Published online May 20, 2015. doi: 10.5493/wjem.v5.i2.84
Peer-review started: October 5, 2014
First decision: November 14, 2014
Revised: February 7, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: May 20, 2015
Processing time: 229 Days and 11.5 Hours
Gastric cancer remains one of the most common cancers worldwide and one of the leading cause for cancer-related deaths. Gastric adenocarcinoma is a multifactorial disease that is genetically, cytologically and architecturally more heterogeneous than other gastrointestinal carcinomas. The aberrant activation of the Wnt/β-catenin signaling pathway is involved in the development and progression of a significant proportion of gastric cancer cases. This review focuses on the participation of the Wnt/β-catenin pathway in gastric cancer by offering an analysis of the relevant literature published in this field. Indeed, it is discussed the role of key factors in Wnt/β-catenin signaling and their downstream effectors regulating processes involved in tumor initiation, tumor growth, metastasis and resistance to therapy. Available data indicate that constitutive Wnt signalling resulting from Helicobacter pylori infection and inactivation of Wnt inhibitors (mainly by inactivating mutations and promoter hypermethylation) play an important role in gastric cancer. Moreover, a number of recent studies confirmed CTNNB1 and APC as driver genes in gastric cancer. The identification of specific membrane, intracellular, and extracellular components of the Wnt pathway has revealed potential targets for gastric cancer therapy. High-throughput “omics” approaches will help in the search for Wnt pathway antagonist in the near future.
Core tip: Available data indicate that Wnt signaling substantially impacts gastric tumorigenesis, prognosis, and resistance to therapy. Loss of Wnt signaling inhibitors (such as APC) by promoter hypermethylation, allelic loss or other mechanisms appear to be particularly important in activating Wnt/β-catenin signaling. Gastric cancer is an entity characterized by its heterogeneity in various aspects, and much remains to be learned about the molecular aspects that determine the process of cancer development in this organ. The role that plays the canonical Wnt pathway in gastric carcinogenesis is reviewed.
- Citation: Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med 2015; 5(2): 84-102
- URL: https://www.wjgnet.com/2220-315X/full/v5/i2/84.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i2.84
Gastric cancer incidence and mortality have been declining over the past 50 years in most Western countries, but remains the fifth most common cancer and the third leading cause of cancer-related death in both sexes worldwide after lung and breast cancer (723000 deaths, 8.8% of the total in 2012)[1,2]. Gastric cancer incidence varies substantially worldwide, being the highest (more than two-thirds) observed in East Asia, Eastern Europe, and the Andean region of South America, while North America, Northern Europe and North and East Africa show the lowest recorded rates[1]. More than 70% of cases in 2012 (677000 cases) occur in developing countries, and half of worldwide cases occur in Eastern Asia (mainly in China)[3]. The highest estimated mortality rates are in Eastern Asia (24 per 100000 in men, 9.8 per 100000 in women), the lowest in Northern America (2.8 and 1.5, respectively)[3]. Latin American countries and nations from Central and Eastern Europe display some of the highest mortality rates in both sexes worldwide[3].
Diagnosis is usually made after the disease reaches an advanced stage because early gastric cancer produces few symptoms. Therefore, most gastric cancer patients are diagnosed in advanced-stage disease with a poor prognosis. The main treatment option is the gastrectomy combined with chemotherapy and radiation therapy protocols. The poor understanding of the pathogenic mechanisms of gastric cancer and etiological factors, and the lack of effective treatment are reflected in the late diagnosis and high mortality of this disease.
Gastric cancer is a multifactorial disease resulting from the interaction between genetic and environmental factors at the stomach mucosa level. These complex interplays can promote gastric cancer development as result of the deregulation of a number of potentially cell oncogenic signaling pathways, leading to acquisition of malignant phenotypes, including increased cell proliferation, evasion of apoptosis and enhanced invasiveness[4].
The most common histologic variant of gastric adenocarcinoma is the intestinal type. A multistep cascade model for the development of intestinal-type gastric adenocarcinoma consists of a progression from chronic superficial (non atrophic) gastritis, to chronic atrophic gastritis to intestinal metaplasia, followed by dysplasia and finally, gastric adenocarcinoma[5]. This model assumes the sequence of precancerous lesions as a dynamic process from an initial superficial inflammation caused by Helicobacter pylori (H. pylori) infection to a fully malignant neoplasm of the stomach. Thus, the chronic infection of the gastric mucosa by the H. pylori, which colonize approximately 50% of the human population, is considered the strongest known risk factor for the development of gastric cancer[6,7].
Ooi et al[8] identified three signaling pathways (nuclear factor-κB, Wnt/β-catenin, and proliferation/stem cell) that were deregulated in more than 70% of the patients diagnosed with gastric cancer, resulting in increased inflammatory cytokine production, abnormal apoptosis, undesirable epithelial cell proliferation/differentiation, and epithelial cell transformation. However, a complete knowledge of the relative importance, temporal activation and crosstalking between these signaling pathways is not well understood yet. Particularly, activation of the Wnt/β-catenin signaling is found in about 30% to 50% of gastric cancer tissues and in many kinds of gastric cancer cell lines[8-10]. In this review, the involvement of the Wnt/β-catenin signaling pathway and its biological significance in gastric carcinogenesis will be discussed.
The Wnt signaling pathways, named for its ligands Drosophila wingless (wg) and the mouse homolog Int-1 (Wnt-1)[11], are a group of signal transduction pathways which play a fundamental role in the cell fate specification during early embryonic development, proliferation, body axis patterning, survival, apoptosis, and in tissue homeostasis in adults[12].
Wnt signaling pathways can be activated upon binding of Wnt ligands (19 known members in humans) to their cell surface receptor complex, which includes 10 different members of the Frizzled (Fz) family of atypical heptahelical G-protein-coupled receptors[13-15]. Moreover, selective recruitment and participation of other single-pass transmembrane proteins function as co-receptors influencing the Wnt signaling, such as lipoprotein receptor-related protein (LRP)5/6, receptor-like tyrosine kinase (Ryk) and receptor tyrosine kinase-like orphan receptor (ROR)-1/2[16-18].
Up to date, the three best characterized Wnt signaling pathways are the canonical Wnt pathway or β-catenin dependent which involves the stabilization of the proto-oncogene β-catenin (armadillo in Drosophila), the planar cell polarity (PCP) pathway, involved in ciliogenesis, and the Wnt/Ca2+ pathway, which stimulates the intracellular release of Ca2+ and activates Ca2+-dependent mediators involved in the control of cell movement and behavior[19-21]. These two last pathways are collectively named as “non-canonical” or “β-catenin-independent” pathways, that also include a steadily increasing number of complex signaling routes: Wnt/cAMP, Wnt/Ror, Wnt/Rap, Wnt/Rac and Wnt/Rho pathways[19,22].
The Wnt/β-catenin intracellular signaling pathway is highly conserved among metazoan species[20,23]. β-catenin is a multifunctional protein that was found as a E-cadherin-binding protein involved in the regulation of cell to cell adhesion, and as a transcriptional regulator in the Wnt signaling pathway[17]. It is assumed that the disruption of cadherin/catenin complexes is involved in the malignant progression of epithelial tumors.
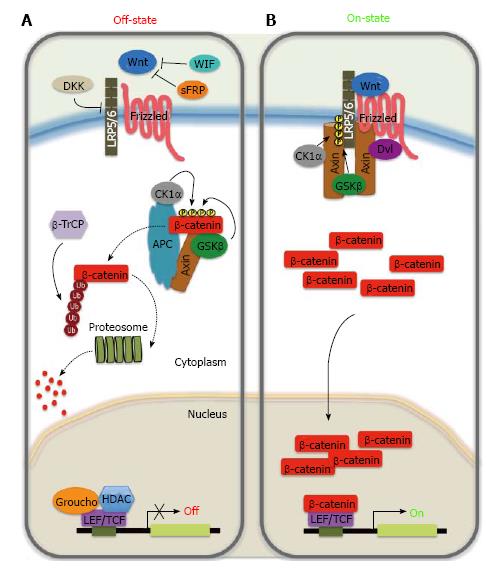
In the absence of Wnt ligands, cytoplasmic β-catenin is bound by the destruction complex composed of the scaffolding proteins Axin, adenomatous polyposis coli (APC), and the serine/threonine kinases, Casein kinase 1 alpha (CK1α) and glycogen synthase kinase 3β (GSK3β)[24]. Phosphorylated β-catenin is targeted to proteosomal degradation after recognition by the E3 ubiquitin ligase β-TrCP resulting in low cytosolic levels of β-catenin[25]. As a consequence, the transcription factor T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) binds to transcriptional inhibitors of the Groucho family, that recruit histone deacetylase to mediate transcriptional repression through chromatin compaction[26,27].
In contrast, when the secreted Wnt ligands bind to their Fz/LRP receptor complex, the dissociation of the destruction complex occurs by recruiting Dishevelled (Dvl) to the cytoplasmic domain of Fz. This association results in LRP phosphorylation and the delocalization of cytoplasmic Axin by its recruitment to the receptors. These events prevent phosphorylation and subsequent degradation of β-catenin, which accumulates in the cytoplasma and translocates to the nucleus. For its nuclear action, β-catenin behaves as transcriptional co-regulator by interacting with TCF/LEF complex, displaces Groucho, and activates Wnt target gene expression, such as CCND1 (encoding cyclin D1 protein), gastrin and c-myc[27,28].
Even in the presence of Wnt ligand, the Wnt/β-catenin pathway can be inhibited through the binding of soluble Dickkopf (Dkk1-4) to LRP5/6, or by Wnt-inhibitory factor (WIF-1) and the family of secreted Fzd receptor proteins (sFRP1-5) that can interact with Wnt ligands preventing them from binding to receptors[29-31].
Activity of the canonical Wnt signaling pathway is dependent on controlling the accumulation of β-catenin in the cytoplasmic and nuclear compartments (Figure 1)[20,32,33]. β-catenin is normally constitutively transcribed and translated. It has been suggested that β-catenin is regulated by its cytoplasmic retention by both cadherins and Axin that sequester β-catenin in the plasma membrane and cytoplasm, respectively[34]. Upon receptor activation a signal-induced block in proteolysis leads to rapid rise in cytosolic β-catenin protein levels that subsequently also accumulates in the nucleus. Moreover, β-catenin does not contain any recognizable nuclear localization signal or export signal, and its nuclear entry is independent of classic import factors. Finally, it has been proposed that the nuclear build-up of β-catenin results from a combination of anchoring for β-catenin in the nucleus by nuclear proteins (TCF, LEF-1, Pygopus, and BCL9) and the nuclear export, in which Axin, APC, and RanBP3 have been implicated[35-41].
Homeostasis of the gastrointestinal epithelium is strongly dependent on the balance existing between cell proliferation, cell cycle arrest, and cell migration. The canonical Wnt signaling pathway, in addition to its role in early embryogenesis, plays a crucial role in regulating proliferation, stem cell maintenance and homeostasis in normal gastric mucosa[42-44]. On the other hand, it is recognized that dysregulation of the Wnt pathway plays a critical role in the development of human cancer, with more than 30% of gastric cancer in which activated Wnt/β-catenin signaling can be found. Furthermore, the essential role of Wnt/β-catenin signaling in the self-renewal of gastric cancer stem cells (GCSC) has been demonstrated[45-47]. Several components of the Wnt pathway (Figure 1) may be overexpressed or their function increased by other mechanisms in gastric cancer (Table 1). Moreover, the loss of Wnt inhibitors may play an important role in gastric carcinogenesis (Table 1).
| Upregulation or function increase in gastric cancer | |
| Wnt-1, Wnt-2, Wnt-2B, Wnt-5a, Wnt-6, Wnt-10A[47,69-75,232] | |
| Fzd-3[78] | |
| CTNNB1[9,88-93,96,97] | |
| LRP6[228] | |
| TCF7L2[98-100] | |
| PPN[79] | |
| CDH17[80] | |
| EZH2[81] | |
| HMGA1, HMGA2[210,211] | |
| YY1[86] | |
| TC1 (C8orf4)[201,202] | |
| miR-17-92[161] | |
| mir-10a[168] | |
| has-miR-335[166] | |
| hsa-miR-375[163] | |
| Downregulation or function inhibition in gastric cancer | |
| Downregulation by hypermethylation | Inactivation by miRNAs |
| APC[145,146] | APC[175] |
| sFRP1, sFRP2, sFRP4, sFRP5[78,96,149] | AXIN2[159] |
| WIF-1[144,149] | EZF1[78] |
| Dkk-1, Dkk-2, Dkk-3[59,144,148,149] | HIPK1[161] |
| NKD1[149] | HSulf-1[168] |
| Sox10, Sox17[106,107,112,149] | RUNX2[159] |
| HSulf-1[108] | Inactivation by phosphorylation |
| RUNX3[147] | GSK3β[105] |
| PRDM5[152,153] | Inactivation by overexpression of EZH2 |
| RASSF10[154] | CXXC4[81] |
| OSR1[111,112] | Other or undefined mechanisms |
| Inactivation by protein mislocalization | Sox7[113] |
| RUNX3[147] | RACK1[109] |
| Inactivation by H. pylori virulence factor | ZNRF3[110] |
| RNF138[226,227] | CHD8[131] |
| Deletion or mutation | PLA2G2A[205] |
| APC[92,104,133-137] | has-miR-148a[165] |
| GSK3β[104] | miR-193b[163] |
| β-TrCP[142] | miR-200a[176,177] |
| AXIN1, AXIN2[90,91] | |
Large-scale sequencing studies conducted in many tumor types have identified co-occurrence of mutations in positive and negative regulators of the Wnt pathway. However, the prevalence of these mutations seems to be cell-type specific[48,49]. Moreover, a number of recently published studies exploring gene mutations in gastric cancer using high-throughput methodologies (Next-generation sequencing and genotyping array) have confirmed CTNNB1 (the gene that encodes β-catenin protein) and APC as driver genes. Although with variations in prevalence, these studies revealed somatic mutations in both genes that might be relevant in gastric carcinogenesis[50-55].
In addition to multiple genetic alterations, the initiation and progression of gastric cancer are also associated to epigenetic changes[56,57]. Histone modification and promoter CpG methylation alter cancer-related gene expression and are frequently involved in carcinogenesis[58]. So far, downregulation of Wnt antagonist genes associated to promoter hypermethylation have been identified in a variety of malignancies, such as renal, bladder, lung, breast, colorectal, gastric and neuroblastoma[59-65].
Furthermore, the Wnt/β-catenin pathway genes are found among those affected by dysregulation of microRNAs (miRNAs) in many kinds of cancers, and particularly, expression profiling has shown that certain miRNAs are associated with gastric cancer development, progression and response to therapy[66-68].
Members of the Wnt family protein, such as Wnt-1, Wnt-2 and Wnt-2B have been found upregulated in gastric cancer[47,69-71]. The overexpression of Wnt-2 was associated with cytoplasmic/nuclear β-catenin accumulation both in intestinal- and diffuse-type gastric carcinoma, and positively associated with increased metastatic potential[70]. Furthermore, the upregulation of Wnt-1 ligand was found playing an important role either in cellular proliferation of GCSC and in advanced gastric cancer[47,71]. In this regard, in transgenic mouse models, Oshima et al observed that Wnt-1 expression together with activation of PGE2 pathway result in invasive gastric adenocarcinoma formation by 1 year[72].
Wnt-5a, which is upregulated in gastric cancer regardless of their histological phenotype, has an increased expression in advanced stages of gastric cancer and it is correlated with poor prognosis[73,74]. Furthermore, Yuan et al[75] demonstrated that caveolin-1 (Cav1) and Wnt-6 positively cooperate in promoting chemoresistance of gastric cancer cells to DNA-damaging anthracycline drugs through activation of the canonical Wnt/β-catenin pathway. Caveolin is an integrated plasma membrane protein and is involved in lipid transport, membrane trafficking, gene regulation and signal transduction[76]. Certain cancer cells that express caveolins are more aggressive and metastatic, as well as drug-resistant[77].
The upregulation of Fzd-3, Wnt palmitoyltransferase porcupine (PPN), and CDH17 has been found to be critical for cell proliferation and activation of the Wnt/β-catenin signaling pathway in gastric cancer[78-80]. Moreover, Lu et al[81] have provided evidence indicating that EZH2 (histone methyl-transferase, enhancer of zeste homolog 2) activates Wnt signaling in gastric cancer mainly by downregulating the expression of CXXC4 (CXXC finger protein 4) without involving DNA methylation. It was also confirmed that overexpression of CXXC4 disrupts the association of Dvl with Axin-GSK3β by directly interacting with Dvl, thus functioning as a tumor suppressor[81].
Upregulation of Actin-binding protein anillin (ANLN), a protein involved in the cytokinesis and known to be dysregulated in many cancers, was found responding to the activity of Wnt/β-catenin pathway in gastric cancer[82]. Moreover, elevated expression of ANLN was identified as a molecular predictor of intestinal and proliferative type gastric cancer[82]. Similarly, cyclin-dependent kinase 8 expression and the delocalization of β-catenin expression have shown a significant positive correlation with carcinogenesis and tumor progression, especially lymph node metastasis[83].
On the other hand, the ubiquitously distributed transcription factor Yin Yang 1 (YY1) can act either as a tumor suppressor gene or as an oncogene depending on the type of tumor. This dual behavior might be determined by cell context, oncogenic stimulation or the regulation of its upstream pathways[84,85]. YY1 was found to promote the Wnt signaling pathway in gastric cancer, probably by suppressing Wnt antagonists[86]. YY1 expression is involved in the carcinogenesis of diffuse-type gastric carcinoma, and it was correlated with poor prognosis in patients with early stage gastric cancer[86].
It has been suggested the existence of an interplay between the Wnt signaling cascade and Notch1 signaling pathway in gastric cancer cells, in which the aberrant activation of Wnt/β-catenin signaling overcomes the pro-apoptotic role of Notch in gastric cancer cells[87]. Furthermore, Li et al[87] observed that when the two signaling pathways were simultaneously activated, there was a combined effect of promoting the proliferation of gastric cancer BGC-823 cells by upregulating the expression of c-Myc, cyclin D1, cyclin E and CDK2.
Genomic alterations: In contrast to the colorectal cancer, mutations driving gastric cancer are much less defined. Mutations in CTNNB1 have been frequently detected (approximately 30%) in intestinal- and diffuse-type gastric carcinomas displaying nuclear accumulation of β-catenin[9,88-92]. These mutations occur mainly in exon 3, that encodes for the GSK3β phosphorylation consensus region of the β-catenin gene, resulting in mutants refractory to regulation by the destruction complex, and thus in accumulation of this protein and constitutive activation of the Wnt pathway. In contrast, there are studies examining the incidence of CTNNB1 mutations that report their infrequency, either in intestinal and diffuse-type gastric cancer[93-96].
Wang et al[97] performed a genetic association study (944 patients and 848 cancer-free controls) to analyze the correlation between five tagged single nucleotide polymorphisms (SNP) spanning the CTNNB1 gene (rs4135385, rs1798808, rs1880481, rs11564465 and rs2293303) and gastric cancer risk and prognosis. Their results showed that the SNPs rs1880481, rs4135385, rs11564475 and rs2293303 were significantly associated with gastric cancer susceptibility. In addition, the rs4135385 AG/AA genotypes were associated with a 0.74-fold reduced adjusted hazard ratio for favorable overall 5-year survival of non-cardia gastric cancer.
In the nucleus, free β-catenin binds members of the TCF/LEF family of transcription factors, including TCF7L2 (TCF4), thereby modulating gene transcription[27,28]. Somatic frameshift mutations of TCF7L2 in the repeat sequence A9 have been frequently detected in gastric cancers with microsatellite instability (MSI)[98,99]. On the contrary, no MSI mutations in TCF7L2 were detected in Japanese patients with sporadic gastric tumors[100]. In this sense, about 15% to 50% of gastric carcinomas are categorized into MSI–positive cancers[101].
Considering post-transcriptional modifications, the splicing pattern of numerous genes is altered as cells progress in the tumorigenesis process. It is hypothesized that alternative spliced exons can originate functional protein–protein interactions and establish tissue-specific proteome signatures[102]. The participation of Wnt/β-catenin pathway in alternative splicing in colorectal, lung and gastric cancer was identified by a mechanism involving the up-regulation of the serine/threonine-protein kinase SRPK1 and the splicing factor SRSF1[103]. Furthermore, identification of TCF4 binding sites both in the promoter regions of SRPK1 and SRSF1 makes these two genes apparent Wnt-targets.
Several studies have demonstrated that loss of Wnt inhibitors may play a major role in gastric cancer (Table 1). In mouse models, deletion of APC or GSK3β leads to rapid nuclear β-catenin accumulation and Wnt target gene expression that result in formation of benign lesions, such as intestinal-type adenomas and fundic gland polyps but not tumorigenesis[104]. These molecular changes seem to be required to drive early carcinogenesis events in the stomach, a hypothesis that is supported by studies showing that GSK3β phosphorylation and inactivation is associated with cancer progression in gastric cancer[105]. Different studies have shown downregulation-in occasion by unspecified mechanisms-of various negative regulators of the Wnt/β-catenin signaling involved in proliferation and invasion in gastric cancer, including sFRP, Sox7, Sox10, Sox17, HSulf-1, RACK1, ZNRF3 and OSR1[78,106-113].
Some Wnt-target genes, such as Dkk-1, Axin, Nemo kinase, etc., have shown to cause inhibition of Wnt signaling itself[114,115]. These Wnt-antagonists are initially upregulated in response to Wnt signaling activation, initiating a negative feedback loop[116]. However, in certain tumor cells, the epigenetic silencing of Dkk-1 by promoter hypermethylation or loss of heterozygosity, produces an imbalance of Wnt/Dkk negative feedback, therefore contributing to persistent activation of Wnt/β-catenin signaling[117,118]. Moreover, recently it was reported that Polycomb group protein BMI1, which is transcriptionally regulated by the Wnt-target gene c-Myc, act as an activator of the Wnt pathway by repressing Dkk protein family (particularly Dkk-1) as part of positive feedback loop[119].
Conversely, as despite Dkk-1 being a Wnt pathway inhibitor, this protein has been found upregulated in several human cancers, such as lung, esophageal, breast, myeloma multiple and gastric cancer[120-123], indicating that Dkk-1 could has a potential oncogenic role in these tumors rather than acting as a tumor suppressor by antagonizing Wnt signaling. That elevation of Dkk-1 expression is likely to be caused by some epigenetic alterations including the loss of promoter methylation of the Dkk-1 gene[123,124]. Moreover, the oncogenic activities of Dkk-1 could be mediated, at least in part, by non-canonical Jun-mediated Wnt pathways activation.
RUNX3 suppresses the canonical Wnt signaling pathway by forming a ternary complex of RUNX3, TCF4 and β-catenin, that inhibits the binding of the complex to the Wnt target gene promoter, thereby suppressing Wnt signaling[125]. This transcription factor has been originally identified as a tumor suppressor, and RUNX3 loss is seen as an early event in gastric cancer development due to aberrant Wnt signaling, causing spontaneous epithelial-mesenchymal transition (EMT) and producing a tumorigenic stem cell-like subpopulation[126-128]. Furthermore, recent evidence suggests that RUNX3 can either suppress or activate Wnt signaling activity in gastric cancer by binding to the TCF4⁄β-catenin complex, depending of (or regulated by) cell context-dependent mechanisms[129]. Similarly, sFRP1, which is classically considered as an inhibitor of Wnt/β-catenin signaling, has been observed playing in promoting gastric tumourigenesis. This double-edged role seems depend on the strong link between sFRP1 expression and TGFβ signalling activation. In gastric cancer cells high sFRP1 levels are correlated with the activation of TGFβ signaling[130].
A recent study demonstrated that loss of CHD8 expression, commonly observed in gastric cancer, represents an indicator for the biological aggressiveness of gastric cancer[131]. Nishiyama et al[132] suggested that CHD8 (chromodomain helicase DNA-binding) negatively regulates β-catenin function by recruiting histone H1 to the promoters of Wnt target genes, thereby functioning as a tumor suppressor.
Genomic alterations:APC mutations have been detected in both precancerous and malignant gastric lesions with up to 25% of gastric adenomas exhibiting somatic mutations in this gene[133,134]. Moreover, deletions of APC at the chromosome locus 5q21-22 have also been found in 20%-60% of gastric cancer[135-137]. Ogasawara et al[92] observed that gastric tumors had mutations of the APC gene comparable to the frequency of CTNNB1 mutation. They suggested that such APC mutations explain the abnormal β-catenin localization and accumulation that could cause activation of Wnt/β-catenin signaling in some cases. Furthermore, in a murine model heterozygous for E-cadherin gene deletion, loss of heterozygosity of APC synergistically enhanced gastric tumor initiation[138]. The fact that APC gene deletions were mainly observed in advanced tumors suggests that it might occur in the progression but not in the initiation of gastric cancers[137]. In addition, loss of the mesenchymal transcription factor Foxl1 leads to an earlier tumor initiation due to accelerated loss of heterozygosity at the APC locus in colon and stomach[139].
Axin1 and Axin2 proteins negatively regulate the Wnt signaling pathway based in their roles as scaffolding proteins involved in the assembly of the β-catenin destruction complex. Therefore, mutations in their genes have been proposed as critical defects in some cancers[140]. Moreover, Axin2 is a transcriptional target of the LEF/TCF-β-catenin transcription factor complex[141]. A highly mutable G mononucleotide repeats present in exon 7 of Axin2 frequently has a frameshift mutation (1 bp deletion) in gastric cancer with MSI and nuclear β-catenin expression[90,98]. Pan et al[90] also identified in their study in gastric cancer five SNPs (334 C>T, 874 C>T, 1396 G>A, 1690 C>T and 1942 T>G) and frameshift mutations in Axin1.
Missense mutations in β-TrCP, a component of the ubiquitin ligase complex targeting β-catenin for proteasomal degradation, also has been suggested to contribute to the development of gastric cancer through β-catenin stabilization[142]. Finally, somatic mutations in Wilms’ tumor gene on the X chromosome (WTX), which is also considered a negative regulator of Wnt/β-catenin signaling, have been only detected in colorectal cancer, but not in gastric carcinomas[143].
Epigenetic modifications: Hypermethylation of Wnt-antagonist and tumor suppressor genes may be one of the key mechanisms for the translocation of β-catenin to nucleus and subsequent aberrant activation of canonical Wnt/β-catenin pathway involved in the pathogenesis of gastric cancer[144,145]. Indeed, APC is one of the genes commonly hypermethylated in gastric cancer[145,146]. Moreover, hypermethylation of APC promoter contributing to moderate activation of Wnt signaling was associated to the development and progression of gastric adenomas, which are considered as premalignant lesions of gastric adenocarcinoma[145]. This process seems to be accelerated by infection with H. pylori[145].
Several other tumor suppressor genes that function through modulating Wnt/β-catenin signaling were found silenced in gastric cancer, such as members of the sFRP and Dkk gene families. CpG methylation-depending silencing of sFRP1, sFRP2, sFRP4, sFRP5, HSulf-1, WIF-1, RUNX3 as well as, Dkk-1, Dkk-2 and Dkk-3 has been frequently observed among gastric cancer cells lines and primary specimens[59,96,108,144,147,148]. In addition, Yu et al[148] showed by multivariate analysis that Dkk-3 methylation was associated significantly and independently with poor disease survival in gastric cancer, but not in colorectal cancer. Recently, DNA methylation status in 49 gastric cancer samples was analyzed by a bead array with 485512 probes[149]. This study revealed that the Wnt pathway was potentially activated by aberrant methylation of its negative regulators, such as Dkk-3, NKD1, Sox17, WIF-1 and sFRP1. Moreover, DNA methylation and extensive mutation analyses in gastric cancers revealed an association between the CpG island methylator phenotype and oncogenic mutations in CTNNB1, ERBB2, KRAS and PIK3CA[150].
Downregulation of Sox10 by promoter methylation has also been detected in gastric cancer tissues[112]. Sox (SRY-related-HMG-box) familiy members are transcription factors that regulate canonical Wnt signaling through different mechanisms, including protein-protein interactions and binding to Wnt-target gene promoters[151]. Sox10 is an important DNA binding transcription factor that recruits β-catenin to repress Wnt/β-catenin signaling in cancer progression[112].
PRDM5, an epigenetic modifier gene, was found inactivated by promoter methylation in 76% of gastric cancer[152,153]. While knockdown of PRDM5 expression induced cell proliferation, ectopic PRDM5 expression significantly inhibited tumor cell clonogenicity, accompanied by the downregulation of TCF/β-catenin-dependent transcription of CDK4, TWIST1 and MDM2 oncogenes[153].
Moreover, silencing of RASSF10, a member of the Ras association domain family proteins that are considered as tumor suppressor genes, was recently found associated with promoter hypermethylation in gastric cancer cell lines[154]. This study also demonstrated that RASSF10 over-expression inhibited the Wnt/β-catenin signaling pathway in gastric cancer cells.
Odd-skipped related 1 (OSR1), a gene encoding a zinc-finger transcription factor, was found preferentially methylated in gastric cancer by genome-wide methylation screening[111]. The tumor suppressive activity of OSR1 in gastric cancer is in part due to inhibition on the expression of LRP6, CTNNB1, TCF-1, LEF1, and Axin2 in the Wnt/β-catenin signaling pathway. In this work, OSR1 methylation was also considered as an independent predictor of poor survival of patients at early stage of gastric cancer[111].
Role of microRNAs in Wnt/β-catenin signaling in the gastric cancer
MiRNAs are short noncoding RNA molecules of 19-25 nt that regulate gene expression at the post-translational level leading to mRNA degradation or the inhibition of translation[155]. MicroRNAs are involved in the regulation of different biological processes, including apoptosis, proliferation, metabolism, and as modulators of development they can promote either self-renewal or differentiation in stem cells to determine their fates[155,156]. Moreover, miRNA expression profiling in many kind of cancers has shown that certain miRNAs can function as oncogenes or tumor suppressors, and their misexpression and dysregulation contribute to the tumorigenic process[157].
A miRNA expression profile in normal gastric tissues have been described[158]. Moreover, an increasing number of studies have shown dysregulation of miRNAs in gastric cancer tissues and their potential use as biomarker for this disease[66-68]. Although with some controversies, these studies have resulted in the identification of hundreds of differentially expressed miRNAs, some of them involved in regulation of the Wnt/β-catenin signaling[159,160].
Wu et al[161] reported that members of the miR-17-92 cluster (miR-19b, miR-20a and miR-92a) act regulating the self-renewal of GCSC by targeting E2F1 and HIPK1, that are known to suppress Wnt/β-catenin signaling. As a consequence, miR-17-92 subsequently activates Wnt/β-catenin signaling[161]. Other genes involved in Wnt pathway were revealed as potential target of miR-30a (Runx2, CDH1, Wnt5A) and miR-103 (Axin2) in expression profiling studies in gastric cancer[160]. Moreover, hsa-miR-375 upregulation and hsa-miR-142-5p downregulation have been indicated as predictor for recurrence risk in gastric cancer patients following surgical resection, probably by regulating several Wnt/β-catenin target genes[162].
In Chinese patients with gastric cancer other miRNA genes that regulate Wnt/β-catenin target genes, such as miR-144, hsa‐miR‐148a and miR-193b were found downregulated[163-165]. While miR-193b can inhibit tumor invasion and metastasis[163], hsa-miR-148a has been suggested as biomarker for early gastric cancer[165]. Moreover, also in Chinese patients, the survival analyses results showed that patients with high expression levels of hsa-miR-335 had reduced overall survival rate and high recurrence risk of gastric cancer[166]. Among hsa-miR-335 targeted genes is Fzd-3, a member of the FZD receptor family, upregulated in several cancers[167].
Metastatic potential of gastric cancer cells have been associated with changes in the expression profile of miRNAs involved in regulating Wnt signaling pathway. MiR-10a upregulation and miR-516a-3p decreased expression were proven to play a pivotal role in tumor invasion and metastasis from primary gastric cancer to lymph nodes or peritoneum, respectively[168,169]. Using proteomics approaches, Takey et al[169] showed that miR-516a-3p targets extracellular sulfatase 1 (HSulf-1). HSulf-1 removes 6-O sulfates from heparan sulfate proteo-glycans chains to liberate Wnt ligands, and thus, enable them for binding to Frizzled receptors and subsequently activate the Wnt canonical pathway[170]. However, as it was mentioned above, loss or downregulation of HSulf-1 is associated with carcinogenesis in several tissues.
Tang et al[171] identified a novel role for GSK3β in the regulation of miRNAs biogenesis in gastric cancer cells. They showed that GSK3β inhibits the expression of miR-96, miR-182 and miR-183 through the β-catenin/TCF/LEF-1 pathway. GSK3β protein levels are decreased in human gastric cancer tissue compared with surrounding normal gastric tissue, coinciding with increased levels of β-catenin and miR-96, miR-182, miR-183. In addition, overexpression of β-catenin enhances the expression of miR-96, miR-182 and miR-183 in human gastric cancer cells[171].
A recent study identified the overexpression of miR-200b and miR-22 as the mechanism related to the anti-tumor effects of Diallyl disulfide in gastric cancer cells[172]. Furthermore, Wnt-1 was identified as a target of both miR-200b and miR-22. Moreover, MAPRE1, which acts as an activator of the Wnt/β-catenin pathway may be a direct and functional target of miRNA-10b in gastric cancers[173,174].
MiR-27 has been identified as modulator of Wnt signaling by interacting with APC and also promoting human gastric cancer cell metastasis by inducing EMT[175]. Finally, it was recently revealed that the upregulation of miR-200a increases E-cadherin and suppresses the Wnt/β-catenin pathway by targeting CTNNB1, ZEB1 and ZEB2 in gastric cancer[176,177].
Wnt/β-catenin signaling in the gastric cancer stem cells
Cancer stem cells (CSCs) are a group of heterogeneous cells representing a fraction of certain tumors that share many properties with somatic stem cells, such as the capacity for self-renewal and multipotent differentiation potential[178]. CSCs are involved in tumor development, and form the basis for tumor proliferation and metastasis[179-181]. Researchers proved the existence of CSCs in many solid tumors, including in various gastrointestinal neoplasms such as colon, gastric pancreas, liver and gastroesophageal tumors[181-184]. Moreover, CSCs are thought to be responsible for tumor recurrence, which is one of the biggest problems in cancer therapy.
Although the mechanism by which CSCs develop remains unclear several studies have investigated the role of dysregulation of the Wnt/β-catenin, transformation growth factor-β (TGF-β) and Hedgehog pathways in generation of CSC[185-187]. Abnormal activation of Wnt/β-catenin signaling pathway strongly correlates with tumorigenesis and progression through maintaining CSCs[188]. In this regard, Zhu et al[189] identified that Dkk-1 secreted by mesenchymal stem cells was able to inhibit proliferation of human carcinoma cell lines through the canonical Wnt signaling pathway. Moreover, knockdown of β-catenin resulted in inhibition of CSC in lung cancer[190].
The development of gastric cancer is closely associated with GCSC. Clinicopathological analysis in gastric cancer has demonstrated that the expression of stem cell markers CD44 and CD133 is highly associated with the degree of malignancy and tumor grading[191-193]. A few studies have explored the role of Wnt/β-catenin signaling in GCSC self-renewal, proliferation and sensibility to treatment.
The squamo-columnar junction (SCJ) region of the normal mouse stomach contains a gland consisting of CD44+ cells, of which a few are quiescent or slow-cycling stem cell-like gastric cells[45]. These CD44+ cells in the SCJ region can be expanded by the cooperative effect of PGE2-mediated inflammatory signaling and Wnt signaling, suggesting a mechanism to development of gastric tumors in mice[45]. Moreover, Mao et al[47] observed that stem-related genes Wnt-1 and CD44 are overexpressed in gastric cancer specimens. They found that the proliferation rate of GCSC was dramatically inhibited by knockdown of Wnt-1. Also, Salinomycin, an antibiotic thought to suppress Wnt/β-catenin signal transduction can inhibit GCSC[47,194]. Finally, the importance of the Wnt/β-catenin pathway for the self-renewal of CSCs in human gastric cancer have also been suggested by proving that stimulation with Dkk-1 could decrease the self-renewal ability of cancer stem-like cells[46].
Wnt/β-catenin signaling and metastatic potential in gastric cancer cells
A distinctive feature of gastric cancer is early invasiveness into adjacent tissues and the peritoneal cavity. It has been hypothesized that Wnt/β-catenin signaling could contribute to tumor progression by enhancing the proliferation and invasiveness of gastric cancer cells. Furthermore, it is thought that the Wnt/β-catenin pathway is one of the major signaling pathways involved in EMT and therefore, playing a critical role in metastasis[195]. Indeed, disruption of Wnt/β-catenin pathway has shown anti-metastatic activity in gastric cancer cells[196].
In this regard, an important player is Wnt-5a that stimulates cell migration and invasion of gastric cancer cells through regulation of focal adhesion complexes by activating focal adhesion kinase and small GTP-binding protein Rac[74]. Wnt-5a also contributes to gastric cancer progression by inducing expression of laminin γ2, thus increasing metastatic potential[197].
On the other hand, nuclear β-catenin is correlated with an invasive phenotype in intestinal-type tumors[198]. This increase in tumor invasion is likely mediated by the upregulation of Wnt transcriptional targets, such as EphA2 (erythropoietin-producing hepatocellular) and membrane type 3 matrix metalloproteinase (MT3-MMP) genes[199,200]. Furthermore, evidence provided by Kim et al[201,202] suggests that TC1 (C8orf4), which is upregulated in high-grade gastric cancers, coordinates the upregulation of Wnt/β-catenin target genes involved in the aggressive biological behavior and poor clinical outcome observed in advanced gastric cancer[202,203]. TC1 enhances the signaling pathway by relieving the antagonistic regulation of Chibby (Cby) on β-catenin-mediated transcription[203]. Cby was first identified as an interactor with β-catenin in humans, which suggested it could be a tumor suppressor gene[204].
Silencing of PLA2G2A (phospholipase A2) enhances the invasive ability of gastric cancer cells[205]. Surprisingly, PLA2G2A, which is an independent predictor of favorable outcome for patients with gastric cancer[206,207], is also a direct Wnt target gene and it has been found upregulated in early stages of gastric cancer. Ganesan et al[205] have proposed that in late-stage tumors, Wnt signaling is still active driving expression of proto-oncogenes; however, PLA2G2A expression is decreased possibly by epigenetic inactivation and/or genomic deletions, resulting tumors with a highly aggressive clinical phenotype. Moreover, the overexpression of the Wnt signalling regulator sFRP1 has been correlated with activation of TGFβ signalling pathway, thereby it may induce cell proliferation, EMT and invasion[130].
HMGA (high mobility group protein) proteins contain DNA binding domains that mediate binding to AT-rich regions of chromatin, having the ability to modulate transcription of their target genes by altering the chromatin structure at the promoter and/or enhancers[208]. Members of HMGA family, HMGA1 and HMGA2, are highly expressed during embryonic development and are linked to malignant transformation and progression in human cancers, including gastric cancer[208,209]. HMGA1, is induced by the Wnt/β-catenin pathway and maintains proliferation of gastric cancer cells[210]. Moreover, HMGA2 activates the Wnt/β-catenin pathway and causes EMT in gastric cancer cells by suppressing the expression of Axin1, thereby protecting β-catenin from phosphorylation degradation[211]. HMGA2 also promotes β-catenin translocation by increasing expression of TWIST1, which can repress E-cadherin expression[211].
Role of infections in enhancing Wnt/β-catenin pathway signaling in gastric cancer
H. pylori virulence factors contributing to gastric cancer development include cytotoxin-associated gene product (cagA), vacuolating cytotoxin A (vacA), blood group antigen binding adhesion 2 (BabA), outer inflammatory protein (oipA) and induced by contact with epithelium (iceA)[212].
Infection with cagA-positive H. pylori strains increases the risk of peptic ulcers and gastric carcinoma[213]. CagA protein is translocated by a type IV secretion system into gastric epithelial cells where forms a physical complex with the Src homology 2 domain-containing tyrosine phosphatase in a phosphorylation dependent manner[214]. These changes trigger a complex set of alterations in signal transduction that affects various cellular processes[215]. Another H. pylori constituent linked to the development of gastric cancer is the secreted VacA toxin. VacA can cause a wide assortment of alterations in gastric epithelial cells, including cell vacuolation, gastric epithelial cell autophagy and apoptosis[216,217]. VacA can also interfere with T cell activation, which suppresses local immune response[218].
H. pylori infection could cause cancer tumorigenesis and malignant transformation of host cells through the activation of oncogenic pathways[219]. In this sense, evidence has raised the possibility that Wnt/β-catenin pathway plays a role in the pathogenesis of H. pylori infection, including the promotion of pathological changes seen in gastric cancer. Indeed, in vitro H. pylori infection of cells promotes an increase in nuclear levels of β-catenin and TCF/LEF transactivation, with upregulation of the β-catenin target gene cyclin D1 in a CagA-independent manner[220,221].
Among the pleiotropic effects of the vacuolating cytotoxin VacA, Nakayama et al[222] reported that this H. pylori virulence factor induces the Wnt/β-catenin pathway through the activation of the PI3K/Akt signaling pathway. This triggers the phosphorylation and inhibition of GSK3β, and β-catenin translocation to the nucleus after dissociation of GSK3β/β-catenin complex, with subsequent activation of the CCND1 promoter[222].
β-catenin activation by CagA-dependent manner has also been associated with gastric carcinogenesis. H. pylori CagA plays an important role in the development of intestinal metaplasia, a premalignant lesion of gastric epithelial cells from which intestinal-type gastric adenocarcinoma arises[220]. Moreover, CagA deregulates the Wnt/β-catenin pathway in a phosphorylation-independent manner by the interaction with E-cadherin and the destruction of the complex between E-cadherin and β-catenin, which causes cytoplasmic and nuclear accumulation of β-catenin. CagA-deregulated β-catenin then trans-activates cdx1 and p21 genes that are involved in intestinal differentiation of gastric epithelial cells[220]. Additionally, another study has suggested that non-phosphorylated CagA may induce Wnt signaling in a met proto-oncogene (c-Met)-dependent manner[223].
The relationship between H. pylori-induced inflammation and oncogenic mechanisms has been recently assessed by the transgenic expression of CagA in a novel in vivo model of intestinal hyperplasia in zebrafish[224]. This study reported significant CagA-dependent up-regulation of the Wnt target genes CCND1, Axin2 and the zebrafish c-myc ortholog myca. Moreover, CagA infection can lead to advanced gastric carcinoma through the upregulation of cyclin D1[225]. It is thought that CagA promotes the phosphorylation of MUC1, which is a glycoprotein expressed on the apical borders of secretory epithelial cells, thereby facilitating its interaction with β-catenin, and the subsequent transcriptional activation of cyclin D1[225]. In addition, gene expression profile analyzed in H. pylori-infected and uninfected patients undergoing chronic superficial gastritis showed that the E3 ubiquitin-protein ligase RNF138, which is involved in the ubiquitination and degradation of TCF/LEF, was downregulated by H. pylori[226,227].
Among the multiple ways in which H. pylori can induce Wnt signaling, the bacteria also induces phosphorylation of the Wnt co-receptor LRP6 in a T4SS-dependent manner and involving Dvl-2 and Dvl-3 proteins[228].
In gastric tumor mouse models, the cooperation between the canonical Wnt signaling and H. pylori-induced COX-2/PGE2 pathway has been established in promoting gastric carcinogenesis via the metaplasia-carcinoma sequence[72,229]. In addition, the Wnt signaling pathway plays a role in the transcriptional induction of COX2 in gastric cancer cells through a β-catenin/TCF dependent mechanism[230,231]. Furthermore, TNF-α treatment and H. pylori infection might play roles in human gastric cancer cell line MKN45 through upregulation of Wnt-10A[232].
Finally, it is important to mention the correlation between Epstein-Barr virus (EBV) and gastric cancer. In this regard, Kim et al[233] found that p21 and APC loss was positively correlated with EPV positive gastric carcinomas. EBV is accepted as a causative microorganism for gastric carcinoma, accounting for nearly 10% of gastric cancer cases worldwide[234].
The Wnt/β-catenin pathway plays a key role in gastric cancer pathogenesis; therefore, chemotherapeutic approaches for targeting this pathway should be a major effort. However, several factors have hampered progress in this field, such as the number and variety of Wnt ligands, receptors and co-receptors involved in Wnt signaling[14,18]. Furthermore, this complex pathway regulates a broad range of developmental processes, its components have redundancy in other cellular functions, and the transcriptional output of β-catenin is highly context dependent[235,236]. Finally, crosstalk from various non-Wnt factors also intervenes to regulate nuclear β-catenin accumulation.
Wnt inhibitors are the focus of intense investigation in pharmaceutical and academic research, and as a consequence of this effort; potential anticancer agents have emerged that target components of this signaling pathway[235,237-239]. There are currently several therapeutic approaches to modulate the Wnt signaling cascade at various stages of development, as well as natural compounds that have been proved, or are being tested, in different systems[235,240]. Several studies in which potential targets and strategies were tested for gastric cancer treatment targeting Wnt/β-catenin signaling pathway are reviewed below.
The antibacterial potassium ionophore Salinomycin was first described as a selective inhibitor of breast cancer[241]. Thereafter, Salinomycin was found to inhibit Wnt/β-catenin signaling by inducing the degradation of Wnt co-receptor LRP6 in chronic lymphocytic leukemia[242]. Recently, it was also confirmed that Salinomycin inhibits tumor growth by targeting preferably gastric CSCs, at least partially, via suppression of Wnt1 and β-catenin expression[47].
Zhang et al[243] examined the effects of acetyl-11-keto-beta-boswellic acid (AKBA) and its underlying molecular mechanisms on human gastric carcinoma. AKBA is an active component of the Boswellia serrata gum resin[243,244]. In this study, AKBA significantly inhibited human gastric cancer growth without toxicity. This effect was attributed, among other mechanisms, to induction of apoptosis and modulation of the Wnt/β-catenin signaling pathway.
Natural products involving downregulation of the Wnt/β-catenin signaling pathway have also shown antitumor effects against gastric cancer, such as γ-tocotrienol, flavanone, Capsosiphon fulvescens glycoprotein (Cf-GP) and diphyllin[245-248]. Diphyllin is a cytostatic lignan isolated from Cleistanthus collinus that potently inhibits V-ATPases involved in Wnt/β-catenin signaling[245,249]. Shen et al[245] observed in gastric carcinoma treated with diphyllin a decrease in phospho-LRP6, but not in LRP6, accompanied by reduction of β-catenin levels and the expression of its target genes associated with the inhibition of V-ATPase. LRP6 phosphorylation is accompanied by receptor internalization in caveolin-containing vesicles and endocytosis, which is essential for Wnt/β-catenin signaling[250].
Yao et al[251] observed that the combined treatment with the ERK1/2 inhibitor PD98059 and the γ-secretase inhibitor DAPT in gastric cancer originated induction of apoptosis and regulation of β-catenin, c-Myc, and cyclin D1. Although this combined treatment results in a dramatically increased cytotoxicity compared to that observed with any of the drugs alone, the exact mechanism by which these two agents act together to downregulate Wnt/β-catenin signaling pathway remains unknown.
Anti-metastatic activity resulting from disruption of Wnt/β-catenin pathway was recently reported in gastric cancer cells by Hanaki et al[196] using anti-Wnt-5a polyclonal antibody. They suggested that the anti-Wnt5a antibody was capable of suppressing Wnt-5a-dependent internalization of receptors, thus leading to disruption of Wnt/β-catenin pathway. Further investigation with anti-Wnt-5a monoclonal antibody could be useful for the clinical management of cancers with overexpression of Wnt-5a. In this sense, monoclonal antibodies against Wnt-1 and Wnt-2 can induce apoptosis in some human cancers that overexpress these ligands[252,253].
Interestingly, two different approaches targeting the secreted inhibitor of the Wnt/β-catenin pathway Dkk-1 have been reported as potential treatment for gastric cancer[121,254]. As mentioned above, the role of Dkk-1 in cancer appears to be diverse, because it can act as tumor suppressor or metastasis promoter[255]. Thus, Dkk-1 appears to be critical to determining the tumor microenvironment and could has a cancer type and/or patient specific role[255]. Sato et al[121] reported a strategy based on Dkk-1-neutralizing antibodies indicating the direct antitumor effect in cancer cells lines that overexpressed Dkk-1, such as pancreatic, bile duct, breast, cervical and gastric cancer, and hepatocellular carcinoma. Alternatively, Wang et al[254] used a chimeric 5/35 adenovirus-mediated Dkk-1 overexpression strategy to suppress the endogenous Wnt/β-catenin signaling aberrantly activated in GCSC. This study demonstrated that adenoviral expression of Dkk-1 was efficient in modulating activation of Wnt signaling and also proved that CSC-targeting gene therapy could be effective in gastric cancer.
Recently, Zhang et al[256] reported that the proton pump inhibitor pantoprazole treatment inhibited the hyper-activated Wnt/β-catenin signaling and reduced cell invasiveness of chemotherapy-resistant gastric cancer cells and epithelial-mesenchymal transition. This work, besides giving evidence that the enhanced aggressive phenotype appeared to be mediated by activation of the canonical Wnt/β-catenin signaling pathway, it also showed that it is possible to reduce the aggressiveness of gastric cancer with a proton pump inhibitor[256].
Recent studies have identified new components and regulators, as well as confirmed other already known players, of the canonical Wnt/β-catenin pathway involved in the development, progression and metastasis of gastric cancer. These studies have also provided new approaches to therapeutically target Wnt/β-catenin signaling pathway in primary and metastatic gastric cancer. The complexity of the Wnt/β-catenin pathway and the potential influence of the context imply a critical need for understanding the interaction between Wnt regulators and with different oncogenic signaling pathways, to completely elucidate this pathway.
In this regard, the contribution of high throughput methods for functional genomics, epigenomics and for the screening of potential therapeutic molecules certainly will help in the near future. These “new generation” methods could also address the additional challenge that represents the intratumoral and intertumoral (between patients) heterogeneity for effective treatment of gastric tumors.
To N Lander, PhD, for revising the English of the manuscript.
P- Reviewer: Ditzel M, Dalay N, Huerta-Franco MR, Singh SR, Wiemer EAC S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25533] [Article Influence: 1823.8] [Reference Citation Analysis (7)] |
| 3. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013; . |
| 4. | Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, Sung JJ. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010;295:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. [PubMed] |
| 6. | Resende C, Thiel A, Machado JC, Ristimäki A. Gastric cancer: basic aspects. Helicobacter. 2011;16 Suppl 1:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | McNamara D, El-Omar E. Helicobacter pylori infection and the pathogenesis of gastric cancer: a paradigm for host-bacterial interactions. Dig Liver Dis. 2008;40:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503-3506. [PubMed] |
| 10. | Ikenoue T, Ijichi H, Kato N, Kanai F, Masaki T, Rengifo W, Okamoto M, Matsumura M, Kawabe T, Shiratori Y. Analysis of the beta-catenin/T cell factor signaling pathway in 36 gastrointestinal and liver cancer cells. Jpn J Cancer Res. 2002;93:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Baker NE. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489-497. [PubMed] |
| 12. | Croce JC, McClay DR. Evolution of the Wnt pathways. Methods Mol Biol. 2008;469:3-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 15. | Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1132] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 19. | Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4613] [Cited by in RCA: 4499] [Article Influence: 281.2] [Reference Citation Analysis (0)] |
| 21. | Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 498] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 22. | Schulte G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev. 2010;62:632-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009;19:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1536] [Cited by in RCA: 1656] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 25. | Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1938] [Cited by in RCA: 2032] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 26. | Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 539] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Hurlstone A, Clevers H. T-cell factors: turn-ons and turn-offs. EMBO J. 2002;21:2303-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 430] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469-7481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 791] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 30. | Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 564] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 31. | Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1249] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 32. | Berthon A, Martinez A, Bertherat J, Val P. Wnt/β-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol. 2012;351:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4384] [Article Influence: 337.2] [Reference Citation Analysis (0)] |
| 34. | Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E. The way Wnt works: components and mechanism. Growth Factors. 2013;31:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Tolwinski NS, Wieschaus E. Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development. 2001;128:2107-2117. [PubMed] |
| 36. | Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J Cell Sci. 2006;119:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Henderson BR, Galea M, Schuechner S, Leung L. Lymphoid enhancer factor-1 blocks adenomatous polyposis coli-mediated nuclear export and degradation of beta-catenin. Regulation by histone deacetylase 1. J Biol Chem. 2002;277:24258-24264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep. 2002;3:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA. 2004;101:2882-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Hendriksen J, Fagotto F, van der Velde H, van Schie M, Noordermeer J, Fornerod M. RanBP3 enhances nuclear export of active (beta)-catenin independently of CRM1. J Cell Biol. 2005;171:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1563] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 43. | Byun T, Karimi M, Marsh JL, Milovanovic T, Lin F, Holcombe RF. Expression of secreted Wnt antagonists in gastrointestinal tissues: potential role in stem cell homeostasis. J Clin Pathol. 2005;58:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1192] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 45. | Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, Baba H, Saya H, Nagano O. CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 2010;101:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Cai C, Zhu X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol Med Rep. 2012;5:1191-1196. [PubMed] |
| 47. | Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 48. | The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6641] [Article Influence: 510.8] [Reference Citation Analysis (0)] |
| 49. | Song J, Wang Z, Ewing RM. Integrated analysis of the Wnt responsive proteome in human cells reveals diverse and cell-type specific networks. Mol Biosyst. 2014;10:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 629] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 51. | Lee J, van Hummelen P, Go C, Palescandolo E, Jang J, Park HY, Kang SY, Park JO, Kang WK, MacConaill L. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS One. 2012;7:e38892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Nagarajan N, Bertrand D, Hillmer AM, Zang ZJ, Yao F, Jacques PÉ, Teo AS, Cutcutache I, Zhang Z, Lee WH. Whole-genome reconstruction and mutational signatures in gastric cancer. Genome Biol. 2012;13:R115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 54. | Holbrook JD, Parker JS, Gallagher KT, Halsey WS, Hughes AM, Weigman VJ, Lebowitz PF, Kumar R. Deep sequencing of gastric carcinoma reveals somatic mutations relevant to personalized medicine. J Transl Med. 2011;9:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Fassan M, Simbolo M, Bria E, Mafficini A, Pilotto S, Capelli P, Bencivenga M, Pecori S, Luchini C, Neves D. High-throughput mutation profiling identifies novel molecular dysregulation in high-grade intraepithelial neoplasia and early gastric cancers. Gastric Cancer. 2014;17:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Vogiatzi P, Vindigni C, Roviello F, Renieri A, Giordano A. Deciphering the underlying genetic and epigenetic events leading to gastric carcinogenesis. J Cell Physiol. 2007;211:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Nakamura J, Tanaka T, Kitajima Y, Noshiro H, Miyazaki K. Methylation-mediated gene silencing as biomarkers of gastric cancer: a review. World J Gastroenterol. 2014;20:11991-12006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415-428. [PubMed] |
| 59. | Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28:2459-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Veeck J, Wild PJ, Fuchs T, Schüffler PJ, Hartmann A, Knüchel R, Dahl E. Prognostic relevance of Wnt-inhibitory factor-1 (WIF1) and Dickkopf-3 (DKK3) promoter methylation in human breast cancer. BMC Cancer. 2009;9:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Costa VL, Henrique R, Ribeiro FR, Carvalho JR, Oliveira J, Lobo F, Teixeira MR, Jerónimo C. Epigenetic regulation of Wnt signaling pathway in urological cancer. Epigenetics. 2010;5:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Zhang YW, Miao YF, Yi J, Geng J, Wang R, Chen LB. Transcriptional inactivation of secreted frizzled-related protein 1 by promoter hypermethylation as a potential biomarker for non-small cell lung cancer. Neoplasma. 2010;57:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Rawson JB, Sun Z, Dicks E, Daftary D, Parfrey PS, Green RC, Gallinger S, McLaughlin JR, Wang PP, Knight JA. Vitamin D intake is negatively associated with promoter methylation of the Wnt antagonist gene DKK1 in a large group of colorectal cancer patients. Nutr Cancer. 2012;64:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Varol N, Konac E, Onen IH, Gurocak S, Alp E, Yilmaz A, Menevse S, Sozen S. The epigenetically regulated effects of Wnt antagonists on the expression of genes in the apoptosis pathway in human bladder cancer cell line (T24). DNA Cell Biol. 2014;33:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Zhang J, Zhou B, Liu Y, Chen K, Bao P, Wang Y, Wang J, Zhou Z, Sun X, Li Y. Wnt inhibitory factor-1 functions as a tumor suppressor through modulating Wnt/β-catenin signaling in neuroblastoma. Cancer Lett. 2014;348:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ. MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark. 2012;11:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Song J, Bai Z, Zhang Z. MicroRNAs are implicated in the initiation and progression of gastric cancer. Chin Med J (Engl). 2014;127:554-559. [PubMed] |
| 68. | Liu HS, Xiao HS. MicroRNAs as potential biomarkers for gastric cancer. World J Gastroenterol. 2014;20:12007-12017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Katoh M, Kirikoshi H, Terasaki H, Shiokawa K. WNT2B2 mRNA, up-regulated in primary gastric cancer, is a positive regulator of the WNT- beta-catenin-TCF signaling pathway. Biochem Biophys Res Commun. 2001;289:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Li H. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett. 2005;223:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Zhang H, Xue Y. Wnt pathway is involved in advanced gastric carcinoma. Hepatogastroenterology. 2008;55:1126-1130. [PubMed] |
| 72. | Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Boussioutas A, Li H, Liu J, Waring P, Lade S, Holloway AJ, Taupin D, Gorringe K, Haviv I, Desmond PV. Distinctive patterns of gene expression in premalignant gastric mucosa and gastric cancer. Cancer Res. 2003;63:2569-2577. [PubMed] |
| 74. | Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439-10448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 75. | Yuan G, Regel I, Lian F, Friedrich T, Hitkova I, Hofheinz RD, Ströbel P, Langer R, Keller G, Röcken C. WNT6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells. Oncogene. 2013;32:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Boscher C, Nabi IR. Caveolin-1: role in cell signaling. Adv Exp Med Biol. 2012;729:29-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 77. | Gupta R, Toufaily C, Annabi B. Caveolin and cavin family members: dual roles in cancer. Biochimie. 2014;107 Pt B:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | To KF, Chan MW, Leung WK, Yu J, Tong JH, Lee TL, Chan FK, Sung JJ. Alterations of frizzled (FzE3) and secreted frizzled related protein (hsFRP) expression in gastric cancer. Life Sci. 2001;70:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Mo ML, Li MR, Chen Z, Liu XW, Sheng Q, Zhou HM. Inhibition of the Wnt palmitoyltransferase porcupine suppresses cell growth and downregulates the Wnt/β-catenin pathway in gastric cancer. Oncol Lett. 2013;5:1719-1723. [PubMed] |
| 80. | Qiu HB, Zhang LY, Ren C, Zeng ZL, Wu WJ, Luo HY, Zhou ZW, Xu RH. Targeting CDH17 suppresses tumor progression in gastric cancer by downregulating Wnt/β-catenin signaling. PLoS One. 2013;8:e56959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Lu H, Sun J, Wang F, Feng L, Ma Y, Shen Q, Jiang Z, Sun X, Wang X, Jin H. Enhancer of zeste homolog 2 activates wnt signaling through downregulating CXXC finger protein 4. Cell Death Dis. 2013;4:e776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Pandi NS, Manimuthu M, Harunipriya P, Murugesan M, Asha GV, Rajendran S. In silico analysis of expression pattern of a Wnt/β-catenin responsive gene ANLN in gastric cancer. Gene. 2014;545:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Kim MY, Han SI, Lim SC. Roles of cyclin-dependent kinase 8 and β-catenin in the oncogenesis and progression of gastric adenocarcinoma. Int J Oncol. 2011;38:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Nicholson S, Whitehouse H, Naidoo K, Byers RJ. Yin Yang 1 in human cancer. Crit Rev Oncog. 2011;16:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Tang S, Mishra M, Frazier DP, Moore ML, Inoue K, Deora R, Sui G, Dubey P. Positive and negative regulation of prostate stem cell antigen expression by Yin Yang 1 in prostate epithelial cell lines. PLoS One. 2012;7:e35570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Kang W, Tong JH, Chan AW, Zhao J, Dong Y, Wang S, Yang W, Sin FM, Ng SS, Yu J. Yin Yang 1 contributes to gastric carcinogenesis and its nuclear expression correlates with shorter survival in patients with early stage gastric adenocarcinoma. J Transl Med. 2014;12:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Li H, Mo J, Jia G, Liu C, Luan Z, Guan Y. Activation of Wnt signaling inhibits the pro-apoptotic role of Notch in gastric cancer cells. Mol Med Rep. 2013;7:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, Kim SY, Lee HK, Kim PJ, Oh ST. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 1999;59:4257-4260. [PubMed] |
| 89. | Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001;95:108-113. [PubMed] |
| 90. | Pan KF, Liu WG, Zhang L, You WC, Lu YY. Mutations in components of the Wnt signaling pathway in gastric cancer. World J Gastroenterol. 2008;14:1570-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Ebert MP, Fei G, Kahmann S, Müller O, Yu J, Sung JJ, Malfertheiner P. Increased beta-catenin mRNA levels and mutational alterations of the APC and beta-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis. 2002;23:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Ogasawara N, Tsukamoto T, Mizoshita T, Inada K, Cao X, Takenaka Y, Joh T, Tatematsu M. Mutations and nuclear accumulation of beta-catenin correlate with intestinal phenotypic expression in human gastric cancer. Histopathology. 2006;49:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Candidus S, Bischoff P, Becker KF, Höfler H. No evidence for mutations in the alpha- and beta-catenin genes in human gastric and breast carcinomas. Cancer Res. 1996;56:49-52. [PubMed] |
| 94. | Sasaki Y, Morimoto I, Kusano M, Hosokawa M, Itoh F, Yanagihara K, Imai K, Tokino T. Mutational analysis of the beta-catenin gene in gastric carcinomas. Tumour Biol. 2001;22:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Tong JH, To KF, Ng EK, Lau JY, Lee TL, Lo KW, Leung WK, Tang NL, Chan FK, Sung JJ. Somatic beta-catenin mutation in gastric carcinoma--an infrequent event that is not specific for microsatellite instability. Cancer Lett. 2001;163:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Nojima M, Suzuki H, Toyota M, Watanabe Y, Maruyama R, Sasaki S, Sasaki Y, Mita H, Nishikawa N, Yamaguchi K. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene. 2007;26:4699-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 97. | Wang S, Tian Y, Wu D, Zhu H, Luo D, Gong W, Zhou Y, Zhou J, Zhang Z. Genetic variation of CTNNB1 gene is associated with susceptibility and prognosis of gastric cancer in a Chinese population. Mutagenesis. 2012;27:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Kim MS, Kim SS, Ahn CH, Yoo NJ, Lee SH. Frameshift mutations of Wnt pathway genes AXIN2 and TCF7L2 in gastric carcinomas with high microsatellite instability. Hum Pathol. 2009;40:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Duval A, Iacopetta B, Ranzani GN, Lothe RA, Thomas G, Hamelin R. Variable mutation frequencies in coding repeats of TCF-4 and other target genes in colon, gastric and endometrial carcinoma showing microsatellite instability. Oncogene. 1999;18:6806-6809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Saeki H, Tanaka S, Tokunaga E, Kawaguchi H, Ikeda Y, Maehara Y, Sugimachi K. Genetic alterations in the human Tcf-4 gene in Japanese patients with sporadic gastrointestinal cancers with microsatellite instability. Oncology. 2001;61:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Simpson AJ, Caballero OL, Pena SD. Microsatellite instability as a tool for the classification of gastric cancer. Trends Mol Med. 2001;7:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Ellis JD, Barrios-Rodiles M, Colak R, Irimia M, Kim T, Calarco JA, Wang X, Pan Q, O’Hanlon D, Kim PM. Tissue-specific alternative splicing remodels protein-protein interaction networks. Mol Cell. 2012;46:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 103. | Thorsen K, Mansilla F, Schepeler T, Øster B, Rasmussen MH, Dyrskjøt L, Karni R, Akerman M, Krainer AR, Laurberg S. Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway. Mol Cell Proteomics. 2011;10:M110.002998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 104. | Radulescu S, Ridgway RA, Cordero J, Athineos D, Salgueiro P, Poulsom R, Neumann J, Jung A, Patel S, Woodgett J. Acute WNT signalling activation perturbs differentiation within the adult stomach and rapidly leads to tumour formation. Oncogene. 2013;32:2048-2057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Zheng HC, Xu XY, Xia P, Yu M, Takahashi H, Takano Y. Involvement of inactive GSK3beta overexpression in tumorigenesis and progression of gastric carcinomas. Hum Pathol. 2010;41:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Du YC, Oshima H, Oguma K, Kitamura T, Itadani H, Fujimura T, Piao YS, Yoshimoto T, Minamoto T, Kotani H. Induction and down-regulation of Sox17 and its possible roles during the course of gastrointestinal tumorigenesis. Gastroenterology. 2009;137:1346-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 107. | Ye YW, Wu JH, Wang CM, Zhou Y, Du CY, Zheng BQ, Cao X, Zhou XY, Sun MH, Shi YQ. Sox17 regulates proliferation and cell cycle during gastric cancer progression. Cancer Lett. 2011;307:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Li J, Mo ML, Chen Z, Yang J, Li QS, Wang DJ, Zhang H, Ye YJ, Li HL, Zhang F. HSulf-1 inhibits cell proliferation and invasion in human gastric cancer. Cancer Sci. 2011;102:1815-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY, Li G, Ji XD, Shi S, Guan DX. RACK1 suppresses gastric tumorigenesis by stabilizing the β-catenin destruction complex. Gastroenterology. 2012;142:812-823.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Zhou Y, Lan J, Wang W, Shi Q, Lan Y, Cheng Z, Guan H. ZNRF3 acts as a tumour suppressor by the Wnt signalling pathway in human gastric adenocarcinoma. J Mol Histol. 2013;44:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Otani K, Dong Y, Li X, Lu J, Zhang N, Xu L, Go MY, Ng EK, Arakawa T, Chan FK. Odd-skipped related 1 is a novel tumour suppressor gene and a potential prognostic biomarker in gastric cancer. J Pathol. 2014;234:302-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Tong X, Li L, Li X, Heng L, Zhong L, Su X, Rong R, Hu S, Liu W, Jia B. SOX10, a novel HMG-box-containing tumor suppressor, inhibits growth and metastasis of digestive cancers by suppressing the Wnt/β-catenin pathway. Oncotarget. 2014;5:10571-10583. [PubMed] |
| 113. | Cui J, Xi H, Cai A, Bian S, Wei B, Chen L. Decreased expression of Sox7 correlates with the upregulation of the Wnt/β-catenin signaling pathway and the poor survival of gastric cancer patients. Int J Mol Med. 2014;34:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 114. | Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520-8526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 115. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3831] [Cited by in RCA: 4049] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 116. | Hu Y, Wan R, Yu G, Shen J, Ni J, Yin G, Xing M, Chen C, Fan Y, Xiao W. Imbalance of Wnt/Dkk negative feedback promotes persistent activation of pancreatic stellate cells in chronic pancreatitis. PLoS One. 2014;9:e95145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Rawson JB, Manno M, Mrkonjic M, Daftary D, Dicks E, Buchanan DD, Younghusband HB, Parfrey PS, Young JP, Pollett A. Promoter methylation of Wnt antagonists DKK1 and SFRP1 is associated with opposing tumor subtypes in two large populations of colorectal cancer patients. Carcinogenesis. 2011;32:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 118. | Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, García JM, Muñoz A, Esteller M, González-Sancho JM. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116-4121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 119. | Cho JH, Dimri M, Dimri GP. A positive feedback loop regulates the expression of polycomb group protein BMI1 via WNT signaling pathway. J Biol Chem. 2013;288:3406-3418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 120. | Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70:5326-5336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 122. | Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119:1728-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 123. | Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96:646-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 124. | Krause U, Ryan DM, Clough BH, Gregory CA. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Dis. 2014;5:e1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 125. | Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell. 2008;14:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 126. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 835] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 127. | Ito K, Chuang LS, Ito T, Chang TL, Fukamachi H, Salto-Tellez M, Ito Y. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology. 2011;140:1536-46.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 128. | Voon DC, Wang H, Koo JK, Nguyen TA, Hor YT, Chu YS, Ito K, Fukamachi H, Chan SL, Thiery JP. Runx3 protects gastric epithelial cells against epithelial-mesenchymal transition-induced cellular plasticity and tumorigenicity. Stem Cells. 2012;30:2088-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Ju X, Ishikawa TO, Naka K, Ito K, Ito Y, Oshima M. Context-dependent activation of Wnt signaling by tumor suppressor RUNX3 in gastric cancer cells. Cancer Sci. 2014;105:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Qu Y, Ray PS, Li J, Cai Q, Bagaria SP, Moran C, Sim MS, Zhang J, Turner RR, Zhu Z. High levels of secreted frizzled-related protein 1 correlate with poor prognosis and promote tumourigenesis in gastric cancer. Eur J Cancer. 2013;49:3718-3728. [PubMed] |
| 131. | Sawada G, Ueo H, Matsumura T, Uchi R, Ishibashi M, Mima K, Kurashige J, Takahashi Y, Akiyoshi S, Sudo T. CHD8 is an independent prognostic indicator that regulates Wnt/β-catenin signaling and the cell cycle in gastric cancer. Oncol Rep. 2013;30:1137-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 132. | Nishiyama M, Skoultchi AI, Nakayama KI. Histone H1 recruitment by CHD8 is essential for suppression of the Wnt-β-catenin signaling pathway. Mol Cell Biol. 2012;32:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 133. | Horii A, Nakatsuru S, Miyoshi Y, Ichii S, Nagase H, Kato Y, Yanagisawa A, Nakamura Y. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res. 1992;52:3231-3233. [PubMed] |
| 134. | Nakatsuru S, Yanagisawa A, Furukawa Y, Ichii S, Kato Y, Nakamura Y, Horii A. Somatic mutations of the APC gene in precancerous lesion of the stomach. Hum Mol Genet. 1993;2:1463-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Rhyu MG, Park WS, Jung YJ, Choi SW, Meltzer SJ. Allelic deletions of MCC/APC and p53 are frequent late events in human gastric carcinogenesis. Gastroenterology. 1994;106:1584-1588. [PubMed] |
| 136. | Sanz-Ortega J, Sanz-Esponera J, Caldes T, Gomez de la Concha E, Sobel ME, Merino MJ. LOH at the APC/MCC gene (5Q21) in gastric cancer and preneoplastic lesions. Prognostic implications. Pathol Res Pract. 1996;192:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 137. | Fang Z, Xiong Y, Li J, Liu L, Zhang W, Zhang C, Wan J. APC gene deletions in gastric adenocarcinomas in a Chinese population: a correlation with tumour progression. Clin Transl Oncol. 2012;14:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Smits R, Ruiz P, Diaz-Cano S, Luz A, Jagmohan-Changur S, Breukel C, Birchmeier C, Birchmeier W, Fodde R. E-cadherin and adenomatous polyposis coli mutations are synergistic in intestinal tumor initiation in mice. Gastroenterology. 2000;119:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 139. | Perreault N, Sackett SD, Katz JP, Furth EE, Kaestner KH. Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev. 2005;19:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Mazzoni SM, Fearon ER. AXIN1 and AXIN2 variants in gastrointestinal cancers. Cancer Lett. 2014;355:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 141. | Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1402] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 142. | Kim CJ, Song JH, Cho YG, Kim YS, Kim SY, Nam SW, Yoo NJ, Lee JY, Park WS. Somatic mutations of the beta-TrCP gene in gastric cancer. APMIS. 2007;115:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 143. | Yoo NJ, Kim S, Lee SH. Mutational analysis of WTX gene in Wnt/ beta-catenin pathway in gastric, colorectal, and hepatocellular carcinomas. Dig Dis Sci. 2009;54:1011-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 144. | Guo Y, Guo W, Chen Z, Kuang G, Yang Z, Dong Z. Hypermethylation and aberrant expression of Wnt-antagonist family genes in gastric cardia adenocarcinoma. Neoplasma. 2011;58:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 145. | Wang ZK, Liu J, Liu C, Wang FY, Chen CY, Zhang XH. Hypermethylation of adenomatous polyposis coli gene promoter is associated with novel Wnt signaling pathway in gastric adenomas. J Gastroenterol Hepatol. 2012;27:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 146. | Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 147. | Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743-7750. [PubMed] |
| 148. | Yu J, Tao Q, Cheng YY, Lee KY, Ng SS, Cheung KF, Tian L, Rha SY, Neumann U, Röcken C. Promoter methylation of the Wnt/beta-catenin signaling antagonist Dkk-3 is associated with poor survival in gastric cancer. Cancer. 2009;115:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 149. | Yoda Y, Takeshima H, Niwa T, Kim JG, Ando T, Kushima R, Sugiyama T, Katai H, Noshiro H, Ushijima T. Integrated analysis of cancer-related pathways affected by genetic and epigenetic alterations in gastric cancer. Gastric Cancer. 2015;18:65-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 150. | Kim JG, Takeshima H, Niwa T, Rehnberg E, Shigematsu Y, Yoda Y, Yamashita S, Kushima R, Maekita T, Ichinose M. Comprehensive DNA methylation and extensive mutation analyses reveal an association between the CpG island methylator phenotype and oncogenic mutations in gastric cancers. Cancer Lett. 2013;330:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 151. | Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 152. | Duan Z, Person RE, Lee HH, Huang S, Donadieu J, Badolato R, Grimes HL, Papayannopoulou T, Horwitz MS. Epigenetic regulation of protein-coding and microRNA genes by the Gfi1-interacting tumor suppressor PRDM5. Mol Cell Biol. 2007;27:6889-6902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 153. | Shu XS, Geng H, Li L, Ying J, Ma C, Wang Y, Poon FF, Wang X, Ying Y, Yeo W. The epigenetic modifier PRDM5 functions as a tumor suppressor through modulating WNT/β-catenin signaling and is frequently silenced in multiple tumors. PLoS One. 2011;6:e27346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 154. | Wei Z, Chen X, Chen J, Wang W, Xu X, Cai Q. RASSF10 is epigenetically silenced and functions as a tumor suppressor in gastric cancer. Biochem Biophys Res Commun. 2013;432:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 155. | Gartel AL, Kandel ES. miRNAs: Little known mediators of oncogenesis. Semin Cancer Biol. 2008;18:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 156. | Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014;15:565-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 157. | Adams BD, Kasinski AL, Slack FJ. Aberrant regulation and function of microRNAs in cancer. Curr Biol. 2014;24:R762-R776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 158. | Ribeiro-dos-Santos Â, Khayat AS, Silva A, Alencar DO, Lobato J, Luz L, Pinheiro DG, Varuzza L, Assumpção M, Assumpção P. Ultra-deep sequencing reveals the microRNA expression pattern of the human stomach. PLoS One. 2010;5:e13205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 159. | Wang JL, Hu Y, Kong X, Wang ZH, Chen HY, Xu J, Fang JY. Candidate microRNA biomarkers in human gastric cancer: a systematic review and validation study. PLoS One. 2013;8:e73683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 160. | Shrestha S, Hsu SD, Huang WY, Huang HY, Chen W, Weng SL, Huang HD. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med. 2014;3:878-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 161. | Wu Q, Yang Z, Wang F, Hu S, Yang L, Shi Y, Fan D. MiR-19b/20a/92a regulates the self-renewal and proliferation of gastric cancer stem cells. J Cell Sci. 2013;126:4220-4229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 162. | Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui J, Liu Y, Gao Z, Li J, Shen L. Combination of hsa-miR-375 and hsa-miR-142-5p as a predictor for recurrence risk in gastric cancer patients following surgical resection. Ann Oncol. 2011;22:2257-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 163. | Zhou H, Wang K, Hu Z, Wen J. TGF-β1 alters microRNA profile in human gastric cancer cells. Chin J Cancer Res. 2013;25:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 164. | Liu D, Hu X, Zhou H, Shi G, Wu J. Identification of Aberrantly Expressed miRNAs in Gastric Cancer. Gastroenterol Res Pract. 2014;2014:473817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 165. | Zheng G, Xiong Y, Xu W, Wang Y, Chen F, Wang Z, Yan Z. A two-microRNA signature as a potential biomarker for early gastric cancer. Oncol Lett. 2014;7:679-684. [PubMed] |
| 166. | Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z, Chen F, Zheng G. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 167. | Ueno K, Hirata H, Hinoda Y, Dahiya R. Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. Int J Cancer. 2013;132:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 168. | Chen W, Tang Z, Sun Y, Zhang Y, Wang X, Shen Z, Liu F, Qin X. miRNA expression profile in primary gastric cancers and paired lymph node metastases indicates that miR-10a plays a role in metastasis from primary gastric cancer to lymph nodes. Exp Ther Med. 2012;3:351-356. [PubMed] |
| 169. | Takei Y, Takigahira M, Mihara K, Tarumi Y, Yanagihara K. The metastasis-associated microRNA miR-516a-3p is a novel therapeutic target for inhibiting peritoneal dissemination of human scirrhous gastric cancer. Cancer Res. 2011;71:1442-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 170. | Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 171. | Tang X, Zheng D, Hu P, Zeng Z, Li M, Tucker L, Monahan R, Resnick MB, Liu M, Ramratnam B. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the β-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014;42:2988-2998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 172. | Tang H, Kong Y, Guo J, Tang Y, Xie X, Yang L, Su Q, Xie X. Diallyl disulfide suppresses proliferation and induces apoptosis in human gastric cancer through Wnt-1 signaling pathway by up-regulation of miR-200b and miR-22. Cancer Lett. 2013;340:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 173. | Liu M, Yang S, Wang Y, Zhu H, Yan S, Zhang W, Quan L, Bai J, Xu N. EB1 acts as an oncogene via activating beta-catenin/TCF pathway to promote cellular growth and inhibit apoptosis. Mol Carcinog. 2009;48:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 174. | Kim K, Lee HC, Park JL, Kim M, Kim SY, Noh SM, Song KS, Kim JC, Kim YS. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics. 2011;6:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 175. | Zhang Z, Liu S, Shi R, Zhao G. miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer Genet. 2011;204:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 176. | Su J, Zhang A, Shi Z, Ma F, Pu P, Wang T, Zhang J, Kang C, Zhang Q. MicroRNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int J Oncol. 2012;40:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 177. | Cong N, Du P, Zhang A, Shen F, Su J, Pu P, Wang T, Zjang J, Kang C, Zhang Q. Downregulated microRNA-200a promotes EMT and tumor growth through the wnt/β-catenin pathway by targeting the E-cadherin repressors ZEB1/ZEB2 in gastric adenocarcinoma. Oncol Rep. 2013;29:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 178. | Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 179. | Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2621] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 180. | Williams SA, Anderson WC, Santaguida MT, Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 2013;93:970-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 181. | Yang L, Lai D. Ovarian cancer stem cells enrichment. Methods Mol Biol. 2013;1049:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 182. | Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 183. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3045] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 184. | Alison MR. Characterization of the differentiation capacity of rat-derived hepatic stem cells. Semin Liver Dis. 2003;23:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 185. | Janssens N, Janicot M, Perera T. The Wnt-dependent signaling pathways as target in oncology drug discovery. Invest New Drugs. 2006;24:263-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 186. | Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295-309. [PubMed] |
| 187. | Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1033] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 188. | Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 893] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 189. | Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23:925-933. [PubMed] |
| 190. | Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 191. | Sun M, Zhou W, Zhang YY, Wang DL, Wu XL. CD44(+) gastric cancer cells with stemness properties are chemoradioresistant and highly invasive. Oncol Lett. 2013;5:1793-1798. [PubMed] |
| 192. | Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 193. | Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, Chen JP, Zhou ZG, Mo XM, Hu JK. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One. 2013;8:e59154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 194. | Zhi QM, Chen XH, Ji J, Zhang JN, Li JF, Cai Q, Liu BY, Gu QL, Zhu ZG, Yu YY. Salinomycin can effectively kill ALDH(high) stem-like cells on gastric cancer. Biomed Pharmacother. 2011;65:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 195. | Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3:117-136. [PubMed] |
| 196. | Hanaki H, Yamamoto H, Sakane H, Matsumoto S, Ohdan H, Sato A, Kikuchi A. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol Cancer Ther. 2012;11:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 197. | Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W, Kikuchi A. Laminin gamma2 mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology. 2009;137:242-252, 252.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 198. | Miyazawa K, Iwaya K, Kuroda M, Harada M, Serizawa H, Koyanagi Y, Sato Y, Mizokami Y, Matsuoka T, Mukai K. Nuclear accumulation of beta-catenin in intestinal-type gastric carcinoma: correlation with early tumor invasion. Virchows Arch. 2000;437:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 199. | Lowy AM, Clements WM, Bishop J, Kong L, Bonney T, Sisco K, Aronow B, Fenoglio-Preiser C, Groden J. beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res. 2006;66:4734-4741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 200. | Huang J, Xiao D, Li G, Ma J, Chen P, Yuan W, Hou F, Ge J, Zhong M, Tang Y. EphA2 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer cells. Oncogene. 2014;33:2737-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 201. | Kim B, Koo H, Yang S, Bang S, Jung Y, Kim Y, Kim J, Park J, Moon RT, Song K. TC1(C8orf4) correlates with Wnt/beta-catenin target genes and aggressive biological behavior in gastric cancer. Clin Cancer Res. 2006;12:3541-3548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 202. | Kim B, Bang S, Lee S, Kim S, Jung Y, Lee C, Choi K, Lee SG, Lee K, Lee Y. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003;63:8248-8255. [PubMed] |
| 203. | Jung Y, Bang S, Choi K, Kim E, Kim Y, Kim J, Park J, Koo H, Moon RT, Song K. TC1 (C8orf4) enhances the Wnt/beta-catenin pathway by relieving antagonistic activity of Chibby. Cancer Res. 2006;66:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 204. | Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 205. | Ganesan K, Ivanova T, Wu Y, Rajasegaran V, Wu J, Lee MH, Yu K, Rha SY, Chung HC, Ylstra B. Inhibition of gastric cancer invasion and metastasis by PLA2G2A, a novel beta-catenin/TCF target gene. Cancer Res. 2008;68:4277-4286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 206. | Xing XF, Li H, Zhong XY, Zhang LH, Wang XH, Liu YQ, Jia SQ, Shi T, Niu ZJ, Peng Y. Phospholipase A2 group IIA expression correlates with prolonged survival in gastric cancer. Histopathology. 2011;59:198-206. [PubMed] |
| 207. | Wang X, Huang CJ, Yu GZ, Wang JJ, Wang R, Li YM, Wu Q. Expression of group IIA phospholipase A2 is an independent predictor of favorable outcome for patients with gastric cancer. Hum Pathol. 2013;44:2020-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 208. | Ozturk N, Singh I, Mehta A, Braun T, Barreto G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev Biol. 2014;2:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 209. | Nam ES, Kim DH, Cho SJ, Chae SW, Kim HY, Kim SM, Han JJ, Shin HS, Park YE. Expression of HMGI(Y) associated with malignant phenotype of human gastric tissue. Histopathology. 2003;42:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 210. | Akaboshi S, Watanabe S, Hino Y, Sekita Y, Xi Y, Araki K, Yamamura K, Oshima M, Ito T, Baba H. HMGA1 is induced by Wnt/beta-catenin pathway and maintains cell proliferation in gastric cancer. Am J Pathol. 2009;175:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 211. | Zha L, Zhang J, Tang W, Zhang N, He M, Guo Y, Wang Z. HMGA2 elicits EMT by activating the Wnt/β-catenin pathway in gastric cancer. Dig Dis Sci. 2013;58:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 212. | Shiota S, Suzuki R, Yamaoka Y. The significance of virulence factors in Helicobacter pylori. J Dig Dis. 2013;14:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 213. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [PubMed] |
| 214. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [PubMed] |
| 215. | Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 216. | Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 411] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 217. | Boquet P, Ricci V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012;20:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 218. | Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 219. | Aituov B, Duisembekova A, Bulenova A, Alibek K. Pathogen-driven gastrointestinal cancers: Time for a change in treatment paradigm? Infect Agent Cancer. 2012;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 220. | Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Azuma T. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 360] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 221. | Sokolova O, Bozko PM, Naumann M. Helicobacter pylori suppresses glycogen synthase kinase 3beta to promote beta-catenin activity. J Biol Chem. 2008;283:29367-29374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 222. | Nakayama M, Hisatsune J, Yamasaki E, Isomoto H, Kurazono H, Hatakeyama M, Azuma T, Yamaoka Y, Yahiro K, Moss J. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J Biol Chem. 2009;284:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 223. | Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 224. | Neal JT, Peterson TS, Kent ML, Guillemin K. H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis Model Mech. 2013;6:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 225. | Udhayakumar G, Jayanthi V, Devaraj N, Devaraj H. Interaction of MUC1 with beta-catenin modulates the Wnt target gene cyclinD1 in H. pylori-induced gastric cancer. Mol Carcinog. 2007;46:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 226. | Yang ZM, Chen WW, Wang YF. Gene expression profiling in gastric mucosa from Helicobacter pylori-infected and uninfected patients undergoing chronic superficial gastritis. PLoS One. 2012;7:e33030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 227. | Yamada M, Ohnishi J, Ohkawara B, Iemura S, Satoh K, Hyodo-Miura J, Kawachi K, Natsume T, Shibuya H. NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF). J Biol Chem. 2006;281:20749-20760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 228. | Gnad T, Feoktistova M, Leverkus M, Lendeckel U, Naumann M. Helicobacter pylori-induced activation of beta-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol Cancer. 2010;9:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 229. | Oshima H, Oguma K, Du YC, Oshima M. Prostaglandin E2, Wnt, and BMP in gastric tumor mouse models. Cancer Sci. 2009;100:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 230. | Takasu S, Tsukamoto T, Cao XY, Toyoda T, Hirata A, Ban H, Yamamoto M, Sakai H, Yanai T, Masegi T. Roles of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 expression and beta-catenin activation in gastric carcinogenesis in N-methyl-N-nitrosourea-treated K19-C2mE transgenic mice. Cancer Sci. 2008;99:2356-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 231. | Nuñez F, Bravo S, Cruzat F, Montecino M, De Ferrari GV. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS One. 2011;6:e18562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 232. | Kirikoshi H, Sekihara H, Katoh M. Up-regulation of WNT10A by tumor necrosis factor alpha and Helicobacter pylori in gastric cancer. Int J Oncol. 2001;19:533-536. [PubMed] |
| 233. | Kim B, Byun SJ, Kim YA, Kim JE, Lee BL, Kim WH, Chang MS. Cell cycle regulators, APC/beta-catenin, NF-kappaB and Epstein-Barr virus in gastric carcinomas. Pathology. 2010;42:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 234. | Fukayama M, Hino R, Uozaki H. Epstein-Barr virus and gastric carcinoma: virus-host interactions leading to carcinoma. Cancer Sci. 2008;99:1726-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 235. | Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 833] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 236. | Rosenbluh J, Wang X, Hahn WC. Genomic insights into WNT/β-catenin signaling. Trends Pharmacol Sci. 2014;35:103-109. [PubMed] |
| 237. | Chen W, Chen M, Barak LS. Development of small molecules targeting the Wnt pathway for the treatment of colon cancer: a high-throughput screening approach. Am J Physiol Gastrointest Liver Physiol. 2010;299:G293-G300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 238. | Ewan K, Pajak B, Stubbs M, Todd H, Barbeau O, Quevedo C, Botfield H, Young R, Ruddle R, Samuel L. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010;70:5963-5973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 239. | Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1384] [Cited by in RCA: 1523] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 240. | Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 241. | Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 1905] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 242. | Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108:13253-13257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 243. | Zhang YS, Xie JZ, Zhong JL, Li YY, Wang RQ, Qin YZ, Lou HX, Gao ZH, Qu XJ. Acetyl-11-keto-β-boswellic acid (AKBA) inhibits human gastric carcinoma growth through modulation of the Wnt/β-catenin signaling pathway. Biochim Biophys Acta. 2013;1830:3604-3615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 244. | Liu HP, Gao ZH, Cui SX, Wang Y, Li BY, Lou HX, Qu XJ. Chemoprevention of intestinal adenomatous polyposis by acetyl-11-keto-beta-boswellic acid in APC(Min/+) mice. Int J Cancer. 2013;132:2667-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 245. | Shen W, Zou X, Chen M, Liu P, Shen Y, Huang S, Guo H, Zhang L. Effects of diphyllin as a novel V-ATPase inhibitor on gastric adenocarcinoma. Eur J Pharmacol. 2011;667:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 246. | Park CH, Hahm ER, Lee JH, Jung KC, Yang CH. Inhibition of beta-catenin-mediated transactivation by flavanone in AGS gastric cancer cells. Biochem Biophys Res Commun. 2005;331:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 247. | Li Y, Sun WG, Liu HK, Qi GY, Wang Q, Sun XR, Chen BQ, Liu JR. γ-Tocotrienol inhibits angiogenesis of human umbilical vein endothelial cell induced by cancer cell. J Nutr Biochem. 2011;22:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 248. | Kim YM, Kim IH, Nam TJ. Capsosiphon fulvescens glycoprotein inhibits AGS gastric cancer cell proliferation by downregulating Wnt-1 signaling. Int J Oncol. 2013;43:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 249. | Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 250. | Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 251. | Yao J, Qian C, Shu T, Zhang X, Zhao Z, Liang Y. Combination treatment of PD98059 and DAPT in gastric cancer through induction of apoptosis and downregulation of WNT/β-catenin. Cancer Biol Ther. 2013;14:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 252. | He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 253. | You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170-6174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 254. | Wang B, Liu J, Ma LN, Xiao HL, Wang YZ, Li Y, Wang Z, Fan L, Lan C, Yang M. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44+ gastric cancer cells via attenuating Wnt signaling. J Gastroenterol. 2013;48:798-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 255. | Menezes ME, Devine DJ, Shevde LA, Samant RS. Dickkopf1: a tumor suppressor or metastasis promoter? Int J Cancer. 2012;130:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 256. | Zhang B, Yang Y, Shi X, Liao W, Chen M, Cheng AS, Yan H, Fang C, Zhang S, Xu G. Proton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial-mesenchymal transition. Cancer Lett. 2015;356:704-712. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |