Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.105485
Revised: March 18, 2025
Accepted: April 25, 2025
Published online: September 20, 2025
Processing time: 200 Days and 19.7 Hours
Atrial fibrillation (AF) is a prevalent cardiac arrhythmia associated with signi
To support clinicians in navigating the complexities of anticoagulation in this high-risk population, ensuring optimal outcomes.
The present review followed PRISMA guidelines. Data extraction was conducted using a standardized template that captured key study characteristics: Population demographics, renal function metrics, anticoagulant dosing strategies, and primary and secondary outcomes. For quality assessment, we employed the Cochrane Risk of Bias 2.0 tool for randomized controlled trials. Observational studies were appraised using the Newcastle-Ottawa Scale.
We analyze data from 16 studies to provide recommendations on optimal anticoagulation strategies, balancing thrombotic and bleeding risks. Current evidence supports the preferential use of apixaban in moderate chronic kidney disease and cautiously in end-stage renal disease, emphasizing the importance of individualized therapy.
The management of anticoagulation in AF patients with renal dysfunction is challenging but critical for reducing stroke risk.
Core Tip: Atrial fibrillation (AF) is a common arrhythmia linked to significant morbidity, especially in patients with renal dysfunction. Anticoagulation therapy mitigates thromboembolic risk but poses challenges due to altered drug metabolism and bleeding risks. This systematic review, following PRISMA guidelines, analyzes 16 studies to guide anticoagulant use in this high-risk group. Data extraction covered demographics, renal function, dosing strategies, and outcomes. Quality assessment utilized Cochrane’s Risk of Bias tool and the Newcastle-Ottawa Scale. Evidence favors apixaban for moderate chronic kidney disease and cautious use in end-stage renal disease, stressing individualized treatment. Effective anticoagulation management is crucial for optimizing outcomes in AF patients with renal impairment.
- Citation: Kumar A, Kumar C, Kumar A, Kumari S, Aneela, Rai R, Kumar A, Kumar K, Gyaneshwari, Aslam H, Jawed I, Alam F, Rizvi SAFA, Umair M, Mirza AM. Management of anticoagulation in patients with atrial fibrillation and renal dysfunction: A systematic review. World J Exp Med 2025; 15(3): 105485
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/105485.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.105485
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia, affects millions worldwide, with its prevalence expected to rise due to aging populations and an increasing burden of comorbidities such as hypertension, diabetes, and chronic kidney disease (CKD)[1,2]. It is also associated with an increased risk of stroke, heart failure, and overall mortality[3].
While renal dysfunction is a common comorbidity in patients with AF, affecting approximately one-third of this population[4]. CKD and end-stage renal disease (ESRD) amplify the complexity of managing AF due to altered drug metabolism, heightened bleeding risk, and the potential for thromboembolic complications[5,6].
The interplay between AF and renal dysfunction creates a challenging therapeutic landscape. Vitamin K antagonists (VKAs) like warfarin have been the mainstay of anticoagulation for decades. However, warfarin therapy is fraught with challenges, including narrow therapeutic windows, significant drug and dietary interactions, and the need for regular monitoring[1]. These limitations are exacerbated in patients with CKD due to fluctuating renal function, which affects drug clearance and increases the risk of over-anticoagulation and bleeding[7].
The advent of direct oral anticoagulants (DOACs) has revolutionized anticoagulation therapy by offering predictable pharmacokinetics, fewer drug interactions, and no routine monitoring requirements[2,3]. DOACs, including dabigatran, apixaban, rivaroxaban, and edoxaban, have demonstrated comparable or superior efficacy and safety to VKAs in the general AF population[8]. However, in the current literature, their use in patients with moderate to severe CKD and ESRD remains controversial, as these patients were often excluded from pivotal clinical trials[5].
The pharmacokinetic properties of DOACs differ substantially, particularly concerning renal clearance. For instance, dabigatran is primarily renally excreted, making it less suitable for patients with severe renal dysfunction[1]. In contrast, apixaban, with a lower degree of renal excretion, has emerged as a promising option for these patients due to its favorable safety profile in observational studies[9]. Nonetheless, real-world data and randomized controlled trials (RCTs) in CKD and ESRD populations are limited, highlighting a critical gap in evidence[10].
Adding to the complexity, the risk of bleeding, particularly gastrointestinal bleeding, is higher in patients with renal dysfunction necessitates careful risk stratification[11]. Moreover, emerging biomarkers like D-dimer and cystatin C offer potential for more precise thrombotic and bleeding risk assessment, paving the way for personalized anticoagulation strategies[6].
This study differs from previous meta-analyses by incorporating a broad spectrum of studies, providing a more comprehensive assessment of anticoagulation strategies in AF patients with CKD, ensuring a more nuanced understanding of anticoagulant use in renal impairment[12,13]. There are controversies in guideline recommendations, differences in expert opinions regarding optimal anticoagulation strategies, and gaps in RCTs data, particularly in patients with advanced CKD and ESRD, where evidence remains limited and real-world data plays a crucial role in clinical decision-making.
This review synthesizes the current evidence on anticoagulation management in patients with AF and renal dysfunction by using a systematic review approach aiming to provide a comprehensive overview of therapeutic options while addressing gaps in knowledge and highlighting areas for future research.
We conducted a systematic review of studies published between 2009 and 2024, focusing on anticoagulation in AF patients with renal dysfunction, as per the guidelines of PRISMA. The PICO strategy has been applied for the study selection. P: Patients with AF and documented renal dysfunction. I: Use of DOACs for stroke prevention and anticoagulation management. C: Use of VKA, such as warfarin, as an alternative anticoagulation strategy. O: Clinical endpoints, including stroke prevention, thromboembolic events, major bleeding, and all-cause mortality.
Databases searched included PubMed, Scopus, and the Cochrane Library. Search terms used were "atrial fibrillation", "renal dysfunction", "anticoagulation", "direct oral anticoagulants", "DOACs", and "vitamin K antagonists". Filters were applied to include peer-reviewed articles published in English, focusing on RCTs, observational studies, and meta-analyses (Supplementary Table 1). The present review followed PRISMA guidelines.
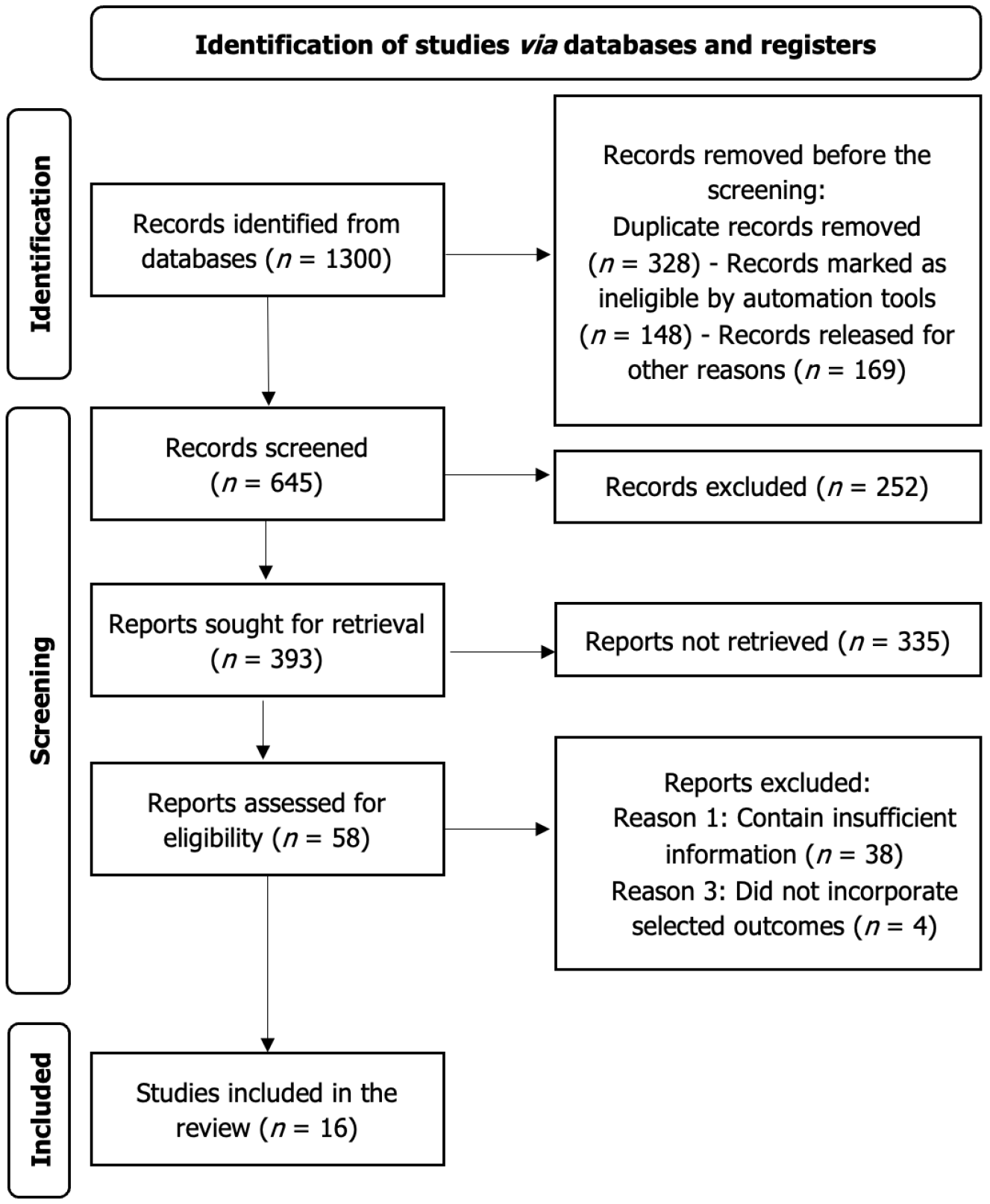
The PRISMA flow diagram illustrates the systematic selection process of studies included in the review (Figure 1). Records were retrieved from databases such as PubMed, Scopus, and the Cochrane Library in the identification phase, with duplicates removed. Two authors (Aslam H and Mirza AMW) independently searched the literature to retrieve studies using the pre-specified search criteria and screened the studies. Screening of titles, abstracts, and full texts was done independently by Aslam H and Mirza AMW while a third reviewer Kumar C resolved the conflicts. 1300 results were retrieved, and 645 records were left after removing duplicates and ineligible studies. 252 studies were removed from the primary screening based on title and abstract. Due to the non-retrieval of 335 studies, only 58 studies could be included in the secondary screening, out of which 42 studies were excluded as they were not reporting the outcomes of interest or reported insufficient information. Finally, we included 16 studies in our systematic review. This structured approach ensures transparency, minimizes bias and enhances the reproducibility of the study selection process. Grey literature was excluded due to concerns about incomplete data reporting and lack of peer review, which may affect study quality and reliability. However, to mitigate publication bias, conference abstracts/preprints were screened and excluded. This systematic review has been submitted for PROSPERO registration (ID 1002988) and is awaiting approval.
Studies were included if they: (1) Investigated anticoagulation in patients with AF and documented renal dysfunction, including CKD stages 3-5 and ESRD; (2) Reported outcomes related to stroke prevention, thromboembolism, major bleeding, and mortality; (3) Utilized DOACs or VKAs as the primary intervention; and (4) Provided sufficient data for risk stratification or subgroup analysis of renal function.
Exclusion criteria included: (1) Studies lacking clear renal function stratification; (2) Articles focusing solely on populations without AF or anticoagulation therapy; (3) Case reports, reviews, or editorials without original data; and (4) Non-English language studies or unpublished data.
Data extraction was conducted using a standardized template that captured key study characteristics: Population demographics, renal function metrics, anticoagulant dosing strategies, and primary and secondary outcomes. Two independent reviewers performed data extraction, with disagreements resolved by consensus or third-party adjudication.
For quality assessment, we employed the Cochrane Risk of Bias 2.0 tool for RCTs (Table 1), evaluating domains such as randomization processes, deviations from intended interventions, and measurement of outcomes. Observational studies were appraised using the Newcastle-Ottawa Scale (NOS) (Table 2), focusing on selection, comparability, and outcome assessment. Studies scoring below a threshold for methodological rigor were excluded to maintain review integrity (Table 2).
| Ref. | Random bias | Blinding bias | Deviations from intended interventions | Missing outcome data | Outcome measurement bias | Selective reporting bias | Overall risk of bias |
| Connolly et al[1] | Low | Low | Low | Low | Low | Low | Low |
| Granger et al[2] | Low | Low | Low | Low | Low | Low | Low |
| Patel et al[3] | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Giugliano et al[33] | Low | Moderate | Low | Low | Low | Low | Low |
| Harel et al[10] | High | High | Moderate | Moderate | Moderate | Low | High |
The Cochrane Risk of Bias 2.0 tool identified potential biases in randomization and blinding across RCTs. Studies with a high risk of bias in more than two domains were excluded. For observational studies, NOS assessments flagged issues with confounding variables and follow-up adequacy. High-quality studies consistently adjusted for renal function and comorbidities, ensuring reliable results. Observational studies were assessed using the NOS, with studies scoring below 5 out of 9 considered high risk of bias and excluded from analysis. This threshold ensured inclusion of studies with robust methodological quality, minimizing confounding and enhancing the reliability of findings on anticoagulation in AF patients with CKD.
A narrative synthesis approach was employed due to heterogeneity in study designs, populations, and outcomes. Subgroup analyses examined anticoagulant efficacy and safety in moderate CKD, advanced CKD, and ESRD populations. Results were stratified based on anticoagulant type, renal function, and primary outcomes, such as thromboembolic events and bleeding rates.
To address heterogeneity, sensitivity analyses were performed, excluding studies with high bias or incomplete data. These methods enhanced the robustness of conclusions drawn from diverse study designs.
The 16 studies included (Table 3) varied in design, patient population, and outcome measures[1-3]. DOACs had a net clinical benefit in AF patients with CKD. Dabigatran reduced the risk of stroke as compared to warfarin but increased the risk of bleeding in moderate CKD patients[1]. In the case of severe CKD, apixaban was found to have lower bleeding incidents[2], while rivaroxaban and edoxaban had comparable effectiveness to warfarin[3,8].
| Ref. | Study design | Sample size | Follow-up duration (year) | Population | Intervention | Key outcomes |
| Chan et al[14] | Retrospective Cohort Study | 2740 | 1.6 | End-stage renal disease patients with atrial fibrillation | Warfarin (vs nonuse), clopidogrel (vs noanuse), aspirin (vs nonuse) | End-stage renal disease patients with coexistent atrial fibrillation are at a significantly higher risk of suffering from a stroke when using warfarin |
| Connolly et al[1] | Randomized Controlled Noninferiority Trial | 18113 | 2.0 | Atrial fibrillation patients, moderate renal impairment | Dabigatran vs Warfarin | Demonstrated that dabigatran significantly reduced the risk of stroke compared to warfarin in atrial fibrillation patients with moderate renal impairment, though increased bleeding risk was observed |
| Granger et al[2] | Randomized Controlled Trial, double-blind | 18201 | 1.8 | Atrial fibrillation patients, all chronic kidney disease stages | Apixaban vs Warfarin | Showed that apixaban reduced bleeding risk and mortality compared to warfarin, including in chronic kidney disease subgroups, making it a safer alternative for atrial fibrillation patients with renal dysfunction |
| Patel et al[3] | Randomized Controlled Trial, double-blind | 14264 | 1.9 | Atrial fibrillation patients, moderate renal impairment | Rivaroxaban vs Warfarin | Highlighted rivaroxaban's comparable efficacy to warfarin in preventing stroke in atrial fibrillation patients, with bleeding risks affected by renal impairment and dosing strategies |
| Giugliano et al[33] | Randomized Controlled Trial, double-blind, double-dummy trial | 21105 | 2.8 | Atrial fibrillation patients, moderate to severe chronic kidney disease | Edoxaban vs Warfarin | Reported edoxaban as an effective alternative to warfarin in atrial fibrillation patients with moderate to severe chronic kidney disease, but with a need for careful dose adjustment to minimize bleeding risks |
| Harel et al[10] | Randomized Controlled Trial, open-label, pilot study | 151 | 0.5 | Dialysis patients with Atrial fibrillation | Direct oral anticoagulants vs Vitamin K antagonists | A pilot randomized controlled trial exploring direct oral anticoagulants use in dialysis patients, indicating potential efficacy but underscoring the importance of individualized therapy in this complex subgroup |
| Siontis et al[9] | Retrospective Cohort Study | 25523 | 0.4 | End-stage renal disease patients with atrial fibrillation | Apixaban use | Revealed lower bleeding rates with apixaban use compared to warfarin in end-stage renal disease patients, supporting its safety and effectiveness in advanced chronic kidney disease populations |
| Weitz et al[20] | Randomized Controlled Trial, single blind | 1242 | 0.1 | Venous thromboembolism prevention population | Milvexian for thromboembolism prevention | Focused on milvexian for venous thromboembolism prevention but emphasized the potential applicability of novel anticoagulants in chronic kidney disease-related atrial fibrillation management |
| Pisters et al[11] | Retrospective Cohort Study | 3978 | 1.0 | Atrial fibrillation patients with bleeding risk | HAS-BLED score | Developed the HAS-BLED score as a bleeding risk prediction tool, with potential for modification to better suit chronic kidney disease-specific bleeding risk assessment |
| Van Bulck et al[19] | Retrospective Mortality Study | 390 | NA | Congenital heart disease patients | Patterns of care in terminal illness | Explored causes of death and care patterns in congenital heart disease patients, indirectly addressing the challenges of anticoagulation in comorbid conditions like chronic kidney disease |
| Yao et al[7] | Retrospective Cohort Study | 14865 | 0.3 | Atrial fibrillation patients with renal dysfunction | Direct oral anticoagulants dosing strategies | Examined direct oral anticoagulants dosing adherence in atrial fibrillation patients with renal dysfunction, identifying inappropriate dosing as a significant contributor to adverse outcomes |
| Lai et al[16] | Retrospective Cohort Study | 399 | 2.5 | Patients with chronic kidney disease and nonvalvular atrial fibrillation | Warfarin (vs nonuse) | Warfarin use was not associated with increase in the incidence of major bleeding events in the chronic kidney disease patients. There was no statistically significant difference between bleeding episodes in both groups |
| Matusik et al[6] | Prospective Cohort Study | 180 | 2.0 | Atrial fibrillation and stage 4 chronic kidney disease patients | Biomarkers and thromboembolism risk | Assessed biomarkers like cystatin C in predicting thromboembolism and bleeding risks in stage 4 chronic kidney disease, advocating for precision medicine in anticoagulation therapy |
| Liabeuf et al[18] | Post-hoc Analysis | 6559 | 3.0 | Moderate-to-advanced chronc kidney disease with Atrial fibrillation | Direct oral anticoagulants prescriptions | Explored direct oral anticoagulants prescription patterns in moderate-to-advanced chronic kidney disease, identifying underuse due to clinician concerns about bleeding risks and patient monitoring challenges |
| Winkelmayer et al[15] | Retrospective Cohort Study | 2313 | 1.8 | End-stage renal disease patients with atrial fibrillation | Warfarin (vs nonuse) | Warfarin use in the end-stage renal disease patients who had a history of atrial fibrillation was associated significantly with hemorrhagic stroke; however, it lowered the risk of ischemic stroke |
| Akbar et al[17] | Retrospective Cohort Study | 88 | 0.9 | End-stage renal disease patients with atrial fibrillation | Warfarin (vs nonuse) | Use of warfarin in hemodialysis patients with atrial fibrillation was not associated with lower risk of death, stroke incidence, and hemorrhagic stroke. However, the risk of myocardial infarction was reduced significantly |
Moderate CKD: Stroke and bleeding outcomes: Connolly et al[1] noted that 110 mg of dabigatran was non-inferior to warfarin (RR: 0.91, P < 0.001) while 150 mg was superior (RR: 0.66, P < 0.001). They concluded that dabigatran reduced the risk of stroke compared to warfarin but can increase the chances of bleeding in patients with moderate renal dysfunction.
Severe CKD: Bleeding and adjustment of dosage: Granger et al[2] demonstrated that apixaban was superior to warfarin in preventing hemorrhagic stroke (HR: 0.51, P < 0.001), caused less bleeding (HR: 0.69, P < 0.001), and resulted in lower mortality (HR: 0.89, P = 0.047). Moreover, its 110mg dose was related to a reduced risk of major bleeding with an RR of 0.80 (P = 0.003). Thus, apixaban has significantly lower bleeding rates than warfarin in patients who had severe renal impairment.
Comparable efficacy: Patel et al[3] demonstrated that rivaroxaban had similar efficacy as compared to warfarin. Rivaroxaban was non-inferior to warfarin (HR: 0.88, P < 0.001), and it had a lower incidence of intracranial hemorrhage (0.5% vs 0.7%, P = 0.02) and fatal bleeding (0.2% vs 0.5%, P = 0.003). They further reported that dose adjustments must be made based on the renal function of the patients.
ESRD/dialysis: Safety: Siontis et al[9] proposed that apixaban can be a safe choice in ESRD patients as compared to warfarin. Apixaban showed a significantly lower risk of major bleeding (HR: 0.72, P < 0.001). Five milligrams was associated with significantly lower risks of stroke/systemic embolism (HR: 0.61, P = 0.04) and death (HR: 0.64, P = 0.01) as compared with reduced-dose apixaban and warfarin (HR: 1.11, P = 0.49 for stroke/systemic embolism; HR: 1.07, P = 0.52 for death).
According to Chan et al[14] and Winkelmayer et al[15], ESRD patients with coexistent AF had a higher risk of hemorrhagic strokes with an HR of 1.93 (P = 0.02) and 2.38 (c-statistic= 0.68) respectively, while Lai et al[16], reported a decreased risk of thromboembolic events in warfarin use (OR: 0.28, P < 0.0001). However, according to Akbar et al[17], warfarin was not associated with increased hemorrhagic strokes (HR: 0.56, P = 0.689), while the risk of myocardial infarction was reduced significantly (HR: 0.34, P = 0.001).
DOACs were found to have an advantage over warfarin as they consistently reduced the stroke risk, thus decreasing thromboembolic events in all CKD stages compared to VKAs. Two studies reported bleeding complications in advanced CKD patients. Connolly et al[1] and Patel et al[3] observed increasing rates of gastrointestinal bleeding with dabigatran and rivaroxaban usage. However, apixaban was associated with reduced major bleeding events, as reported by Siontis et al[9] Additionally, Shroff et al[8] stated that there was no significant effect of the choice of anticoagulant on CKD progression.
Matusik et al[6] proposed that GDF-15 was positively associated with thrombin formation in high-risk AF patients with coexistent renal dysfunction. Moreover, multivariable analysis predicted that higher levels of some biomarkers can lead to increased risk of bleeding; cystatin C (mg/L, HR: 9.2, P = 0.003), cTnT-hs (ng/L, HR: 1.3, P = 0.0001), GDF-15 (per 100 pg/L, HR: 1.5, P < 0.0001) (Table 3).
Several studies highlighted unique patient populations and outcomes. For example, a pilot RCTs by Harel et al[10] explored anticoagulation in dialysis patients, showing that DOACs might offer similar efficacy to VKAs but emphasized the need for individualized dosing strategies. Similarly, observational data from Yao et al[7] highlighted that inappropriate dosing of DOACs in patients with renal dysfunction increased the risk of adverse outcomes, underscoring the importance of adherence to renal dosing guidelines.
The study by Liabeuf et al[18] investigated the prescribing patterns of DOACs in patients with moderate-to-advanced CKD, revealing significant variability in clinical practice and the need for standardized protocols to optimize outcomes. Moreover, biomarker-based assessments, such as those discussed by Matusik et al[6], are gaining traction as a means to refine anticoagulation decisions and mitigate risks in high-risk populations.
Patients with advanced CKD face unique challenges due to the dual risks of thromboembolism and bleeding[4]. Data from Potpara et al[4] emphasized the importance of integrating patient-specific factors, including comorbid conditions and frailty, into decision-making processes. This holistic approach aligns with findings by Shroff et al[8], who advocated for multidisciplinary care models to enhance treatment outcomes in this complex patient cohort. Studies document patterns of care along with death causes in patients with comorbid congenital heart diseases in addition to chronic cardiovascular patients who need anticoagulation treatment[19]. Similarly, use of novel therapeutic options like milvexian can lead to improved outcomes and better safety profile[20].
Anticoagulation in AF patients with renal dysfunction requires a nuanced approach to balance thromboembolic protection against bleeding risks[21]. Evidence supports the use of DOACs over VKAs in mild to moderate CKD, particularly apixaban, given its favorable safety profile[2,9]. The pharmacokinetic advantages of DOACs, including predictable effects and fewer dietary interactions, make them preferable in many scenarios[22]. Similar results were shown by a systematic review done by Alyassi et al[23], in which they concluded that DOACs were superior to warfarin in terms of stroke prevention, safety profile, and lesser major bleeding events. However, clinicians must carefully adjust doses based on renal function to mitigate bleeding risks while ensuring efficacy.
For patients with advanced CKD or ESRD, therapeutic decision-making becomes more complex[24]. Apixaban’s reduced renal clearance compared to other DOACs provides a relative advantage, supported by observational studies indicating lower bleeding rates[9]. A systematic review by Parker et al[25] reported contrasting findings. They found that the efficacy of DOACs and warfarin was comparable, and there was no significant difference between them [RR: 0.7 (0.4 to 1.1), P value = 0.12]. However, a cohort study reports that apixaban usage reduces recurrent venous thromboembolism (VTE) in ESRD patients who are being treated for acute VTE [HR: 0.6, (0.4 to 0.8)][26]. Nevertheless, these findings are based on real-world data rather than RCTs, highlighting the need for further research in this population[27].
Incorporating biomarkers into anticoagulation management has emerged as a promising strategy to optimize outcomes in AF patients with renal dysfunction[28]. Biomarkers such as D-dimer, cystatin C, and NT-proBNP provide insights into thrombotic and bleeding risks, offering a more personalized approach to therapy. Matusik et al[6] demonstrated that integrating these biomarkers into clinical decision-making enhances risk stratification, particularly in high-risk populations with stage 4 CKD. Increased D-dimer levels in blood refer to prothrombotic activity, reflecting increased endothelial dysfunction, chronic inflammation, and abnormal function of thrombocytes in patients with advanced CKD. Thus, it can serve as a significant assessment biomarker for an individualized approach to intervention in such patients. Similarly, heightened levels of cystatin C refer to an increased risk of renal dysfunction and hemostatic dysregulation, thus depicting abnormal glomerular filtration rate and a more individualized approach towards DOAC treatment.
While the HAS-BLED score remains a widely used tool for bleeding risk assessment, its limitations in CKD patients necessitate adaptations or supplementary tools[29]. For instance, combining HAS-BLED with renal-specific biomarkers could improve predictive accuracy and guide anticoagulation choices more effectively[11].
Recent advances in the understanding of renal pathophysiology in anticoagulation have illuminated critical pathways that may alter drug effectiveness and safety profiles[30]. Studies highlight the role of uremic toxins in modifying the metabolism of anticoagulants, with implications for personalized therapy in ESRD patients[8]. Additionally, the interplay between CKD-induced inflammation and hypercoagulability adds another layer of complexity to risk stratification and treatment planning[4].
Emerging evidence also points to the benefits of machine learning algorithms in predicting individual patient outcomes[31]. These technologies integrate clinical, genetic, and biomarker data to refine anticoagulation strategies, offering a glimpse into the future of precision medicine in this field[18].
Effective management of AF in patients with renal dysfunction often requires a multidisciplinary approach[32]. Collaboration between cardiologists, nephrologists, and primary care physicians ensures comprehensive risk assessment and tailored treatment plans. Shroff et al[8] highlighted the importance of addressing comorbidities, such as hypertension and diabetes, which frequently coexist in this population and influence anticoagulation outcomes. Another study reported edoxaban as an effective alternative to warfarin in AF patients with moderate to severe CKD, but with a need for careful dose adjustment to minimize bleeding risks[33].
The role of patient education and shared decision-making cannot be overstated[34]. Engaging patients in discussions about the risks and benefits of different anticoagulation strategies fosters adherence and improves overall treatment satisfaction. Potpara et al[4] advocated for incorporating patient preferences and values into the decision-making process, particularly in scenarios where the evidence base is limited or conflicting. Moreover, the challenges of anticoagulation in CKD due to other comorbid conditions like cardiovascular diseases have also been discussed[35].
Despite advancements in anticoagulation therapy, several gaps remain in the evidence base for managing AF in patients with renal dysfunction[36]. The exclusion of ESRD patients from many pivotal DOAC trials leaves clinicians reliant on observational data and post hoc analyses. Future RCTs should prioritize this population to provide robust, high-quality evidence[37]. Additionally, exploring the role of novel anticoagulants, such as milvexian, may offer new therapeutic options with improved safety profiles[20,38].
Long-term studies evaluating the impact of anticoagulation on CKD progression are also needed[39]. While current data suggest minimal effects on renal function, a more detailed understanding of these relationships could inform treatment strategies and enhance patient outcomes.
The role of novel imaging modalities and hemodynamic assessments in monitoring anticoagulation efficacy and safety in CKD populations also deserves exploration. Advanced imaging technologies, combined with biomarkers, could provide a more dynamic picture of patient-specific risks, enabling real-time adjustments to therapeutic regimens.
The incorporation of novel pathways, such as the factor XI inhibitors, represents a frontier in anticoagulation therapy. These agents promise to minimize bleeding risks while maintaining thromboembolic protection, offering a potentially transformative approach for high-risk CKD populations[38].
Further investigations should focus on the socio-economic barriers affecting treatment access. Studies reveal disparities in the prescription of DOACs between different demographic groups, often influenced by healthcare accessibility and socio-economic factors. Addressing these disparities through policy reform and targeted interventions could significantly improve outcomes in vulnerable populations.
The integration of wearable technologies capable of real-time monitoring of anticoagulation parameters may also enhance clinical decision-making[40]. These devices, combined with artificial intelligence, could alert healthcare providers to potential complications, facilitating early intervention and reducing adverse events. Moreover, Labovitz et al[41] studied the effect of implementing AI software for treatment adherence on 28 patients diagnosed with ischemic stroke. The intervention group showed 100% adherence over 12 weeks, while 50% was in the control group. Thus showcasing that AI has the potential to monitor patient behavior and change adherence patterns. Research on the efficacy and cost-effectiveness of these innovations in CKD populations should be prioritized.
A study conducted by Lunde et al[42] evaluated the effect of socioeconomic factors on the oral anticoagulation therapy pattern of the patients. They concluded that low education, low income, and living alone affected the treatment initiation in the patients, as these factors were associated with less initiation of the therapy. Moreover, crude inequality was observed, which increased from 6% (4 to 8) in 2011-2013 to 9% (7 to 11) in 2013-2016 for males, and the same trend was seen for females as well. This emphasizes the need to reduce the systematic inequality in society and homogenize the availability of treatment.
Our study is subject to several limitations. The results should be cautiously interpreted and must not be overgeneralized. Observational studies are subject to reporting biased data because of confounding factors, selection bias, information bias, and high heterogeneity due to different study designs (cohort, case-control, cross-sectional), methodologies, and analytical/statistical models. Lack of randomization, with patients having disease of different severity and comorbidities, and lack of adjustment of all confounding factors can lead to less precise results. Thus results cannot be generalized based on limited observational data and small number of clinical trials, especially for the high-risk group like ESRD.
Current evidence supports the preferential use of reduced-dose apixaban for anticoagulation in AF patients with moderate CKD and cautiously in ESRD patients, to reduce the risk of stroke. Biomarker-driven approaches, multidisciplinary care models, and advancements in predictive analytics offer additional avenues for optimizing outcomes. Future studies should address the limitations of existing data by focusing on robust clinical trials on ESRD patients, as current literature relies heavily on observational data. Moreover, the pharmacokinetics and pharmacodynamics of DOACs should be studied in patients with severe renal dysfunction.
| 1. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7917] [Cited by in RCA: 8063] [Article Influence: 503.9] [Reference Citation Analysis (0)] |
| 2. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6075] [Cited by in RCA: 6543] [Article Influence: 467.4] [Reference Citation Analysis (0)] |
| 3. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 6915] [Article Influence: 493.9] [Reference Citation Analysis (2)] |
| 4. | Potpara TS, Ferro C, Lip GYH, Dan GA, Lenarczyk R, Mallamaci F, Ortiz A, Sarafidis P, Ekart R, Dagres N. Management of atrial fibrillation in patients with chronic kidney disease in clinical practice: a joint European Heart Rhythm Association (EHRA) and European Renal Association/European Dialysis and Transplantation Association (ERA/EDTA) physician-based survey. Europace. 2020;22:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Goel N, Jain D, Haddad DB, Shanbhogue D. Anticoagulation in Patients with End-Stage Renal Disease and Atrial Fibrillation: Confusion, Concerns and Consequences. J Stroke. 2020;22:306-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Matusik PT, Leśniak WJ, Heleniak Z, Undas A. Thromboembolism and bleeding in patients with atrial fibrillation and stage 4 chronic kidney disease: impact of biomarkers. Kardiol Pol. 2021;79:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J Am Coll Cardiol. 2017;69:2779-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 8. | Shroff GR, Stoecker R, Hart A. Non-Vitamin K-Dependent Oral Anticoagulants for Nonvalvular Atrial Fibrillation in Patients With CKD: Pragmatic Considerations for the Clinician. Am J Kidney Dis. 2018;72:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, Noseworthy PA, Shah ND, Saran R, Nallamothu BK. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation. 2018;138:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 10. | Harel Z, Smyth B, Badve SV, Blum D, Beaubien-Souligny W, Silver SA, Clark E, Suri R, Mavrakanas TA, Sasal J, Prasad B, Eikelboom J, Tennankore K, Rigatto C, Prce I, Madore F, Mac-Way F, Steele A, Zeng Y, Sholzberg M, Dorian P, Yan AT, Sood MM, Gladstone DJ, Tseng E, Kitchlu A, Walsh M, Sapir D, Oliver MJ, Krishnan M, Kiaii M, Wong N, Kotwal S, Battistella M, Acedillo R, Lok C, Weir M, Wald R. Anticoagulation for Patients with Atrial Fibrillation Receiving Dialysis: A Pilot Randomized Controlled Trial. J Am Soc Nephrol. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2971] [Cited by in RCA: 3357] [Article Influence: 223.8] [Reference Citation Analysis (0)] |
| 12. | Rhee TM, Lee SR, Choi EK, Oh S, Lip GYH. Efficacy and Safety of Oral Anticoagulants for Atrial Fibrillation Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2022;9:885548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 13. | Li Y, Wu S, Zhou J, Zhang J. Efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation combined with chronic kidney disease: a systematic review and meta-analysis. Thromb J. 2024;22:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 15. | Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Lai HM, Aronow WS, Kalen P, Adapa S, Patel K, Goel A, Vinnakota R, Chugh S, Garrick R. Incidence of thromboembolic stroke and of major bleeding in patients with atrial fibrillation and chronic kidney disease treated with and without warfarin. Int J Nephrol Renovasc Dis. 2009;2:33-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Akbar MR, Febrianora M, Iqbal M. Warfarin Usage in Patients With Atrial Fibrillation Undergoing Hemodialysis in Indonesian Population. Curr Probl Cardiol. 2023;48:101104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Liabeuf S, Laville SM, Bieber B, Tu C, Stengel B, Wong MMY, Calice da Silva V, Fliser D, Robinson BM, Pecoits-Filho R, Massy ZA. Prescription of Direct Oral Anticoagulants to Patients With Moderate-to-Advanced CKD: Too Little or Just Right? Kidney Int Rep. 2021;6:2496-2500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Van Bulck L, Goossens E, Morin L, Luyckx K, Ombelet F, Willems R, Budts W, De Groote K, De Backer J, Annemans L, Moniotte S, de Hosson M, Marelli A, Moons P; BELCODAC consortium. Last year of life of adults with congenital heart diseases: causes of death and patterns of care. Eur Heart J. 2022;43:4483-4492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Weitz JI, Strony J, Ageno W, Gailani D, Hylek EM, Lassen MR, Mahaffey KW, Notani RS, Roberts R, Segers A, Raskob GE; AXIOMATIC-TKR Investigators. Milvexian for the Prevention of Venous Thromboembolism. N Engl J Med. 2021;385:2161-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 21. | Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, Goldsmith D. Anticoagulation in Concomitant Chronic Kidney Disease and Atrial Fibrillation: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2204-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 22. | Wiggins BS, Dixon DL, Neyens RR, Page RL 2nd, Gluckman TJ. Select Drug-Drug Interactions With Direct Oral Anticoagulants: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Alyassi A, Iqbal MS, Hamadi RL, Alhabsy SH, Khouri ES, Shamia OHA, Alshamsi FA, Hossain MR, Hussein AM, Saeed AY, Ahmed KAH. Comparative Efficacy of Direct Oral Anticoagulants vs Warfarin in Atrial Fibrillation Patients with Chronic Kidney Disease. J Popul Ther Clin Pharmacol. 2025;32:221-231. [DOI] [Full Text] |
| 24. | Rosansky SJ, Schell J, Shega J, Scherer J, Jacobs L, Couchoud C, Crews D, McNabney M. Treatment decisions for older adults with advanced chronic kidney disease. BMC Nephrol. 2017;18:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Parker K, Hartemink J, Saha A, Mitra R, Lewis P, Power A, Choudhuri S, Mitra S, Thachil J. A systematic review of the efficacy and safety of anticoagulants in advanced chronic kidney disease. J Nephrol. 2022;35:2015-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Wetmore JB, Herzog CA, Yan H, Reyes JL, Weinhandl ED, Roetker NS. Apixaban versus Warfarin for Treatment of Venous Thromboembolism in Patients Receiving Long-Term Dialysis. Clin J Am Soc Nephrol. 2022;17:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Garrison LP Jr, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report. Value Health. 2007;10:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 494] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 28. | Koniari I, Artopoulou E, Velissaris D, Ainslie M, Mplani V, Karavasili G, Kounis N, Tsigkas G. Biomarkers in the clinical management of patients with atrial fibrillation and heart failure. J Geriatr Cardiol. 2021;18:908-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 29. | Truong B, Zheng J, Hornsby L, Fox B, Chou C, Qian J. Development and Validation of Machine Learning Algorithms to Predict 1-Year Ischemic Stroke and Bleeding Events in Patients with Atrial Fibrillation and Cancer. Cardiovasc Toxicol. 2024;24:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Scicchitano P, Tucci M, Bellino MC, Cortese F, Cecere A, De Palo M, Massari F, Caldarola P, Silvestris F, Ciccone MM. The Impairment in Kidney Function in the Oral Anticoagulation Era. A Pathophysiological Insight. Cardiovasc Drugs Ther. 2021;35:505-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Chekroud AM, Bondar J, Delgadillo J, Doherty G, Wasil A, Fokkema M, Cohen Z, Belgrave D, DeRubeis R, Iniesta R, Dwyer D, Choi K. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry. 2021;20:154-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 32. | Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C. Cardio-Pulmonary-Renal Interactions: A Multidisciplinary Approach. J Am Coll Cardiol. 2015;65:2433-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4144] [Cited by in RCA: 3805] [Article Influence: 317.1] [Reference Citation Analysis (0)] |
| 34. | Narva AS, Norton JM, Boulware LE. Educating Patients about CKD: The Path to Self-Management and Patient-Centered Care. Clin J Am Soc Nephrol. 2016;11:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1083] [Article Influence: 270.8] [Reference Citation Analysis (2)] |
| 36. | Gopinathannair R, Chen LY, Chung MK, Cornwell WK, Furie KL, Lakkireddy DR, Marrouche NF, Natale A, Olshansky B, Joglar JA; American Heart Association Electrocardiography and Arrhythmias Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; and the Stroke Council. Managing Atrial Fibrillation in Patients With Heart Failure and Reduced Ejection Fraction: A Scientific Statement From the American Heart Association. Circ Arrhythm Electrophysiol. 2021;14:HAE0000000000000078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 37. | Kovesdy CP. Clinical trials in end-stage renal disease-priorities and challenges. Nephrol Dial Transplant. 2019;34:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Perera V, Abelian G, Li D, Wang Z, Zhang L, Lubin S, Bello A, Murthy B. Single-Dose Pharmacokinetics of Milvexian in Participants with Normal Renal Function and Participants with Moderate or Severe Renal Impairment. Clin Pharmacokinet. 2022;61:1405-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Hughes S, Szeki I, Nash MJ, Thachil J. Anticoagulation in chronic kidney disease patients-the practical aspects. Clin Kidney J. 2014;7:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Xie Y, Lu L, Gao F, He SJ, Zhao HJ, Fang Y, Yang JM, An Y, Ye ZW, Dong Z. Integration of Artificial Intelligence, Blockchain, and Wearable Technology for Chronic Disease Management: A New Paradigm in Smart Healthcare. Curr Med Sci. 2021;41:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using Artificial Intelligence to Reduce the Risk of Nonadherence in Patients on Anticoagulation Therapy. Stroke. 2017;48:1416-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 42. | Lunde ED, Joensen AM, Fonager K, Lundbye-Christensen S, Johnsen SP, Larsen ML, Lip GYH, Riahi S. Socioeconomic inequality in oral anticoagulation therapy initiation in patients with atrial fibrillation with high risk of stroke: a register-based observational study. BMJ Open. 2021;11:e048839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |