Published online Aug 20, 2014. doi: 10.5493/wjem.v4.i3.27
Revised: May 29, 2014
Accepted: July 17, 2014
Published online: August 20, 2014
Processing time: 129 Days and 0 Hours
Multiple sclerosis (MS) is a complex disease with many different immune cells involved in its pathogenesis, and in particular T cells as the most recognized cell type. Recently, the innate immune system has also been researched for its effect on the disease. Hence, cells of the immune system play vital roles in either ameliorating or exacerbating the disease. The genetic and environmental factors, as well as the etiology and pathogenesis are of utmost importance for the development of MS. An insight into the roles play by T cells, B cells, natural killer cells, and dendritic cells in MS and the animal model experimental autoimmune encephalomyelitis, will be presented. Understanding the mechanisms of action for current therapeutic modalities should help developing new therapeutic tools to treat this disease and other autoimmune diseases.
Core tip: The role played by various immune cells in either ameliorating or exacerbating multiple sclerosis is discussed.
- Citation: Høglund RA, Maghazachi AA. Multiple sclerosis and the role of immune cells. World J Exp Med 2014; 4(3): 27-37
- URL: https://www.wjgnet.com/2220-315X/full/v4/i3/27.htm
- DOI: https://dx.doi.org/10.5493/wjem.v4.i3.27
Recent developments in multiple sclerosis (MS) research have been staggering, including numerous new therapies either approved or at the brink of approval, new oral therapies and leaps made in genetics. This article will focus on the role that immune cells play in MS, and their involvement in mediating the effects of drugs used to treat relapsing-remitting (RR) MS patients.
The basic pathology of MS was recognized almost 150 years ago by Charcot who described a disease caused by sclerotic plaques in the central nervous system (CNS) of those affected[1]. There were other reports describing the pathology before this time, but failed to recognize it as a distinct disease[2]. It is reasonable to believe that MS has been prevalent also before these descriptions, as the disease often arises early in life[3]. Today, we know that MS is a chronic inflammatory, autoimmune, demyelinating and degenerative disease of the CNS[4]. Substantial discoveries have been made regarding its therapeutics, pathogenesis and genetics. Yet, there is no cure and no recognized definite cause, be it genetic, microbial or environmental. Although no definite cause of MS has yet been identified, substantial amounts of research point to a dual multifactorial influence of both genetics and environmental elements contributing to the development of the disease[5,6].
The genetic component was first described after family studies were performed, with a family recurrence rate of about 20%-33%, and about 10-12 fold risk increase in first degree and three folds in second degree relatives[7,8]. For siblings or children of patients with MS, the overall risk was estimated to be 3%-5%[9], but twin studies show a higher risk (25%) if the sibling is a monozygotic twin[10,11].
The first risk allele to be identified was the human leukocyte antigen (HLA) class II haplotype HLA-DRB 1501 in the early 1970’s[12], which is also the allele with the strongest association with the disease[13]. The road to discovering additional alleles was complicated and hampered by insufficient methods[4,14]. In recent years large genome wide association studies (GWAS) using single nucleotide polymorphisms and comparing diseased to healthy individuals have made it possible to identify numerous alleles associated with MS with sufficient power and significance, most of these with low to modest MS risk association[5,15]. Researchers with the help from a huge GWAS consortium[13] identified over 50 susceptible loci, many of which are associated with the immune system, including genes encoding receptors for interleukin (IL)-2 and IL-7[15]. Other genes important for cytokine pathways include CXCR5, IL-12A, IL-12β, IL-12Rβ1 among others associated with the cytokine tumor necrosis factor α, as well as genes associated with co-stimulatory molecules such as CD80, CD86, CD37 were also described. GWAS studies have even identified risk association with genes that are important for current and new MS therapies including IL-2Rα (daclizumab) and VCAM1 (natalizumab), as well as genes related to environmental risk factor vitamin D3[13]. Such knowledge while adding new information supports much of what is known about MS pathogenesis. Still, further research into these risk alleles is needed to improve our understanding of their associations with the disease.
While family studies support a genetic association, they also show that genetics alone are not enough to develop MS, as shown by homozygous twins not both acquiring disease, even though one of them is diagnosed with MS. Other information supporting environmental effects include the geographic increase of MS with increasing latitude[16], and how individuals migrating from low-risk to high-risk areas acquire higher susceptibility if the migration occurs during childhood. Hence, the incidence of developing MS correlates with the risk in areas of childhood residence[4]. Additionally, studies have shown risk association during months of birth as spring births have higher risk of MS than autumn births[17].
Several environmental factors have been investigated. One hypothesis is that vitamin D3 deficiency increases the risk of MS, as increased latitude is also correlated with lower blood vitamin D3 levels. For instance, ecological studies showed the amount of exposure to sunlight was inversely correlated with the risk of MS, both by regional distribution and association with altitude, as well as by individual exposure to sunlight[18]. Sunlight is the main source of human vitamin D3 through conversion of 7-dehydrocholesterol to previtamin D3 in the skin, and through further metabolic steps to active hormone 1,25-Dihydroxyvitamin D3[19]. It was also shown how vitamin D3 intake may reduce the risk of MS because of latitude dependent deficiency, for instance in communities which consume higher amounts of vitamin D3 rich fish[20]. These studies also point out the difficulties of such concepts, as confounding factors may be quite prevalent. There is also association with the experimental autoimmune encephalomyelitis (EAE) model, an animal model for MS, as dosing of 1,25(OH)2D3 prevented the disease[21,22]. These effects may be induced by vitamin D3 on the adaptive immune system not yet fully understood. Definite effects of supplementing patients with vitamin D3 have not yet been shown, but some studies indicate that serum concentrations of vitamin D3 may affect disease severity[19]. However, this field is quickly developing, and is being investigated for possible future prospects, for instance in preventing MS[23].
Another risk factor with strong association to MS is Epstein Barr virus (EBV) infection[24,25]. A major finding was that individuals who were seronegative for EBV had very low, almost no risk of acquiring MS when compared to seropositive individuals[24]. However, those patients who at some point infected with EBV are not necessarily at risk of developing MS[6]. Further, there was an increased risk of MS if a history of infectious mononucleosis and a temporal increase of EBV antibodies serum titers were present[26], and that MS patients were more often infected with EBV at later ages when compared to controls[27]. It has been hypothesized that EBV may mimic myelin basic protein (MBP) pathogenic antigens by presentation on HLA-DRB1*1501, hence, providing links to both environmental and genetic risk factors[28]. Although the association with MS is well investigated, the role of EBV in its pathology remains uncertain. However, EBV infection and mononucleosis as a priming or initiating factors for developing MS are seemingly likely[24]. Finally, many other factors have shown association with increased MS risk but are in need of further research to be conclusive. These include cigarette smoking, a diet rich in saturated but low in polyunsaturated fats, sex hormones, and socioeconomic status, among others[18,29]. Viruses other than EBV have also been implicated in the etiology of MS[30].
Patients with MS show a wide variety of symptoms caused by lesions in the CNS affecting motor, sensory, visual or even autonomic functions. Lesions appear mainly in the white matter, but also appear in the grey matter. Hence, symptoms show great heterogeneity in both inter- and intra-individually ranging from slight tingling in the fingers to extreme fatigue or complete monocular loss of sight, depending on the lesion(s) localization[4]. Commonly, the patients present with a clinically isolated syndrome (CIS), followed by serial sub-acute relapses with varying time intervals. The number of annual relapses and time intervals between them varies among patients, but more than 1.5 relapses/year is a rare occasion[4]. Not all patients have subsequent disease activity after a single CIS, but risk of another episode is increased if white matter lesions are detected by MRI[31]. Between relapses the patients revert spontaneously to normal or near normal neurological function. This clinical subtype of MS is referred to as RR MS, and most patients are present with this form of MS[30]. The clinical course may later convert to a more progressive stage in about 65% of the patients, with fewer or no relapse activity and increasing irreversible disability. Patients have a mean age of about 40 years when they enter this stage, referred to as secondary progressive (SP) MS[32,33]. A minority of patients (about 10%-15%) may present with progressive disability and no relapsing activity, this is called primary progressive (PP) MS[34]. This subtype usually has a later onset than RRMS, at a mean age of around 40 years[33].
Patients with MS experience decades of disease and eventual progression. Due to many complications which occur alongside increased disability, the average life expectancy is reduced by about 5-10 years, and has a median time of 30 years between onset and death[35]. It remains to be seen whether current or future therapeutic methods may extend the time until irreversible disability occurs.
An important finding in MS lesion is the disruption of the blood brain barrier (BBB), and hence a basic understanding of this barrier is essential to understand the pathogenesis of the disease[36]. The BBB is a functional and anatomical barrier separating the blood from neurons in the CNS. It consists of both the vascular wall, CNS astrocytes covering these with glia limitans, and the perivascular space in between[37]. The BBB has several important functions vital for the brain to function properly, including maintenance of proper ionic concentrations through dynamic regulation. The BBB may be viewed as a concept rather than an actual barrier. Even though the word barrier has a certain static ring to it, the BBB is rather dynamic allowing for instance immunological surveillance[38,39]. Lesions occur when leukocytes migrate into the brain and inflammation ensues. This is a two-step process, involving an initial migration across post-capillary venules into perivascular space, and further migration through the glia limitans into the brain parenchyma[37]. The perivascular space allows normal immunosurveillance by monocytes, as this space also serves as the brains’ lymphatic drainage[37]. There are at least two additional routes for leukocytes to enter the CNS, as shown by accumulation of immune cells at these sites in animal models, which include the blood-cerebrospinal fluid (CSF) barrier in the choroid plexus, and through meningeal arteries[39].
The role of T cells has always been considered central in MS pathogenesis, much due to the experimental animal model EAE, but also due to the strong genetic association with HLA class II genes[5]. EAE is a widely used animal model for MS, and is induced by immunizing mice or other rodents with myelin peptides, or by adoptively transferring myelin-reactive T-cells[6]. Hence, they cause a T cell mediated acute autoimmune reaction against myelin in the rodents CNS, with signs and symptoms that are similar to those seen in MS. The cellular pathogenesis of MS is however, more complicated than this[40]. For a long time auto-reactive CD4+ T cells secreting interferon-gamma (IFN-γ), were thought to be the main mediators of the inflammation causing MS lesions[4]. Further research suggests numerous other cell types and cell subsets are also involved, with key roles assigned to T helper 17 (Th17) cells[41]. These T cells secrete the pro-inflammatory cytokines IL-17, IL-6 and are regulated by IL-23[42,43]. It is commonly believed that disease occurs when these inflammatory cells and/or other cell types become deregulated and a transition from physiological surveillance to a pathological immune response occurs[4,30].
Since EAE is considered a model of acute inflammation, it has been used to explore what cells are important for this process. It was shown in mice with EAE that CD4+ Th17 cells are necessary to develop EAE[44]. Studies of lesions from MS patients confirmed an overwhelming presence of CD4+ cells secreting IL-17 in active lesions. However, in MS lesions both CD4+ and CD8+ cells express IL-17[45]. Chemokine receptor CCR6 expressed on Th17 cells facilitates transport through the choroid plexus into the CSF and perivascular space by interacting with CCL20/MIP-3α expressed on endothelium[46]. Th17 cells may also produce GM-CSF promoted by resident antigen presenting cells (APCs) secreting IL-23, which then initiates a positive feedback loop as the same APCs are stimulated by GM-CSF[30,47,48]. Th17 cells may further increase permeability of the BBB by disrupting the endothelial tight junctions due to the secretion of IL-17 and IL-22, and through interactions with endothelium allowing further attraction of CD4+ subsets as well as other immune cells. Consequently, initiating pathological cascade of inflammation, perivascular infiltrates and damage to neurons and glia cells[30,45]. One identified damaging molecule is Granzyme B secreted by the same Th17 cells[49]. However, to gain access into the parenchyma, the cells must traverse the glia limitans. This is thought to be mediated by perivascular APCs and macrophages secreting matrix metalloproteinases (MMP) 2 and MMP-9 which are gelatinases able to cleave dystroglycan, a transmembrane receptor anchoring astrocytes end feet to the parenchymal basement membrane. When mice were knocked down for MMP-2 and MMP-9, they became resistant to EAE as T-cells became trapped in perivascular space[50]. In summary, this cascade may be viewed as a stepwise model, with an initial attraction and migration of Th17 cells into the CSF and perivascular spaces, and later increased permeability of the BBB allowing additional inflammatory cells access, and later a complete disruption of the BBB with damage to the CNS occurs, resulting in active lesions and potential clinical exacerbation of the disease[25,30].
Although our understanding of how inflammation may be initiated has increased with the advent of the Th17 hypothesis, much remains to be discovered of how T cells and other cell types are recruited into the CNS during inflammation. It is known that chemokines are important for recruiting these cells across the BBB. Chemokines are classified into subfamilies, consisting of CXC, CC, C and CX3C. While some are constitutively expressed, others are up-regulated during inflammation[51]. In MS lesions, chemokines expressed on post-capillary venule endothelial walls and bind chemokine receptors expressed on T cells, allowing extravasation. Chemokine concentration gradients in tissues allow the cells to be further guided towards the sites of inflammation[52].
We explored the role of chemokine receptors expression, which include CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR9, CCR10, CXCR1, CXCR3, CXCR4, CXCR5 and CXCR6 on four T cell clones isolated from the blood or CSF of MS patient who was treated with the drug glatiramer acetate (GA). We observed that only four chemokine receptors warranted further study. These were CCR4, CCR5, CCR6 and CXCR3[53]. The fact that all four clones from both peripheral blood and CSF expressed similar patterns of chemokine receptors could be due to migration of the same clones from blood into the CSF, plausibly using CCR5 or CCR6 for entry (Table 1). When these cells were activated, all chemokine receptors expression with the exception of CCR4 was markedly reduced. The migration pattern corresponded well with the expression of receptors observed, with reduced migration observed when receptors were reduced or no longer expressed. Similarly after activation, migration towards chemokines was less robust. These observations support the hypothesis of diminished effects of GA due to inflammation and premature activation of GA-reactive cells. As migration into the CNS is a necessary step of the bystander suppression hypothesis[24], knowledge of how these cells migrate into the CNS is important in order to possibly enhance the effectiveness of this drug.
