Revised: October 16, 2012
Accepted: January 17, 2013
Published online: February 20, 2013
Processing time: 99 Days and 16.1 Hours
Trypanosoma cruzi (T. cruzi), the etiological agent of Chagas disease, affects nearly 18 million people in Latin America and 90 million are at risk of infection. The parasite presents two stages of medical importance in the host, the amastigote, intracellular replicating form, and the extracellular trypomastigote, the infective form. Thus infection by T. cruzi induces a complex immune response that involves effectors and regulatory mechanisms. That is why control of the infection requires a strong humoral and cellular immune response; hence, the outcome of host-parasite interaction in the early stages of infection is extremely important. A critical event during this period of the infection is innate immune response, in which the macrophage’s role is vital. Thus, after being phagocytized, the parasite is able to develop intracellularly; however, during later periods, these cells induce its elimination by means of toxic metabolites. In turn, as the infection progresses, adaptive immune response mechanisms are triggered through the TH1 and TH2 responses. Finally, T. cruzi, like other protozoa such as Leishmania and Toxoplasma, have numerous evasive mechanisms to the immune response that make it possible to spread around the host. In our Laboratory we have developed a vaccination model in mice with Trypanosoma rangeli, nonpathogenic to humans, which modulates the immune response to infection by T. cruzi, thus protecting them. Vaccinated animals showed an important innate response (modulation of NO and other metabolites, cytokines, activation of macrophages), a strong adaptive cellular response and significant increase in specific antibodies. The modulation caused early elimination of the parasites, low parasitaemia, the absence of histological lesions and high survival rates. Even though progress has been made in the knowledge of some of these mechanisms, new studies must be conducted which could target further prophylactic and therapeutic trials against T. cruzi infection.
- Citation: Basso B. Modulation of immune response in experimental Chagas disease. World J Exp Med 2013; 3(1): 1-10
- URL: https://www.wjgnet.com/2220-315X/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5493/wjem.v3.i1.1
Trypanosoma cruzi (T. cruzi), the etiological agent of Chagas disease, affects nearly 2 500 000 people in Argentina and 18 million in Latin America. The parasite presents two stages of medical importance in the host, the amastigote, intracellular replicating form, and the extracellular trypomastigote, the infective form. That is why control of the infection requires a strong humoral and cellular immune response; hence, the outcome of host-parasite interaction in the early stages of infection is extremely important. In humans the disease presents different clinical and immunological periods: the acute period, characterized by the presence of trypomastigotes in the bloodstream, associated with immunosuppressive phenomena[1], which is asymptomatic in 95% of cases[2] and remits spontaneously, to enter in a second indeterminate phase, after 3 or 4 mo, which can last the rest of the host’s life with no clinical signs. It is characterized by low parasitaemia and positive serology and, in later years, approximately 30% of infected people develop some degree of cardiac or digestive pathology in the chronic period of infection. This is attributed to direct action of the parasite, or to autoimmune reactions induced by T. cruzi. Gironès et al[3] critically reviewed the evidence in favour of and against autoimmunity through molecular mimicry as responsible for Chagas disease pathology from clinical, pathological and immunological perspectives. Also in this sense, Bonney et al[4] observed that vaccination with heat-killed T. cruzi induces the development of autoimmunity via molecular mimicry and other mechanisms and potentially fatal cardiomyopathy. Their results show that exposure to T. cruzi antigen alone is sufficient to induce autoimmunity and cardiac damage, yet additional immune factors, including a dominant TH1/TH17 immune response, are likely required to induce cardiac inflammation.
Immune response to T. cruzi is highly complex and involves many components, both effectors and regulators. The unspecific immunosupression that occurs during the first stage of the infection and T. cruzi’s capacity to adapt and evade this response allow it to invade cells and spread, which means that the parasite may remain indefinitely in the host’s tissues because it is not completely eliminated[1].
The process in which trypomastigotes enter the host’s cells involves several stages: initial parasite-cell contact, trypomastigote adhesion, early induction of immune response, which causes modifications to the membrane proteins. The parasite has been claimed to enter the host’s cell using a variety of mechanisms: (1) it enters professional phagocytic cells by phagocytosis; (2) the cellular membrane emits pseudopodia, modifications are produced in the actin filaments, and protein tyrosine kinases such as PI-3 are activated; this process culminates with the formation of a parasitophorous vacuole and soon after lysosomes and endosomes are recruited[5]; (3) it enters non phagocytic cells by means of endocytosis but there is no emission of pseudopodia in the host’s cell; and (4) another mechanism involves direct penetration by the parasite in the cell by means of membrane invagination, with an important intake of energy[6].
After entering, the parasite lodges in the cytoplasmic vacuolar compartment where a gradual differentiation process occurs from trypomastigote to amastigote[7,8], and the latter divide by means of binary fission, to then become trypomastigotes once again; they leave the cell to spread via lymph and blood, and infect other cells in which they once again go through the replication cycle. T. cruzi primarily infects cells belonging to the reticuloendothelial system, nerve and muscle tissue, including cardiac fibres[9].
In order to progress with regard to knowledge of the immune response set off by T. cruzi infection and to analyze whether it is possible to modulate this complex response, several experimental models have been developed. A model for vaccinating mice with Trypanosoma rangeli (T. rangeli), a parasite closely related to T. cruzi, but nonpathogenic to humans[10-12], has been designed in our laboratory[13]. T. rangeli shares areas of geographical distribution, epidemiological characteristics, and antigenic and immunogenic components with T. cruzi. Specific diagnosis becomes difficult by means of classical serological methodologies because it induces a response of crossed antibodies. Moreover, both parasites cannot be morphologically differentiated[14-16]. The antigenic similitude between T. rangeli and T. cruzi has been shown by means of different methods by numerous research groups[17-20].
T. rangeli presents an enzyme, sialidase, with neuraminidase activity which is fundamentally expressed in the epimastigote stage and, unlike T. cruzi, does not present transialidase. Recent studies show that the sialidase system is very complex and can take on different expressions in different strains of the parasite, owing to genetic mutations[21]. In addition, it induces a complex modulation of the immunological mechanisms of the infected vector (Rhodnius genus) causing a reduction in the production of soluble mediators such as nitric oxide, oxygen free radicals, and the inhibition of phagocytosis as well as humoral response, among others, which favours the development of the parasite and results in the death of the vector[11].
The strategy of vaccinating with a parasite that is nonpathogenic to humans is based on the fact that, in the event of the future development of a vaccine for human use, and accepting the role played by autoimmune mechanisms in the pathology of Chagas disease, the possible induction of auto-aggression due to vaccination must be avoided[3,4].
In our experimental model, two groups of mice were used, one vaccinated with T. rangeli (at least n = 6 in each experiment) and then challenged by T. cruzi, and another group of control animals (n = 6), which were only infected with T. cruzi. A fixed number of 1500 virulent parasites were used to infect and the starting time of the infection was determined.
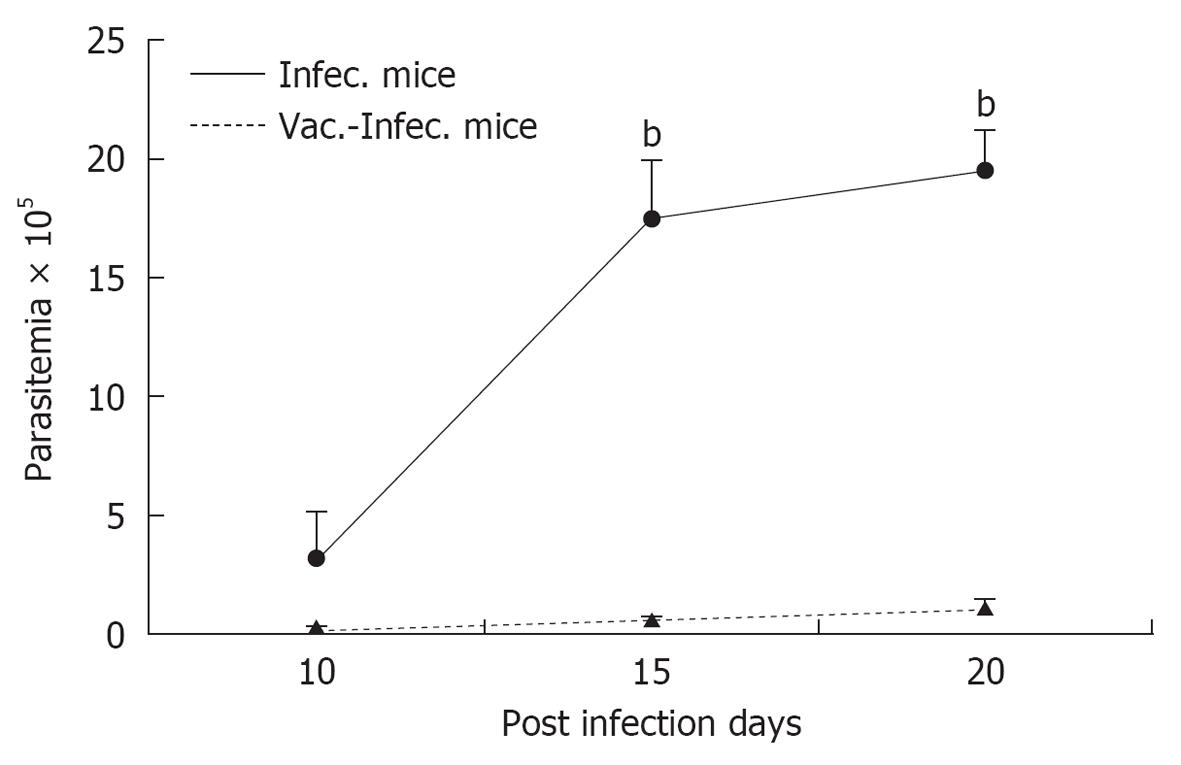
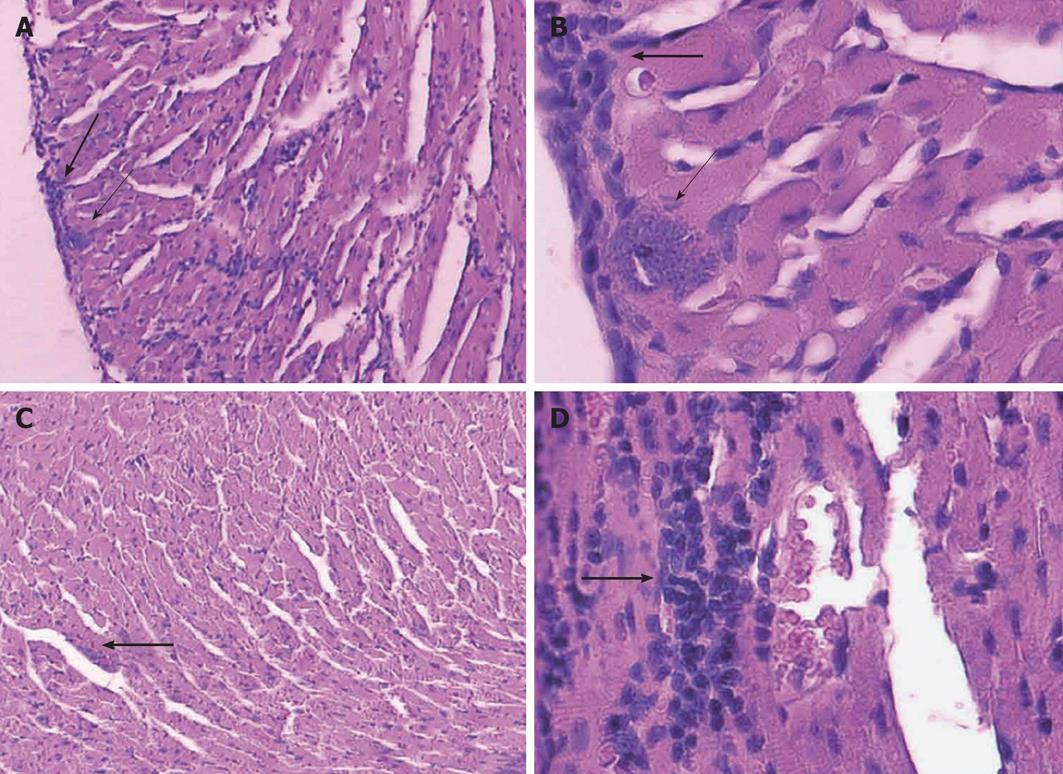
We observed that previously vaccinated mice showed very low parasitaemia, high survival rates and an absence of histological and autoimmune lesions, while mice that were only infected showed high parasitaemia, high mortality and severe histopathological alterations in the heart, skeletal muscle, spleen and liver[13,22,23]. For histological studies, mice from each group: vaccinated with T. rangeli and afterward challenged with T. cruzi (V) (n = 6) and non-vaccinated but infected with T. cruzi (I) (n = 6), were killed with ether anesthesia. Hearth, spleen, liver and skeletal muscles from the quadriceps were immediately removed from each mouse, fixed in buffered, 10% formalin (pH 7.0), and embedded in paraffin wax. One-half of each organ was cut into 5-μm-thick sections, and they were stained with haematoxylin-eosin. At least 20 areas from each section were checked for parasites and histopathology under a 40-x objective in a blind study.
