Published online Aug 20, 2013. doi: 10.5493/wjem.v3.i3.43
Revised: July 3, 2013
Accepted: August 20, 2013
Published online: August 20, 2013
Processing time: 194 Days and 6.3 Hours
AIM: To investigate the role of matrix-degrading metalloproteinases 9, 12 (MMPs), as mediators of functional connective tissue damage in actinic cheilitis.
METHODS: Thirty five formalin-fixed, paraffin embedded specimens of actinic cheilitis, and twelve specimens of normal lower lip vermillion, which were obtained by the archives of the Department of Oral Medicine and Maxillofacial Pathology, were examined. From each block, 5 μm thick sections were cut and routinely stained with Hematoxylin and Eosin. Immunohistochemical studies were performed on 4-μm thick sections of formalin-fixed paraffin embedded actinic cheilitis lesions and of normal lower lip vermillion, for MMP-9 and MMP-12 in serial sections of our specimens. Appropriate positive and negative controls were performed to confirm the specificity of the staining reaction. MMP immunohistochemistry was evaluated using a semiquantitative immunoreactive score.
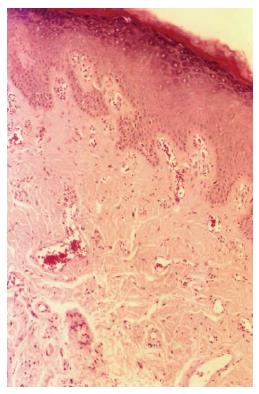
RESULTS: Haematoxylin and eosin staining revealed in actinic cheilitis lesions atrophic stratified squamous cell epithelium, or focally and irregularly hyperplastic of variable thickness, in some areas was observed marked keratin production. Varying degrees of epithelial dysplasia were noticed with a wide spectrum of change within the same specimen. Characteristic was the appearance of chronic inflammatory infiltration, and a band of amorphous acellular, basophilic change like solar elastosis (elastin replacement of collagen). In normal lower lip specimens weak and scanty positive expression of MMP-9 and MMP-12 was observed. Anti-MMP-9 antibody showed a weak reaction, in actinic cheilitis lesions, focal in the elastotic material, in chronic inflammatory cells and mostly in macrophages and neutrophils. Strong and in some cases diffused immunohistochemical expression of MMP-12 was detected in actinic cheilitis lesions in the areas of the fragmented, distorted and thickened elastic fibers. MMP-12 was also expressed in chronic inflammatory cells and mostly macrophages. MMP-12 was significantly higher in actinic cheilitis specimens compared with the normal lower lip specimens (P = 0.0029).
CONCLUSION: Our results suggest that especially MMP-12 may play an important role in remodeling events occurring in the connective tissue during long-term exposure to sunlight in the actinic cheilitis lesions.
Core tip: Actinic cheilitis is a chronic inflammatory disorder affecting mainly the lower lip, and it is caused by chronic and excessive exposure of the lips to the ultraviolet radiation in sunlight. Histologic features for actinic cheilitis include epithelial and connective tissue alterations. The matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidases, which are responsible for a wide range of proteolytic events. The aim of this study was to investigate the role and the expression of MMP-9, -12 as mediators of functional connective tissue damage in actinic cheilitis.
- Citation: Poulopoulos AK, Andreadis D, Markopoulos AK. Expression of matrix metalloproteinases 9 and 12 in actinic cheilitis. World J Exp Med 2013; 3(3): 43-49
- URL: https://www.wjgnet.com/2220-315X/full/v3/i3/43.htm
- DOI: https://dx.doi.org/10.5493/wjem.v3.i3.43
The matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidases, which are capable of digesting extracellular matrix and basement membrane components. The MMPs are responsible for a wide range of proteolytic events. Under physiological conditions, the MMPs are involved in many processes including cell proliferation, differentiation, migration, apoptosis, and angiogenesis. Moreover the MMPs are often up-regulated in groups forming activation cascades both in the inflammatory and malignant diseases[1].
MMP-9 is a member of the matrixin family of metallo-endopeptidases. MMP-9 is historically referred as gelatinase B because of its ability to cleave gelatin, a denatured form of collagen, in vitro[2]. MMP-9 differs from other MMPs because it contains three fibronectin type II repeats that have high binding affinity for collagen. These repeats are thought to mediate binding of MMP-2 and -9 to collagen[3]. This binding interaction brings the catalytic pocket of the MMP in proximity to collagen, thereby enhancing its rate of hydrolysis[4]. Despite the above mentioned biochemical interactions, MMP-9 is also able to cleave a number of other proteins and may have a rather wide range of physiologic substrates[5].
Much of our understanding of the biological function of MMP-9 comes from the study of mice lacking this gene[6]. Studies on this mice indicated that MMP-9-deficient mice are resistant to dermal blistering in a bullous pemphigoid model, an effect that has been attributed to the inability of these mice to cleave the SEPRIN a-1 proteinase inhibitor[7]. Other work in the same model for multistage carcinogenesis indicated that MMP-9 is part of the angiogenic “switch” that is essential for tumor growth[8]. Furthermore other reports suggested that MMP-9 may play a role in inflammation in the nervous system[9].
Human macrophage metalloelastase (MMP-12) is an MMP that was initially found in alveolar macrophages of cigarette smokers[10] . On a molar basis, it is clearly the most active MMP against elastin[11]. MMP-12 has a broad substrate specificity, however being able to degrade also type IV collagen, laminin, fibronectin vitronectin entactin, heparan and chondroitin sulfates[12]. In vivo MMP-12 has been shown to participate in the degradation of elastic fibers in the pathogenesis of atherosclerosis and in emphysema[13]. MMP-12 mRNA and protein are also expressed by accumulations of macrophages in granulomatous skin disorders[14].
In addition to degrading elastic tissue, MMP-12 has been shown to aid macrophage migration in other tissues; macrophages from MMP-12 deficient mice are unable to penetrate reconstituted basement membranes (BMs) in vivo and in vitro[15]. Additional findings on MMP-12 expression in macrophages under the shedding intestinal epithelium in inflammatory bowel diseases support its role in the degradation of BM components. Furthermore, MMP-12 expression may also be associated with macrophage migration through BM in certain inflammatory skin diseases such as dermatitis herpetiformis or pityriasis lichenoides[14].
Long term sun exposure causes to the lower lip vermillion a lesion known as actinic cheilitis (AC), the histologic examination of which reveals loss of collagen with concomitant accumulation of elastotic material[16].
The aim of this study was to investigate the role and the expression of MMP-9, -12 as mediators of functional connective tissue damage in actinic cheilitis.
Biopsies of the lower lip vermilion from 35 patients with AC (32 males and 3 females, mean age 54.7 ± 12.1) were obtained from the archives of the Department of Oral Medicine and Maxillofacial Pathology. Normal lip vermilion biopsies from 12 patients (11 males and 1 females, mean age 53.6 ± 14.8) were used as controls. Cases of squamous cell carcinoma of the lower lip with previous diagnosis of AC were excluded. The demographic information of the patients, their occupation (sun exposure), as well as their habits of smoking and drinking are presented in Table 1.
| n(%) | |
| Males | 32 (91.42) |
| Females | 3 (8.57) |
| Mean age (yr) | 54.7 ± 12.1 |
| Cigarette smokers | 23 (65.7) |
| Drinking | 15 (42.85) |
| Out-door occupation | 24 (68.57) |
| Out-door occupation + smoking | 18 (51.42) |
| Out-door occupation + drinking | 13 (37.14) |
| Out-door occupation + smoking + drinking | 22 (62.85) |
The tissue specimens were fixed in 10% buffered formalin for a maximum of 24 h, followed by paraffin embedding. From each block, 5-μm thick sections were cut and routinely stained with hematoxylin and eosin (HE). Histopathological characteristics that were evaluated included the presence and the degree of epithelial keratinization, the thickness of the spinous cell layer, the presence and the degree of epithelial dysplasia, connective tissue changes, such as inflammation and elastosis. All study participants gave informed consent, the whole study was approved by the appropriate ethics committee and was performed according to Helsinki II Declaration.
Immunohistochemical studies were performed on 4-μm thick sections of formalin-fixed paraffin embedded actinic cheilitis lesions and of normal lower lip vermillion, which were mounted on 3-amino-propyltriethoxy-silane (organo-silane; Sigma, United Kingdom)-coated slides dewaxed in xylene and rehydrated in graded ethanols according to standard procedures. Endogenous peroxidase activity was blocked by pretreatment of slides with 3% hydrogen peroxidase for 10 min. Lyophilized Mouse Monoclonal Antibody Matrix Metalloproteinase-9 (NCL-MMP9-439) (Novocastra) was used as a primary antibody for the MMP-9, and Rabbit Monoclonal Antibody Matrix Metalloproteinase-12 (ab52897) for the MMP-12 respectively. Slides were incubated with the primary antibodies diluted in Tris -buffered solution (50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.4) under conditions as described in Table 2 followed by incubation with Dako Envision™ Peroxidase (Dako Diagnostica), for 30 min at room temperature. For visualization of the antigen 3,3’-diaminobenzidine tetrahydrochloride (Dako) was added for 20 min at room temperature. Slides were counterstained with Mayer’s haematoxylin. The applied antibodies and the staining conditions are summarized in Table 3. Appropriate positive and negative controls, including the use of an irrelevant antibody, and the omission of various layers, were performed to confirm the specificity of the staining reaction.
| Score | Percentage of immunopositive cells (PS) | Staining intensity |
| 0 | 0 | Negative |
| 1 | < 10% | Weak |
| 2 | 10%-50% | Moderate |
| 3 | > 50% | Strong |
| Antibody | Source | Dilution | Antigen retrieval | Incubation time |
| Anti-MMP-9 | Novocastra/Leica | 1:50 | Microwave | Room temperature |
| NCL-MMP9-439 | 1 h | |||
| Anti-MMP-12 | Epitomics | 1:50 | Microwave | Room temperature |
| ab52897 | 1 h |
MMP immunohistochemistry was evaluated using a semiquantitative immunoreactive score from 0 to 6 based on the percentage of positive stained cells (PS) (0-3 points) and their staining intensity (SI) (0-3 points), as described in previously established protocols[17], and shown in Table 2. The total score (TS) was calculated by adding the PS and SI scores, and the mean of the TS was used for the statistical analysis.
Proportion scoring was performed only if the intensity of the cells staining was more than that of the internal controls limiting errors in semiquantitation as a consequence of nonspecific background staining. Sections were examined by two of the authors independently of each other. The slides then were reviewed by the examiners as a group and discussion was occasionally necessary to establish uniformity.
Statistical analysis was performed on the immune scores derived, by using the Mann-Whitney test and the statistical package SPSS 20.0 (Statistical Package for the Social Sciences) (Chigago Illinois, United States) for Windows 7.
HE staining revealed in actinic cheilitis lesions atrophic stratified squamous cell epithelium, or focally and irregularly hyperplastic of variable thickness, in some areas was observed marked keratin production (Figure 1). Varying degrees of epithelial dysplasia were noticed with a wide spectrum of change within the same specimen. Characteristic was the appearance of chronic inflammatory infiltration, and a band of amorphous acellular, basophilic change like solar elastosis (elastin replacement of collagen). The histopathological findings of actinic cheilitis patients are presented in Table 4.
| Histopathological characteristics | Negligible | Mild | Moderate | Severe |
| Increased thickness of keratin layer | 4 (11.42) | 13 (37.14) | 11 (31.42) | 7 (20) |
| Parakeratosis/orthokeratosis | 12 (34.28) | 2 (5.71) | 5 (14.28) | 16 (45.71) |
| Increased thickness of spinous cell layer | 8 (22.85) | 12 (34.28) | 9 (25.71) | 6 (17.14) |
| Atrophy of the spinous cell layer | 0 | 0 | 2 (5.71) | 0 |
| Epithelial dysplasia | 3 (8.57) | 11 (31.42) | 13 (37.14) | 8 (22.85) |
| Connective tissue inflammation | 3 (8.57) | 11 (31.42) | 12 (34.28) | 9 (25.71) |
| Perivascular inflammation | 17 (48.57) | 7 (20) | 6 (17.14) | 5 (14.28) |
| Elastosis | 1 (2.85) | 9 (25.71) | 15 (42.8) | 10 (28.57) |
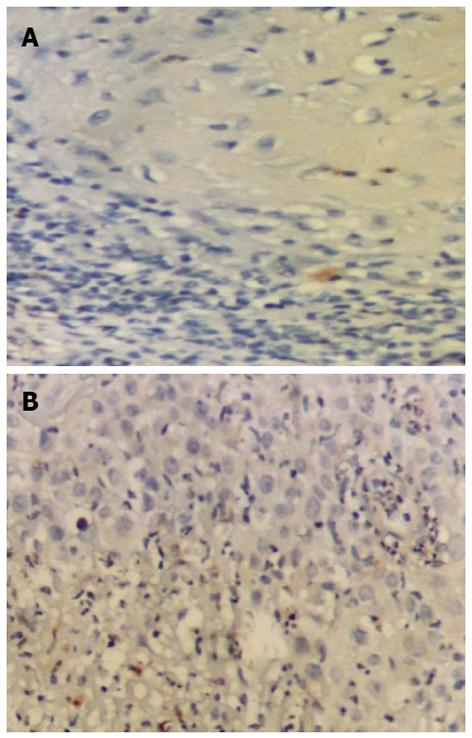
In normal lower lip specimens minimal to negative expression of MMP-9 (Figure 2A) and MMP-12 (Figure 2B) was observed.
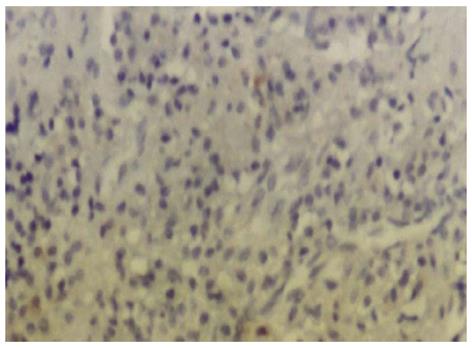
The negative controls confirmed the specificity of the staining reaction (Figure 3).
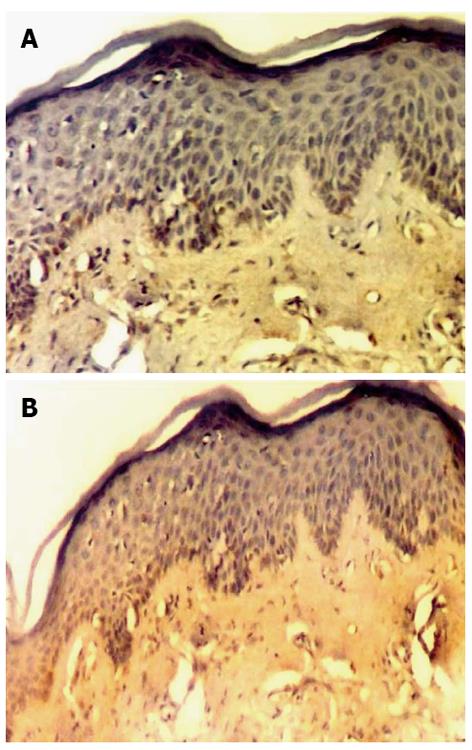
Anti-MMP-9 antibody showed a weak reaction, in actinic cheilitis lesions, focal in the elastotic material, in chronic inflammatory cells and mostly in macrophages and neutrophils (Figure 4A).
Furthermore strong and in some cases diffused immunohistochemical expression of MMP-12 was detected in actinic cheilitis lesions (Figure 4B), in the areas of the fragmented, distorted and thickened elastic fibers. MMP-12 was also expressed in chronic inflammatory cells and mostly macrophages (Figure 5).
MMP-12 was significantly higher in actinic cheilitis specimens compared with the normal lower lip specimens (P = 0.0029). The immune scores of MMPs expressed as mean total score are presented in Table 5.
Chronic exposure to ultraviolet (UV) radiation causes degenerative alterations to the lower lip vermillion, clinically characterized by erosions and atrophy[18]. The major histological changes in actinically damaged lip are the accumulation of basophilic fibers in the upper part of the connective tissue, referred to as basophilic degeneration or as actinic elastosis[19]. Previous histological and biochemical studies on the nature of the accumulated fibers have demonstrated that altered elastin is the primary component of actinic elastosis[20].
Disappearance of normal elastic fibers is a feature of many skin diseases and actinic cheilitis[21]. Little research has been done, however, to reveal the pathomechanisms behind this phenomenon. Elastin is critical to the structural integrity of a variety of connective tissues. Elastin is highly resistant to proteolytic degradation, but several enzymes capable of solubilizing this matrix constituent have been found. Because of their role in tissue remodelling at sites of inflammation and injury, inflammatory cells have been of particular interest for their elastase activity[20].
The participation of several proteases in photoaging has been reported. It has been reported that in neutrophil elastase -deficient mice, elastic fibers in the skin are unaffected by UVB irradiation, whereas an increase in elastic fibers occurs in normal mice[22]. It has been demonstrated that multiple exposures of the skin to UV radiation result in elevated levels of MMPs in vivo and in vitro[23]. These previous findings suggest that the elevated expression of certain MMPs may be associated with the elastotic material in sun-damaged skin.
Histologically, photoaging causes accumulation of so-called elastotic material, composed of elastin and versican, in the upper and mid connective tissue[24]. This is accompanied by degeneration of surrounding collagenous meshwork, but due to the inability of MMP-12 and MMP-7 to degrade fibrillar collagens, they probably do not participate in that event.
We found abundant immunostaining for MMP-12 in the areas of abnormal elastotic fibers in actinic cheilitis lesions. Furthermore we have not find any immunoreactivity for MMP-12 in normal lower lip vermillion specimens, suggesting that MMP-12 does not bind to normal elastin or that in healthy lower lip vermillion no abnormal accumulations of macrophages exist, as possible sources of MMP-12. Our findings are in accordance to previous immunohistochemical study for the accumulation of MMP-12 in actinic damage skin[25].
It has been proposed that the elastotic material accumulating in photoaged skin results from direct UV-mediated damage to elastotic fibers and fibroblasts[26]. MMP-12 possibly takes part in a reparative remodeling process by trying to cleave this abnormal elastotic material or fibrillin[27]. Granulocyte-macrophage colony stimulating factor, induced at least in keratinocytes by UV light, is able to upregulate MMP-12 production by macrophages[28], and could thus be one of the candidate cytokines to stimulate MMP-12 in solar damage. UV irradiation causing abnormal changes in elastin might lead to accumulation of macrophages that try to cleave both abnormal elastin by secreting elastases as well as activate elastin/collagen synthesis by releasing, e.g., transforming growth factor (TGF)-β[29]. Interestingly, abundant staining for latent TGF-β binding protein-1 and TGF-β colocalize with MMP-12 staining in solar elastosis and keratosis[30]. TGF-β is not likely to upregulate MMP-12 expression, as it usually downregulates MMP function. We cannot however exclude that MMP-12 participates in the proteolytic release of this growth factor from the extracellular matrix. TGF-β could further augment the deposition of abnormal elastin, which is unable to assemble into functional elastic fibers due to influence of proteolytic enzymes and UV radiation.
Elevated expression of MMP-9 in UV irradiated skin has been demonstrated previously[31]. Recently it has been reported that MMP-9 was strongly expressed in SCCs of the lip and moderately expressed in ACs and control samples[32]. Whereas another study detected positive expression of MMP-9 in actinic cheilitis lesions with no statistical differences in the pattern of expression in comparison with squamous cell carcinomas[33]. Despite the above mentioned previous findings, in our study we were not able to detect strong immunoreactivity for MMP-9 in elastotic material in actinic lesions. Our findings are in accordance with similar findings for MMP-9 in actinic elastosis of sun damaged skin[21]. This may possibly be because enhanced expression of MMP-9 does not lead to the accumulation or the incorporation of MMP-9 in the elastotic material. MMPs are secretary enzymes and cannot be detected in the extracellular spaces in normal tissue. In addition, cells potentially producing MMP-9 are limited to keratinocytes, monocytes, alveolar macrophages, PMN leukocytes and SV40-transformed human lung fibroblasts, and secretion of this enzyme is not detected in any normal cell strain of fibroblast origin[34]. This restricted distribution of potential MMP-9 producing cells may explain the diminished immunoreactivity of MMP-9 in elastotic materials in actinic cheilitis.
In conclusion, our findings suggest that enhanced MMP-12 expression was detected in abnormal elastic fibers in actinic cheilitis lesions. Thus MMP-12may play a role in the remodeling events occurring in the connective tissue during long term exposure to sunlight in actinic cheilitis.
Actinic cheilitis is a common inflammatory disorder caused by solar ultraviolet radiation that affects the lip vermilion. The lesion is potentially malignant and may transform into squamous cell carcinoma. The progression of actinic cheilitis is associated with the expression of matrix metalloproteinases (MMPs).
The MMPs are a large family of zinc-dependent endopeptidases, which are often up-regulated in groups forming activation cascades both in the inflammatory and malignant diseases. However, the role of MMPs is not entirely clear in actinic cheilitis lesions. In this study, enhanced MMP-12 expression was detected in actinic cheilitis lesions.
Recent and previous reports investigated the presence of Metalloproteinases in a spectrum of preinvasive and invasive neoplastic epithelial lesions that included actinic cheilitis and squamous cell carcinoma. This is the first study to focus specifically in actinic cheilitis lesions and to demonstrate that the alterations in MMP-12 expression may play a role in the remodeling events occurring in the connective tissue during long term exposure to sunlight in actinic cheilitis.
By understanding how metalloproteinases and especially MMP-12 are induced and by preventing extracellular matrix damage and activation of MMPs, and inhibition of MMP expression (e.g., by retinoids) and activity (e.g., by natural and synthetic inhibitors), this study may represent a future strategy for therapeutic intervention in the treatment of actinic cheilitis.
MMP-9 is a member of the matrixin family of metallo-endopeptidases. MMP-9 is historically referred as gelatinase B because of its ability to cleave gelatin, a denatured form of collagen, in vitro. MMP-12 is able to degrade extracellular matrix components such as elastin and is involved both in inflammatory and malignant diseases.
The authors investigated the role and the expression of MMP-9, -12 in actinic cheilitis lesions. Enhanced immunohistochemical expression of MMP-12 was detected in actinic cheilitis lesions. The results are interesting and suggested that MMP-12may play a role in the remodeling events occurring in the connective tissue during long term exposure to sunlight in actinic cheilitis.
P- Reviewers Chen SS, Rojas IG S- Editor Wen LL L- Editor A E- Editor Liu XM
| 1. | Chen Y, Zhang W, Geng N, Tian K, Jack Windsor L. MMPs, TIMP-2, and TGF-beta1 in the cancerization of oral lichen planus. Head Neck. 2008;30:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3284] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 4. | Swarnakar S, Mishra A, Chaudhuri SR. The gelatinases and their inhibitors: the structure-activity relationships. EXS. 2012;103:57-82. [PubMed] |
| 5. | Fridman R, Toth M, Chvyrkova I, Meroueh SO, Mobashery S. Cell surface association of matrix metalloproteinase-9 (gelatinase B). Cancer Metastasis Rev. 2003;22:153-166. [PubMed] |
| 6. | Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Littlepage LE, Sternlicht MD, Rougier N, Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI, Werb Z. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 2011;8:206-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268:23824-23829. [PubMed] |
| 11. | Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, Hartland SN, Vernon M, Duffield JS, Benyon RC. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Gronski TJ, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272:12189-12194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5:265-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998;152:1005-1014. [PubMed] |
| 15. | Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA. 1996;93:3942-3946. [PubMed] |
| 16. | Markopoulos A, Albanidou-Farmaki E, Kayavis I. Actinic cheilitis: clinical and pathologic characteristics in 65 cases. Oral Dis. 2004;10:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Gonzalez LO, Junquera S, del Casar JM, González L, Marín L, González-Reyes S, Andicoechea A, González-Fernández R, González JM, Pérez-Fernández R. Immunohistochemical study of matrix metalloproteinases and their inhibitors in pure and mixed invasive and in situ ductal carcinomas of the breast. Hum Pathol. 2010;41:980-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Cavalcante AS, Anbinder AL, Carvalho YR. Actinic cheilitis: clinical and histological features. J Oral Maxillofac Surg. 2008;66:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Kaugars GE, Pillion T, Svirsky JA, Page DG, Burns JC, Abbey LM. Actinic cheilitis: a review of 152 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Rojas IG, Martínez A, Pineda A, Spencer ML, Jiménez M, Rudolph MI. Increased mast cell density and protease content in actinic cheilitis. J Oral Pathol Med. 2004;33:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Ohnishi Y, Tajima S, Akiyama M, Ishibashi A, Kobayashi R, Horii I. Expression of elastin-related proteins and matrix metalloproteinases in actinic elastosis of sun-damaged skin. Arch Dermatol Res. 2000;292:27-31. [PubMed] |
| 22. | Starcher B, Conrad M. A role for neutrophil elastase in solar elastosis. Ciba Found Symp. 1995;192:338-346; discussion 346-347. [PubMed] |
| 23. | Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998;3:61-68. [PubMed] |
| 24. | Bernstein EF, Fisher LW, Li K, LeBaron RG, Tan EM, Uitto J. Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab Invest. 1995;72:662-669. [PubMed] |
| 25. | Saarialho-Kere U, Kerkelä E, Jeskanen L, Hasan T, Pierce R, Starcher B, Raudasoja R, Ranki A, Oikarinen A, Vaalamo M. Accumulation of matrilysin (MMP-7) and macrophage metalloelastase (MMP-12) in actinic damage. J Invest Dermatol. 1999;113:664-672. [PubMed] |
| 26. | Bernstein EF, Chen YQ, Tamai K, Shepley KJ, Resnik KS, Zhang H, Tuan R, Mauviel A, Uitto J. Enhanced elastin and fibrillin gene expression in chronically photodamaged skin. J Invest Dermatol. 1994;103:182-186. [PubMed] |
| 27. | Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999;340:171-181. [PubMed] |
| 28. | Kumar R, Dong Z, Fidler IJ. Differential regulation of metalloelastase activity in murine peritoneal macrophages by granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor. J Immunol. 1996;157:5104-5111. [PubMed] |
| 29. | Quaglino D, Nanney LB, Kennedy R, Davidson JM. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990;63:307-319. [PubMed] |
| 30. | Karonen T, Jeskanen L, Keski-Oja J. Transforming growth factor beta 1 and its latent form binding protein-1 associate with elastic fibres in human dermis: accumulation in actinic damage and absence in anetoderma. Br J Dermatol. 1997;137:51-58. [PubMed] |
| 31. | Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 947] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 32. | Souza Freitas V, de Andrade Santos PP, de Almeida Freitas R, Pereira Pinto L, de Souza LB. Mast cells and matrix metalloproteinase 9 expression in actinic cheilitis and lip squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Hernández-Pérez M, El-hajahmad M, Massaro J, Mahalingam M. Expression of gelatinases (MMP-2, MMP-9) and gelatinase activator (MMP-14) in actinic keratosis and in in situ and invasive squamous cell carcinoma. Am J Dermatopathol. 2012;34:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2113] [Cited by in RCA: 2145] [Article Influence: 67.0] [Reference Citation Analysis (0)] |