| Receptor | Main role | Ligands | CD4+ subsets | PBL-74 | PBL-78 | CSF-25 | CSF-26 | ||||||
| Th1 | Th2 | Naive | Unact | Act | Unact | Act | Unact | Act | Unact | Act | |||
| CXC family | |||||||||||||
| CXCR1 | I | CXCL6, CXCL7, CXCL8 | |||||||||||
| CXCR2 | I | CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8 | |||||||||||
| CXCR3 | I | CXCL9, CXCL10, CXCL11 | H | H | M | H | M | H | M | H | L | ||
| CXCR4 | C | CXCL12 | H | ||||||||||
| CXCR5 | C | CXCL13 | M | ||||||||||
| CXCR6 | I | CXCL16 | |||||||||||
| CXCR7 | D | CXCL11, CXCL12 | |||||||||||
| CC family | |||||||||||||
| CCR1 | I | CCL3, CCL5, CCL7, CCL8, CCL15, CCL16, CCL23 | M | ||||||||||
| CCR2 | I | CCL2, CCL7, CCL8, CCL13, CCL16 | H | H | |||||||||
| CCR3 | I | CCL5 ,CCL7, CCL11, CCL13, CCL15, CCL24, CCL26, CCL28 | H | ||||||||||
| CCR4 | D | CCL17, CCL22 | H | H | H | H | M | H | H | L | |||
| CCR5 | I | CCL3, CCL4, CCL5, CCL8, CCL11, CCL14, CCL16 | H | H | M | L | H | M | |||||
| CCR6 | D | CCL20 | M | M | L | ||||||||
| CCR7 | C | CCL19, CCL21 | H | ||||||||||
| CCR8 | I | CCL1, CCL16 | H | ||||||||||
| CCR9 | D | CCL25, | |||||||||||
| CCR10 | I | CCL27, CCL28 | L | L | |||||||||
| Other | |||||||||||||
| XCR1 | I | XCL1, XCL2 | |||||||||||
| CX3CR1 | D | CX3CL1 | |||||||||||
These results could in part explain why aggressive immunosuppression followed by maintenance therapy is very effective. Such combination therapy is relatively new to MS treatment, but has been used in other autoimmune diseases. Inspired by this, researchers applied combination therapies which resulted in a better outcome for RRMS patients. Several combinations have been tested, some show more promise than others. It was for instance described how patients treated with a short induction of mitoxantrone followed by longer term GA therapy showed a reduction in new relapses compared to GA alone, as well promising results regarding gadolinium enhanced lesion load[54-57]. This suggests possible synergistic effects among these drugs. Mitoxantrone is a powerful immunosuppressive drug that is approved for treatment of RRMS and secondary progressive MS (SPMS) in the United States. By suppressing inflammation induced by autoreactive T cells such as Th17 and/or Th1, this drug may pave the way for GA-reactive cell migration into the CNS upon reconstitution of the immune system. This way, the cells may mediate their bystander suppression without being impeded by an inflammatory environment. However, while initial studies of this showed important clinical efficacy, further studies are warranted in order to determine whether this treatment regimen is better suited than newer therapies such as alemtuzumab and other future immunomodulators.
The presence of inflammatory T cells in perivascular space and parenchyma triggers recruitment of more T cells, as well as B cells, dendritic cells, microglia and natural killer (NK) cells. The cytokines secreted may by themselves cause damage to the surrounding tissues[58]. Complement depositions, opsonization and local activation of microglia and macrophages causing demyelination[59], and neuronal cell death are few examples of the effects of cytokines[60]. Axonal damage is also present[61,62], and the degree of this was associated with abundance of microglia and CD8+ T cells[63]. Clonally expanded CD8+ T cells are present usually at the lesion edge, as well as in the perivascular infiltrates, indicating a specific antigen response and also possibly responsible for damages to neurons through cell dependent cytotoxicity[64-67]. It was also demonstrated that clonal γδ T cells are present in MS lesions[68]. When the lesion acute phase is over, removal of cellular debris starts, and simultaneously remyelination occurs within the MS plaque. A minority of the patients (about 20%) show more abundant remyelination[69].
In addition, a study showed that MS patients had a significant loss of effector function in the regulatory CD4+CD25+ subset of T cells (Treg), when compared to healthy individuals[70]. Another study showed reduced frequency of this subset in general as well as reduced expression of transcription factor FOXP3[71]. Although circulating numbers may be the same in patients and healthy individuals, the suppressive potential of Treg cells in MS patients was reduced[72]. Interestingly, these T cells were also able to produce IL-17 when stimulated with APCs in the presence of IL-2 and IL-15[73], but retained their suppressive abilities dependent on the surrounding cytokine environment[74]. Although this regulatory subset may inhibit auto reactive T cells, it is not certain where such inhibition may occur[41].
Finally, it may be that MS is not initially caused by the adaptive immune system at all, but rather as a response to an intrinsic CNS neurodegeneration, indicating that inflammation may follow initial axonal degeneration caused by a currently unknown factor. This is supported by the fact that progression of disease is mainly caused by the amount of lost neurons[30]. In addition, there seems to be a correlation between age at onset than initial clinical course on the disease progression[33].
The most obvious argument for B cells being involved in MS pathogenesis is the presence of immunoglobulins such as immunoglobulin G1 in the CSF, as detected by isoelectric focusing or gel electrophoresis in as many as 95% of diagnosed patients. Although myelin reactive antibodies have been detected, their relevance is not certain[25], much like the role of antigens. In MS lesions, there have been findings of both immunoglobulin and complement which may suggest a pathogenic role[75,76]. Additionally, B lymphoid follicles, T cells and APCs were identified in the meninges of patients at later stages of MS (SPMS but not PPMS)[76,77]. B cells may contribute to the pathology through antigen presentation, cell interactions or production of immunoglobulins from plasma cells, although B cell activity may represent a response to the autoimmune reaction, rather than to a primary inducer[25].
In EAE, the antigens responsible for the disease have been described. By immunizing the animal with myelin proteins such as MBP, proteolipid protein or myelin oligodendrocyte glycoprotein (MOG), disease can be induced[78]. In humans, such common antigens remain unknown in spite of several attempts to identify them[25,30]. Several candidate antigens are being or have been investigated, but no single candidate has been targeted as the one responsible. Myelin or peptides derived from myelin were thought to be good candidates due to the similarity between EAE and MS, but the responses to these antigens have proven to be unspecific and may suggest that several antigens are involved and/or an extensive epitope spreading occurring after initiation of the disease[30,41,79]. One recently suggested antigen is αβ-crystallin, which contrary to previous candidates is not present in human myelin, but rather is detected in early active MS lesions, and patients have antibodies against it in their CSF[6]. When the gene encoding αβ-crystallin was knocked down in mice, a more intense inflammatory EAE with higher cytokine load occurred[80]. This suggests a protective role that may be disrupted by a pathogenic immune response. Another candidate not directly associated with myelin is neurofascin, expressed on neuronal axons. As antibodies have been detected in patients with MS, this may contribute to axonal damage[81].
NK cells are large granular lymphocytes that possess the ability to spontaneously lyse target cells without a prior sensitization[82]. NK cells also have immunoregulatory features, including secretion of cytokines, chemokines and cell to cell contact[83,84]. Functionally, these cells are important in immune responses to viral infections as well as controlling tumor growths[85,86]. The activities of these cells are regulated by activating and inhibitory receptors, which by intracellular integration of challenges and inhibitions determine the cell course of action[87]. NK cells recognize and are activated by cells that are in distress by detecting stress induced ligands on target cells through natural cytotoxicity receptors, such as NKp30, NKp44, or NKp46, and the C-type lectin receptor NKG2D, among others[87,88]. In healthy cells however, activating factors are held in equilibrium by inhibiting signals. NK cells express numerous receptors that inhibit activation, including members of the killer-cell immunoglobulin-like receptor (KIR) family that interact with HLA-I molecules and CD94-NKG2A that interact with HLA-E. In the absence of these “self” ligands, NK cells are activated and consequently, kill target cells[89].
In human blood, NK cells constitute about 2% to 18% of the total circulating lymphocytes[90,91], and can be classified into subsets based on expression of surface receptors. CD16+CD56- constitute about 85%-90% of circulating NK cells, are highly cytotoxic but produce little cytokines, while CD16-CD56+ cells are less cytotoxic but effective cytokine producers[92]. NK cells may also be classified based on their cytokine expression. Similar to Th1/Th2 subsets, these cells were divided into NK1 expressing IFN-γ, or NK2 expressing IL-5 and IL-13[93]. Further research divided these NK cells into even smaller subsets, recognized as NK22 secreting IL-22 and found in lymphoid tissues of the gastrointestinal tract[94], or NK17/NK1 cells secreting IL-17 and IFN-γ when stimulated with IL-2[95].
The role of NK cells in autoimmune diseases is being investigated, and is highly debated as both positive and negative associations have been observed[96-99]. In the EAE model, depletion of NK cells resulted in a severe relapsing EAE, and more pronounced CNS pathology[100-103]. This suggests protective effects for NK cells, especially since the depletion was associated with increased CD4+ T cell activity and hence, may be associated with direct killing of these cells[88,104]. Also in EAE, mice deficient in the chemokine receptor CX3CR1 had more severe EAE. This receptor is necessary for the recruitment of NK cells into the CNS, providing evidence showing that NK cell infiltrating into the CNS represents an important event in ameliorating and controlling the disease[105]. In the same mice there were increased responses from Th17 cells locally in CNS, suggesting a plausible role for NK cells in curbing these cells[106]. Conflicting with these results, it was found that depletion of NK cells in MOG-induced EAE actually ameliorated the disease[107]. Additionally, by stimulating NK cells to produce IFN-γ, these cells may also cause inflammation in part by stimulating Th1 cell response[108,109]. These findings represent detrimental effects of NK cells eliciting inflammatory lesions and exacerbating the inflammatory response. Much of the confusion regarding conflicting findings may be attributed to failure to recognize different effects by different subsets, which should encourage further investigation into this field.
The functional activity of NK cells is variable and is generally lower in MS patients than in healthy individuals[110]. Periods of reduced NK cell numbers in the blood was associated with a higher relapse tendency after. The same study also found a correlation between high mean NK cell activity and total lesion load determined by MRI[111]. As reduced numbers of NK cells were thought to be mediated by migration into tissues including the CNS, this could indicate a pathological role for NK cells. However, it could also be viewed as a risk factor for new attacks due to reduced activity of NK cells. In a more recent study, researchers found a reduced number of CD8lowCD56+CD3-CD4- cells in untreated patients with CIS, when compared to healthy controls[112].
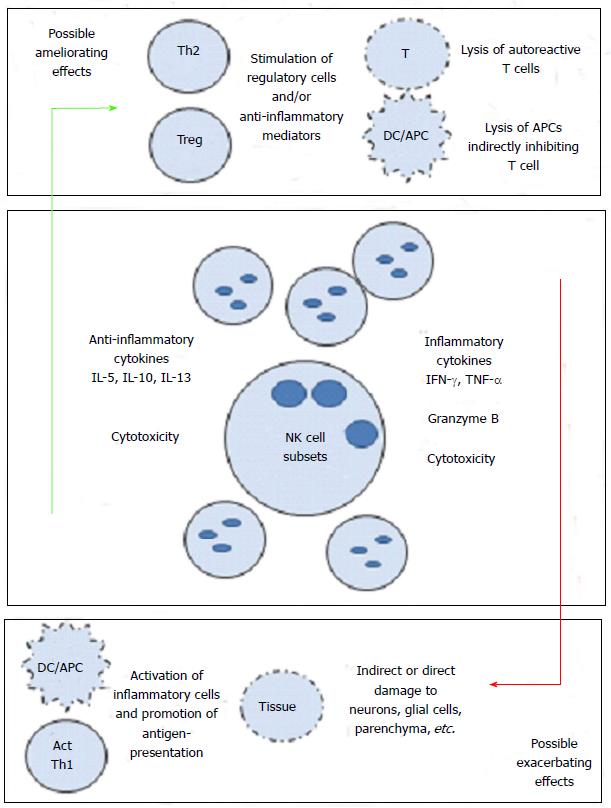
Along these associations, there have been speculations as to how exactly NK cells mediate their effects, be it positive or negative. One suggested pathway was interactions between NK cells and dendritic cells[97,101]. The cognate and non-cognate interactions among NK cells and DCs have been previously described. Activated NK cells are also able to kill immature (i) DCs, but mature (m) DCs are spared. How and where these cells interact are not clear, but it was hypothesized that such interactions may occur in inflamed tissues. Our work on the effects of GA on NK cells in MS patients and healthy individuals shows that GA influences this cross-talk between DCs and NK cells. Consequently, we hypothesized that this may impede antigen presentation to auto reactive T cells (Th17 or Th1), and may further reduce inflammation. We described that NK cells stimulated in vitro with GA became more cytotoxic towards autologous and allogeneic iDCs and mDCs[113]. Furthermore, we described how NK cells from EAE mice treated with GA were more cytotoxic than NK cells isolated from EAE mice treated with vehicle alone, which was associated with ameliorating the disease[114]. While these findings support a mechanism of action for GA on NK cells, it had to be further addressed in humans with in vivo exposure to the drug. Consequently, we examined the activities of NK cells and DCs in MS patients receiving the drug GA for one year. In this study, we compared the cytotoxicity levels before treatment with those observed after treatment. We demonstrated that NK cells isolated from GA-dosed MS patients had significantly increased cytotoxicity against K562 tumor cells. Trends of significantly increased cytotoxicity were also observed against both iDC and mDC in the same patients[115]. In summary, it seems that GA increases the cytotoxic activity of NK cells when compared to pretreatment levels. The increased cytotoxicity correlated well with the elevated expression of activating NK cells cytotoxicity receptors. Figure 1 represents our current knowledge regarding the role that NK cells might play in MS.
The evidence for a complex immunopathology has become ever clearer with the reveal of numerous genes associated with the immune system carrying risk of developing MS, novel cell subsets being discovered and shown to possibly be related to the pathogenesis, and knowledge of how disease modifying therapies (DMT) target several immune cells rather than single subsets. All of these call for continuous bedside-to-bench research into how the newer DMTs enhance, inhibit or modulate the immune system of patients resulting in a better clinical course than without such treatment. The introduction of the new oral therapeutics teriflunomide (Aubagio®), fingolimod (Gilenya®) and dimethyl fumarate (Tecfidera®), all with incomplete understandings of their mechanisms of action on the immune system, will be particularly interesting to follow in this regard.
So far, research into how immune cells act in MS patients, EAE models and in vitro have primarily focused on single subsets or categorically on either adaptive- or innate immune cells. Recent knowledge of how NK cells may belong in a grey area between these traditional groupings challenges this concept[116]. For instance, while our own research focused on the interactions between NK and dendritic cells, similar interactions between NK and B cells would be of interest, as B cells also have antigen presenting abilities. NK cells also have the ability to modify or lyse T cells, and hence investigations into how these cells interact in MS patients or in the EAE model could improve our understanding of the regulation or dysregulation the immune systems of MS patients.
P- Reviewer: Sotelo J, Sivak S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Charcot J. Histologie de la sclerose en plaques. Gazette des hopitaux. 1968;41:554-555. |
| 2. | Compston A. The 150th anniversary of the first depiction of the lesions of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1988;51:1249-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Holmøy T, Hestvik AL, Vartdal F. Management of progressive multifocal leukoencephalopathy associated with natalizumab--possibilities and responsibility. Eur J Neurol. 2006;13:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3540] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 5. | Gourraud PA, Harbo HF, Hauser SL, Baranzini SE. The genetics of multiple sclerosis: an up-to-date review. Immunol Rev. 2012;248:87-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Holmøy T, Hestvik AL. Multiple sclerosis: immunopathogenesis and controversies in defining the cause. Curr Opin Infect Dis. 2008;21:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Robertson NP, Clayton D, Fraser M, Deans J, Compston DA. Clinical concordance in sibling pairs with multiple sclerosis. Neurology. 1996;47:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Carton H, Vlietinck R, Debruyne J, De Keyser J, D’Hooghe MB, Loos R, Medaer R, Truyen L, Yee IM, Sadovnick AD. Risks of multiple sclerosis in relatives of patients in Flanders, Belgium. J Neurol Neurosurg Psychiatry. 1997;62:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Sadovnick AD, Yee IM, Ebers GC, Risch NJ. Effect of age at onset and parental disease status on sibling risks for MS. Neurology. 1998;50:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA. 2003;100:12877-12882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 327] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Mumford CJ, Wood NW, Kellar-Wood H, Thorpe JW, Miller DH, Compston DA. The British Isles survey of multiple sclerosis in twins. Neurology. 1994;44:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 202] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Svejgaard A. The immunogenetics of multiple sclerosis. Immunogenetics. 2008;60:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1953] [Cited by in RCA: 2108] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 14. | Sawcer S. Bayes factors in complex genetics. Eur J Hum Genet. 2010;18:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1249] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 16. | Simpson S, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Grytten N, Torkildsen Ø, Aarseth JH, Benjaminsen E, Celius EG, Dahl OP, Holmøy T, Løken-Amsrud K, Midgard R, Myhr KM. Month of birth as a latitude-dependent risk factor for multiple sclerosis in Norway. Mult Scler. 2013;19:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 19. | Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 20. | Brustad M, Sandanger T, Aksnes L, Lund E. Vitamin D status in a rural population of northern Norway with high fish liver consumption. Public Health Nutr. 2004;7:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 351] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861-7864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 519] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 23. | Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1255] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 24. | Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288-299. [PubMed] |
| 25. | Hestvik AL. The double-edged sword of autoimmunity: lessons from multiple sclerosis. Toxins (Basel). 2010;2:856-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, Olek MJ, Hankinson SE, Hunter DJ. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA. 2001;286:3083-3088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 27. | Martyn CN, Cruddas M, Compston DA. Symptomatic Epstein-Barr virus infection and multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 396] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 29. | Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Dolei A, Garson JA, Arru G, Clerici M, Germi R, Marche PN, Perron H. Multiple sclerosis-associated retrovirus and related human endogenous retrovirus-W in patients with multiple sclerosis. J Neuroimmunol. 2014;266:87-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, Thompson AJ, Miller DH. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131:808-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 607] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 32. | Weinshenker BG. The natural history of multiple sclerosis: update 1998. Semin Neurol. 1998;18:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 347] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 34. | Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 35. | Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain. 2004;127:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Engelhardt B. The blood-central nervous system barriers actively control immune cell entry into the central nervous system. Curr Pharm Des. 2008;14:1555-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 38. | Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389-8394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 432] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 39. | Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 756] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 40. | Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 41. | McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 750] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 42. | Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2961] [Cited by in RCA: 3168] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 43. | Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1230] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 45. | Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 966] [Cited by in RCA: 916] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 46. | Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 932] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 47. | El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568-575. [PubMed] |
| 48. | Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 967] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 49. | Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1391] [Cited by in RCA: 1320] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 50. | Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 438] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 51. | Bruserud Ø. The chemokine system in experimental and clinical hematology. Preface. Curr Top Microbiol Immunol. 2010;341:v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Cheng WJ, Chen GJ. Chemokines and Chemokine Receptors in Multiple Sclerosis. Mediat inflamm. 2014;2014:659206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Høglund RA, Hestvik AL, Holmøy T, Maghazachi AA. Expression and functional activity of chemokine receptors in glatiramer acetate-specific T cells isolated from multiple sclerosis patient receiving the drug glatiramer acetate. Hum Immunol. 2011;72:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 54. | Vollmer T, Panitch H, Bar-Or A, Dunn J, Freedman MS, Gazda SK, Campagnolo D, Deutsch F, Arnold DL. Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult Scler. 2008;14:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Ramtahal J, Jacob A, Das K, Boggild M. Sequential maintenance treatment with glatiramer acetate after mitoxantrone is safe and can limit exposure to immunosuppression in very active, relapsing remitting multiple sclerosis. J Neurol. 2006;253:1160-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Boggild M. Immunosuppression followed by immunomodulation. J Neurol Sci. 2009;277 Suppl 1:S50-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Arnold DL, Campagnolo D, Panitch H, Bar-Or A, Dunn J, Freedman MS, Gazda SK, Vollmer T. Glatiramer acetate after mitoxantrone induction improves MRI markers of lesion volume and permanent tissue injury in MS. J Neurol. 2008;255:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Zajicek JP, Wing M, Scolding NJ, Compston DA. Interactions between oligodendrocytes and microglia. A major role for complement and tumour necrosis factor in oligodendrocyte adherence and killing. Brain. 1992;115:1611-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Prineas JW, Graham JS. Multiple sclerosis: capping of surface immunoglobulin G on macrophages engaged in myelin breakdown. Ann Neurol. 1981;10:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 188] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Breij EC, Brink BP, Veerhuis R, van den Berg C, Vloet R, Yan R, Dijkstra CD, van der Valk P, Bö L. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 242] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 61. | Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2859] [Cited by in RCA: 2743] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 62. | Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1033] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 63. | Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 519] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 64. | Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 315] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schröder R, Deckert M, Schmidt S. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 670] [Cited by in RCA: 672] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 66. | Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA. 2004;101:2428-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 67. | Junker A, Ivanidze J, Malotka J, Eiglmeier I, Lassmann H, Wekerle H, Meinl E, Hohlfeld R, Dornmair K. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 68. | Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA. 1992;89:4588-4592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 207] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Brück W, Lucchinetti C, Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 595] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 70. | Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1287] [Cited by in RCA: 1350] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 71. | Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 72. | Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 323] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 73. | Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 612] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 74. | Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 75. | Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 298] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 76. | Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 986] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 77. | Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65:124-141. [PubMed] |
| 78. | Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2010;Chapter 15:Unit 15.1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 79. | McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 415] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 81. | Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 82. | Herberman RB. Natural killer cells. Prog Clin Biol Res. 1989;288:161-167. [PubMed] |
| 83. | Maghazachi AA. Chemokines, G proteins and natural killer cells. Arch Immunol Ther Exp (Warsz). 2000;48:65-72. [PubMed] |
| 84. | Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol. 2010;341:37-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 85. | Maghazachi AA, Al-Aoukaty A. Chemokines activate natural killer cells through heterotrimeric G-proteins: implications for the treatment of AIDS and cancer. FASEB J. 1998;12:913-924. [PubMed] |
| 86. | Yang Q, Goding SR, Hokland ME, Basse PH. Antitumor activity of NK cells. Immunol Res. 2006;36:13-25. [PubMed] |
| 87. | Maghazachi AA. Insights into seven and single transmembrane-spanning domain receptors and their signaling pathways in human natural killer cells. Pharmacol Rev. 2005;57:339-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Kaur G, Trowsdale J, Fugger L. Natural killer cells and their receptors in multiple sclerosis. Brain. 2013;136:2657-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Oberg L, Johansson S, Michaëlsson J, Tomasello E, Vivier E, Kärre K, Höglund P. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo - role of KARAP/DAP12-dependent and -independent pathways. Eur J Immunol. 2004;34:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Maghazachi AA. Compartmentalization of human natural killer cells. Mol Immunol. 2005;42:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Maghazachi AA. G protein-coupled receptors in natural killer cells. J Leukoc Biol. 2003;74:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Rolin J, Maghazachi AA. Implications of chemokines, chemokine receptors, and inflammatory lipids in atherosclerosis. J Leukoc Biol. 2014;95:575-585. [PubMed] |
| 93. | Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821-5824. [PubMed] |
| 94. | Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1031] [Cited by in RCA: 1050] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 95. | Pandya AD, Al-Jaderi Z, Høglund RA, Holmøy T, Harbo HF, Norgauer J, Maghazachi AA. Identification of human NK17/NK1 cells. PLoS One. 2011;6:e26780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 97. | Maghazachi AA. On the role of natural killer cells in neurodegenerative diseases. Toxins (Basel). 2013;5:363-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Tian Z, Gershwin ME, Zhang C. Regulatory NK cells in autoimmune disease. J Autoimmun. 2012;39:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Segal BM. The role of natural killer cells in curbing neuroinflammation. J Neuroimmunol. 2007;191:2-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | French AR, Yokoyama WM. Natural killer cells and autoimmunity. Arthritis Res Ther. 2004;6:8-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 101. | Maghazachi AA. Role of natural killer cells in multiple sclerosis. ISRN Immunology. 2012;2012:Article ID 795075. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 301] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 103. | Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681-1688. [PubMed] |
| 104. | Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, Xiang R, La Cava A, Van Kaer L, Shi FD. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 105. | Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;163:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 107. | Winkler-Pickett R, Young HA, Cherry JM, Diehl J, Wine J, Back T, Bere WE, Mason AT, Ortaldo JR. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495-4506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 2005;174:2696-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Benczur M, Petrányl GG, Pálffy G, Varga M, Tálas M, Kotsy B, Földes I, Hollán SR. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin Exp Immunol. 1980;39:657-662. [PubMed] |
| 110. | Kastrukoff LF, Morgan NG, Zecchini D, White R, Petkau AJ, Satoh J, Paty DW. A role for natural killer cells in the immunopathogenesis of multiple sclerosis. J Neuroimmunol. 1998;86:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Grunebaum E, Malatzky-Goshen E, Shoenfeld Y. Natural killer cells and autoimmunity. Immunol Res. 1989;8:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | De Jager PL, Rossin E, Pyne S, Tamayo P, Ottoboni L, Viglietta V, Weiner M, Soler D, Izmailova E, Faron-Yowe L. Cytometric profiling in multiple sclerosis uncovers patient population structure and a reduction of CD8low cells. Brain. 2008;131:1701-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 113. | Sand KL, Knudsen E, Rolin J, Al-Falahi Y, Maghazachi AA. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell Mol Life Sci. 2009;66:1446-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Al-Falahi Y, Sand KL, Knudsen E, Damaj BB, Rolin J, Maghazachi AA. Splenic natural killer cell activity in two models of experimental neurodegenerative diseases. J Cell Mol Med. 2009;13:2693-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 115. | Høglund RA, Holmøy T, Harbo HF, Maghazachi AA. A one year follow-up study of natural killer and dendritic cells activities in multiple sclerosis patients receiving glatiramer acetate (GA). PLoS One. 2013;8:e62237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 117. | Zlotnik A, Yoshie O. The Chemokine Superfamily Revisited. Immunity. 2012;36:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 908] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 118. | Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2 mediated responses. Immunol Today. 2008;19:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 677] [Article Influence: 25.1] [Reference Citation Analysis (0)] |