Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.107220
Revised: April 17, 2025
Accepted: June 3, 2025
Published online: September 20, 2025
Processing time: 140 Days and 16.6 Hours
The World Health Organization defined long coronavirus disease 2019 (COVID-19) as the continuation or development of new symptoms 3 months after the initial severe acute respiratory syndrome coronavirus 2 infection, with these symptoms lasting for at least 2 months with no other explanation.
To evaluate the potential laboratory and instrumental findings (short-term and long-term) resulting from COVID-19.
This longitudinal observational COVID-19 cohort study (March 1, 2020-March 1, 2021) was carried out on patients ≥ 18 years old who were admitted to the University Hospitals of Pisa, Siena and Careggi and the Azienda USL Toscana Nord Ovest, Sud Est and USL Centro Toscana and were subjected to follow-up. Follow-up was conducted between 0 day and 89 days, 90 days and 179 days, 180 days and 269 days, 270 days and 359 days, and more than 360 days after hospitalization.
Of 2887 patients (58.5% males, average age 66.2 years) hospitalized in the study period (March 1, 2020-March 1, 2021) carrying out at least one follow-up examination within 12 months of discharge, a total of 1739 patients (705 males, average age 66 years) underwent laboratory tests, of whom 714 patients (470 males, average age 63 years) underwent spirometry. Some laboratory test results remained above the threshold even at follow-up beyond 360 days (C-reactive protein: 36%, fibrin degradation fragment: 48.8%, gamma-glutamyl transferase: 16.8%), while others showed a return to normal range more quickly in almost all patients. Alterations in liver enzymes, he
Alterations in liver enzymes, hematocrit or hemoglobin, lymphocytes and neutrophils were associated with risk outcomes (need for oxygen therapy or spirometry alterations). These imbalanced conditions may contribute to pulmonary dysfunction.
Core Tip: Long coronavirus disease 2019 (COVID-19) is a chronic condition that occurs after the initial severe acute respiratory syndrome coronavirus 2 infection. This longitudinal observational cohort study evaluates the short-term and long-term laboratory and instrumental findings in COVID-19 patients, and was conducted in hospitals in Tuscany between March 1, 2020, and March 1, 2021. The results indicated that alterations in liver enzymes, hematocrit or hemoglobin, lymphocytes and neutrophils were associated with a risk of requiring oxygen therapy or experiencing spirometry ab
- Citation: Silvestri C, Stasi C, Profili F, Bartolacci S, Sessa E, Tacconi D, Villari L, Carrozzi L, Dotta F, Bargagli E, Donnini S, Masotti L, Rasero L, Lavorini F, Pistelli F, Chimera D, Sorano A, D'alessandro M, Pacifici M, Milli C, Voller F. Evaluation of short and long-term laboratory and instrumental findings in COVID-19 patients hospitalized in Tuscany. World J Exp Med 2025; 15(3): 107220
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/107220.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.107220
World Health Organization defined long coronavirus disease 2019 (COVID-19) as “the continuation or development of new symptoms 3 months after the initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with these symptoms lasting for at least 2 months with no other explanation”[1]. Chen et al[2] estimated a global pooled prevalence of the post-COVID-19 condition of 0.43 (95%CI: 0.39-0.46), with a pooled prevalence of 0.54 hospitalized and 0.34 non-hospitalized patients. Long COVID-19 has heterogeneous signs and symptoms with variable frequencies. The more complex symptoms appear to be neurological and neuropsychiatric[3]. The most frequent symptoms were mental health, gastrointestinal, cardiopulmonary and neurological issues, and pain[3-6]. Therefore, long COVID-19 includes single or multiple diagnosable conditions, including interstitial lung disease and hypoxemia[7].
A recent meta-analysis[4] on a total of 211 eligible studies, covering a population of 13368074 individuals, showed that the most frequently reported persistent symptoms up to 3 months from infection were fatigue, post-traumatic stress, anxiety, dyspnea and depression. Several studies have described the impact of long-term conditions of COVID-19 infection. Ottiger et al[5] assessed the post-COVID-19 impact on the work ability and return-to-work of subjects pre
However, it is impossible to generalize the impact of COVID-19 on the social and professional life of those affected.
The main objective of this study was to evaluate the potential laboratory and instrumental findings resulting from COVID-19. Here we describe short-term and long-term blood tests performed after the acute clinical picture of SARS-CoV-2 infection (long COVID-19) and what the results may indicate in terms of the progression of the disease, in particular the risk of presenting abnormal spirometry and needing oxygen therapy.
The Regional Health Agency of Tuscany (ARS), in collaboration with the Operational Healthcare Units and University Hospitals of Tuscany, promoted a prospective-retrospective observational cohort study entitled "prospective and retrospective study on outcomes and complications from COVID-19 in a cohort of hospitalized patients in Tuscany (SPRINT)”.
This was a prospective cohort study conducted on patients aged ≥ 18 years discharged (March 1, 2020-March 1, 2021) from hospitals in Tuscany with a COVID-19 diagnosis (reverse transcription-polymerase chain reaction positive), who underwent follow-up. For the retrospective study, see Silvestri et al[8]. Patients were admitted to the University Hospitals of Pisa, Siena and Careggi and the Azienda USL Toscana Nord Ovest, Sud Est and USL Centro Toscana. Follow-up was conducted between 0-89 days, 90-179 days, 180-269 days, 270-359 days, and more than 360 days after discharge. Statistical analysis utilized multiple time points to evaluate forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio changes during follow-up.
The COVID-19 diagnosis was selected using the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) codes indicated in the Decree of the Ministry of Health of October 28, 2020.
The study was submitted to and approved by the regional Ethics Committee and the company task forces (No. 17508). The researchers obtained consent from all participants to participate in this research project.
The study protocol included the execution of laboratory tests and spirometry at follow-up, including measuring the performance of forced expiration maneuvers. In the different centers, spirometry was performed according to the American Thoracic Society/European Respiratory Society recommendations[9]. Spirometry measurements were obtained pre-bronchodilator. The FVC identifies the total volume of air expelled after maximal inspiration, and the FEV1 indicates the volume of air exhaled in the first second of forced expiration. These parameters can be used to identify a possible obstructive functional deficit or restrictive or mixed-type deficit.
In this prospective study, the following laboratory parameters were taken into consideration: (1) Aspartate aminotransferase (AST) (UI/L); (2) Alanine aminotransaminase (ALT) (UI/L); (3) Gamma-gamma-glutamyl transferase (GT) (UI/L); (4) D-Dimer (mg/L); (5) Hematocrit (%); (6) Hemoglobin (g/dL); (7) Ferritin (ng/mL); (8) Estimated glomerular filtration rate (mL/minute/1.73 m²); (9) White globules (× 109/L); (10) Red cells (× 1012/L); (11) Lymphocytes (× 109/L); (12) Monocytes (× 109/L); (13) Neutrophils (× 109/L); (14) Platelets (× 109/L); and (15) Protein C reactive (mg/dL).
Comorbidities were identified by matching the universal code (IDUNI) attributed by the Regione Toscana to each person with hospital admission codes identified by the ICD-9-CM.
The IDUNI were used to extract the fields (laboratory tests, spirometry and oxygen therapy) from the medical records required by the protocol and for the subsequent pseudonymization of the data. The Information and Telecommunications Unit of the ARS has specifically set up a protected channel for sending anonymized databases to the ARS. The pseudonymization procedure allowed the ARS to link the records between the databases, and thus create a clinical and symptomatic continuum between hospitalization and monitoring period, protecting the patient's personal information.
An analysis was performed on the influence of laboratory parameters and outcomes (oxygenation or a FEV1/FVC ratio < 70%) in patient follow-up. We also analyzed the main risk factors (age, gender, body mass index, chronic pathologies at admission, smoking habits) considered in follow-up. The instrumental and laboratory parameters were considered significant when they exceeded a predetermined clinically relevant threshold. For each risk factor, the relative risk (with a 95%CI) of the individual outcomes was calculated, crude and adjusted for the effect of age, gender and any other confounding risk factors (when possible, based on the amount of data). Adjusted relative risk estimates were calculated using generalized linear regression models for binomial data.
As previously described[8], patients enrolled in the study period (March 1, 2020-March 1, 2021) who underwent at least one follow-up examination within 12 months of discharge were 2887 (58.5% males, average age 66.2 years) patients. A total of 1739 (705 males, average age 66 years) patients underwent laboratory tests and a total of 714 patients (470 males, average age 63 years) underwent spirometry at follow-up. The data gap of patients undergoing laboratory/instrumental tests at follow-up is due to the lack of data recorded and sent during patient monitoring period.
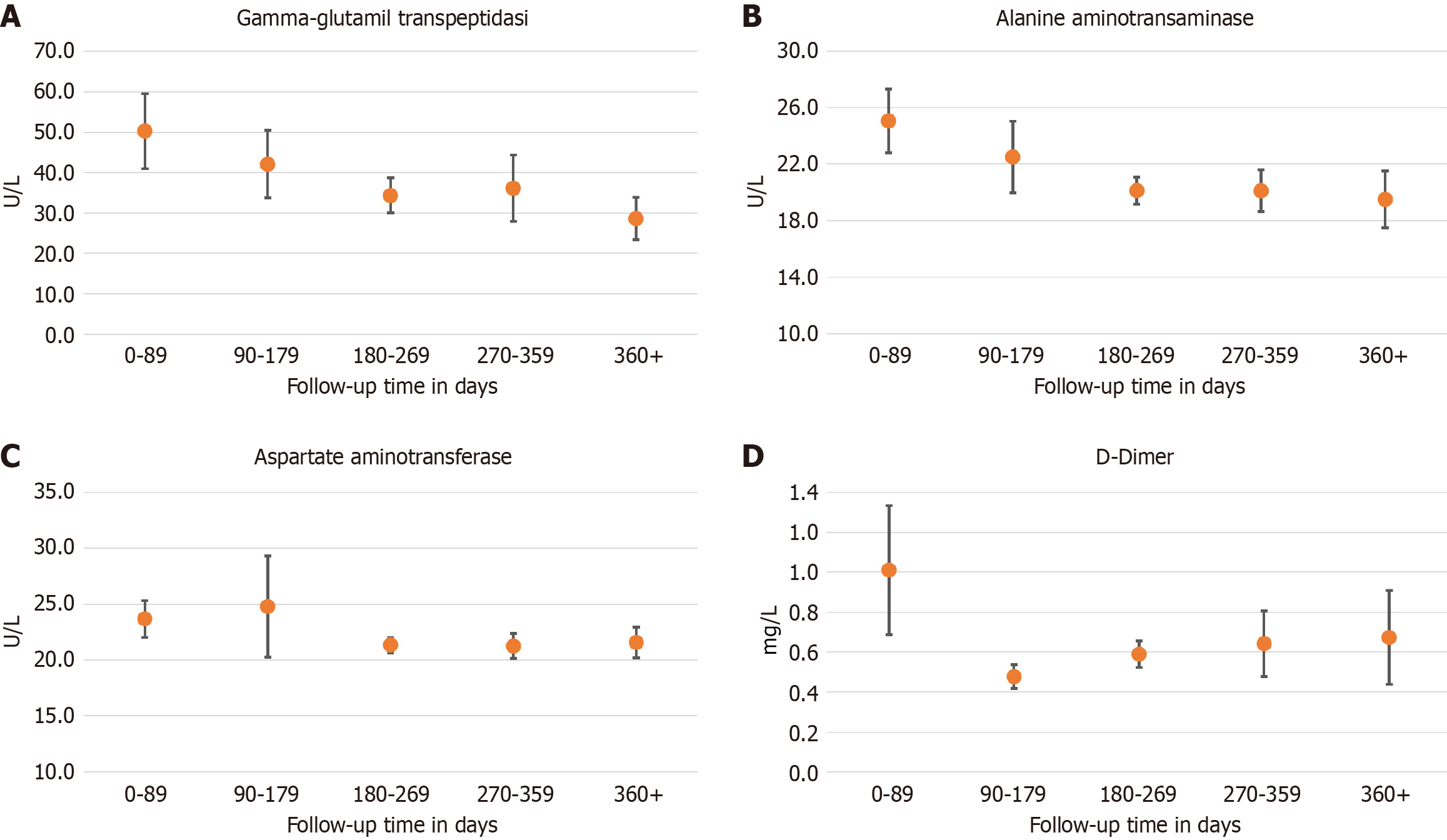
Considering the laboratory data, the majority of patients were discharged with some blood test values outside the range, in particular those concerning the inflammation indices. Above-threshold values were observed in particular for ferritin (73.8%) and C-reactive protein (CRP) values (54.3%). D-Dimer (56%), AST (15.6%), ALT (48.6%), and gamma-GT (41.6%) were also above the threshold. Figure 1 shows the trend of liver enzymes and D-Dimer at a 360-day follow-up. In patients with altered liver enzymes, diabetes had a prevalence of 8.3%, cirrhosis of 0.2%, viral hepatitis 1.5%, and hypertension 10.2%.
Many of these tests remained above the threshold even in follow-ups beyond 360 days (CRP: 36%, D-Dimer: 48.8%, gamma-GT: 16.8%), while the other laboratory tests returned to the normal range more quickly in almost all enrolled patients.
At 180-239 days’ follow-up, 17.1% of patients had a FEV1/FVC ratio < 0.70.
Among the laboratory tests, alterations of liver enzymes, in hematocrit or hemoglobin, lymphocytes and neutrophils were associated with a risk of requiring oxygen therapy or abnormal FEV1/FVC ratio in follow-up (Table 1).
| Blood tests | Level | Raw RR | RR adjusted for age and gender | RRs adjusted for age and gender RRs adjusted for age. gender. days after discharge | |||||||||||||
| RR | Min | Max | P value | RR | Min | Max | P value | RR | Min | Max | P value | ||||||
| Alteration of liver enzymes (aspartate aminotransferase, alanine transaminase, Gamma-gamma-glutamyl transferase) | None | ||||||||||||||||
| 1 or more impaired during hospitalization | 0.63 | 0.36 | 1.09 | 0.099 | 0.66 | 0.38 | 1.15 | 0.144 | 0.70 | 0.40 | 1.22 | 0.207 | |||||
| Altered at discharge | 1.61 | 1.09 | 2.39 | 0.017 | 1.61 | 1.07 | 2.42 | 0.021 | 1.55 | 1.03 | 2.32 | 0.034 | |||||
| D-Dimer | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.73 | 0.19 | 2.85 | 0.652 | 0.65 | 0.16 | 2.59 | 0.543 | 0.74 | 0.18 | 3.09 | 0.684 | |||||
| Altered at discharge | 0.76 | 0.46 | 1.23 | 0.263 | 0.76 | 0.47 | 1.23 | 0.264 | 0.77 | 0.46 | 1.30 | 0.328 | |||||
| Hematocrit | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.92 | 0.53 | 1.57 | 0.751 | 0.76 | 0.46 | 1.28 | 0.305 | 0.77 | 0.52 | 1.12 | 0.169 | |||||
| Altered at discharge | 0.55 | 0.37 | 0.83 | 0.005 | 0.46 | 0.30 | 0.68 | 0.000 | 0.47 | 0.33 | 0.67 | 0.000 | |||||
| Hemoglobin | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 1.13 | 0.71 | 1.79 | 0.610 | 0.84 | 0.53 | 1.33 | 0.464 | 0.86 | 0.56 | 1.33 | 0.505 | |||||
| Altered at discharge | 0.73 | 0.50 | 1.07 | 0.108 | 0.58 | 0.39 | 0.86 | 0.007 | 0.60 | 0.41 | 0.89 | 0.011 | |||||
| Ferritin | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | |||||||||||||||||
| Altered at discharge | 1.12 | 0.65 | 1.92 | 0.689 | 1.09 | 0.64 | 1.85 | 0.765 | 0.99 | 0.58 | 1.70 | 0.979 | |||||
| Glomerular filtration rate | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.83 | 0.45 | 1.54 | 0.563 | 0.52 | 0.28 | 0.98 | 0.043 | 0.45 | 0.25 | 0.82 | 0.009 | |||||
| Altered at discharge | 1.30 | 0.76 | 2.20 | 0.339 | 0.84 | 0.50 | 1.43 | 0.531 | 0.75 | 0.48 | 1.18 | 0.213 | |||||
| White globules | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 1.12 | 0.68 | 1.82 | 0.661 | 0.96 | 0.60 | 1.56 | 0.884 | 0.91 | 0.58 | 1.42 | 0.674 | |||||
| Altered at discharge | 1.01 | 0.53 | 1.91 | 0.984 | 0.98 | 0.53 | 1.84 | 0.960 | 0.92 | 0.52 | 1.65 | 0.789 | |||||
| Red cells | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.97 | 0.58 | 1.62 | 0.904 | 0.83 | 0.50 | 1.35 | 0.451 | 0.85 | 0.53 | 1.35 | 0.484 | |||||
| Altered at discharge | 0.76 | 0.52 | 1.12 | 0.168 | 0.59 | 0.40 | 0.87 | 0.008 | 0.59 | 0.41 | 0.87 | 0.007 | |||||
| Lymphocytes | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.62 | 0.33 | 1.17 | 0.143 | 0.55 | 0.30 | 1.02 | 0.058 | 0.57 | 0.31 | 1.05 | 0.070 | |||||
| Altered at discharge | 0.87 | 0.49 | 1.54 | 0.635 | 0.55 | 0.31 | 0.97 | 0.037 | 0.56 | 0.32 | 0.99 | 0.045 | |||||
| Monocytes | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 1.05 | 0.61 | 1.79 | 0.871 | 1.04 | 0.61 | 1.77 | 0.883 | 1.05 | 0.62 | 1.79 | 0.850 | |||||
| Altered at discharge | 1.75 | 0.93 | 3.32 | 0.085 | 1.53 | 0.82 | 2.86 | 0.180 | 1.69 | 0.89 | 3.21 | 0.109 | |||||
| Neutrophils | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.97 | 0.55 | 1.69 | 0.907 | 0.83 | 0.49 | 1.43 | 0.507 | 0.81 | 0.48 | 1.39 | 0.451 | |||||
| Altered at discharge | 0.75 | 0.41 | 1.38 | 0.351 | 0.50 | 0.27 | 0.92 | 0.026 | 0.42 | 0.23 | 0.77 | 0.004 | |||||
| Platelets | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 0.70 | 0.39 | 1.26 | 0.237 | 0.63 | 0.36 | 1.12 | 0.118 | 0.64 | 0.36 | 1.13 | 0.126 | |||||
| Altered at discharge | 0.74 | 0.34 | 1.57 | 0.429 | 0.69 | 0.33 | 1.43 | 0.317 | 0.73 | 0.36 | 1.50 | 0.392 | |||||
| Protein C reactive | None | 1.00 | 1.00 | 1.00 | |||||||||||||
| 1 or more impaired during hospitalization | 2.05 | 0.50 | 8.43 | 0.319 | 1.56 | 0.39 | 6.18 | 0.529 | 1.53 | 0.39 | 6.09 | 0.544 | |||||
| Altered at discharge | 3.44 | 0.88 | 13.50 | 0.077 | 2.58 | 0.68 | 9.78 | 0.164 | 2.31 | 0.61 | 8.79 | 0.219 | |||||
In our cohort, both FVC and FEV1/FVC ratios have a statistically significant difference between the start of follow-up and follow-up over 300 days, with an FEV1/FVC ratio ranging from 78.8% within 2 months of discharge and 81.7% more than 300 days after discharge (Table 2).
| Time elapsed between follow-up and discharge date (days) | FEV1 | FVC | FEV1/FVC ratio | ||||||
| Patients (n) | Average value | SD | Patients (n) | Average value | SD | Patients (n) | Average value | SD | |
| 0-59 | 3 | 2 | 2 | ||||||
| 60-119 | 283 | 98.4 | 15.8 | 279 | 97.9 | 16.1 | 280 | 78.8 | 7.5 |
| 120-179 | 144 | 97.9 | 16.2 | 141 | 96.9 | 17.4 | 137 | 78.8 | 8.3 |
| 180-239 | 160 | 94.8 | 19.9 | 159 | 95.5 | 19.1 | 158 | 76.5 | 9.7 |
| 240-299 | 31 | 99.0 | 16.1 | 31 | 95.4 | 17.9 | 28 | 79.2 | 7.4 |
| 300+ | 93 | 96.4 | 17.7 | 91 | 90.7 | 19.2 | 88 | 81.7 | 6.5 |
| Test Anova P value: 0.242 | Test Anova P value: 0.017 | Test Anova P value: 0.001 | |||||||
Certain risk factors in particular were found to indicate the need for oxygen therapy and/or the presence of an FEV1/FVC ratio < 0.70 (indicative of airflow obstruction) (Table 3).
| Variable | Level | Raw RR | RR adjusted for age and gender | RRs adjusted for age and gender RRs adjusted for age, gender, days after discharge | |||||||||
| RR | Min | Mx | P value | RR | Min | Mx | P value | RR | Min | Mx | P value | ||
| Class of age | 18-49 | 1.00 | 1.00 | 1.00 | |||||||||
| 50-59 | 1.22 | 0.66 | 2.27 | 0.521 | 1.18 | 0.63 | 2.18 | 0.606 | 1.17 | 0.63 | 2.16 | 0.617 | |
| 60-69 | 1.45 | 0.80 | 2.63 | 0.218 | 1.40 | 0.77 | 2.54 | 0.269 | 1.43 | 0.79 | 2.59 | 0.234 | |
| 70-79 | 2.95 | 1.71 | 5.10 | 0.000 | 2.93 | 1.70 | 5.07 | 0.000 | 2.81 | 1.63 | 4.85 | 0.000 | |
| 80+ | 3.61 | 1.99 | 6.54 | 0.000 | 3.60 | 1.99 | 6.52 | 0.000 | 3.25 | 1.81 | 5.84 | 0.000 | |
| Gender | Male | 1.00 | 1.00 | 1.00 | |||||||||
| Female | 0.84 | 0.62 | 1.12 | 0.234 | 0.77 | 0.57 | 1.03 | 0.078 | 0.82 | 0.62 | 1.09 | 0.165 | |
| Chronic illnesses | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.17 | 0.88 | 1.54 | 0.282 | 0.93 | 0.70 | 1.24 | 0.629 | 1.00 | 0.76 | 1.31 | 0.983 | |
| Chronic obstructive pulmonary disease | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 2.19 | 1.35 | 3.55 | 0.001 | 1.62 | 0.99 | 2.64 | 0.053 | 1.46 | 0.92 | 2.32 | 0.109 | |
| Cardiovascular diseases | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.24 | 0.77 | 1.98 | 0.376 | 0.97 | 0.61 | 1.54 | 0.890 | 0.97 | 0.61 | 1.55 | 0.914 | |
| Diabetes | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.22 | 0.82 | 1.81 | 0.322 | 1.13 | 0.76 | 1.67 | 0.545 | 1.13 | 0.76 | 1.66 | 0.552 | |
| Hypertension | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 0.91 | 0.63 | 1.32 | 0.620 | 0.80 | 0.56 | 1.15 | 0.227 | 0.87 | 0.61 | 1.25 | 0.456 | |
| Neurological diseases | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 0.62 | 0.09 | 4.06 | 0.616 | 0.36 | 0.05 | 2.38 | 0.289 | 0.45 | 0.07 | 3.03 | 0.412 | |
| Tumors | No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.47 | 0.74 | 2.89 | 0.270 | 1.35 | 0.70 | 2.59 | 0.371 | 1.36 | 0.71 | 2.63 | 0.355 | |
| Smoking | No | 1.00 | 1.00 | 1.00 | |||||||||
| Ex smoker | 0.59 | 0.32 | 1.10 | 0.099 | 0.55 | 0.30 | 1.01 | 0.054 | 0.56 | 0.33 | 0.95 | 0.033 | |
| Smoker | 0.52 | 0.28 | 0.97 | 0.039 | 0.58 | 0.32 | 1.06 | 0.074 | 0.61 | 0.35 | 1.07 | 0.087 | |
| Days to discharge | < 90 | 1.00 | 1.00 | 1.00 | |||||||||
| 90-179 | 0.42 | 0.29 | 0.61 | 0.000 | 0.52 | 0.37 | 0.74 | 0.000 | 0.52 | 0.37 | 0.74 | 0.000 | |
| 180-269 | 0.26 | 0.18 | 0.39 | 0.000 | 0.31 | 0.22 | 0.46 | 0.000 | 0.31 | 0.22 | 0.46 | 0.000 | |
| 270-359 | 0.26 | 0.14 | 0.51 | 0.000 | 0.33 | 0.18 | 0.63 | 0.001 | 0.33 | 0.18 | 0.63 | 0.001 | |
| 360+ | 0.16 | 0.07 | 0.40 | 0.000 | 0.21 | 0.08 | 0.51 | 0.001 | 0.21 | 0.08 | 0.51 | 0.001 | |
| Body mass index | Normal weight/underweight | 1.00 | 1.00 | 1.00 | |||||||||
| Overweight | 0.38 | 0.22 | 0.63 | 0.000 | 0.38 | 0.23 | 0.64 | 0.000 | 0.38 | 0.24 | 0.62 | 0.000 | |
| Obese | 0.85 | 0.54 | 1.36 | 0.512 | 1.02 | 0.64 | 1.63 | 0.922 | 0.93 | 0.63 | 1.37 | 0.714 | |
In fact, in addition to the factor of age > 70 years, certain abnormal test results at discharge were also found to predict more significant pulmonary impairment.
From the hematochemical and instrumental tests performed during the follow-up phase, the first observation is that a large number of the patients discharged with a diagnosis of COVID-19 presented clinical characteristics at follow-up that suggested the risk of progression towards a form of long COVID-19. Globally, in our cohort, approximately 60% of patients underwent laboratory tests after discharge. Gamma-GT, CRP and D-dimer remained above the threshold even in follow-up beyond 360 days in 16.8%, 36% and 48.8% of patients, respectively. Spirometry value alterations persisted in about 17% of patients undergoing tests at approximately 180-239 days’ follow-up. It appears, however, that chronic obstructive pulmonary disease (COPD) is associated with a decrease in FEV1/FVC ratio and/or with the need for oxygen therapy in these patients, although it does not reach statistical significance for the adjusted relative risks.
Altered values of D-dimer, which is a degradation product of fibrin, may suggest altered coagulation mechanisms, which could cause the formation of clots (thrombi), which leads to the onset of dangerous pathological conditions.
In 2021, Townsend et al[10] observed increased levels of D-dimer (> 500 ng/mL) in 25.3% of patients (up to 4 months after SARS-CoV-2 infection), demonstrating that a sustained increase in D-dimers was a common finding that occurs more frequently in patients with severe acute illness and older adults. The same study found persistent symptoms consistent with long COVID-19, with fatigue occurring in 51% of patients. The guidelines suggest monitoring D-dimer levels even after discharge from the hospital but only in patients with persistent respiratory symptoms (long COVID-19)[11].
The suprathreshold levels observed in blood gamma-GT values more than 360 days after discharge suggests hepatic involvement not directly due to COVID-19. In fact, upon admission, a high number of enrolled patients displayed suprathreshold levels of both transaminase and gamma-GT.
In our cohort, the role played by pre-existing chronic viral hepatitis does not seem to be prominent (1.5%), while other causes such as alcohol-related fatty liver disease, non-alcoholic fatty liver disease, obesity, diabetes (8.3%) or a combination of these clinical conditions requires better characterization. It should be underlined that these patients were potentially subjected to drug-induced liver injury during hospitalization. In fact, in our cohort, the systemic use of corticosteroids was 19.3% during the first phase and 51.9% in the second phase[8].
Despite the lack of data on symptoms, laboratory tests show the persistence of an inflammation state with concomitant alterations in spirometry values. In particular, the risk of requiring oxygen therapy or having FEV1/FVC < 70% was displayed by patients with alterations in liver enzymes, hematocrit, hemoglobin, lymphocytes and neutrophils. Although immune cells represent the body's first line of defense, they can also overreact, contributing to a pro-inflammatory state, probably associated with airway remodeling, which contributes to pulmonary dysfunction.
Although the definition of long-term COVID-19 was not yet known at the time, many studies have confirmed the failure of patients affected by COVID-19 to return to a well-being state before acute infection. The manifestations of long COVID-19 are highly variable and include organ-specific clinical manifestations, such as pulmonary and gastrointestinal clinical manifestations[12].
A study conducted on 50402 COVID-19 patients[13] showed a prevalence of long COVID-19 in 3.4% of cases. In multivariable regression analysis, lipid metabolism disorders and obesity, but not diabetes, were identified as strong risk factors for long COVID-19. Therefore, in agreement with our results, diabetes is not among the risk factors influencing the short-term and long-term outcomes of COVID-19. Although in our study there was no data relating to lipid levels, we can hypothesize an alteration of lipid levels in association with an alteration of liver enzymes (including gamma-GT) in the case of hepatic steatosis. Other hematological alterations (red blood cells, hemoglobin) have frequently been found in subjects affected by COVID-19, with persistent symptoms even 4-8 months after discharge[14].
Kalaivani et al[15] observed the persistence of high D-dimer levels up to 6 months after discharge, which is associated with short-term and long-term consequences of COVID-19. During the acute phase of SARS-CoV-2 infection, the immune response and cytokine storm activate the coagulation pathway, leading to a prothrombotic state and excessive inflammatory response[16]. Red blood cells, by storing and releasing several cytokines, including the pro-inflammatory cytokines, could be crucial in the cytokine storm[17]. Although the number of patients undergoing follow-up pro
The limitation of this study was the small size of our sample, which, as is evident, reduces the precision of the estimates (see width of the confidence intervals). The low proportion of patients undergoing laboratory/instrumental tests at follow-up is due to the lack of data recorded and sent during patient monitoring, and is not indicative of patients requiring tests or not after discharge. Furthermore, the lack of data relating to pre-existing pathologies, particularly respiratory conditions (asthma, COPD) associated with spirometric impairment, must be kept in mind, as well as the variability in the data collected in different centers for different case series.
Lo et al[18] studied the relationship between small airway dysfunction and static lung hyperinflation in patients with post-acute sequelae of COVID-19, specifically dyspnea and fatigue. They found that airway dysfunction and dysregulated T-cell immune response correlated with static lung hyperinflation may contribute to the development of dyspnea and fatigue in patients with post-acute sequelae of COVID-19.
Several factors can be indicative of spirometry alterations and the need for oxygen therapy. In particular, we found that alterations in liver enzymes, hematocrit, hemoglobin, lymphocytes and neutrophils were associated with a risk of requiring oxygen therapy or abnormal spirometry results. These imbalanced conditions may contribute to pulmonary dysfunction and warrant further investigation to define potential pulmonary abnormalities.
We thank the physicians for their support in the retrieval of patient records and laboratory data utilized in this study, particularly the SPRINT study group (Marrelli Filomena, Manuela La Torre, Giovanna Manfredini, Elisa Iacopini, Ferruccio Aquilini, Miriana d'Alessandro, Paolo Cameli, Laura Bergantin, Carla Amato, Lorenzo Giovannoni, Luca Livi, Cecilia Guidotti) and Giulia Chiarini for resource management.
| 1. | World Health Organization. Post COVID-19 condition (Long COVID). 2022. Available from: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition. |
| 2. | Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis. 2022;226:1593-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 788] [Article Influence: 262.7] [Reference Citation Analysis (0)] |
| 3. | Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, Rathod S, Halabi S, Elliot K, Shi JQ, Phiri P. A systematic review and meta-analysis of long COVID symptoms. Syst Rev. 2023;12:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 4. | Luo D, Mei B, Wang P, Li X, Chen X, Wei G, Kuang F, Li B, Su S. Prevalence and risk factors for persistent symptoms after COVID-19: a systematic review and meta-analysis. Clin Microbiol Infect. 2024;30:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 5. | Ottiger M, Poppele I, Sperling N, Schlesinger T, Müller K. Work ability and return-to-work of patients with post-COVID-19: a systematic review and meta-analysis. BMC Public Health. 2024;24:1811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Buonsenso D, Gualano MR, Rossi MF, Valz Gris A, Sisti LG, Borrelli I, Santoro PE, Tumminello A, Gentili C, Malorni W, Valentini P, Ricciardi W, Moscato U. Post-Acute COVID-19 Sequelae in a Working Population at One Year Follow-Up: A Wide Range of Impacts from an Italian Sample. Int J Environ Res Public Health. 2022;19:11093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Ely EW, Brown LM, Fineberg HV; National Academies of Sciences, Engineering, and Medicine Committee on Examining the Working Definition for Long Covid. Long Covid Defined. N Engl J Med. 2024;391:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 85] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 8. | Silvestri C, Stasi C, Profili F, Bartolacci S, Sessa E, Tacconi D, Villari L, Carrozzi L, Dotta F, Bargagli E, Donnini S, Masotti L, Rasero L, Lavorini F, Pistelli F, Chimera D, Sorano A, Pacifici M, Milli C, Voller F; Sprint Study Group. Retrospective Study on the Features and Outcomes of a Tuscany COVID-19 Hospitalized Patients Cohort: Preliminary Results. J Clin Med. 2024;13:4626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Oropez CE, Rosenfeld M, Stanojevic S, Swanney MP, Thompson BR. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70-e88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 909] [Cited by in RCA: 2306] [Article Influence: 461.2] [Reference Citation Analysis (0)] |
| 10. | Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, Bergin C, O'Farrelly C, Conlon N, Bourke NM, Ward SE, Byrne M, Ryan K, O'Connell N, O'Sullivan JM, Ni Cheallaigh C, O'Donnell JS. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 11. | Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl JP, Yahav D. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. 2022;28:955-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 12. | Rapporto ISS. COVID-19 n. 15/2021. Indicazioni ad interim sui principi di gestione del Long-COVID. Available from: https://www.iss.it/documenti-in-rilievo/-/asset_publisher/btw1J82wtYzH/content/rapporto-iss-covid-19-n.-15-2021-indicazioni-ad-interim-sui-principi-di-gestione-del-long-covid.-versione-del-1%C2%B0-luglio-2021. |
| 13. | Loosen SH, Jensen BO, Tanislav C, Luedde T, Roderburg C, Kostev K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: a cross-sectional study from 50,402 COVID-19 patients. Infection. 2022;50:1165-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Lechuga GC, Morel CM, De-Simone SG. Hematological alterations associated with long COVID-19. Front Physiol. 2023;14:1203472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 15. | Kalaivani MK, Dinakar S. Association between D-dimer levels and post-acute sequelae of SARS-CoV-2 in patients from a tertiary care center. Biomark Med. 2022;16:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | Lazzaroni MG, Piantoni S, Masneri S, Garrafa E, Martini G, Tincani A, Andreoli L, Franceschini F. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021;46:100745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 17. | Karsten E, Breen E, Herbert BR. Red blood cells are dynamic reservoirs of cytokines. Sci Rep. 2018;8:3101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Lo PC, Feng JY, Hsiao YH, Su KC, Chou KT, Chen YM, Ko HK, Perng DW. Long COVID symptoms after 8-month recovery: persistent static lung hyperinflation associated with small airway dysfunction. Respir Res. 2024;25:209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |