Published online Nov 20, 2016. doi: 10.5493/wjem.v6.i4.63
Peer-review started: July 1, 2016
First decision: September 5, 2016
Revised: September 19, 2016
Accepted: October 17, 2016
Article in press: October 19, 2016
Published online: November 20, 2016
Processing time: 140 Days and 13.3 Hours
To investigate the effect of two ways of lipofuscin production (lipid peroxidation and glycation) on lipofuscin fluorescence characteristics and phototoxicity and to compare them with the properties of natural lipofuscin.
Model lipofuscins were prepared on the basis of bovine photoreceptor outer segments (POS) with bisretinoid A2E addition. One set of samples was prepared from POS modified by lipid peroxidation, while another set from POS modified by glycation with fructose. Fluorescent properties and kinetics of photoinduced superoxide generation of model lipofuscins and human retinal pigment epithelium (RPE) lipofuscin were compared. The fluorescence spectra of samples were measured at 365 nm excitation wavelength and 380-650 emission wavelength.
The fluorescence spectra of model lipofuscins are almost the same as the spectrum of natural lipofuscin. Visible light irradiation of both model lipofuscins and natural lipofuscin isolated from RPE cells leads to decrease of a fluorescence maximum at 550 nm and to appearance of a distinct, new maximum at 445-460 nm. The rate of photogeneration of reactive oxygen forms by both model lipofuscins was almost the same and approximately two times less than that of RPE lipofuscin granules.
These data suggest that fluorescent characteristics and phototoxicity of lipofuscin granules depend only to an insignificant degree on the oxidative modification of POS proteins and lipids, and generally are defined by the bisretinoid fluorophores contained in them.
Core tip: The aim of this work is to investigate the influence of different ways of protein-lipid modification of photoreceptor outer segments (POS) on the spectral characteristics and toxicity of lipofuscin. Therefore, model lipofuscins were prepared by protein-lipid modification of POS with products of lipid peroxidation or glycation reaction and the subsequent addition of the fluorophore A2E. The type of photoreceptor modification has no effect on model lipofuscins phototoxicity.
- Citation: Dontsov A, Koromyslova A, Ostrovsky M, Sakina N. Lipofuscins prepared by modification of photoreceptor cells via glycation or lipid peroxidation show the similar phototoxicity. World J Exp Med 2016; 6(4): 63-71
- URL: https://www.wjgnet.com/2220-315X/full/v6/i4/63.htm
- DOI: https://dx.doi.org/10.5493/wjem.v6.i4.63
Fluorescent (aging pigment) lipofuscin accumulates during lifetime and is one of the most important factors limiting the life of a cell. Lipofuscin is found in tissues of various organs such as brain, heart, liver, kidneys and skin. Especially a lot of lipofuscin accumulates in aging postmitotic tissues such as nerve and muscle. Lipofuscin is insoluble and cannot to be utilized by neither lysosomal enzymes nor proteasomal system of a cell[1].
In the eye lipofuscin is largely accumulated in the retinal pigment epithelium cells (RPE)[2], being one of the key biomarkers of aging and oxidative stress. It also plays an important role in the development of retinal pathologies[3].
RPE lipofuscin granules are composed of a complex mixture of fluorophores, which determine fluorescent characteristics of a granule[4]. The most studied fluorophore of lipofuscin granules is pyridinium bisretinoid - A2E[5].
Both lipofuscin granules and fluorophore A2E are toxic to cells. Upon irradiation with visible light, they are able to generate reactive oxygen species[6-8], enforce damage of membrane structures[9-11], inhibit lysosomal and proteasome degradation of proteins[12-14] and lipids[15], as well as to induce apoptosis of RPE cells[16,17].
It is generally accepted that lipofuscin granules are formed as a result of incomplete digestion during phagocytosis of exhaust disks of photoreceptor outer segments (POS) in RPE cells. Inability of proteins and lipids in lipofuscin granules to be utilized is associated with the processes of oxidation and formation of intra- and inter-molecular covalent cross-linking. This modification of molecules in granules is considered to be mainly connected to reactions of lipid peroxidation that can actively pass in the retina and RPE tissues[18]. The reactive electrophilic aldehydes produced by process of lipid peroxidation, especially malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), react readily with amino acid residues of lysine, histidine, cysteine and arginine, as well as with amino groups of phospholipids. This results in damage and disruption of their structure and catabolism[19]. Indeed, most of the proteins identified in lipofuscin from human RPE are modified in reactions with MDA and 4-HNE[19]. Proteins modified by lipid peroxidation reactions are called advanced lipoxidation end products (ALE).
Reduced susceptibility of phagolysosome proteins to proteolysis after their modification by malondialdehyde and 4-hydroxynonenal is supposed to be an important factor in lipofuscinogenesis[20].
However, it is well known that covalently crosslinked proteins and lipids are also produced under conditions of hyperglycaemia resulting from the Maillard reaction[21]. These so-called advanced glycation end products (AGE) are believed to play an important role in the development of diabetic complications[22] and various eye pathologies[23]. AGE products were found to accumulate with age in cadaver eyes within the RPE in Bruch’s membrane and drusen[24,25]. AGE accumulation leads to the reduced activity of lysosomal enzymes and, consequently, to the increased accumulation of lipofuscin[25].
AGE products were also found directly in lipofuscin granules. Horie et al[26] has first shown that lipofuscin from brain tissue of elderly people is formed not only in reactions of lipid peroxidation, but also in glycation reactions. Apparently, this can also occur in the lipofuscin granules of the RPE. For instance, AGE-modified proteins were also found in lipofuscin granules of human RPE[19].
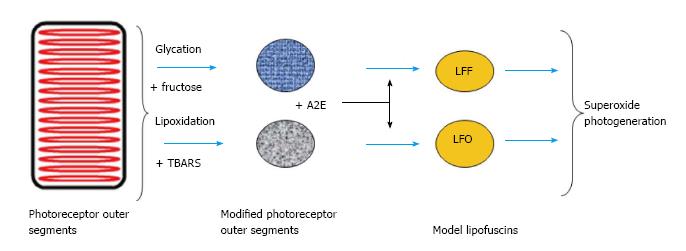
All in all, that suggests that during lipofuscinogenesis modification of proteins and lipids in phagolysosome of RPE cells can occur either as a result of lipid peroxidation and ALE products formation, or by glycation reactions and formation of AGE products. The later can happen if there is an increased content of reducing sugars in the cell. There is currently no information about the properties of lipofuscin granules formed under the conditions of hyperglycemia. The aim of this work is to investigate the influence of different ways of protein-lipid modification on the spectral characteristics and toxicity of lipofuscin. It is assumed that the fluorescence of RPE lipofuscin determines the fundus autofluorescence (FAF) in vivo[27,28]. Monitoring FAF is noninvasive diagnostic method used in detection retinal degenerative diseases such as recessive Stargardt disease, Best macular dystrophy, age-related macular degeneration (AMD) and Diabetic Retinopaty[3]. So the model systems of lipofuscin study may be important for FAF interpretation. Therefore, model lipofuscin was prepared on the basis of POS and the fluorophore A2E. Model lipofuscin preparing was accomplished by corporating modified POS and the fluorophore A2E. Modification of POS was carried out either by the reaction with products of lipid peroxidation (LPO-lipofuscin model), or by glycation reaction in the presence of fructose (LFF-lipofuscin model). Properties of the model lipofuscins were compared with natural lipofuscin from human eye RPE.
Cadaver eyes from 40-75 years old donors without any ophthalmic diseases were kindly provided by the Eye Tissue Bank of the Sv. Fyodorov Eye Microsurgery Institute, Moscow. Experiments on tissue isolated from human-cadaver eyes were performed in compliance with officially accepted procedures, in particular Russian Federation law N 4180-I dated 22.12.1992, “on human organs or tissue transplantation” (with modifications and additions dated 20.06.2000, 16.10.2006, 09.02.2007, and 29.11.2007); section II “removal of organs or tissue from dead bodies”; clause 8 “presumption of consent for removal of organs or tissue”; clause 10 “permission to remove organs or tissue from dead bodies”. According to section II (clause 8) of this law, consent from the donor or the next of kin to use organs or tissue is not required[29]. On the basis of the Russian Federal Service on Surveillance in Healthcare (Roszdravnadzor) licenses No. 99-01-005317 dated 30.04.2008 and No. FS-99-01-008251 dated 18.02.2013, the Eye Tissue Bank located in the Sv. Fyodorov Eye Microsurgery Complex (Beskudnikovsky bld. 59a, Moscow, Russia, 127486, http://www.mntk.ru/mntk-moscow/Scientific-units/biology/glaznoy_bank/) obtains human cadaver eyes from the mortuary departments of the Moscow Forensic Medical Examination Bureau. These licenses permit the use of tissue isolated from human-cadaver eyes for transplantation and scientific research. Permission was obtained from the chief medical officer of the Sv. Fyodorov Eye Microsurgery Complex, under a scientific collaboration agreement between the Complex and the Emanuel Institute of Biochemical Physics dated 11.01.2011, to perform scientific research in the Laboratory of Physical and Chemical Bases of Vision at the Institute with RPE from cadaver eyes. Cadaver eyes, after removal of corneas for transplantation, were delivered by the Eye Tissue Bank to the Laboratory by road in a box for human organs. Cadaver eyes after isolation of RPE were returned to the Eye Tissue Bank for use, in compliance with officially accepted procedures.
Lipofuscin granules were isolated from RPE cells by a modified technique described in[30]. The obtained granules were dissolved in phosphate buffer and stored at -70 °C.
POS were isolated from bovine retinas by a modified method of McDowell[31]. Obtained POS were dissolved in 0.1 mol/L potassium phosphate buffer (pH 7.6) and stored at -20 °C.
A2E samples were synthesized and purified by the method described in[32]. Briefly, a mixture of all-trans-retinal and ethanolamine (Sigma) in a ratio of 2.3:1 in absolute ethanol was stirred in the presence of acetic acid (1 eq.) at room temperature in the dark for 2.5 d. The mixture was then evaporated under vacuum; the pellet was dissolved in chloroform (Himmed, Russia) and chromatographed on a silica gel-chloroform column (200-400 mesh, 60 A, Sigma). Purified A2E was dissolved in methanol (Sigma). Purity of final A2E was monitored by HPLC on a chromatograph Knauer (Germany), fitted with a Diasfer-110 C18 reverse phase analytical column, and with the mobile phase consisting of acetonitrile/water (84% of acetonitrile in mobile phase) + 0.1% trifluoroacetic acid (TFA)[33].
The model lipofuscins were prepared from POS[34].
Model of lipofuscin from oxidized POS: POS from bovine eyes were subjected to the process of autoxidation. A suspension of POS containing 10-12 mg protein per 1 mL K-phosphate buffer was incubated at 4 °C in the dark for 30-45 d. The concentration of TBA-reactive substances in the autooxidised POS was 12.0 ± 1.0 nmol/mg protein. Autooxidised POS were dissolved in 0.1 mol/L K-phosphate buffer, pH 7.6 (final concentration 1.3-1.5 mg protein/mL) and incubated at 37 °C in the dark with constant stirring for 72 h. After incubation, modified POS were dialyzed against phosphate buffer to remove unreacted low molecular weight molecules. For dialysis, Float-A-Lyser (SPECTRUM Labs) cellulose-ester membranes with a Molecular Weight Cut-Off of approximately 3.5 kDa were used. Dialysis was carried out for 1.5 d at 6 °C. Solution of the A2E in methanol was added to the purified modified POS to a final concentration of 60-70 nmol/mL. The prepared complex was centrifuged at 10000 g in a Beckman Allegra 64R centrifuge for 20 min. The pellet was resuspended in 0.1 mol/L K-phosphate buffer (LFO-lipofuscin).
Model lipofuscin from weakly oxidized POS: Freshly prepared POS from bovine eyes (concentration of TBA-reactive substances did not exceed 0.5-0.7 nmol/mg protein) were dissolved in 0.1 mol/L K-phosphate buffer containing 50 mmol/L fructose (Sigma), to a final concentration of 1.3-1.5 mg protein/mL and incubated with constant stirring at 37 °C for 72 h in the dark. No fructose was added to the control samples. After incubation, modified samples were treated in the same way as oxidized POS. The final pellet was resuspended in 0.1 mol/L K-phosphate buffer (LFF-lipofuscin).
To determine the wet weight of natural and model lipofuscin aliquots of samples were subjected to centrifugation using Amicon-Ultra-0.5 filters at 10000 g for 30 min. The concentration of samples was calculated in mg wet weight per 1 mL. For our samples it was the following: LFO-lipofuscin approximately 110 ± 15 mg/mL, LFF-lipofuscin approximately 80 ± 12 mg/mL and for RPE lipofuscin approximately 100 ± 20 mg/mL. A2E content in model lipofuscins was approximately 0.8-1.0 nmol/mg of wet weight. А2Е concentration was calculated by using ε = 3.1 × 104/mol/L per centimeter at 430 nm wavelength.
The fluorescence spectra of samples were measured on a spectrofluorometer (Shimadzu RF-5301) at 365 nm excitation wavelength and 380-650 emission wavelength. All experiments were made using pure solvent as a reference.
For irradiation of samples an incandescent lamp KGM 24-150, 150 watt, equipped with a focusing system and heat filter was used. Irradiation energy was 80 mW/cm2. Illumination was carried out under constant stirring at room temperature, monitoring the initial sample volume. The spectral range of irradiation was set to 390-700 nm.
The concentration of peroxidation products was determined by the accumulation of the reaction products with thiobarbituric acid (Sigma) (TBA-reactive substances - TBARS), which was measured by absorption at a wavelength of 532 nm[35].
The superoxide concentration was measured by cytochrome c reduction (Fe3+), inhibited by superoxide dismutase by a modified method from[36]. The reaction mixture contained Hanks buffer supplemented with 20 mmol/L sodium bicarbonate, pH 7.6, 100 mmol/L cytochrome c, 50 μg/mL catalase and 0.05% cetyltrimethylammonium bromide. The concentration of model lipofuscins and lipofuscin from human RPE in the samples was 3-5 mg/mL. All reagents used for this method were obtained from Sigma. The mixture was irradiated with visible light (irradiation energy 80 mW/cm2) under constant stirring. Superoxide concentration was measured in a spectrophotometer (Shimadzu UV-1700) by the increase in maximum absorbance at 550 nm (molar absorption 21/mol/L per centimeter)[37].
The data were expressed as the mean ± SD. For the statistics, Student’s t-test was used. P < 0.05 was considered as statistically significant.
The preparation of model lipofuscin samples included two stages. First stage - modification of proteins and lipids from POS during incubation at 37 °C in the presence of either lipid autoxidation products (oxidation way) or fructose (glycation way).
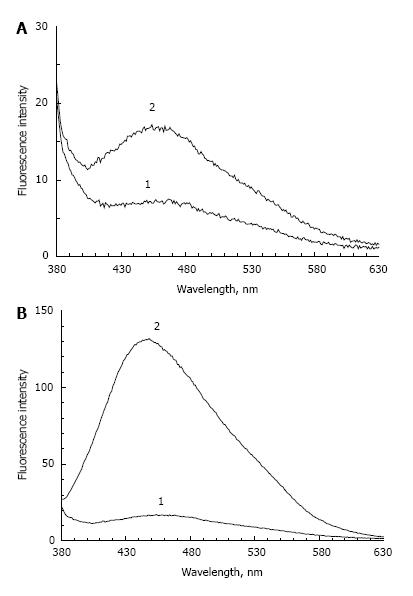
Figure 1 shows the fluorescence spectrum of POS samples containing a small concentration of peroxidation products and incubated in the absence (Figure 1A) or presence (Figure 1B) of fructose. In the absence of fructose, a 72-h incubation causes an approximately two-fold increase in the fluorescence maximum. In the presence of fructose, the intensity of POS fluorescence at 445-450 nm increased about 8 times. At the same time, an increase of absorption at a wavelength of 320 nm was observed (data not shown). It appears that optical absorption and fluorescence increases due to the glycation reaction and formation of fluorescent Schiff bases in proteins and lipids. In the absence of sugar, this process is very slow and could be explained by residual products of lipid peroxidation.
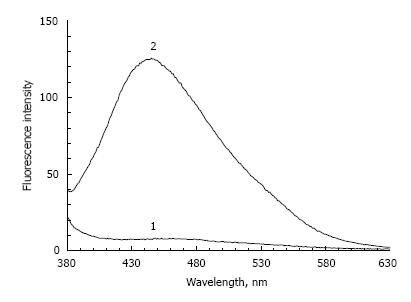
In addition, following the incubation of POS samples with a high concentration of lipid peroxidation products, a significant increase in fluorescence intensity occurs (almost 15 times) irrespective of the fructose presence (Figure 2). In this case, the emission wavelength maximum practically coincides with the emission wavelength maximum of POS samples incubated with fructose.
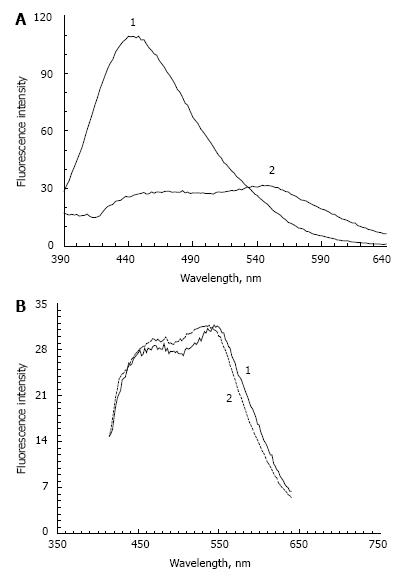
The second stage of model lipofuscin preparation involves mixing modified and dialyzed POS with A2E followed by precipitation of the complex and solubilization of the precipitate in phosphate buffer, as described in Materials and Methods. This procedure leads to a significant change in spectral fluorescent characteristics of the modified POS. There was a significant decrease in fluorescence intensity at a wavelength of 445-450 nm (the major fluorescence peak observed before the addition of A2E, Figure 3A, curve 1) and an appearance of the emission maximum at 550 nm (Figure 3A, curve 2). The fluorescence maximum at 550 nm is typical for A2E and could be explained by the presence of these molecules in the model lipofuscin samples.
The reason for the sharp decrease of the fluorescence maximum at 445-450 nm is not fully understood. It could be due to fluorescence quenching of Schiff bases by A2E molecules or by their destruction.
Our model lipofuscins and lipofuscin from human RPE have very similar fluorescence spectra. Figure 3B shows the fluorescence spectra of LFO lipofuscin (curve 1) and natural lipofuscin (dashed curve 2) at an excitation wavelength of 365 nm. The spectra are almost completely identical. It should be appreciated, however, that the fluorescence emission spectra of lipofuscin from human RPE can considerably vary at excitation 360-365 nm, producing emission maxima between 460 and 630 nm[38].
This dispersion appears to depend on many factors, including age and diet quality[39]. The fluorescence spectra of the LFO and LFF lipofuscins in these conditions were almost identical. These results show that the fluorescence spectrum of the lipofuscin model is largely determined by its fluorophores content; in this case, the fluorophore A2E.
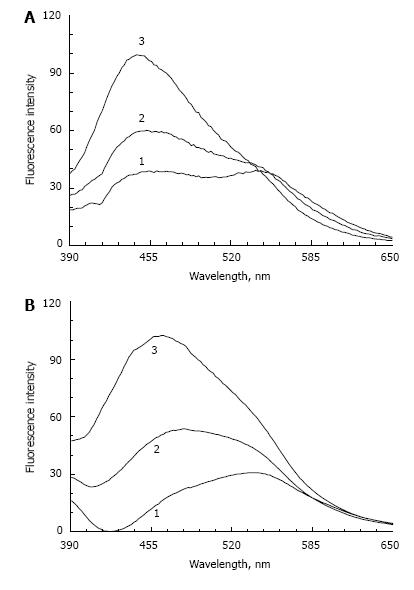
Figure 4 shows the fluorescence spectra of LFF lipofuscin (Figure 4A) and lipofuscin from RPE (Figure 4B) upon irradiation with visible light with different exposures. It can be seen that in both cases the 550 nm peak disappears while the fluorescence amplitude significantly increases in the short wavelength region. Emission maximum after a 3-h exposure is shifted to 445-450 nm for LFF and to 460 nm for RPE lipofuscin. Apparently, the reason for this shift is associated with photodestruction of fluorophore A2E, leading to the formation of its oxidized derivatives[4]. The fluorescence spectra of these oxidized compounds are shifted to a shorter wavelength range.
It is also possible that A2E destruction reveals the underlying fluorescence of modified POS proteins and lipids. Because the model lipofuscin contains only fluorophore A2E, but RPE lipofuscin contains a mixture of different fluorophores, we can assume that, in natural RPE lipofuscin fluorophore A2E plays an important role in determining the total fluorescence.
Lipofuscin phototoxicity is known to be primarily determined by the ability of lipofuscin to generate reactive oxygen species. We have previously shown that phototoxicity of RPE lipofuscin is higher than toxicity of just its A2E content[11]. This is probably due to the presence of other more active fluorophores in lipofuscin granules.
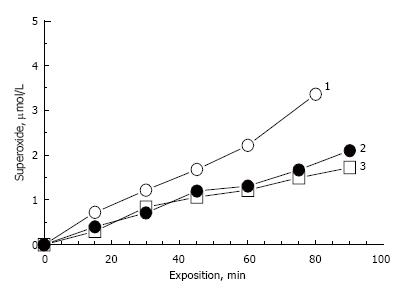
Model lipofuscins, prepared in this study, contain only one fluorophore A2E. Figure 5 shows the comparative kinetics of photogenerated superoxide upon irradiation of model and RPE lipofuscins. Model lipofuscins contained about 0.8-1.0 nmol A2E per 1 mg wet weight of sample.
Irradiation of LFO and LFF lipofuscin samples exhibited almost the same rate of superoxide generation (curves 2 and 3). A2E content in RPE lipofuscin can be roughly estimated according to the data from[40]. In this study quantitative analysis of bisretinoids in human RPE lipofuscin showed that the average content of A2E and iso-A2E is 380 pmol per 5.5 × 107 lipofuscin granules, which corresponds to 6.4 × 10-18 mol/granule. The weight of one lipofuscin granule is 1.3 ± 0.2 pg[41], which gives the value of about 5 nmol/mg of A2E content in human RPE lipofuscin granules. This roughly corresponds to the A2E content in our model lipofuscin, assuming that the dry weight of the model pellets is about 20% of wet weight.
However, the rate of superoxide photogeneration catalyzed by RPE lipofuscin (curve 1, Figure 5) was significantly higher compared to that of model lipofuscin (curves 2 and 3, Figure 5). Average rates of superoxide photogeneration in all experiments are presented in the Table 1. The higher rate of superoxide photogeneration by RPE lipofuscin compared to A2E-containig model lipofuscins could be explained by the presence of other photoactive fluorophores[11,42-44] because the concentration of A2E in RPE lipofuscin does not exceed the concentration of the fluorophore in the model lipofuscins.
| Sample name | Superoxide generation rate nmole/min mg wet weight |
| Model lipofuscin from oxidized POS (LPO-lipofuscin) | 8.5 ± 0.6 |
| Model lipofuscin obtained from not oxidized POS in the presence of fructose (LFF-lipofuscin) | 9.2 ± 0.4 |
| Natural lipofuscin from human eye | 15.7 ± 0.8 |
As noted earlier, lipid peroxidation products are undoubtedly important for the formation of lipofuscin. Although the process of AGE products accumulation during normal aging has been well studied, much less is known about the role of advanced glycation end products in the formation of lipofuscin, which accumulates with age in RPE cells[45].
An important role in this process belongs to fructose. Although its concentration in the blood is significantly lower than the glucose concentration (approximately 35 μmol/L, while the concentration of glucose is approximately 5 mmol/L), the cellular concentration of fructose can be much higher. This increase could be induced by hyperglycaemic conditions when fructose formation through the polyol pathway is activated[46]. An elevated concentration of glucose is known to activate the cellular enzyme aldose reductase, which catalyzes the transformation of glucose into sorbitol. Sorbitol is then oxidized to fructose in a reaction catalyzed by the polyol dehydrogenase enzyme. It has been established that the retina and RPE are characterized by high expression of aldose reductase under hyperglycaemia[47-49].
Fructose is a much more effective glycation agent in Maillard reactions than glucose. This is probably due to the fact that the open form of glucose molecules, which is directly involved in the Maillard reaction, is present at only 0.0002% of the content of the inactive cyclic form, whereas the fructose open form reaches 0.7% of the cyclized form[50]. A significant difference between glucose and fructose in the induction of carbonylation of target molecules may be explained by the formation of glyceraldehyde during fructose metabolism, while during glucose metabolism glyceraldehyde-3-phosphate is formed, which is much less active in Maillard reactions[51].
Hyperglycaemia and polyol path activation greatly increase retinal sensitivity to oxidative stress[52]. All these facts suggest that aging and diabetes create conditions in the retina and in RPE cells that promote development of advanced glycation reactions. Therefore, in lipofuscin, cross-links in proteins and lipids may form, not only by their interaction with reactive carbonyls produced by lipid peroxidation, but also by reactions with products of glycation. Lipofuscin thus should be considered as fluorescent pigment generated by modification of lipids and proteins through the action of lipid and carbohydrate reaction pathways. Schema of such processes generation of RPE lipofuscin from modified POS and fluorophore A2E is shown in Figure 6.
Our experiments indicate that fluorescent characteristics of the modified POS are essentially independent of the method of their modification. And various modifications of POS seem to have no or only a small influence on the spectral properties of obtaining lipofuscin.
Processes of fructozylation (glycation) and lipid peroxidation in POS cause fluorescence with the same maximum emission (445-450 nm) at an excitation wavelength of 365 nm. However, binding of the A2E fluorophore by modified POS led to a significant decrease of the original short wavelength emission maximum of modified POS. That also led to the appearance of a long-wave fluorescent peak, characteristic of the fluorescence profile of A2E. It also suggests that the fluorescence of lipofuscin is only slightly dependent on the nature of the fluorescent compounds arising from the modification of proteins and lipids. The fluorescence profile is mainly determined by the bisretinoid fluorophores present in lipofuscin granules.
Model lipofuscins prepared by fructozylation and lipid peroxidation contained approximately equal concentrations of fluorophore A2E and showed the same rate of superoxide photogeneration. Consequently, we hypothesize that the lipofuscin toxicity is independent from paths of protein and lipid modification. Instead, it mainly depends on the bisretinoid content.
The authors would like to thank Professor Glickman RD from The University of Texas HSC at San Antonio for the generous help and for much constructive criticism, helpful discussions and language editing.
Lipofuscinogenesis in the retinal pigment epithelium (RPE) cells is connected in many respects with formation of the modified proteins and the lipids incapable of degradation by lysosomal enzymes. Modification of proteins and lipids is carried out by their interaction with the active carbonyl compounds accumulated in reactions of lipid peroxidation and glycation. These two different ways of modification are used for preparing of model lipofuscin systems in this study.
The authors examined the effect of different ways of protein-lipid modification of photoreceptor outer segments (POS) on the fluorescence characteristics and toxicity of model lipofuscins and compared them with properties of natural lipofuscin.
The results of this study are proposed that fluorescent characteristics and phototoxicity of lipofuscin granules depend only to an insignificant degree on the oxidative modification of proteins and lipids and generally are defined by bisretinoid fluorophores containing in them.
It is supposed that the fluorescence of RPE lipofuscin determines the fundus autofluorescence (FAF) in vivo. Monitoring FAF is noninvasive diagnostic method used in detection some retinal degenerative diseases such as recessive Stargardt disease, best macular dystrophy, and age-related macular degeneration. Preparation of model lipofuscins with predetermined fluorescent characteristics, defining by the relative content of the modified proteins and lipids, as well as the ratio of oxidized and reduced forms of fluorophores, is important for understanding the character of fluorescence ocular fundus and diagnostics associated pathologies. So the study of lipofuscin model systems may be an important factor for FAF interpretation.
Lipofuscin is fluorescent pigment that accumulates in aging postmitotic tissues. In the eye lipofuscin is mainly accumulated in the RPE, being one of the key biomarkers of aging and oxidative stress. Lipofuscin also plays significant role in the development of retinal pathologies.
This is an interesting manuscript.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): C
Grade E (Poor): 0
P- Reviewer: Schoenhagen P, Tangvarasittichai S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Boulton ME. Studying melanin and lipofuscin in RPE cell culture models. Exp Eye Res. 2014;126:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 450] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Sakai N, Decatur J, Nakanishi K, Eldred GE. Ocular age pigment A2E: an unprecedented pyridinium bisretinoid. J Am Chem Soc. 1996;118:1559-1560. [RCA] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993;19:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Kanofsky JR, Sima PD, Richter C. Singlet-oxygen generation from A2E. Photochem Photobiol. 2003;77:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Rózanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998;24:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | De S, Sakmar TP. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J Gen Physiol. 2002;120:147-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Sokolov VS, Sokolenko EA, Sokolov AV, Dontsov AE, Chizmadzhev YA, Ostrovsky MA. Interaction of pyridinium bis-retinoid (A2E) with bilayer lipid membranes. J Photochem Photobiol B. 2007;86:177-185. [PubMed] |
| 11. | Dontsov AE, Sakina NL, Ostrovsky MA. Comparative study of the dark and light-induced toxicity of lipofuscin granules from human retinal pigment epithelium and their chromophore A2E on the cardiolipin liposome model. Russ Chem B. 2012;61:442-448. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Holz FG, Schütt F, Kopitz J, Eldred GE, Kruse FE, Völcker HE, Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737-743. [PubMed] |
| 13. | Höhn A, Jung T, Grimm S, Catalgol B, Weber D, Grune T. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radic Biol Med. 2011;50:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci USA. 2002;99:3842-3847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Powell SR, Wang P, Divald A, Teichberg S, Haridas V, McCloskey TW, Davies KJ, Katzeff H. Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Radic Biol Med. 2005;38:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Suter M, Remé C, Grimm C, Wenzel A, Jäättela M, Esser P, Kociok N, Leist M, Richter C. Age-related macular degeneration. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J Biol Chem. 2000;275:39625-39630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PubMed] |
| 19. | Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663-3668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Burcham PC, Kuhan YT. Diminished susceptibility to proteolysis after protein modification by the lipid peroxidation product malondialdehyde: inhibitory role for crosslinked and noncrosslinked adducted proteins. Arch Biochem Biophys. 1997;340:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 924] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 22. | Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Glenn JV, Stitt AW. The role of advanced glycation end products in retinal ageing and disease. Biochim Biophys Acta. 2009;1790:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Yamada Y, Ishibashi K, Ishibashi K, Bhutto IA, Tian J, Lutty GA, Handa JT. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Glenn JV, Mahaffy H, Wu K, Smith G, Nagai R, Simpson DA, Boulton ME, Stitt AW. Advanced glycation end product (AGE) accumulation on Bruch’s membrane: links to age-related RPE dysfunction. Invest Ophthalmol Vis Sci. 2009;50:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, Kurokawa K. Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer’s disease and aged neurons. Biochem Biophys Res Commun. 1997;236:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718-729. [PubMed] |
| 28. | Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855-1866. [PubMed] |
| 29. | Russian Federation law N 4180-I dated 22. 12.1992. “On human organs or tissue transplantation” (with modifications and additions). Available from: http://base.garant.ru/136366/. |
| 30. | Boulton M, Marshall J. Repigmentation of human retinal pigment epithelial cells in vitro. Exp Eye Res. 1985;41:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | McDowell JH. Preparing rod outer segment membranes, regenerating rhodopsin, and determining rhodopsin concentration. In: Hargrave PA, editor. Methods in Neurosciences. Academic Press NY. 1993;15:123-130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA. 1998;95:14609-14613. [PubMed] |
| 33. | Yakovleva MA, Sakina NL, Kononikhin AS, Feldman TB, Nikolaev EN, Dontsov AE, Ostrovsky MA. Detection and study of the products of photooxidation of N-retinylidene-N-retinylethanolamine, the fluorophore of lipofuscin granules from retinal pigment epithelium of human donor eyes. Doklady Biochem Biophys. 2006;409:223-225. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Katz ML, Gao CL, Rice LM. Formation of lipofuscin-like fluorophores by reaction of retinal with photoreceptor outer segments and liposomes. Mech Ageing Dev. 1996;92:159-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymology. 1978;52:302-310. [DOI] [Full Text] |
| 36. | McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049-6055. [PubMed] |
| 37. | Yamashita T. Selective inhibition by the sulfhydryl reagent maleimide of zymosan particle phagocytosis by neutrophils. FEBS Lett. 1982;141:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Eldred GE, Miller GV, Stark WS, Feeney-Burns L. Lipofuscin: resolution of discrepant fluorescence data. Science. 1982;216:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 103] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Bilinska B, Janikowska G, Zawada Z, Wylegala E, Dontsov A, Sakina N. Fluorescence spectra of lipofuscin isolated from human RPE cells. Polish J Med Phys Eng. 2003;9:121-135. |
| 40. | Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS, Kim SR, Rózanowska MB, Bonilha VL, Rayborn ME. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Warburton S, Southwick K, Hardman RM, Secrest AM, Grow RK, Xin H, Woolley AT, Burton GF, Thulin CD. Examining the proteins of functional retinal lipofuscin using proteomic analysis as a guide for understanding its origin. Mol Vis. 2005;11:1122-1134. [PubMed] |
| 42. | Glickman RD. Ultraviolet phototoxicity to the retina. Eye Contact Lens. 2011;37:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Wielgus AR, Chignell CF, Ceger P, Roberts JE. Comparison of A2E cytotoxicity and phototoxicity with all-trans-retinal in human retinal pigment epithelial cells. Photochem Photobiol. 2010;86:781-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Wielgus AR, Roberts JE. Retinal photodamage by endogenous and xenobiotic agents. Photochem Photobiol. 2012;88:1320-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Schalkwijk CG, Stehouwer CD, van Hinsbergh VW. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab Res Rev. 2004;20:369-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Huang SP, Palla S, Ruzycki P, Varma RA, Harter T, Reddy GB, Petrash JM. Aldo-keto reductases in the eye. J Ophthalmol. 2010;2010:521204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Sato S, Lin LR, Reddy VN, Kador PF. Aldose reductase in human retinal pigment epithelial cells. Exp Eye Res. 1993;57:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Vinores SA, Campochiaro PA, Williams EH, May EE, Green WR, Sorenson RL. Aldose reductase expression in human diabetic retina and retinal pigment epithelium. Diabetes. 1988;37:1658-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Laroque D, Inisan C, Berger C, Vouland E, Dufosse L, Guerard F. Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem. 2008;111:1032-1042. [RCA] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 51. | Tessier FJ, Monnier VM, Sayre LM, Kornfield JA. Triosidines: novel Maillard reaction products and cross-links from the reaction of triose sugars with lysine and arginine residues. Biochem J. 2003;369:705-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |