Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.108025
Revised: May 16, 2025
Accepted: July 16, 2025
Published online: September 20, 2025
Processing time: 131 Days and 17.9 Hours
Smoking is a leading cause of carcinogenesis in the head and neck region, representing a critical public health issue. Identifying genotoxic damage in smokers can provide valuable insights for developing preventive interventions.
To assess genotoxic damage through the micronucleus assay in exfoliated buccal mucosa cells from users of conventional tobacco, reverse smoking, cannabis, electronic cigarettes, and non-smokers.
A cross-sectional study was conducted with 100 participants divided into five groups: 20 conventional tobacco smokers, 20 reverse smokers, 20 electronic cigarette users, 20 cannabis users, and 20 non-smokers. Exfoliated buccal mucosa cells were analyzed using Giemsa and Papanicolaou staining to identify micronuclei (MN) as markers of genotoxic damage.
MN were present in 86% of the samples. Statistically significant differences were observed in the median micronucleus count between conventional, reverse, and electronic cigarette smokers compared to non-smokers (P < 0.001), while no significant difference was found for cannabis smokers (P = 0.89). A significant correlation was identified between the presence of oral lesions and micronucleus count (P = 0.03). Regression analysis ruled out alcohol as a confounding factor.
This study identified genotoxic damage associated with various smoking habits, except for cannabis use, highlighting the need for public health interventions to reduce smoking and mitigate its genotoxic effects. These findings provide a foundation for future research and the implementation of preventive policies.
Core Tip: This study reveals significant genotoxic damage in buccal mucosa cells among conventional, reverse, and electronic cigarette smokers, but not cannabis users, using the micronucleus assay. A strong correlation was found between smoking-related oral lesions and micronucleus frequency, independent of alcohol use. These findings underscore the urgent need for targeted public health strategies to reduce smoking-induced DNA damage and prevent oral carcinogenesis. The results provide critical evidence for policymakers and future research on tobacco-related harm.
- Citation: Álvarez-Martínez E, Porto-Puerta IE, Ardila CM. Genotoxic damage assessment using the micronucleus assay in buccal mucosa of different types of smokers: A cross-sectional study. World J Exp Med 2025; 15(3): 108025
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/108025.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.108025
Cancer is a complex group of chronic diseases characterized by uncontrolled cellular proliferation, tissue invasion, and metastatic potential. Collectively, these diseases are one of the leading causes of global mortality[1]. The Pan American Health Organization reported 1.4 million cancer deaths in the Americas during 2022, 45.1% of which occurred prematurely in individuals under 69 years of age[2]. Current epidemiological projections indicate the regional cancer burden will grow from 4.2 million incident cases in 2022 to approximately 6.7 million by 2045 due to both demographic transitions and persistent exposure to modifiable risk factors[1-3].
Tobacco consumption remains the predominant preventable carcinogen exposure and accounts for approximately 22% of cancer-related deaths worldwide[2]. The combustion of conventional tobacco generates over 70 known carcinogens, and smokers are at 5–25 times greater risk for aerodigestive cancers than non-smokers[3,4]. Despite extensive tobacco-control measures, including taxation, advertising restrictions, and public health campaigns, smoking remains prevalent worldwide due to nicotine addiction’s potent neurobiological mechanisms and the tobacco industry’s evolving strategies for targeting younger demographics[3-5]. This persistent challenge highlights critical gaps in the understanding of tobacco-related carcinogenesis and the need for innovative prevention approaches.
The emergence of electronic nicotine delivery systems (ENDS) has significantly increased the variety of tobacco products available. ENDS aerosolize liquid solutions containing propylene glycol, vegetable glycerin, flavor additives, and frequently high nicotine concentrations[3]. Recent surveillance data from the United States show alarming threefold annual increases in adolescent ENDS use, and prevalence estimates exceed 20% among high school populations[1,4]. While the long-term oncogenic risks of ENDS remain undefined, emerging evidence associates their use with respiratory symptoms, vascular dysfunction, and histopathological pulmonary changes[5]. This uncertainty underscores the importance of assessing potential genotoxic effects using molecular epidemiology.
Cannabis sativa is another increasingly smoked substance with debated carcinogenic potential. With nearly 50% of adults reporting lifetime cannabis use, cannabis is the most widely consumed illicit drug globally, and consumption continues to increase[6]. The literature contains contradictory findings regarding cannabis smoke and aerodigestive carcinogenesis. Some studies have implicated cannabis combustion products in causing oral epithelial dysplasia through mechanisms analogous to those of tobacco smoke[7]. Conversely, large consortium studies like INHANCE have not demonstrated significant cancer associations among cannabis users who abstain from tobacco and alcohol[7]. Investigators have even proposed therapeutic applications for cannabinoids in cancer management, citing their analgesic, antiemetic, and anti-inflammatory properties[7,8].
Reverse smoking, a culturally entrenched practice among specific Afro-descendant and Southeast Asian populations, presents unique public health challenges. This practice involves igniting the lit end of tobacco products inside the oral cavity, generating intense local heat and concentrated smoke exposure[8]. While observational studies suggest associations between the practice and mucosal carcinomas, cultural barriers and small population sizes hamper rigorous investigation[8]. The persistent uncertainty surrounding various smoking modalities and their carcinogenic potentials creates significant obstacles to evidence-based prevention strategies.
Molecular epidemiology offers powerful tools for addressing the uncertainties surrounding smoked substances and smoking modalities. Salivary biomarkers such as Cyclin D1, p53, and matrix metalloproteinases can be used for non-invasive diagnosis, but high costs and technical requirements constrain their use[10,11]. The micronucleus assay of exfoliated oral mucosa cells has emerged as a practical alternative, demonstrating 90% sensitivity and 94% specificity for detecting genotoxic damage in oral carcinogenesis[12-14]. Micronuclei (MN) formation reflects chromosomal instability and correlates strongly with p53 mutations and thus serves as a sensitive indicator of both local and systemic genotoxic stress[12]. While this technique cannot distinguish between specific etiologies of DNA damage, its cost-effectiveness and technical accessibility make it particularly valuable for population-level studies in resource-limited settings[13,14].
The present study addresses critical evidence gaps regarding different smoking modalities’ comparative genotoxic risks. Our primary objective was to quantify micronucleus frequencies in buccal mucosal cells in five distinct groups: Conventional tobacco smokers, reverse tobacco smokers, cannabis users, electronic cigarette users, and non-smokers. Our secondary aims included comprehensively recording sociodemographic factors, systematically documenting oral mucosal lesions, and analyzing potential correlations between micronucleus indices and clinically detectable pathology. We hypothesized that the exposure groups would differ significantly in genotoxic damage, with traditional tobacco smokers demonstrating the highest micronucleus frequencies. The null hypothesis posits no intergroup differences in genotoxic damage and no association between micronucleus counts and oral lesions.
A cross-sectional observational study was conducted. Sample collection took place from June 2021 to December 2023, and data analysis was completed in March 2024.
The sample size was determined using the formula for finite populations, considering an estimated total population of two million smokers in our country. A hypothetical frequency of 50% ± 5% was assumed due to the lack of prior evi
Based on the results obtained from OpenEpi version 3, the required sample size for an 85% confidence level was 100 subjects. Thus, the study included 100 individuals, selected through non-probabilistic convenience sampling.
Participants were recruited from the Faculty of Dentistry at the University and from individuals receiving medical and dental care in urban and rural areas of the country. Recruitment was conducted verbally in dental and maxillofacial surgery consultations, as well as through mass media and social networks.
Subjects were divided into five equal groups of 20 participants: (1) Conventional tobacco smokers; (2) Reverse smokers; (3) Electronic cigarette smokers; (4) Cannabis users; and (5) Control group (non-smokers).
All subjects had to be over 18 years old. This criterion ensured that participants had the legal capacity to provide informed consent and that their smoking habits were well-established.
Informed consent: Participants were required to provide written informed consent, detailing the study objectives, procedures, potential risks and benefits, and data confidentiality.
Active smoking practice: Conventional tobacco smokers: Individuals who had been daily smokers of traditional cigarettes for at least two years.
Reverse smokers: Individuals practicing reverse smoking, a culturally specific form of smoking, for at least two years.
Electronic cigarette smokers: Regular users of electronic cigarettes with a daily use history of at least two years.
Cannabis users: Individuals who regularly smoked cannabis for at least two years.
Non-smokers: A control group composed of individuals who had never smoked.
Multiple substance use: Individuals engaging in more than one type of smoking simultaneously to avoid confounding results from combined substance effects.
Use of unstudied substances: Smokers of substances not included in the study, such as opioids or methamphetamines, which could alter research outcomes.
History of malignant neoplasms: Individuals diagnosed and/or treated for any type of cancer before inclusion in the study, to prevent potential confounding effects of oncological treatments and disease impact.
Autoimmune diseases: Subjects with diagnosed or suspected autoimmune diseases, as these conditions could affect immune responses and measured parameters.
Cardiac diseases: Individuals with diagnosed heart disease, as these conditions may influence general health and biomarker levels.
Neurological and/or psychiatric disorders: Participants with diagnosed neurological or psychiatric disorders, as these conditions could interfere with study comprehension and results.
Exposure to environmental genotoxic agents: Individuals with a history of significant exposure to genotoxic agents in occupational or residential settings (e.g., radiation or industrial chemicals), which could influence micronucleus formation and confound results.
These inclusion and exclusion criteria were designed to ensure sample homogeneity and result validity, allowing proper comparison between smoking groups and the control group.
A meticulous and standardized data collection process was conducted by two experienced clinicians specialized in pathology and maxillofacial surgery. These professionals underwent prior retraining and calibration in stomatological diagnosis to ensure consistency and precision in subject evaluation. The calibration process resulted in high inter-examiner agreement, with an intraclass correlation coefficient ranging from 0.90 to 0.98, indicating excellent measurement reliability.
Informed consent: Before sample collection, all participants signed an informed consent form. This document provided detailed information on the study’s objectives, procedures, potential risks and benefits, and confidentiality guarantees. It ensured that participants fully understood the study and voluntarily agreed to participate.
Structured interview: Each participant underwent a structured interview designed to obtain detailed in-formation on smoking habits, alcohol consumption, medical history, and demographic background. The interview included specific questions regarding smoking frequency, duration, substance type, and other relevant risk factors.
Comprehensive stomatological examination: Clinicians performed a systematic and thorough stomatological examination on each subject. This assessment included visual inspection and palpation of the oral cavity to detect lesions, abnormalities, or signs of oral diseases. The evaluations were conducted in accredited dental offices, and findings were recorded in a standardized form to ensure data consistency.
Pilot study and protocol validation: Before clinical sample collection, a pilot study was conducted to design and validate the sample collection, fixation, staining, and microscopic analysis protocol. This protocol was based on the method described by Walsh et al[13] and was adapted to ensure sample viability and high quality. During this phase, tongue depressors were discarded due to their low efficiency in collecting viable cells, and Ziehl-Neelsen staining was excluded due to its poor micronucleus marking.
Exfoliative cytology of the oral mucosa: Exfoliative cytology was performed on the buccal mucosa to obtain epithelial cell samples. The procedure involved gently scraping the buccal mucosa with a cytology brush, making five 360-degree rotations. The collected cells were smeared onto two clean slides with alcohol fixation.
Sample fixation: The prepared slides were air-dried and fixed with 95% alcohol for 30 minutes to preserve cellular integrity and prevent degradation.
Sample transport and storage: Fixed samples were transported in airtight containers to a specialized laboratory, ensuring appropriate storage conditions to prevent contamination or deterioration.
Staining and microscopic analysis: In the laboratory, samples were stained with 5% Giemsa and Papanicolaou techniques, which allowed clear visualization of nuclear and cytoplasmic characteristics. Micronucleus analysis was performed using an optical microscope with 40 × objective magnification and a 10 × ocular lens, employing a coded slide system to maintain evaluator blinding.
Quality control: Strict quality control measures were implemented throughout the process, from sample collection to microscopic analysis, to ensure data accuracy and reliability. Clinicians conducted periodic reviews and recalibrations to maintain high inter-examiner agreement.
Slide coding: To maintain evaluator blinding and prevent bias, all slides were coded, ensuring that analysts had no information regarding sample origin during analysis.
Cell observation: A total of 4000 cells per individual were examined. This number was selected to provide a representative sample of exfoliated cells and ensure accuracy in micronucleus detection.
Micronucleus measurement and recording: Results were expressed as the median number of MN per 1000 cells, providing a robust representation that minimizes susceptibility to outliers.
The laboratory analysis was conducted by a team of highly trained professionals specializing in micronucleus assay interpretation. This team belonged to the Colegio Mayor de Antioquia University Institution, ensuring a high level of precision and re-liability in the results obtained. Samples from Bolívar were directly transported to the institution by the clinicians, adhering to strict quality control protocols.
This meticulous approach to sample collection and analysis guarantees the validity and reproducibility of the data, contributing significantly to understanding the impact of smoking and other habits on oral health.
Exfoliative cytology samples from the oral mucosa were classified into three categories based on the MN observed per 1000 cells. This classification was developed by the authors based on prior studies such as those by Walsh et al[13] and Titenko et al[15]:
Low (1 to 2 MN): Samples in this category exhibited a minimal MN, potentially indicating low exposure to genotoxic agents.
Moderate (3 to 5 MN): Samples classified in this range showed an intermediate MN, suggesting moderate exposure to genetic damage-inducing factors.
Abundant (6 to 8 MN): These samples exhibited a high MN, reflecting increased exposure to genotoxic agents and a heightened risk of genetic damage.
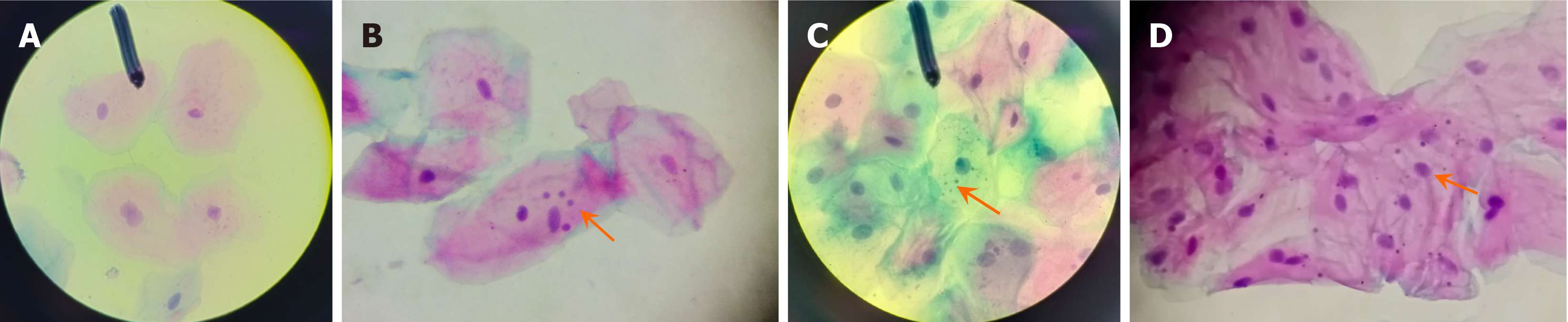
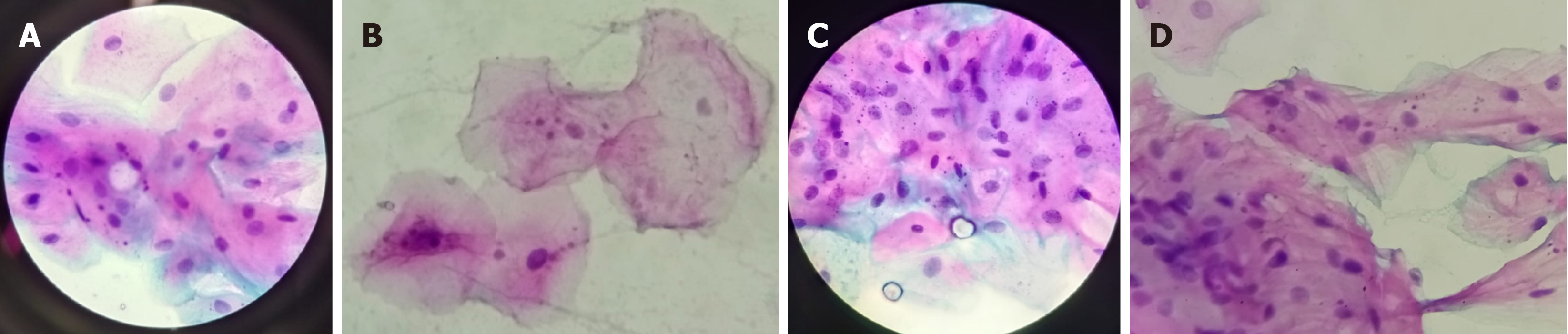
Only cells that were intact, non-fragmented, non-overlapping, and free from ac-cumulation were considered. Cells undergoing degeneration processes such as karyorrhexis or karyolysis were excluded from the analysis to ensure accuracy and consistency. The criteria for identifying MN followed the recommendations of Titenko et al[15], ensuring the validity and reliability of the observations (Figure 1).
The primary variable of interest was the presence and quantity of MN in exfoliative cytology samples from the oral mucosa. This served as an indicator of genetic damage in epithelial cells.
The secondary variables encompassed various sociodemographic characteristics, habits, and other factors potentially influencing micronucleus presence. These were classified as follows.
Sociodemographic characteristics: (1) Gender: Male or female; (2) Socioeconomic status: Classified according to national socioeconomic stratification standards; (3) Educational level: Highest education level attained (primary, secondary, tertiary); (4) Living area: Urban or rural; and (5) Origin: Place of residence.
Alcohol consumption habits: Alcohol consumption intensity: Categorized as occasional, moderate, or heavy, based on frequency and quantity consumed.
Smoking type: (1) Conventional tobacco smokers: Individuals who consume traditional cigarettes; (2) Reverse smokers: Individuals practicing reverse smoking; (3) E-cigarette users: Individuals using electronic smoking devices; and (4) Cannabis users: Individuals who regularly smoke cannabis.
Smoking intensity: (1) Daily consumption: Number of cigarettes, reverse smoking sessions, e-cigarette use, or cannabis consumption per day; and (2) Duration of habit: Years the participant has engaged in smoking.
General health status: Presence of chronic diseases: Previous diagnoses of chronic diseases that may in-fluence study outcomes.
Quantitative variables: (1) Age: Measured on a ratio scale; (2) Duration of smoking habit: Number of years participants have been smoking; and (3) Median micronucleus count: Median MN observed per 1000 examined cells.
Potential effect modifiers considered included age, gender, general health status, and socioeconomic status. Alcohol consumption was evaluated as a possible confounding factor due to its potential impact on cellular health and susceptibility to genetic damage.
To minimize information bias, standardized data collection instruments were employed, including structured interviews and standardized sample collection and analysis procedures. This systematic approach ensured data consistency and accuracy, enabling robust assessments of the associations between different smoking types and micronucleus presence.
The collected data were analyzed using various statistical techniques to ensure a comprehensive and accurate interpretation of the results.
Data description: For quantitative variables, data were expressed as medians, means, interquartile ranges, and standard deviations, allowing for the assessment of central tendency and dispersion. For categorical variables, absolute and relative frequencies were used to represent the proportion of participants in each category.
Bivariate analysis: A bivariate analysis was conducted to identify associations and correlations between primary and secondary variables, exploring direct relationships between smoking type and micronucleus count.
Normality test: The Kolmogorov-Smirnov normality test was applied to determine data distribution. The results yielded values below 0.05, leading to the rejection of the null hypothesis of normality. This indicated that the data did not follow a normal distribution, justifying the use of non-parametric statistical methods.
Median comparison: To test hypotheses and compare median micronucleus counts among groups, the Mann-Whitney U test was applied for pairwise comparisons. Additionally, the Krus-kal-Wallis test with Dunn’s post hoc analysis was employed for multiple subgroup comparisons. A P value < 0.05 was considered statistically significant, ensuring the robustness of the conclusions.
Risk Factor Analysis: The Spearman correlation coefficient was used to evaluate the relationship between alcohol consumption duration and smoking habit with micronucleus quantification, revealing a significant correlation. Finally, a logistic regression model was constructed to assess the odds ratio (OR) of micronucleus presence, adjusting for age, residence area, alcohol consumption, and smoking habits. Data analysis was conducted using Stata Version 16.0.
The study was reviewed and approved by the Institutional Ethics Committee of the Faculty of Dentistry at the University of Antioquia (Resolution of Approval number 105-2022), ensuring compliance with all applicable ethical guidelines and standards. All participants signed an informed consent form detailing the study's objectives, procedures, potential risks and benefits, and guarantees of data anonymity and confidentiality. This ensured that participants were fully informed and voluntarily participated.
Additionally, the research adhered to the ethical principles outlined in the Declaration of Helsinki and the 2005 UNESCO Universal Declaration on Bioethics and Human Rights, ensuring the protection of participants' rights and well-being. Collected information remained confidential, with coded identifiers used to protect participant identities, ensuring that personal data were not disclosed.
The study included multiple phases of participant selection and analysis. In the initial phase, 150 potentially eligible individuals were considered. Of these, 130 were assessed for inclusion, and 120 were confirmed as eligible. Ultimately, 100 participants were enrolled in the study and completed follow-up, forming the final analytical sample. During the selection process, 20 individuals were excluded due to the presence of exclusion criteria, such as previously undiagnosed autoimmune diseases and a history of exposure to environmental genotoxic agents.
The sample consisted of 60% women and 40% men, with a mean age of 42.58 years and a median age of 38 years. The majority of participants (73%) were 30 years or older. Regarding educational attainment, 28% had completed higher education, while only 2% were foreign nationals. Most participants (60%) resided in urban areas, and socioeconomic stratum 2 was the most prevalent (46%). The prevalence of systemic diseases and obesity was 33%, and the most frequently reported reason for smoking was pleasure (36%). Detailed sociodemographic characteristics and their distribution among groups are presented in Table 1.
| Variable | NS | CS | MS | ES | RS | Total |
| Age1 | 32.55 ± 9.75 | 51.3 ± 12.49 | 30.6 ± 7.25 | 30.4 ± 9.47 | 68.05 ± 12.45 | 32.55 ± 9.75 |
| Sex1 | ||||||
| Female | 14 (70) | 9 (45) | 2 (10) | 10 (50) | 19 (95) | 54 |
| Male | 6 (30) | 11 (55) | 18 (90) | 10 (50) | 1 (5) | 46 |
| Education al level1 | ||||||
| Illiterate | 0 (0) | 0 (0) | 5 (25) | 0 (0) | 16 (80) | 21 |
| Primary | 0 (0) | 4 (20) | 2 (10) | 0 (0) | 4 (20) | 10 |
| Secondary | 3 (15) | 9 (45) | 10 (50) | 0 (0) | 0 (0) | 22 |
| University | 9 (45) | 5 (25) | 3 (15) | 11 (55) | 0 (0) | 28 |
| Postgraduate | 8 (40) | 2 (10) | 0 (0) | 9 (45) | 0 (0) | 19 |
| Place of origin | ||||||
| Domestic | 20 (100) | 20 (100) | 18 (90) | 20 (100) | 20 (100) | 98 |
| Foreign | 0 (0) | 0 (0) | 2 (10) | 0 (0) | 0 (0) | 2 |
| Residence area1 | ||||||
| Rural | 0 (0) | 6 (30) | 10 (50) | 4 (20) | 0 (0) | 20 |
| Urban | 20 (100) | 14 (70) | 10 (50) | 16 (80) | 20 (100) | 80 |
| Socioeconomic status1 | ||||||
| 1 | 0 (0) | 7 (35) | 6 (30) | 0 (0) | 0 (0) | 13 |
| 2 | 5 (25) | 7 (35) | 11 (55) | 3 (15) | 20 (100) | 46 |
| 3 | 9 (45) | 4 (20) | 1 (5) | 6 (30) | 0 (0) | 16 |
| 4 | 2 (10) | 0 (0) | 2 (10) | 7 (35) | 0 (0%) | 11 |
| 5 | 4 (20) | 2 (10) | 0 (0) | 4 (20) | 0 (0) | 10 |
| Comorbidities1 | ||||||
| Healthy | 19 (95) | 17 (85) | 15 (75) | 9 (45) | 17 (85) | 77 |
| Controlled systemic disease | 0 (0) | 2 (10) | 3 (15) | 9 (45) | 2 (10) | 16 |
| Uncontrolled systemic disease | 0 (0) | 0 (0) | 2 (10) | 1 (5) | 0 (0) | 3 |
| Obesity | 1 (5) | 1 (5) | 0 (0) | 1 (5) | 1 (5) | 4 |
| Reason for habit | ||||||
| Pleasure | - | 13 (65) | 18 (90) | 2 (10) | 3 (15) | 36 |
| Anxiety | - | 0 (0) | 0 (0) | 6 (30) | 2 (10) | 8 |
| Social | - | 7 (35) | 2 (10) | 12 (60) | 1 (5) | 22 |
| Cultural | - | 0 (0) | 0 (0) | 0 (0) | 14 (70) | 14 |
Among smokers, the mean duration of smoking was 18.19 years, with a median of 10 years. Sixty percent of participants consumed alcohol, primarily in a "social" or infrequent manner, with a mean duration of alcohol use of 9.55 years and a median of 5 years. Smokers who also consumed alcohol comprised 53% of the participants. The characteristics of substance use in the study population are detailed below.
The analysis of consumption characteristics revealed notable differences across the groups. The mean duration of use was highest among Reverse Smokers at 45.9 years, followed by Cigarette Smokers at 27.3 years, Marijuana Smokers at 13.4 years, and Electronic Cigarette Smokers at 4.45 years. The overall mean duration of use across all groups was 18.8 years, accounting for 44.2% of the total sample.
In terms of habit intensity, the majority of marijuana smokers (55%) exhibited severe habits, while 40% of electronic cigarette smokers and 85% of reverse smokers also fell into the severe category. Moderate habit intensity was most common among electronic cigarette smokers (40%) and marijuana smokers (35%), whereas mild habits were primarily observed in electronic cigarette smokers (40%) and reverse smokers (15%). Overall, 40% of the total sample reported severe habits, 45% moderate, and 15% mild.
Regarding alcohol consumption, the majority of participants across all groups reported social drinking, with the highest proportion among electronic cigarette smokers (85%) and cigarette smokers (80%). Frequent alcohol consumption was rare, reported by only 15% of marijuana smokers and 5% of electronic cigarette smokers. No participants in any group reported dependent alcohol use. Notably, 95% of the total sample denied regular alcohol consumption, while 5% reported social drinking.
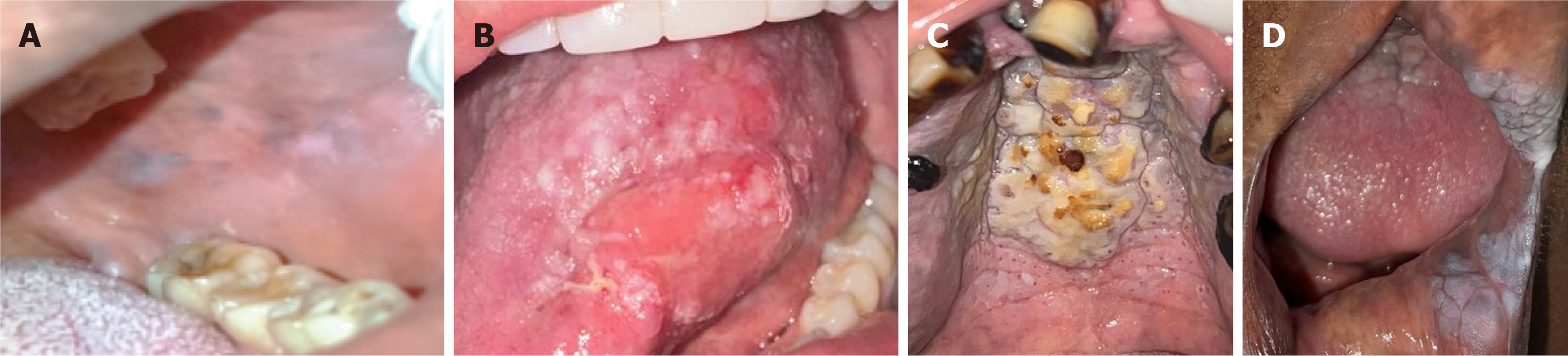
Oral lesions were diagnosed in 41% of the subjects, with 19% presenting a single lesion and 22% exhibiting two or more lesions. No lesions were detected among non-smokers. Regarding the study groups, the most common lesions in cigarette smokers were melanosis and leukoedema, with five cases each, primarily affecting the gingiva. Among marijuana smokers, leukoedema was the most frequently observed lesion, with three cases, exclusively affecting the buccal mucosa. In electronic cigarette users, two cases of white lesions associated with smoking habits, resembling leukoplakia, were identified, along with one notable case of carcinoma in situ. The affected sites in this group included the palate, oropharynx, and lingual mucosa. Lastly, among reverse smokers, a wide variety of oral lesions were observed, with the palate being affected in all 20 subjects in this category. The most common disorder in this group was smoker’s palate, identified in 16 cases. Table 2 provides a detailed description of the types of lesions found and the affected anatomical sites for each group. Figure 2 illustrates some of the most prominent lesions observed in this population.
| Variables | NS | CS | MS | ES | RS | Total | |
| Type of lesion (presumptive diagnosis) | Absent | 20 | 7 | 16 | 15 | 0 | 58 |
| White lesions (leukoplakia) | 0 | 3 | 1 | 2 | 6 | 12 | |
| Red and/or heterogeneous lesions (erythroplasia/Leukoerythroplasia) | 0 | 0 | 0 | 0 | 3 | 3 | |
| Reverse smoker’s palate | 0 | 0 | 0 | 0 | 16 | 14 | |
| Carcinoma in situ | 0 | 0 | 0 | 1 | 1 | 2 | |
| Melanosis | 0 | 5 | 0 | 0 | 14 | 21 | |
| Leukoedema | 0 | 5 | 3 | 2 | 2 | 12 | |
| Anatomical site | Palate | 0 | 5 | 0 | 2 | 20 | 27 |
| Tongue | 0 | 1 | 0 | 1 | 4 | 6 | |
| Buccal mucosa | 0 | 6 | 4 | 2 | 10 | 22 | |
| Gingiva | 0 | 8 | 0 | 0 | 3 | 11 | |
| Oropharynx | 0 | 0 | 0 | 2 | 0 | 2 | |
| Lips | 0 | 2 | 0 | 0 | 1 | 3 |
Micronucleus were detected in 86% of the collected samples. Based on the MN, the samples were classified as follows: 45% of samples were classified as having a low count (1 to 2 MN); 15% of samples were classified as moderate (3 to 5 MN); 6% of samples were categorized as abundant (> 6 MN); 14% of samples did not present micronucleus (absence of MN).
The mean MN was 2.65, with a median of 2. Regarding smoking habits, reverse smokers exhibited the highest mean micronucleus count, followed by conventional cigarette smokers, electronic cigarette users, cannabis smokers, and, finally, non-smokers. The mean micronucleus count in reverse smokers was four times higher than in non-smokers. The micronucleus test results varied across the different smoking categories. Among non-smokers, 4 individuals showed no MN (absent), while 16 exhibited sparse MN. None of the non-smokers had moderate or abundant MN. In contrast, cigarette smokers displayed a higher prevalence of MN, with 9 individuals showing sparse levels, 7 moderate, and 4 abundant. No cigarette smokers had absent MN.
Marijuana smokers had 6 individuals with absent MN, 10 with sparse, and 4 with moderate levels. None of the marijuana smokers exhibited abundant MN. Electronic cigarette smokers showed a similar pattern, with 4 individuals having absent MN, 10 sparse, 4 moderate, and 2 abundant. Reverse smokers had the highest number of individuals with absent MN[14], followed by 10 with sparse, 4 with moderate, and 2 with abundant levels.
Overall, the total distribution of micronucleus test results across all groups was as follows: 28 individuals had absent MN, 45 had sparse, 15 had moderate, and 6 had abundant levels. These findings suggest that smoking status, particularly cigarette and reverse smoking, may be associated with higher frequencies of MN, indicating potential genotoxic effects.
Figure 3 provides examples illustrating MN presence in conventional smokers, marijuana smokers, reverse smokers, and individuals using e-cigarettes.
Micronucleus prevalence was higher in women than in men. Socioeconomic analysis revealed that patients from the lowest socioeconomic level (stratum 1) had the highest mean micronucleus count, followed by strata 2, 4, 5, and finally stratum 3. Regarding educational attainment, the highest micronucleus count was observed in illiterate individuals and those with only primary education, followed by those with secondary education, university degrees, and, lastly, postgraduate education. A higher micronucleus count was also observed in rural populations compared to urban ones.
Smoking intensity influenced the results; patients with moderate or heavy smoking habits had a higher micronucleus count than those with mild smoking habits or non-smokers. Additionally, participants with comorbidities and those presenting oral cavity lesions had a higher mean micronucleus count.
The Kruskal-Wallis test was used to compare median micronucleus counts across different smoker groups and the control group. The results yielded a P value of 0.00 (χ² = 36.2), indicating statistically significant differences among the groups.
Using the Mann-Whitney U test, significant differences in micronucleus frequency were observed between smokers overall and the control group (P = 0.04). Statistically significant differences were also found between cigarette smokers, electronic cigarette users, and reverse smokers compared to non-smokers (P < 0.001), leading to the rejection of the null hypothesis. However, no significant differences were found between marijuana smokers and the control group (P = 0.89), meaning the null hypothesis could not be rejected for this group (Table 3).
| Groups | Micronucleus frequency | Z-Statistic | P value |
| MS | 1.3 ± 1.14 | 0.13 | 0.89 |
| ES | 2.8 ± 2.41 | 0.43 | < 0.001 |
| CS | 3.6 ± 2.25 | -3.96 | < 0.001 |
| RS | 4.4 ± 2.3 | -5.21 | < 0.001 |
| Control | 1.05 ± 0.69 |
Post hoc analysis using Dunn’s test confirmed these findings, showing significant differences between the control group and conventional cigarette smokers (P < 0.001), reverse smokers (P < 0.001), and electronic cigarette users (P < 0.001). Additionally, significant differences were observed between marijuana smokers and conventional cigarette smokers (P < 0.001), reverse smokers (P < 0.001), and electronic cigarette users (P = 0.02), as well as between reverse smokers and electronic cigarette users (P < 0.001) (Table 4).
Spearman’s correlation analysis revealed significant relationships between the duration of substance use and micronucleus quantification. A weak negative correlation was observed between the duration of alcohol consumption and micronucleus quantification (rho = -0.27, P = 0.001), indicating that longer durations of alcohol consumption were associated with lower levels of MN. In contrast, a moderate positive correlation was found between the duration of smoking and micronucleus quantification (rho = 0.48, P = 0.001), suggesting that longer smoking durations were associated with higher levels of MN. Both correlations were statistically significant (P < 0.05).
The regression model confirmed that age, residential area, and alcohol consumption could act as effect modifiers or confounding factors. This analysis ruled out potential interactions between the studied comorbidities and sex (Table 5). The crude model analysis using OR revealed several significant associations. Age showed a significant positive association with the outcome (OR = 3.3, P = 0.03), indicating that older individuals had higher odds of the event of interest. Housing (OR = 0.21, P = 0.03) and alcohol consumption (OR = 0.23, P = 0.04) were both negatively associated with the outcome, suggesting that individuals with certain housing conditions or alcohol consumption habits had lower odds of the event. Sex (OR = 1.8, P = 0.34) and comorbidity (OR = 0.47, P = 0.22) did not show statistically significant associations with the outcome. These findings highlight the potential influence of age, housing, and alcohol consumption on the studied event.
| Variable | Crude OR | Adjusted OR | P value |
| Age | 3.3 | 0.99 | 0.97 |
| Housing | 0.21 | 0.73 | 0.74 |
| Alcohol consumption | 0.23 | 0.37 | 0.25 |
The Mantel-Haenszel test confirmed the transformation of the crude ORs adjusted for age, residential area, and alcohol consumption. This test reduced the significance of all variables, ruling out their role as confounding factors. Age was considered an effect modifier due to its association with the duration of substance use (Table 5).
The study of risk factors associated with carcinogenesis in the head and neck region is essential for improving population quality of life and ensuring the sustainability of healthcare systems. Smoking, identified as the primary risk factor, requires analysis considering sociodemographic, clinical, and molecular variations based on the type of practice, substance used, and duration of the habit[10,16]. This study provides evidence on the relationship between different forms of smoking and genotoxic damage, measured through the presence of MN in buccal mucosa cells.
The differing genotoxic effects observed across smoking modalities warrant particular attention. While conventional tobacco and electronic cigarette users had elevated micronucleus frequencies, cannabis users did not demonstrate comparable genotoxic damage. This difference may be explained by several factors: (1) Cannabinoids like cannabidiol and tetrahydrocannabinol possess antioxidant properties that could mitigate oxidative DNA damage[6-8]; (2) Cannabis users in our cohort had shorter exposure durations than the tobacco groups (13 years vs 27–45 years); and (3) Most of the cannabis consumers reported lower use frequency (social/recreational) than daily tobacco smokers. However, these findings should be interpreted cautiously because, as in prior studies[6,7], concurrent tobacco use in some cannabis consumers is a confounding factor.
The evaluated groups can be categorized according to their sociodemographic characteristics. Conventional cigarette smokers are predominantly urban dwellers, older adults, from a middle/Low socioeconomic background, and with a medium level of education. Their primary reason for smoking is pleasure, with an average consumption duration of 27 years. This finding is supported by the study of Szklo et al[17], conducted in a Brazilian community with similar characteristics[17]. A notable difference is that their study reported an increase in smokers in rural areas, equating to urban populations, which warrants further investigation.
Conversely, reverse smokers are almost exclusively women from rural areas, older adults, from a low socioeconomic background, and with no formal education. Their smoking practice is driven by family and cultural tradition, with an average duration of 45 years. These characteristics have been widely described previously[8,18]. However, contrary to these studies, which identified a higher prevalence of male smokers engaged in fishing activities, this study did not observe such findings, as it did not include insular communities.
Electronic cigarette users are primarily urban residents, young adults, and adolescents from high socioeconomic and educational backgrounds. Their main motivation is social use, with an average consumption of four years, reflecting the recent emergence and rapid growth of this habit. These findings align with descriptive studies from the United States and Europe[19], highlighting the public health challenge of managing this trend. Current efforts focus on re-education in schools and universities.
Regarding marijuana smokers, they may reside in either urban or rural areas, are young adults, from medium/Low socioeconomic and educational backgrounds, and primarily smoke for pleasure and social reasons. Their average consumption duration is 13 years. This is consistent with global literature, which identifies 166 million users worldwide, most under 34 years of age[20]. Trends indicate a decreasing average age of initiation, with an increase in adolescent use (ages 17–21), mirroring trends observed in electronic cigarette users[21].
The median micronucleus count in non-smokers was 1.05 ± 0.69, consistent with current literature. Bonassi et al[22] established a range of 1.37 to 2 MN/1000 cells in non-smokers, Holland et al[23] suggested a range of 0.5 to 2 MN/1000 cells, and Singam et al[24] reported an average of 1 MN/1000 cells. These values, lower than those observed in exposed groups but not negligible, indicate that micronucleus presence in non-smokers may be influenced by lifestyle, diet, comorbidities, alcohol consumption, medications, and environmental exposures.
For conventional cigarette smokers, the median micronucleus count was 3.6 ± 2.25, significantly higher than in non-smokers and marijuana users. This suggests that cigarette smoking induces genotoxicity and cytotoxicity, potentially leading to cancer. These findings align with global literature: Rajabi et al[25] reported an MN average of 3.50 ± 3.832 in cigarette smokers, Thomas et al[26] reported 6.6 ± 0.8, and Mr et al[27] found 3.01 ± 0.7, all with statistically significant differences.
A direct correlation was observed between the presence of oral lesions and an in-creased frequency of MN, with an average of 3.9 ± 2.27 in subjects with oral lesions, which was statistically significant compared to those without lesions. Singam et al. reported a mean micronucleus count of 5.1 ± 1.619 in individuals with leukoplakia, with an increasing trend based on dysplasia severity[24]. Finally, for cases diagnosed with oral squamous cell carcinoma, the highest mean count of 10.1 ± 2.3 was observed, reinforcing the validity of this biomarker.
This study found a linear correlation between smoking duration, intensity, and micronucleus formation. Saito et al[28] assessed tobacco combustion-related variables and the risk of head and neck cancer, reporting an increased risk with longer and more intense smoking histories. They found that risk magnitude rose significantly with smoking durations exceeding 40 years (risk index: 4.22, 95%CI: 1.23–14.51). This risk increase is reflected in this study among reverse smokers, who have an average smoking duration of 45 years and exhibit the highest median micronucleus count.
No correlation was found between alcohol consumption and micronucleus presence in this study, suggesting that alcohol is not a risk factor in this population. This may be attributed to 95% of alcohol-consuming participants engaging in social or infrequent consumption with low severity. Marziliano et al[29] support that the risk of head and neck cancer associated with alcohol consumption depends on its severity, reporting an OR of 1.21 for light alcohol consumption but a substantial increase to 5.21 in heavy or severe drinkers.
Koo et al[30], in a cohort study, confirmed the correlation between alcohol consumption severity and cancer risk, further emphasizing that consumption frequency may be a more critical variable. They reported a relative risk of 0.90 for social drinkers (1–2 times per week) but a significant increase to 1.85 in frequent or dependent drinkers (6–7 times per day), with statistically significant results.
Several other limitations must be considered when interpreting the results. First, the cross-sectional design precludes drawing causal inferences about smoking and genotoxic damage. While we observed associations between smoking modalities and micronucleus formation, the temporal relationship cannot be established without longitudinal follow-up. Future prospective studies should track micronucleus progression and clinical outcomes in exposure groups. Second, diagnoses were based solely on expert clinical evaluations; the lack of confirmatory biopsies for oral lesions may have affected the classification and analysis of oral lesions. Histopathological confirmation would enhance diagnostic accuracy and malignancy risk assessment. Third, the significant demographic variations between smoking groups should be considered when interpreting genotoxic outcomes. The observed differences in age, sex distribution, educational attainment, and socioeconomic status may confound the observed effects by influencing both exposure patterns (e.g., smoking frequency, duration) and biological responses to genotoxic agents.
The findings of this study are consistent with the existing literature, indicating that smoking, in its various forms, is associated with increased genotoxic damage, as evidenced by the presence of MN. The high prevalence of MN among inverted, electronic, and conventional smokers highlights the significant risk associated with these practices. These results reinforce the need for public health interventions aimed at reducing conventional smoking and inverted smoking, both of which show a considerable impact on cellular genotoxicity. This study not only reinforces existing evidence on the genotoxic damage associated with various forms of smoking but also underscores the importance of preventive and regulatory approaches tailored to the specific sociodemographic characteristics of smokers. The identification of subclinical damage through the micronucleus assay may play a crucial role in early detection and timely intervention, thereby contributing to reducing the burden of smoking-related diseases.
| 1. | Yu Z, Bai X, Zhou R, Ruan G, Guo M, Han W, Jiang S, Yang H. Differences in the incidence and mortality of digestive cancer between Global Cancer Observatory 2020 and Global Burden of Disease 2019. Int J Cancer. 2024;154:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 2. | Du E, Mazul AL, Farquhar D, Brennan P, Anantharaman D, Abedi-Ardekani B, Weissler MC, Hayes DN, Olshan AF, Zevallos JP. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope. 2019;129:2506-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Weke A, Holliday R. Electronic cigarettes: an update on products, regulation, public health approaches and oral health. Community Dent Health. 2022;39:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Corey CG, Ambrose BK, Apelberg BJ, King BA. Flavored Tobacco Product Use Among Middle and High School Students--United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Jenssen BP, Boykan R. Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels). Children (Basel). 2019;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Cretu B, Zamfir A, Bucurica S, Scheau AE, Savulescu Fiedler I, Caruntu C, Caruntu A, Scheau C. Role of Cannabinoids in Oral Cancer. Int J Mol Sci. 2024;25:969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Clark TM. Scoping Review and Meta-Analysis Suggests that Cannabis Use May Reduce Cancer Risk in the United States. Cannabis Cannabinoid Res. 2021;6:413-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Ardila C, Jiménez R, Álvarez E. Revisión sistemática de los efectos del hábito de fumar invertido sobre la mucosa oral. Rev Arq Méd Camagüey. 2013;17:336-346. |
| 9. | John HAS, Dakhale R, Sedani S, Ahuja KP. Smoker's Palate: An Often Misunderstood Benign Lesion of the Oral Cavity. Cureus. 2023;15:e48868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kujan O, Shearston K, Farah CS. The role of hypoxia in oral cancer and potentially malignant disorders: a review. J Oral Pathol Med. 2017;46:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Liu J, Huang D, Cai Y, Cao Z, Liu Z, Zhang S, Zhao L, Wang X, Wang Y, Huang F, Wu Z. Saliva diagnostics: emerging techniques and biomarkers for salivaomics in cancer detection. Expert Rev Mol Diagn. 2022;22:1077-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Sommer S, Buraczewska I, Kruszewski M. Micronucleus Assay: The State of Art, and Future Directions. Int J Mol Sci. 2020;21:1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 13. | Walsh T, Macey R, Kerr AR, Lingen MW, Ogden GR, Warnakulasuriya S. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. 2021;7:CD010276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Lim HK, Hughes CO, Lim MJS, Li JJ, Rakshit M, Yeo C, Chng KR, Li A, Chan JSH, Ng KW, Leavesley DI, Smith BPC. Development of reconstructed intestinal micronucleus cytome (RICyt) assay in 3D human gut model for genotoxicity assessment of orally ingested substances. Arch Toxicol. 2022;96:1455-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Titenko-Holland N, Jacob RA, Shang N, Balaraman A, Smith MT. Micronuclei in lymphocytes and exfoliated buccal cells of postmenopausal women with dietary changes in folate. Mutat Res. 1998;417:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Araki Y, Yamamoto N, Tanzawa Y, Higashi T, Kuchiba A, Hayashi K, Takeuchi A, Miwa S, Igarashi K, Endo M, Kobayashi E, Tsuchiya H, Kawai A. Family cancer history and smoking habit associated with sarcoma in a Japanese population study. Sci Rep. 2022;12:17129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Szklo AS, de Souza MC, Szklo M, de Almeida LM. Smokers in Brazil: who are they? Tob Control. 2016;25:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Alvarez GJ, Alvarez E, Jiménez R, Mosquera Y, Gaviria AM, Garcés A, Duque AA, Castaño AZ, González EE, Millán MI, Ossa DR. Reverse smokers's and changes in oral mucosa. Department of Sucre, Colombia. Med Oral Patol Oral Cir Bucal. 2008;13:E1-8. |
| 19. | Gallus S, Lugo A, Liu X, Borroni E, Clancy L, Gorini G, Lopez MJ, Odone A, Przewozniak K, Tigova O, van den Brandt PA, Vardavas C, Fernandez E; TackSHS Project Investigators. Use and Awareness of Heated Tobacco Products in Europe. J Epidemiol. 2022;32:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am J Drug Alcohol Abuse. 2010;36:311-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Noland M, Rayens MK, Wiggins AT, Huntington-Moskos L, Rayens EA, Howard T, Hahn EJ. Current Use of E-Cigarettes and Conventional Cigarettes Among US High School Students in Urban and Rural Locations: 2014 National Youth Tobacco Survey. Am J Health Promot. 2018;32:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Bonassi S, Coskun E, Ceppi M, Lando C, Bolognesi C, Burgaz S, Holland N, Kirsh-Volders M, Knasmueller S, Zeiger E, Carnesoltas D, Cavallo D, da Silva J, de Andrade VM, Demircigil GC, Domínguez Odio A, Donmez-Altuntas H, Gattas G, Giri A, Giri S, Gómez-Meda B, Gómez-Arroyo S, Hadjidekova V, Haveric A, Kamboj M, Kurteshi K, Martino-Roth MG, Montero Montoya R, Nersesyan A, Pastor-Benito S, Favero Salvadori DM, Shaposhnikova A, Stopper H, Thomas P, Torres-Bugarín O, Yadav AS, Zúñiga González G, Fenech M. The HUman MicroNucleus project on eXfoLiated buccal cells (HUMN(XL)): the role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat Res. 2011;728:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 23. | Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, Fenech M. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659:93-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 24. | Singam PK, Majumdar S, Uppala D, Kotina S, Namana M, Ayyagari KR. Evaluation of genotoxicity by micronucleus assay in oral leukoplakia and oral squamous cell carcinoma with deleterious habits. J Oral Maxillofac Pathol. 2019;23:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Rajabi-Moghaddam M, Haji Mirzamohammad M, Yahyazadeh E, Gholinia H, Abbaszadeh H. Comparison of Genotoxic Effect in Buccal Exfoliated Cells between Cigarette and Waterpipe Smokers. Acta Cytol. 2020;64:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Thomas AJ, Nair BJ, Oommen S, Syamkumar V, Raman RK. Comparative Evaluation of Genotoxicity in Tobacco Users versus Nontobacco Users. J Pharm Bioallied Sci. 2021;13:S960-S964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Mr P, Guruprasad Y, Jose M, Saxena K, K D, Prabhu V. Comparative Study of Genotoxicity in Different Tobacco Related Habits using Micronucleus Assay in Exfoliated Buccal Epithelial Cells. J Clin Diagn Res. 2014;8:ZC21-ZC24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Saito N, Sairenchi T, Irie F, Iso H, Iimura K, Watanabe H, Muto T, Ota H. Duration of cigarette smoking is a risk factor for oropharyngeal cancer mortality among Japanese men and women: the Ibaraki Prefectural Health Study (IPHS). Ann Epidemiol. 2013;23:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Marziliano A, Teckie S, Diefenbach MA. Alcohol-related head and neck cancer: Summary of the literature. Head Neck. 2020;42:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Koo HY, Han K, Shin DW, Yoo JE, Cho MH, Jeon KH, Kim D, Hong S, Jun JK. Alcohol Drinking Pattern and Risk of Head and Neck Cancer: A Nationwide Cohort Study. Int J Environ Res Public Health. 2021;18:11204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |