INTRODUCTION
It was in 1921 when abdominal migraine (AM) was first described[1]. Thanks to efforts by previous investigators[2], AM is now regarded as the abdominal equivalent to migraine and has been classified not only in the International Classification of Headache Disorders[3], but also in the Rome III criteria for functional gastrointestinal disorders[4]. To date, the mechanism of AM still remains unknown. In this review, we will present recent studies on AM and speculate its mechanism from both peripheral and central nervous system aspects, to add new knowledge to our understanding of this disease.
CENTRAL SYMPTOMS ACCOMPANYING ABDOMINAL PAIN IN AM
Although the main symptom of AM exists at the peripheral level, as the word “abdominal” indicates in the disease name, previous studies have reported possible dysfunction of central nervous system, including photophobia, phonophobia and abnormal visual evoked response[5,6]. The percentage of visual disturbance and phonophobia in AM were reported to be about 20% and 10%, respectively[5,7]. As expected, these percentages were markedly lower than that of migraine, for which 77% were reported to have photophobia, and 69% had phonophobia[5]. Mortimer and Good showed similar response pattern in visual evoked potential in cases with migraine and AM, e.g., higher amplitude of fast wave activity than healthy controls and the presence of paroxysmal sharp wave activity[6]. The existence of central nervous system symptoms in both AM and migraine indicates that AM is not only a peripheral disorder, but may also carry certain CNS process similar to migraine.
Patients with AM were reported to have central nervous system hypersensitivity. Recently, Hamed reported a case with AM coexisting with Alice in Wonderland syndrome (AWS)[8]. The symptoms are characterized with perceptual abnormalities, distortion of body image, and alternation in subjective time sense, as Alice has in the story written by Lewis Carroll[9]. AWS can also be observed in patients with migraine[10]. The 20-year-old patient, as presented in the case from Hamed, had abdominal colic pain, which fits to diagnostic criteria of AM. This patient had a strong family history of migraine. Hamed stated that, despite normal brain magnetic resonance imaging and electroencephalography, medical examinations including transcranial magnetic stimulation and evoked potentials revealed enhanced cortical excitability in multiple brain lesions[8].
PERIPHERAL SYMPTOM ACCOMPANYING TO ABDOMINAL PAIN IN AM
AM belongs to the category of functional gastrointestinal disorders, which are thought to be related to background visceral hypersensitivity[4]. Recently, we reported a peculiar phenomenon seen in a patient diagnosed with AM who had ecchymosis in the legs and buttocks associated with abdominal pain[11]. No currently known hypothesis could clearly explain the peculiar phenomenon. It is known that a subset of patients with migraine have similar ecchymosis in areas covered with trigeminal nerve, where the main site for migraine headache would be[12,13]. Wesselmann and Lai showed that, in their rat model, insult to a pelvic organ resulted in extravasation in the skin region innerved by the affected nerves[14]. In the process of investigating the mechanism of referred pain from visceral organ, they developed a rat model for pain caused by uterine inflammation. Experimental visceral inflammation in their rats pretreated with evans blue dye resulted in dye extravasation in the skin over the abdomen, groin, lower back, thighs, perineal area and proximal tail. They speculated that the neuronal pathway accounting for the phenomenon may include dichotomizing afferent fibers, afferent-afferent interactions via a spinal cord pathway, or a sympathetic reflex[14]. When viewed in terms of human anatomy, the ecchymosis of legs and buttock can be accounted with neuronal input of visceral pain to sacral segment of spinal cord[15]. Based on these reports, we hypothesize the following mechanism to explain the peculiar skin phenomenon in our case[16]. First, under predisposing visceral hypersensitivity associated with AM, the visceral nerves responsible for abdominal nociception, especially those innerved by the sacral levels of spinal cord, are activated. This change then results in the occurrence of ecchymosis in the legs and buttock.
POSSIBLE MECHANISM OF TRIPTAN TO TREAT AM-RELATED PAIN
Triptan (serotonergic agonists) has been to treat acute symptom of AM[7,11,16]. This agent has been generally thought to act on several regions, e.g., 5-HT1B receptors on the meningeal vasculature, and 5-HT1D receptors on trigeminal nerve terminals projecting to the dural vasculature and to the brain stem trigeminal nuclei[17]. Jeong et al[18] reported that, using their Sprague-Dawley rat model, sumatriptan brought pain relief by inhibiting GABAergic and glutamatergic synaptic transmission within the midbrain periaqueductal grey matter (PAG), a center of pain control, via a 5-hydroxytryptamine (5-HT) 1B and 5-HT1D receptor mediated decrease in neurotransmitter release[18]. Their result supported the central sensitization mechanism of AM pain relief. It is important to note that, central effect of sumatriptan may not be the only way to pain relief from a report of Vera-Portocarrero’s group[19]. They evaluated in two models the effects of systemic/rostral ventromedial medulla (RVM) sumatriptan administration on visceral pain, and the role of RVM, a center of pain modulation, in the process of pain relief by sumatriptan. They developed a rat model for experimental pancreatitis by intravenous injection of dibutyltin dichloride, and another rat model for irritable bowel syndrome by intracolonic instillation of sodium butyrate. They observed the effects of systemic/RVM administration of 5-HT1B/D antagonists on systemic/RVM sumatriptan action. Systemic sumatriptan elicited a dose- and time-related blockade to pain in both models that was blocked by systemic administration of either 5-HT1B or 5-HT1D antagonists, but not by RVM administration of these agents. Sumatriptan administered into the RVM similarly produced dose and time-related blockade of referred hypersensitivity in both models. The pain was blocked by RVM administration of the 5-HT1B antagonists but not the 5-HT1D antagonists. Based on the results, the authors speculated that sumatriptan suppresses either inflammatory or noninflammatory visceral pain, most likely through peripheral 5-HT1B/D receptors. They also mentioned that actions at 5-HT1B receptors within the RVM offer an additional potential site of action for the modulation of visceral pain by triptans.
CONCLUSION
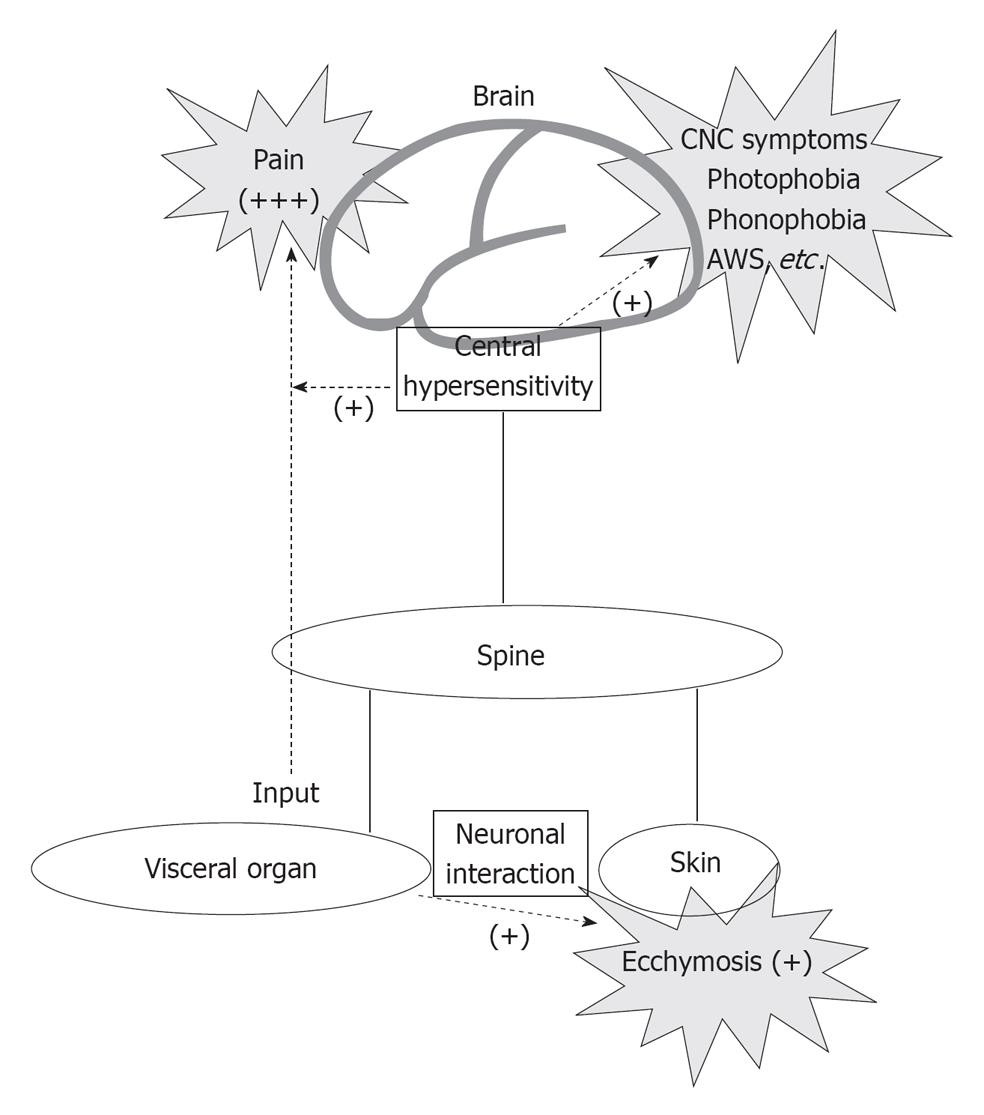
We hypothesize a comprehensive central and peripheral interaction schema to explain the symptoms of AM. As shown in Figure 1, visceral organ produced pain and increased cortical excitability, which in turn induce central symptoms such as photophobia, phonophobia, and AWS; with intricate interactions from the central and peripheral nervous system, effects such as ecchymosis are shown on the peripheral level. Although further investigations and accumulation of AM cases are still needed, we believe that, the schema hypothesized here is helpful to plan further experimental approach to clarify the mechanism of this peculiar disease.
Figure 1 The central and peripheral network for symptoms relating to abdominal migraine is shown.
CNS: Central nervous system; AWS: Alice wonderland syndrome.
ACKNOWLEDGMENTS
The corresponding author dedicates this paper to his wife Haruka and his lovely kids Atsushi and Satomi, and also to his family and colleagues who have always given him intellectual and moral support.
Peer reviewer: Feng Tao, MD, PhD, Assistant Professor, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave. / Ross 355, Baltimore, MD 21025, United States
S- Editor Li JY L- Editor A E- Editor Zheng XM