The Figures 1 and 2 show a representative experiments. Similar results were obtained with two strains of T. rangeli from different origins, isolated in Colombia and Brazil, which revealed that the capacity to protect mice against lethal infection by T. cruzi is a characteristic common to different strains of T. rangeli. This result represents a clear advantage for the future preparation of possible vaccines for animal or human use[24].
On the other hand, it was demonstrated[25] that, in the acute period of experimentally infected mice, T. cruzi induces a response that presents different patterns in each different immune system compartment, splenomegaly, lymphoid subcutaneous tissue expansion, persistent polyclonal activation of lymphocyte T and B, and at the same time, thymus and mesenteric node atrophy.
A critical event during early stages of the infection is the innate immune response, in which the macrophage’s role is vital. Thus, after being phagocytized, the parasite is able to develop intracellularly; however, during later periods, these same cells induce its elimination by means of toxic metabolites. In turn, as the infection progresses, adaptive immune response mechanisms are triggered through the TH1 (cellular) and TH2 (humoral) responses.
Early in the infection, T. cruzi induces an intense inflammatory response, which plays a crucial role in the disease’s pathogenesis. In experimental models, some of the immunological events that take place during the first few hours after infection are known. Indeed, it has been observed that T. cruzi antigens induce activation of the natural killer (NK) cells prior to expansion of T lymphocytes[26]. During this stage the macrophages induce a cascade of cytokines: initially they produce interleukin (IL)-12, which acts on NK cells to induce the production of interferon (IFN)γ, which in turn increases the production of IL-12, tumor necrosis factor (TNF)α and NO in the macrophage, thus contributing to the elimination of the parasite[27]. At the same time, both types of cells synthesize regulatory cytokines such as IL-10 and IL-4 to reduce the harmful effects associated with excess stimulation of the immune system[28]. In very early stages of the infection, components of T. cruzi, including its DNA and membrane glycoconjugates, trigger the innate response through their interaction with Toll like Receptors: TLR2, TLR4 and TLR9 in macrophages and dendritic cells. After activation, both cells secrete cytokines and chemokines, and increase the expression of their co-stimulatory molecules, inducing endocytosis and intracellular death. As mentioned above, an adequate production of proinflammatory cytokines such as IFNγ, TNFα, IL-1, IL-12, IL-6 and IL-18 is essential for controlling infection by intracellular parasites[29]. Meanwhile, it has been observed that IL-17 might regulate the recruitment of inflammatory cells and the differentiation of TH1 in heart tissue[30]. Therefore, in order to resolve the T. cruzi infection, a balance is necessary between the immune response mediated by TH1 and by TH2[31]. TH1 cells are responsible for the production of inflammatory cytokines, while TH2 cells have an anti-inflammatory function and are involved in the antibody mediated response. IL-12 and IL-18 produced by dendritic cells and macrophages promote the development of TH1 cells that produce IFNγ, while IL-4 induce the expansion of TH2 cells and of high amounts of IL-10. As a result of this process, regulation of the cellular response occurs due to a reduction in the activation of dendritic cells and in the macrophage’s microbicidal activity. In addition, IL-4 takes part in inducing transforming growth factor (TGF)β which regulates the activity of the antigen presenting cells and enhance the susceptibility of infection by T. cruzi[32].
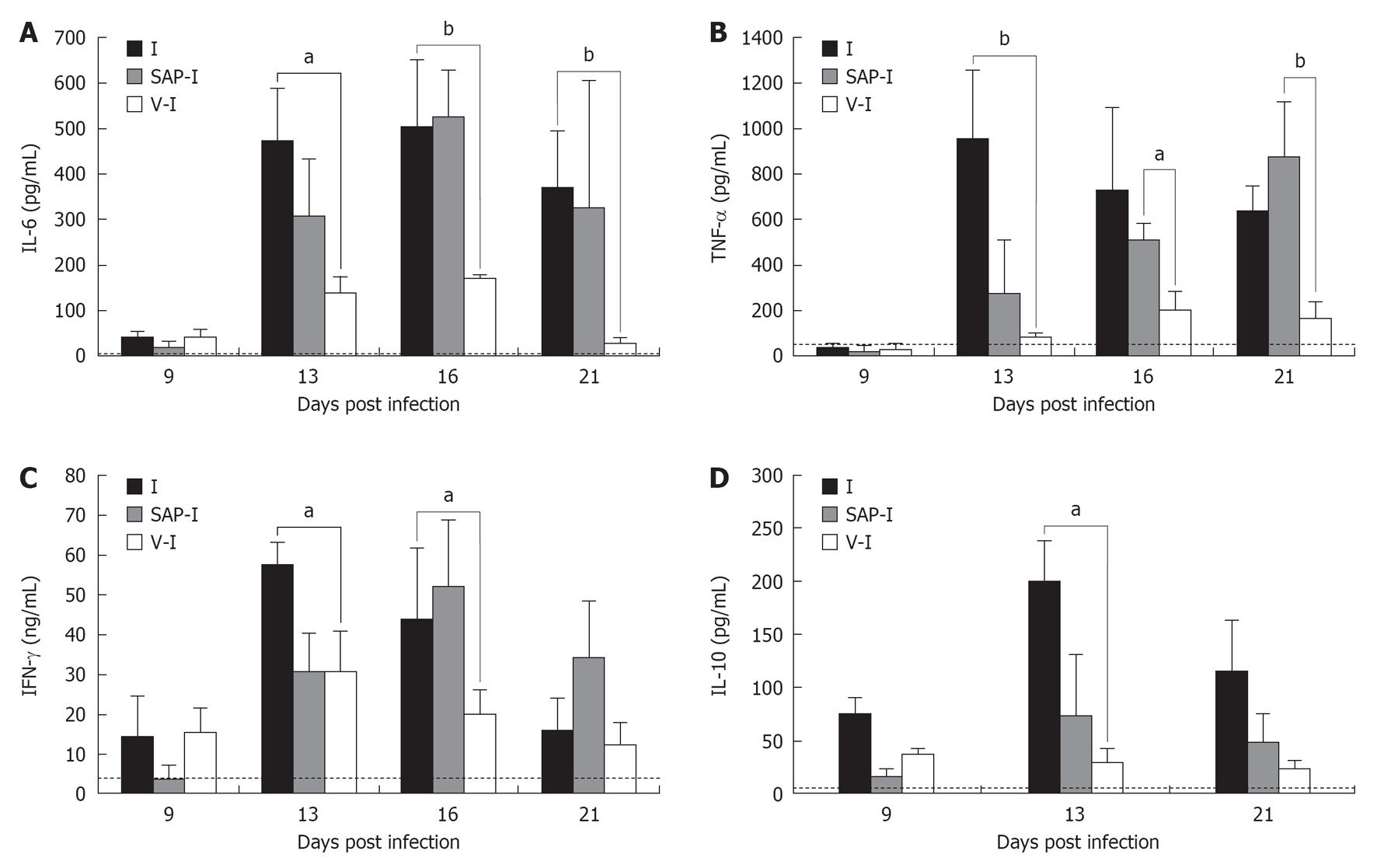
In this sense, we observed in our experimental model[31,33] (n = 6 for each group and each experiment performed) that vaccinated animals had a significant increase of IL-12, down regulation of the proinflammatory cytokines, IL-6, IFNγ, TNFα, and increase of soluble TNF receptors sTNFIR and sTNFIIR, which inhibit the deletereous activity of TNFα, in accord with Camargo et al[27]. Also Chandrasekar et al[34] detected proinflammatory cytokine production (IL-6, TNF-α, IL-1β) in the myocardium of T. cruzi infected mice, which suggests the probable involvement of the production of these molecules in situ in the injury of the myocardial function. The diminished production of proinflamatory cytokines in the immunized group of our model, associated with higher survival rates, suggests that both, IL-6 and TNF-α, are probably involved in the fatal outcome of the infected mice.
The high IFN-γ production during acute T. cruzi infection has been also widely demonstrated[35,36]. This finding is generally associated with protective effects since IFN-γ enhances trypanocidal activity of the macrophages via a nitrogen oxide mediated mechanism[37,38]. In this sense, we have observed a high production of IFN-γ in both vaccinated and control experimental groups. This finding is in agreement with Reed et al[39], who detected high IFN-γ levels in both susceptible and resistant mice. Moreover, in our mentioned works it was observed that in immunized animals, the ratio IFN-γ/IL-10 was greater than in the non immunized-infected mice. Taken together, these results suggest that, in vaccinated - infected animals, the action of protective IFN-γ could be more effective, without the antagonist action of IL-10. On the other hand, in all the experiments performed, the serum concentration of IL-10 correlated with parasitemia levels. These results are consistent with those of Reed et al[39], who detected a lower IL-10 production in resistant mice compared to the susceptible ones. Furthermore, Abrahamsohn et al[40] observed a lower number of parasites and higher IFN-γ production in IL-10 KO mice than in the wild type ones.
The Figure 3 shows the levels of IL-6, IL-10, IFN-γ and TNF-α in a representative experiment, in different groups of mice. From the results obtained in mice treated only with saponina adjuvant and after infected, the effect of the adjuvant alone became evident. In this group of animals a delay in the increase of parasitemia was detected, but the systemic production of IL-6 and TNF-α and the mortality rate were very similar to those in non vaccinated-infected group. This could be due to the unspecific action of the adjuvant on the immune system, which is not sufficient to help control the infection. Additionally, the results of these experiments suggest that, in this experimental model, the levels of IL-6 and TNF-α seem to be earlier markers of fatal outcome than the parasite load[33].
Likewise, the vaccinated group had very low levels of IL-10, which allowed IFNγ to maintain its protective activity, activating macrophages, essential for the elimination of parasites, unlike the control group, which showed high levels if IL-10, which blocks activation of the macrophages and their microbicidal function[31,33]. It was not possible to detect IL-4 or IL-5 in either group with the methodology used. Taken together, these results show that vaccination with T. rangeli made it possible to induce a profile of the cytokines different from that of the non immunized-infected mice, with a delicate balance between TH1 and TH2, suitable for overcome the infection. This modulation of the synthesis and liberation of cytokines and soluble receptors was also observed during the acute period of natural human infection[41].
On the other hand, during the process of invading the host cell, T. cruzi interacts with different receptors of the macrophage to induce its own phagocytosis. Different molecules of the family of Toll type receptors recognize different molecular patterns associated with bacteria, viruses, fungi and protozoa. As a result, innate immune response mechanisms and the development of the subsequent adaptive response are triggered[42].
With regard to NO, it is considered to be the most important early soluble mediator produced by immune system cells. Macrophages recognize antigen microorganisms through their different receptors (Toll-like, NLRs and RIG-like) and trigger the production of inflammatory mediators inducing the activity of the inducible Nitric Oxide synthase enzyme. This enzyme is produced by the antigen presenting cells and may inhibit the expression of class II histocompatibility molecules. However, when effector cells are activated by inflammatory stimuli, important amounts of NO are synthesized, causing modifications in the cellular microenvironment[43].
At high concentrations, NO inhibits the synthesis of IL-12 and apoptosis, contributing to regulating the TH1/TH2 balance since TH1 cells are more susceptible to this process than TH2 cells[44]. In addition, this favours the proliferation of regulatory T cells during the acute experimental infection and inhibits the expression of molecules involved in adhesion and migration of cells. NO exerts its cytotoxic function on T. cruzi, affecting growth factors, for example by nitrosylation of the haem group, reducing the availability of iron. It is also the most important mediator in the destruction of intracellular amastigotes[45]; however, it has been shown that an excess of NO has harmful effects on the host’s tissues[45,46]. In this sense, the results obtained in our studies are in agreement with these authors. In fact vaccinated animals revealed a modulation of NO levels, and the subsequent absence of lesions in the host, unlike the control group, which showed significantly increased levels of this metabolite[31].
Meanwhile, with respect to the cells, macrophages play an indispensable role in the primary response to pathogens but also take part in the resolution process of the inflammation and homeostasis of tissues. The function of macrophages is polarized towards an inflammatory or a regulatory profile, depending on the microenvironment they are in[47]. This cell population can be activated by classical way (type 1) dependent on IFNγ and TNFα, or by an alternative way (type 2) stimulated by IL-4 and IL-13[48]. Classically activated ones are currently grouped in M1, alternatively activated ones in M2a, those that polarize towards a TH2 response in M2b, and those whose stimulation is mediated by glucocorticoids and TGFβ in M2c. Therefore the different types of macrophages are involved in different response to pathogens, tumours and autoimmune diseases, showing markers exclusively associated with the function they play[49]. There is an important consumption of oxygen during the process of phagocytosis. The respiratory burst caused by macrophages and neutrophils is regarded as a powerful microbicidal mechanism. Oxygen free radicals are toxic to pathogens and prevent colonization by microorganisms in tissues. However, most of M1 macrophages’ microbicidal activity is put down to NO and its derivatives like peroxynitrites. NO is produced by activation of iSON, whose substrate is L-arginine. In macrophages, this enzyme is induced by proinflammatory cytokines like TNFα, IFNγ and IL-12.
During the acute phase of the infection by T. cruzi and other protozoa like Leishmania sp[50] and Plasmodium sp, induction to the inflammatory response is necessary to be able to control parasitaemia[51]. However, if the classical activation of the macrophages is not regulated, it can cause severe harm to the host’s tissue, which is why the production of IL-4, IL-10 and TGFβ is very important, because they modulate the action of NO, oxygen reactive metabolites and proinflammatory cytokines. In the early stages of the infection by T. cruzi, the action of soluble mediators like IL-12, IL-18, IFNγ and NO is crucial to inhibit the replication of the parasite.
Induction of arginase has been shown to inhibit the mechanisms involved in eliminating the parasite, among them the activation of T lymphocytes favouring their permanence in tissues. Therefore, evolution of the infection by T. cruzi depends on the magnitude of the TH1 - TH2 response and the macrophages activated classically vs those activated alternatively[52]. Moreover, it has been suggested that the increase in NK cells could act as a “bridge” between the innate and the early adaptive responses[53].
To bring about protection against T. cruzi, CD4+ and CD8+ effector cells need to be generated, which are capable of migrating from lymph nodes to tissues and exert a strong immune response. As it was above mentioned, both types of cells secrete IFNγ, which activates the macrophage in order to exert trypanosomicide activity by means of NO. Antigen presenting cells like macrophages, dendritic cells and B lymphocytes play an essential role in generating effector T lymphocytes, which produce different cytokines involved in the polarization of the TH1 or TH2 immune response[32]. Despite this, T. cruzi is capable of surviving in the host for long periods, which contributes to the development of symptoms and chronic disease[54]. It initially produces immunosupression; however, during infection, large quantities of CD8+ are generated, which circulate towards places where the parasite persists in order to find the antigen; there they exert their effector functions, they acquire an activation phenotype and then become memory cells, responsible for perpetuating the immune response in the face of a second encounter with the antigen[55]. The importance of the function of CD8+ lymphocytes is based on the reduction of the parasite load observed in most of the tissues from which these cells are recruited. On the other hand, the presence of the parasite in tissues might be due to a lack of stimulation for the recruitment of CD8+ or to the fact that the functions of these cells might be inhibited by other populations like CD4+, CD25+ and producers of TGFβ[56].
At a second stage, the immune response mediated by antibodies is very important to control infection. Numerous experimental models with antibody or B cell deficiency have shown high parasitaemia and a low survival rate[57].
T. cruzi infection is known to induce polyclonal activation of B lymphocytes, and as a result hypergammaglobulinemia occurs. Recent studies have shown that most of the activated B lymphocytes do not synthesize specific antibodies during the first days of infection by T. cruzi[58], but they do produce a specific response at the end of the acute stage of the infection. However, this polyclonal activation may also be an important cause of the autoimmune phenomena mediated by autoreactive antibodies. In addition, the proliferation of B cell populations responding specifically to the antigen with poor polyclonal response is associated with resistant strains (C57Bl/6), with IFNγ production and a prevalence of the TH1 profile[59].
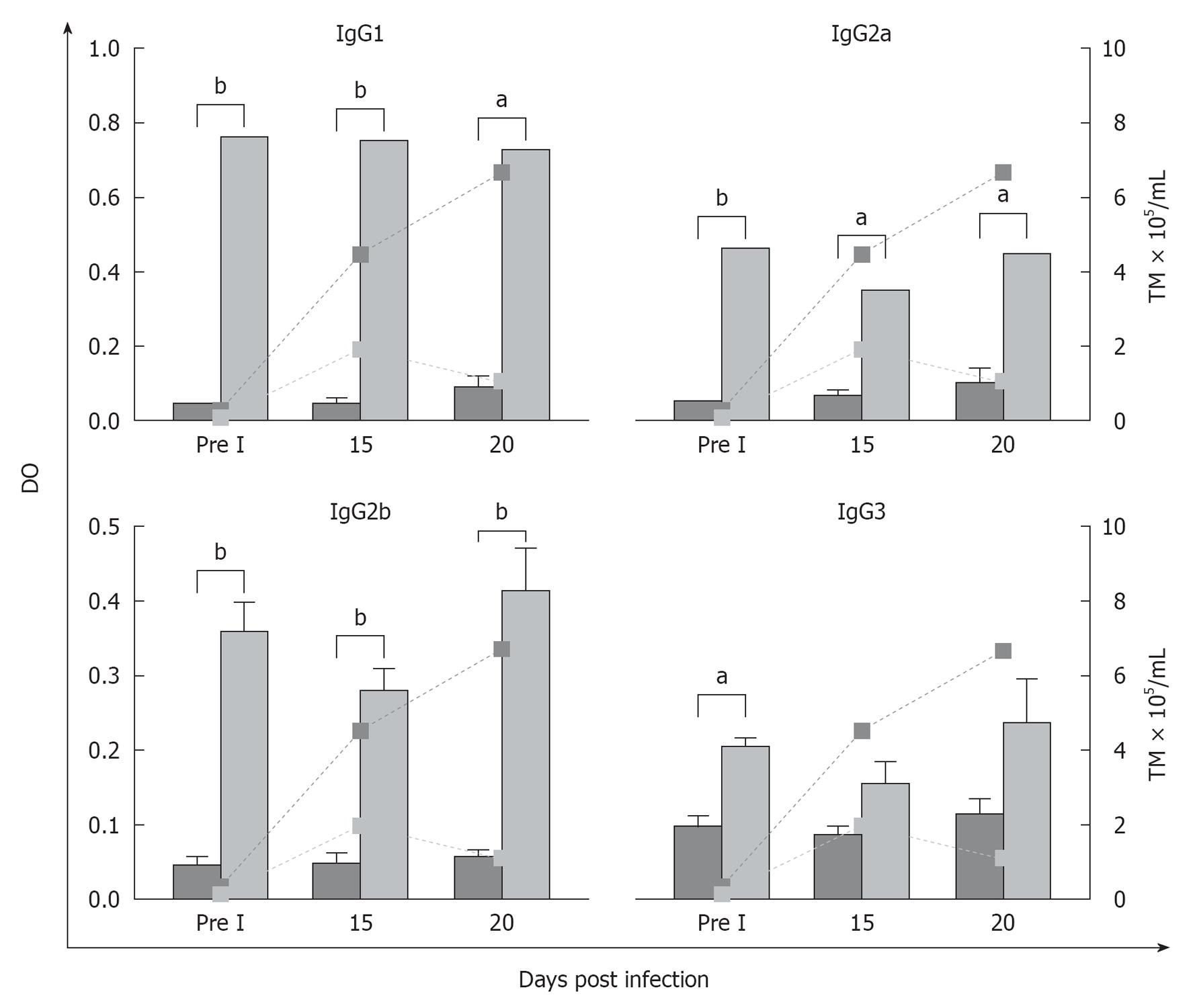
Different immunoglobulin isotypes, mainly IgG subclasses, are involved in the elimination of the parasite at the local and systemic levels by means of mechanisms such as complement fixation, agglutination and cytotoxicity. In this sense, Umekita et al[60] observed that IgG2 might contribute along with other mechanisms to the reduction of parasitaemia in mice infected with T. cruzi upon recognizing the parasitical antigen and acting as opsonin. We observed in our works, in accordance with these authors, that another factor involved in the clearance of the parasite might be associated with high levels of specific antibodies induced by vaccination, which might also act as an early mechanism for controlling the infection[61]. As it is shown the Figure 4, we observed the increase in IgG1 and IgG2 isotypes in peritoneal fluid (the site of the infection) related to different immune response patterns. The ratio IgG1/IgG2a in vaccinated group was 1.6 before infection, 2.1 at 15thpi and 1.6 at 20thpi day. In control mice the ratio was: 0.9; 0.8 and 0.9 respectively. These results showed the importance of TH2, related to antibody response, in vaccinated animals which, together with TH1 response observed through the patterns of cytokines, are both involved in the protection.
Brodskyn et al[62] could reveal the importance of the effector function at the infection site, induced before the challenge, contributing to the reduction of the parasite load. These results are in agreement with those obtained by Gruppi et al[63] who worked with an immunization model using exoantigens of T. cruzi and observed protection associated with increases of IgG1 and IgG2, with low levels of IgG3 at the systemic level. Other authors studying the acute period of the infection detected high levels of IgM, IgG and the isotypic variant IgG1 parallel to the reduction of parasitaemia[64,65]. In our work, IgM, responsible for the specific primary immune response, was high in both experimental groups, in both peritoneal fluid and in plasma. As was expected, the levels detected were always higher in vaccinated animals than in those belonging to the control group[61].
The protective role of the antibodies in the acute phase of the infection is mainly linked to the capacity to induce the elimination of the parasite from circulation, parallel to other cellular events, as we observed in our work, in agreement with others authors[60]. It has been shown that the specific IgG, particularly IgG2, recognizes an important number of parasitical antigens and is able to form microaggregates that fix complement, and increase opsonisation and cytotoxicity mechanisms[66]. In this sense, the neutralization studies developed in our research showed that antibodies and soluble mediators present in the peritoneal fluid of vaccinated mice might be involved in some of the mechanisms responsible for the lysis and reduction in the infectivity of trypomastigotes when these enter the host.
Our findings suggest that the immunogen used in this vaccination model induces an important modulation of the host’s immune response, which are involved in the early clearance of the T. cruzi used in the challenge. Similar results were obtained by Paláu et al[67] and Zuñiga et al[68] when they immunized BALB/c mice with metacyclic trypomastigotes of T. rangeli and later challenged them with a virulent strain of T. cruzi, observing a reduction of the parasitaemia and of the severity of the progress of the infection, with high survival in relation to non immunized and infected mice.
There are also other modulators to the immune response, such as Actinomycetes. Treatment with these actinomycetes significantly reduces acute parasitemia, modifies cell infiltration during acute myocarditis and limits chronic myocarditis in comparison with the infected control group. Similar results were obtained for immunized pregnant mice and then challenged with live T. cruzi[69,70].
These findings are a stimulus to go further in the search for knowledge of immunological events, identifying target cells and molecules, with the goal of advancing in prophylaxis or immuno-intervention, directed towards the development of therapeutic approaches to Chagas disease.
P-Reviewer Catalá A S- Editor Song XX L- Editor A E- Editor Zheng XM
| 1. | Kierszenbaum F, Moretti E, Sztein MB. Molecular basis of Trypanosoma cruzi-induced immunosuppression. Altered expression by activated human lymphocytes of molecules which regulate antigen recognition and progression through the cell cycle. Biol Res. 1993;26:197-207. [PubMed] |
| 2. | Coura JR. Chagas disease: what is known and what is needed--a background article. Mem Inst Oswaldo Cruz. 2007;102 Suppl 1:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Gironès N, Cuervo H, Fresno M. Trypanosoma cruzi-induced molecular mimicry and Chagas’ disease. Curr Top Microbiol Immunol. 2005;296:89-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Bonney KM, Taylor JM, Daniels MD, Epting CL, Engman DM. Heat-killed Trypanosoma cruzi induces acute cardiac damage and polyantigenic autoimmunity. PLoS One. 2011;6:e14571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Vieira M, Dutra JM, Carvalho TM, Cunha-e-Silva NL, Souto-Padrón T, Souza W. Cellular signaling during the macrophage invasion by Trypanosoma cruzi. Histochem Cell Biol. 2002;118:491-500. [PubMed] |
| 6. | de Souza W, de Carvalho TM, Barrias ES. Review on Trypanosoma cruzi: Host Cell Interaction. Int J Cell Biol. 2010;2010:pii: 295394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Ley V, Robbins ES, Nussenzweig V, Andrews NW. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990;171:401-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Andrews NW, Abrams CK, Slatin SL, Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990;61:1277-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 150] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA. Chagas disease. Postgrad Med J. 2006;82:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Grisard EC, Steindel M, Guarneri AA, Eger-Mangrich I, Campbell DA, Romanha AJ. Characterization of Trypanosoma rangeli strains isolated in Central and South America: an overview. Mem Inst Oswaldo Cruz. 1999;94:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Garcia ES, Castro DP, Figueiredo MB, Genta FA, Azambuja P. Trypanosoma rangeli: a new perspective for studying the modulation of immune reactions of Rhodnius prolixus. Parasit Vectors. 2009;2:33. [PubMed] |
| 12. | Vallejo GA, Guhl F, Schaub GA. Triatominae-Trypanosoma cruzi/T. rangeli: Vector-parasite interactions. Acta Trop. 2009;110:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Basso B, Moretti ER, Vottero-Cima E. Immune response and Trypanosoma cruzi infection in Trypanosoma rangeli-immunized mice. Am J Trop Med Hyg. 1991;44:413-419. [PubMed] |
| 14. | Basso B, Moretti E, Vottero-Cima E. Comportamiento antigénicodel Trypanosoma cruzi y del Trypanosoma rangeli frente a sueros de pacientes con Enfermedad de Chagas. Medicina (B. Aires). 1984;44:475-479. |
| 15. | Moretti ER, Gruppi A, Basso B, Vottero-Cima E. Exoantigens of Trypanosoma cruzi. II. Physicochemical properties. Rev Argent Microbiol. 1987;19:139-144. [PubMed] |
| 16. | Chiurillo MA, Crisante G, Rojas A, Peralta A, Dias M, Guevara P, Añez N, Ramírez JL. Detection of Trypanosoma cruzi and Trypanosoma rangeli infection by duplex PCR assay based on telomeric sequences. Clin Diagn Lab Immunol. 2003;10:775-779. [PubMed] |
| 17. | Afchain D, Le Ray D, Fruit J, Capron A. Antigenic make-up of Trypanosoma cruzi culture forms: identification of a specific component. J Parasitol. 1979;65:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Grögl M, Kuhn RE. Identification of antigens of culture forms of Trypanosoma cruzi and Trypanosoma rangeli recognized by sera from patients with chronic Chagas’ disease. J Parasitol. 1984;70:822-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Basso B, Morett ER, Dfontela S, VotteroCima E. Trypanosoma (Schizotrypanum) cruzi and Trypanosoma (Herpetosoma) rangeli. II Overlapping of antigenic spectrum. Rev Lat Microbiol. 1989;31:141-146. |
| 20. | Saldaña A, Sousa OE. Trypanosoma rangeli: epimastigote immunogenicity and cross-reaction with Trypanosoma cruzi. J Parasitol. 1996;82:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Silva MT. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J Leukoc Biol. 2010;87:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Introini MV, Basso B, Moretti E. [Experimental Chagas’ disease: I. Study of different immunization conditions in the infection course]. Bol Chil Parasitol. 1998;53:45-51. [PubMed] |
| 23. | Basso B, Moretti E, Fretes R. Vaccination with epimastigotes of different strains of Trypanosoma rangeli protects mice against Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2008;103:370-374. [PubMed] |
| 24. | Basso B, Moretti E, Fretes R. Vaccination with epimastigotes of different strains of Trypanosoma rangeli protects mice against Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2008;103:370-374. [PubMed] |
| 25. | Cazorla SI, Becker PD, Frank FM, Ebensen T, Sartori MJ, Corral RS, Malchiodi EL, Guzmán CA. Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect Immun. 2008;76:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Brener Z, Gazzinelli RT. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas’ disease. Int Arch Allergy Immunol. 1997;114:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 227] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Camargo MM, Andrade AC, Almeida IC, Travassos LR, Gazzinelli RT. Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania species membranes trigger nitric oxide synthesis as well as microbicidal activity in IFN-gamma-primed macrophages. J Immunol. 1997;159:6131-6139. [PubMed] |
| 28. | Sathler-Avelar R, Vitelli-Avelar DM, Teixeira-Carvalho A, Martins-Filho OA. Innate immunity and regulatory T-cells in human Chagas disease: what must be understood. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Cunha-Neto E, Nogueira LG, Teixeira PC, Ramasawmy R, Drigo SA, Goldberg AC, Fonseca SG, Bilate AM, Kalil J. Immunological and non-immunological effects of cytokines and chemokines in the pathogenesis of chronic Chagas disease cardiomyopathy. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | da Matta Guedes PM, Gutierrez FR, Maia FL, Milanezi CM, Silva GK, Pavanelli WR, Silva JS. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis. 2010;4:e604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Basso B, Cervetta L, Moretti E, Carlier Y, Truyens C. Acute Trypanosoma cruzi infection: IL-12, IL-18, TNF, sTNFR and NO in T. rangeli-vaccinated mice. Vaccine. 2004;22:1868-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Cardillo F, Postol E, Nihei J, Aroeira LS, Nomizo A, Mengel J. B cells modulate T cells so as to favour T helper type 1 and CD8+ T-cell responses in the acute phase of Trypanosoma cruzi infection. Immunology. 2007;122:584-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Cervetta L, Moretti E, Basso B. Experimental Chagas’ disease: the protection induced by immunization with Trypanosoma rangeli is associated with down-regulation of IL-6, TNF-α, and IL-10 synthesis. Acta Parasitol. 2002;47:73-78. |
| 34. | Chandrasekar B, Melby PC, Troyer DA, Freeman GL. Induction of proinflammatory cytokine expression in experimental acute Chagasic cardiomyopathy. Biochem Biophys Res Commun. 1996;223:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Silva JS, Morrissey PJ, Grabstein KH, Mohler KM, Anderson D, Reed SG. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 285] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128-134. [PubMed] |
| 37. | Reed SG. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988;140:4342-4347. [PubMed] |
| 38. | Revelli S, Didoli G, Roggero E, Moreno H, Bernabo J, Wietzerbin J, Bottasso O. Macrophage activity, IL-6 levels, antibody response and heart histology in rats undergoing an attenuated Trypanosoma cruzi acute infection upon treatment with recombinant interferon gamma. Cytokines Cell Mol Ther. 1998;4:153-159. [PubMed] |
| 39. | Reed SG, Brownell CE, Russo DM, Silva JS, Grabstein KH, Morrissey PJ. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol. 1994;153:3135-3140. [PubMed] |
| 40. | Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Moretti E, Basso B, Cervetta L, Brigada A, Barbieri G. Patterns of cytokines and soluble cellular receptors in the sera of children with acute chagas’ disease. Clin Diagn Lab Immunol. 2002;9:1324-1327. [PubMed] |
| 42. | Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 3053] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 43. | Gutierrez FR, Mineo TW, Pavanelli WR, Guedes PM, Silva JS. The effects of nitric oxide on the immune system during Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Xiao BG, Ma CG, Xu LY, Link H, Lu CZ. IL-12/IFN-gamma/NO axis plays critical role in development of Th1-mediated experimental autoimmune encephalomyelitis. Mol Immunol. 2008;45:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Silva JS, Machado FS, Martins GA. The role of nitric oxide in the pathogenesis of Chagas disease. Front Biosci. 2003;8:s314-s325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Stempin CC, Dulgerian LR, Garrido VV, Cerban FM. Arginase in parasitic infections: macrophage activation, immunosuppression, and intracellular signals. J Biomed Biotechnol. 2010;2010:683485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | de Meis J, Morrot A, Farias-de-Oliveira DA, Villa-Verde DM, Savino W. Differential regional immune response in Chagas disease. PLoS Negl Trop Dis. 2009;3:e417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Mylonas KJ, Nair MG, Prieto-Lafuente L, Paape D, Allen JE. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J Immunol. 2009;182:3084-3094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Novais FO, Santiago RC, Báfica A, Khouri R, Afonso L, Borges VM, Brodskyn C, Barral-Netto M, Barral A, de Oliveira CI. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol. 2009;183:8088-8098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Peluffo G, Piacenza L, Irigoín F, Alvarez MN, Radi R. L-arginine metabolism during interaction of Trypanosoma cruzi with host cells. Trends Parasitol. 2004;20:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Stempin C, Giordanengo L, Gea S, Cerbán F. Alternative activation and increase of Trypanosoma cruzi survival in murine macrophages stimulated by cruzipain, a parasite antigen. J Leukoc Biol. 2002;72:727-734. [PubMed] |
| 53. | Vitelli-Avelar DM, Sathler-Avelar R, Massara RL, Borges JD, Lage PS, Lana M, Teixeira-Carvalho A, Dias JC, Elói-Santos SM, Martins-Filho OA. Are increased frequency of macrophage-like and natural killer (NK) cells, together with high levels of NKT and CD4+CD25high T cells balancing activated CD8+ T cells, the key to control Chagas’ disease morbidity. Clin Exp Immunol. 2006;145:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Tzelepis F, de Alencar BC, Penido ML, Gazzinelli RT, Persechini PM, Rodrigues MM. Distinct kinetics of effector CD8+ cytotoxic T cells after infection with Trypanosoma cruzi in naive or vaccinated mice. Infect Immun. 2006;74:2477-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Tzelepis F, Persechini PM, Rodrigues MM. Modulation of CD4(+) T cell-dependent specific cytotoxic CD8(+) T cells differentiation and proliferation by the timing of increase in the pathogen load. PLoS One. 2007;2:e393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Padilla AM, Bustamante JM, Tarleton RL. CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol. 2009;21:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Kumar S, Tarleton RL. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 1998;20:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Bermejo DA, Amezcua-Vesely MC, Montes CL, Merino MC, Gehrau RC, Cejas H, Acosta-Rodríguez EV, Gruppi A. BAFF mediates splenic B cell response and antibody production in experimental Chagas disease. PLoS Negl Trop Dis. 2010;4:e679. [PubMed] |
| 59. | Bryan MA, Guyach SE, Norris KA. Specific humoral immunity versus polyclonal B cell activation in Trypanosoma cruzi infection of susceptible and resistant mice. PLoS Negl Trop Dis. 2010;4:e733. [PubMed] |
| 60. | Umekita LF, Mota I. How are antibodies involved in the protective mechanism of susceptible mice infected with T. cruzi. Braz J Med Biol Res. 2000;33:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Marini V, Moretti E, Bermejo D, Basso B. Vaccination with Trypanosoma rangeli modulates the profiles of immunoglobulins and IL-6 at local and systemic levels in the early phase of Trypanosoma cruzi experimental infection. Mem Inst Oswaldo Cruz. 2011;106:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Brodskyn CI, Silva AM, Takehara HA, Mota I. IgG subclasses responsible for immune clearance in mice infected with Trypanosoma cruzi. Immunol Cell Biol. 1989;67:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Gruppi A, Pistoresi-Palencia MC, Cerban F, Vottero-Cima E. Trypanosoma cruzi exoantigens: can those recognized by sera from chagasic patients trigger a protective immune response in mice. Res Immunol. 1991;142:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Carneiro CM, Martins-Filho OA, Reis AB, Veloso VM, Araújo FM, Bahia MT, de Lana M, Machado-Coelho GL, Gazzinelli G, Correa-Oliveira R. Differential impact of metacyclic and blood trypomastigotes on parasitological, serological and phenotypic features triggered during acute Trypanosoma cruzi infection in dogs. Acta Trop. 2007;101:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Coura-Vital W, Carneiro CM, Martins HR, de Lana M, Veloso VM, Teixeira-Carvalho A, Bahia MT, Corrêa-Oliveira R, Martins-Filho OA, Tafuri WL. Trypanosoma cruzi: immunoglobulin isotype profiles during the acute phase of canine experimental infection with metacyclic or blood trypomastigotes. Exp Parasitol. 2008;120:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Takehara HA, Mota I. The possible mechanism of action of IgG antibodies and platelets protecting against Trypanosoma cruzi infection. Braz J Med Biol Res. 1991;24:759-765. [PubMed] |
| 67. | Paláu MT, Mejía AJ, Vergara U, Zúñiga CA. Action of Trypanosoma rangeli in infections with virulent Trypanosoma cruzi populations. Mem Inst Oswaldo Cruz. 2003;98:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Zuñiga C, Palau T, Penin P, Gamallo C, de Diego JA. Protective effect of Trypanosoma rangeli against infections with a highly virulent strain of Trypanosoma cruzi. Trop Med Int Health. 1997;2:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Fontanella GH, Pascutti MF, Daurelio L, Perez AR, Nocito AL, Wojdyla D, Bottasso O, Revelli SS, Stanford JL. Improved outcome of Trypanosoma cruzi infection in rats following treatment in early life with suspensions of heat-killed environmental Actinomycetales. Vaccine. 2007;25:3492-3500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Davila H, Didoli G, Bottasso O, Stanford J. Maternal immunization with actinomycetales immunomodulators reduces parasitemias in offspring challenged with Trypanosoma cruzi. Immunotherapy. 2011;3:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |