Hepatogastroenterology. 2009;56:321-327. [PubMed] |
| 64. | Yu Y, Chen KC, Chen J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn's disease: a meta-analysis. World J Pediatr. 2019;15:26-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 65. | Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, Bittinger K, Bailey A, Friedman ES, Hoffmann C, Albenberg L, Sinha R, Compher C, Gilroy E, Nessel L, Grant A, Chehoud C, Li H, Wu GD, Bushman FD. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn's Disease. Cell Host Microbe. 2015;18:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 589] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 66. | Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, Garrick V, Russell RK, Blaut M, McGrogan P, Edwards CA. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn's disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 67. | Dunn KA, Moore-Connors J, MacIntyre B, Stadnyk AW, Thomas NA, Noble A, Mahdi G, Rashid M, Otley AR, Bielawski JP, Van Limbergen J. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn's Disease. Inflamm Bowel Dis. 2016;22:2853-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 68. | Walton C, Montoya MP, Fowler DP, Turner C, Jia W, Whitehead RN, Griffiths L, Waring RH, Ramsden DB, Cole JA, Cauchi M, Bessant C, Naylor SJ, Hunter JO. Enteral feeding reduces metabolic activity of the intestinal microbiome in Crohn's disease: an observational study. Eur J Clin Nutr. 2016;70:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Mottawea W, Chiang CK, Mühlbauer M, Starr AE, Butcher J, Abujamel T, Deeke SA, Brandel A, Zhou H, Shokralla S, Hajibabaei M, Singleton R, Benchimol EI, Jobin C, Mack DR, Figeys D, Stintzi A. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn's disease. Nat Commun. 2016;7:13419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 70. | HAAS SV, HAAS MP. The treatment of celiac disease with the specific carbohydrate diet; report on 191 additional cases. Am J Gastroenterol. 1955;23:344-360. [PubMed] |
| 71. | Gottschall E. Breaking the vicious cycle. Baltimore, Ontario, Canada: Kirkton Press, 2012: 205. |
| 72. | Burgis JC, Nguyen K, Park KT, Cox K. Response to strict and liberalized specific carbohydrate diet in pediatric Crohn's disease. World J Gastroenterol. 2016;22:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 73. | Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr. 2014;58:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 74. | Suskind DL, Cohen SA, Brittnacher MJ, Wahbeh G, Lee D, Shaffer ML, Braly K, Hayden HS, Klein J, Gold B, Giefer M, Stallworth A, Miller SI. Clinical and Fecal Microbial Changes With Diet Therapy in Active Inflammatory Bowel Disease. J Clin Gastroenterol. 2018;52:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 75. | Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2014;59:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 76. | Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J. 2014;13:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 77. | Barbalho SM, Goulart RA, Aranão ALC, de Oliveira PGC. Inflammatory Bowel Diseases and Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols: An Overview. J Med Food. 2018;21:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 78. | Barrett JS. Extending our knowledge of fermentable, short-chain carbohydrates for managing gastrointestinal symptoms. Nutr Clin Pract. 2013;28:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 80. | de Roest RH, Dobbs BR, Chapman BA, Batman B, O'Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 81. | Zanetti AJA, Rogero MM, von Atzingen MCBC. Low-FODMAP diet in the management of irritable bowel syndrome. Nutrire. 2018;43:17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Testa A, Imperatore N, Rispo A, Rea M, Tortora R, Nardone OM, Lucci L, Accarino G, Caporaso N, Castiglione F. Beyond Irritable Bowel Syndrome: The Efficacy of the Low Fodmap Diet for Improving Symptoms in Inflammatory Bowel Diseases and Celiac Disease. Dig Dis. 2018;36:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis. 2009;3:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 84. | Maagaard L, Ankersen DV, Végh Z, Burisch J, Jensen L, Pedersen N, Munkholm P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J Gastroenterol. 2016;22:4009-4019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 85. | Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 86. | Pedersen N, Ankersen DV, Felding M, Wachmann H, Végh Z, Molzen L, Burisch J, Andersen JR, Munkholm P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23:3356-3366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (5)] |
| 87. | Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? Eur J Nutr. 2016;55:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 88. | Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Muir JG, Gibson PR. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn's Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin Transl Gastroenterol. 2016;7:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 89. | Sigall-Boneh R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflamm Bowel Dis. 2014;20:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 90. | Sigall Boneh R, Sarbagili Shabat C, Yanai H, Chermesh I, Ben Avraham S, Boaz M, Levine A. Dietary Therapy With the Crohn's Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J Crohns Colitis. 2017;11:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 91. | Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, Kori M, Cohen S, Peleg S, Shamaly H, On A, Millman P, Abramas L, Ziv-Baran T, Grant S, Abitbol G, Dunn KA, Bielawski JP, Van Limbergen J. Crohn's Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology. 2019;157:440-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 92. | Ministry of Health of Brazil. Dietary Guidelines for the Brazilian population. 2nd ed. Brasília: Ministry of Health of Brazil, 2014: 156. |