INTRODUCTION
Radiation-induced liver injury is becoming increasingly familiar with the advancements in technology and increased exposure to low levels of radiation during medical procedures and treatment[1]. Ionizing radiation can induce functional and structural changes in the liver, leading to radiation-induced liver diseases[2]. Using radiation therapy for the treatment of hepatic tumors is rapidly increasing; the application of radiation treatment for tumors in the liver, right lower lung, distal esophagus, and whole abdomen is on the increase, resulting in cell damage to the non-tumor compartments of the liver[3,4]. Clinically, radiation-induced liver diseases have drawn particular attention from physicians recently as the main challenge in delivering curative tumors doses[5]. Novel approaches to prevent radiation-induced liver damage during radiation therapy are urgently needed.
ATP-sensitive K+ (KATP) channels are observed in various cells and tissues[6], including pancreatic β cells[7], skeletal and cardiac muscles[8-11], and the brain[12-14], kidneys[15,16], and liver[17,18]. These channels may close or open depending on the concentration of intracellular ATP, playing a protective role during ischemia[19] by coupling changed energy metabolism via membrane potential[20]. KATP channels are formed by two kinds of subunits, pore-forming subunits (Kir6.1 or Kir6.2) and regulatory subunits, such as sulfonylurea receptors (SUR1, SUR2A, and SUR2B), with a hetero-octamer structure of four pore-forming subunits and four regulatory subunits[21,22]. Different subunit compositions show different physiological and pharmacological functions[23,24]. KATP channels in the hypothalamus are involved in glucose homeostasis by controlling glucagon and catecholamines[25]. The liver has diverse functions, such as producing bile, metabolism of ingested nutrients, eliminating waste products, and synthesizing protein; it is directly involved in glucose metabolism[4], which influences the change in intracellular ATP concentrations to which KATP channels are sensitive. KATP channels regulate the proliferation of primary rat hepatocytes[26]. A previous study revealed that KATP channel subunits are widely localized in hepatocytes, hepatic stellate cells, and Kupffer cells[18].
Radiation exposure is generally classified as low (< 0.5 Gy), moderate (0.5-5 Gy), or high (> 5 Gy)[27]. Radiation causes ultrastructural and biochemical changes in hepatocytes, which are known to depend on lipid metabolism[28]. Radiation exposure is one of the causes of hepatic fibrosis after liver injury recovery[29-31]. However, there is no particular information on the role of KATP channel subunits in radiation-induced liver damage. This study investigated the expression of KATP channel subunits in the liver after different irradiation doses.
MATERIALS AND METHODS
Animals
Twenty adult (8-12 wk old) male C57BL/6 mice (CLEA Japan, Inc.) were applied for this study. The study was approved by the Nagasaki University Japan’s Institutional Animal Care and Use Committee and conducted following the Committee’s protocols. All animal procedures followed the institutional and national guidelines. The mice were kept under constant environmental conditions in group cages with a condition of 12-h light/dark cycle and given food and water ad libitum. The mice were randomly separated into four groups: (1) Control, without γ-ray irradiation (n = 2); (2) lower dose (0.2 Gy) exposure (n = 6); (3) medium dose (1 Gy) exposure (n = 6); and (4) high dose (5 Gy) exposure (n = 6).
Radiation exposure
The mice were placed in a special cage for radiation exposure to assess radiation-induced liver injury corresponding to dose dependency. PS-3100SB (Pony Industry Co., Ltd., Osaka, Japan), a γ-ray irradiation system with a Cs source was used. The mice were exposed to a dose of 0, 0.2, 1, or 5 Gy/min. The mice were not anesthetized before irradiation.
Preparation of samples
After radiation exposure, the mice were sacrificed for experiments at 3 and 24 h later. Mice were euthanized via general anesthesia by intraperitoneal injection of pentobarbital (160 mg/kg), followed by perfusion with cold Zamboni’s fixatives through the heart. The liver was quickly collected and further fixed for 6 h in the same fixative, and then put into 30% sucrose over 12 h until it sank down to the bottom. Cryosections were cut (8 to10 µm thick) with a Leica CM1950 cryostat, thaw-mounted on MAS-coated glass slides (Matsunami Industries, Kishiwada, Japan), and kept at -20 ℃ until use.
Histological and immunohistochemical staining
After air-drying for 30 min, several sections were stained with Mayer hematoxylin and eosin (H&E) for morphological observations. The others sections were pre-incubated with 5% normal donkey serum for 30 min, and then incubated with KATP channel subunit antibodies, which were goat anti-human Kir6.1 and Kir6.2 (Santa Cruz Biotechnology), and rabbit anti-rat SUR1, SUR2A, and SUR2B[18] at 1:500 dilution. After washing in PBS three times, 5 min each, the sections were incubated with biotinylated rabbit anti-goat IgG (BA5000; Vector Laboratories, Inc., Burlingame, CA, United States) or biotinylated goat anti-rabbit IgG (BA1000; Vector Laboratories, Inc.) at 1:200 each for 30 min, and subsequently with the ABC complex (Vectastain ABC Kit; Vector Laboratories, Inc.) according to the manufacturer’s instructions. Immunoreactions were carried out with 3,3’-diaminobenzedine in the presence of 0.003% H2O2, and counterstained with Mayer’s hematoxylin.
To show how the pore-forming subunit Kir6.1 co-localizes with the regulatory subunit SUR2B in the liver after radiation, sections were treated with primary goat anti-human Kir6.1 and rabbit anti-rat SUR2B antibodies, each diluted 1:500, for one night (12 h) at room temperature. After being washed three times in PBS for 15 min, the sections were incubated with a mixture of secondary antibodies labeled with Alexa 488 and Alexa 594 (A21432 and A21207; Molecular Probes, Inc., Eugene, OR), each diluted 1:200. Fluorescence images were acquired using an Olympus microscope (BX51; Tokyo, Japan).
RESULTS
Dose dependency of radiation-induced injury in mouse liver
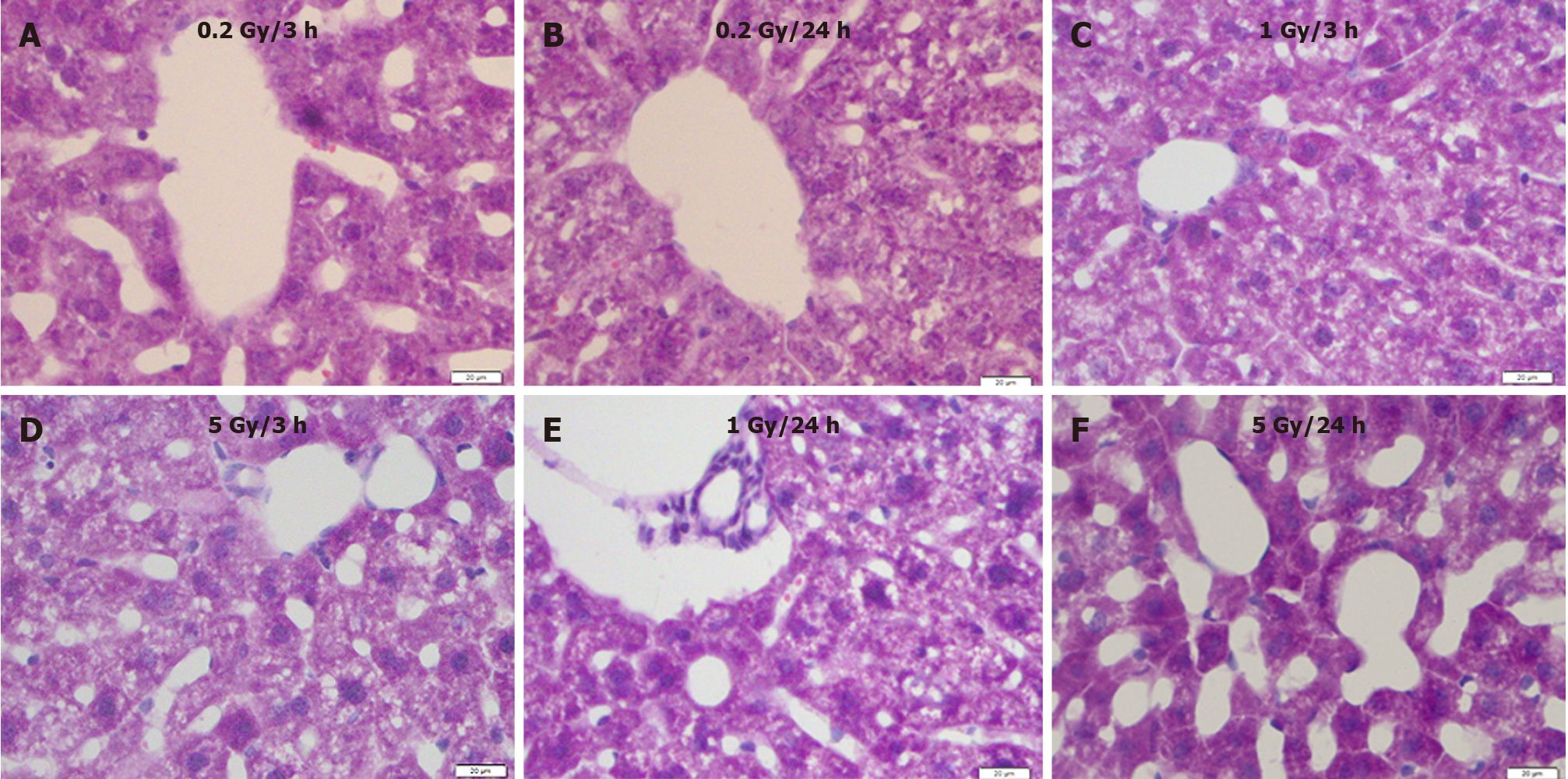
Compared with the control (Figure 1), 0.2 Gy induced hepatocyte atrophy and sinusoid enlargement 3 h after exposure (Figure 2A), which recovered 24 h after irradiation (Figure 2B). Degeneration of hepatocytes, constriction of the hepatocyte cord, and enlarged sinusoids were observed in the 1.0 Gy and 5.0 Gy dose groups 3 h after exposure (Figure 2C and D), with the effects worsening 24 h after γ-ray irradiation (Figure 2E and F).
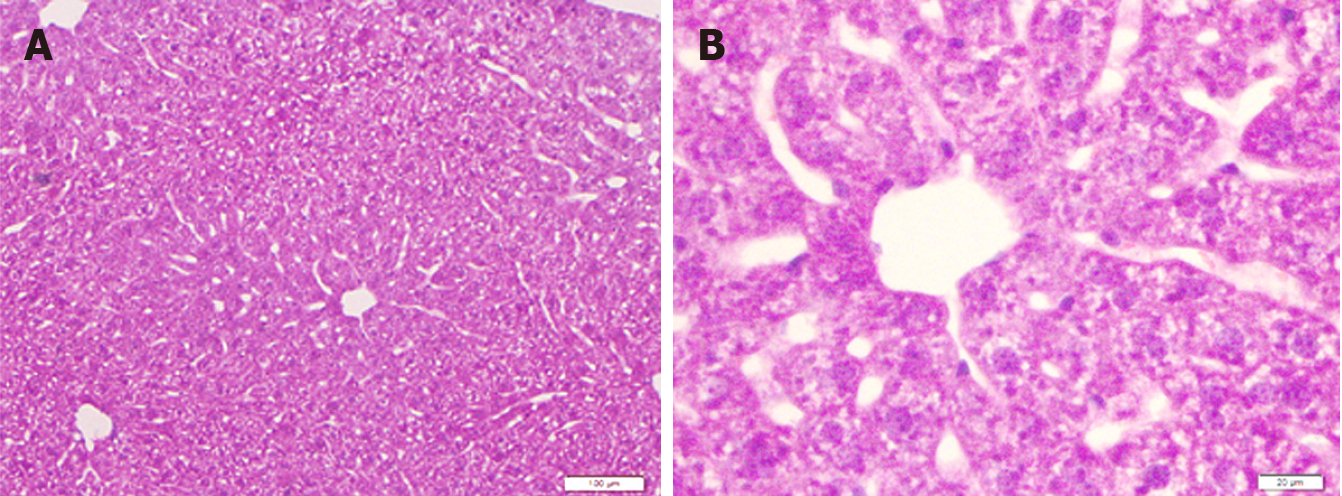
Figure 1 Liver sections of mice in the control group.
A: Low magnification; B: High magnification. H&E staining. Bars: 100 μm (A); 20 μm (B).
Figure 2 Mouse liver sections showing morphological changes with different radiation doses at different time points.
A: 3 h after exposure to low dose (0.2 Gy); B: 24 h after exposure to low dose (0.2 Gy); C: 3 h after exposure to medium dose (1 Gy); D: 3 h after exposure to high dose (5 Gy); E: 24 h after exposure to medium dose (1 Gy); F: 24 h after exposure to high dose (5 Gy). H&E staining. Bars: 20 μm.
Radiation-induced changes in expression of KATP channel subunits
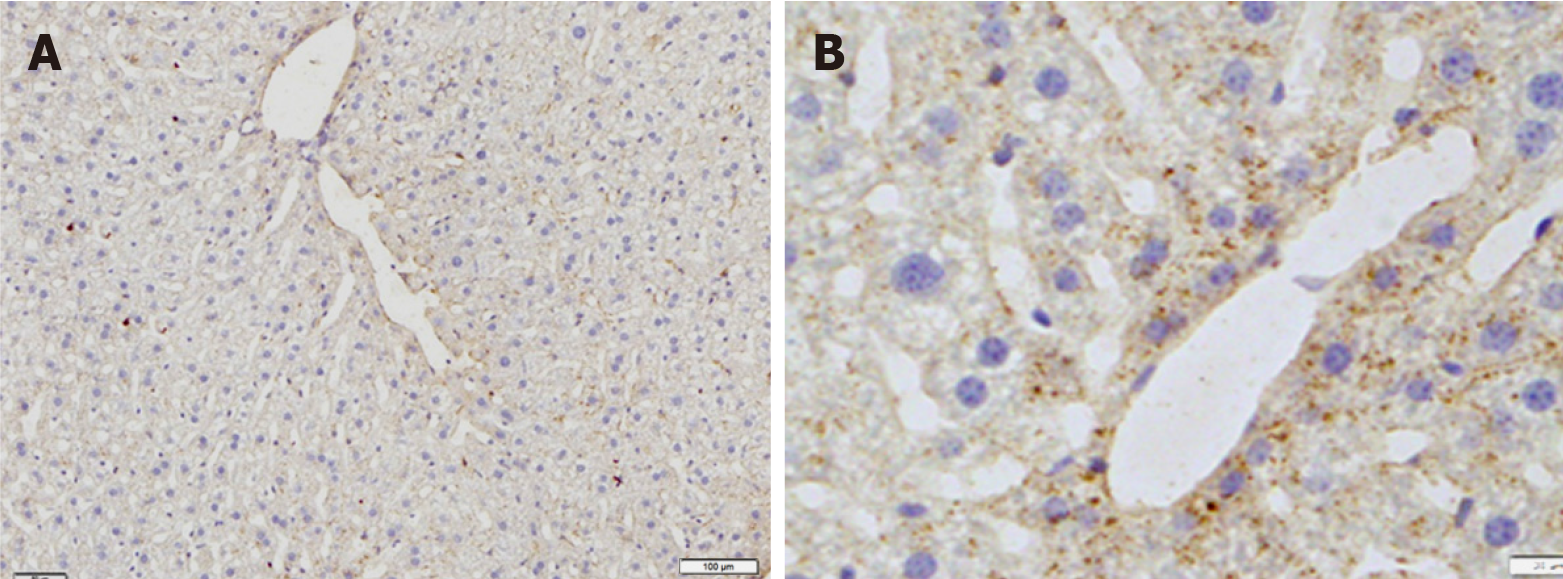
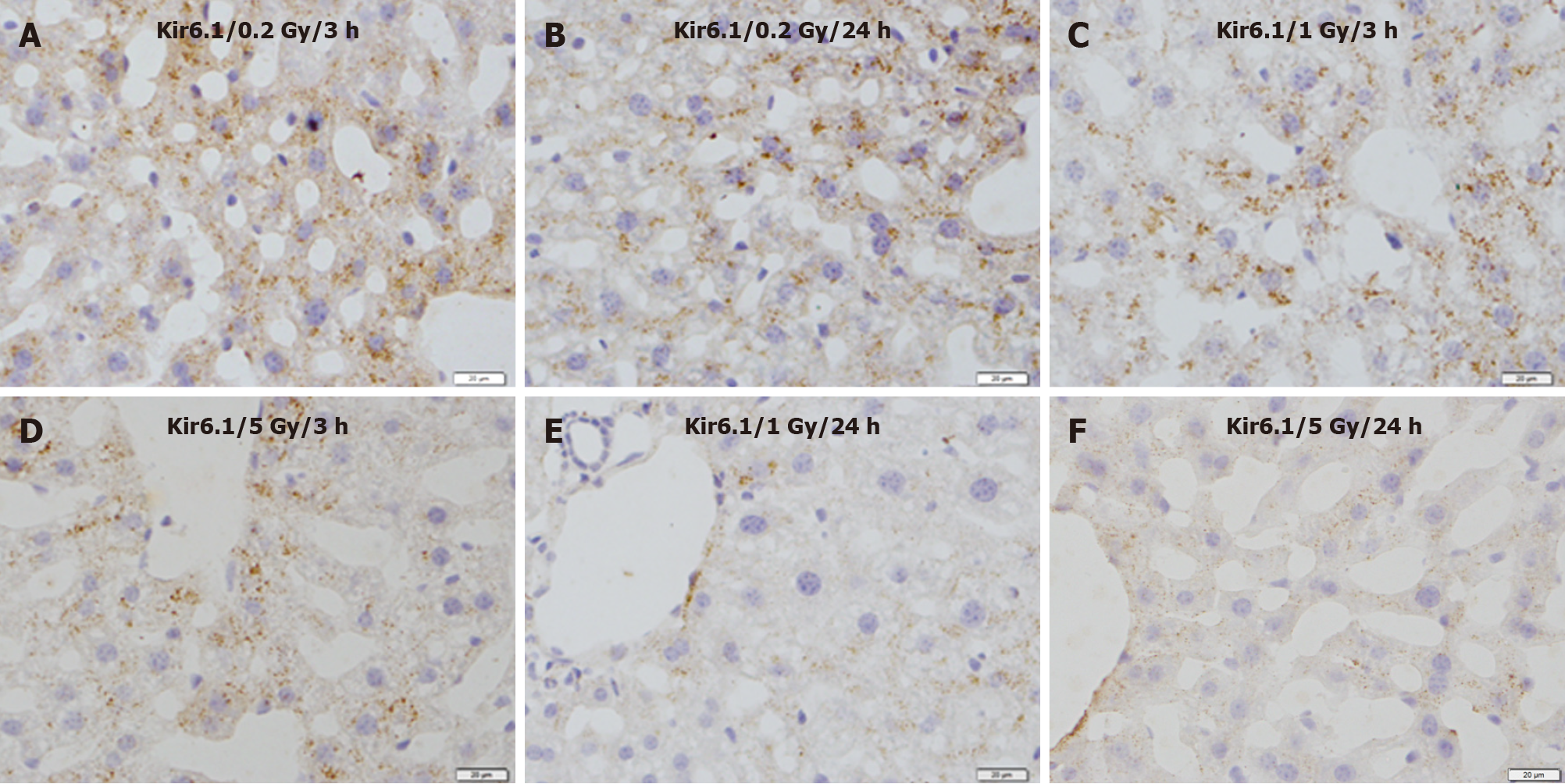
Immunohistochemical staining revealed that immunoreactivity for Kir6.1 was widely observed in hepatocytes in the control group (Figure 3A). The intensity of immunoreactivity was higher in the central vein and lower in the distal area in the cell membrane and cytoplasm of hepatocytes. Under high magnification, punctate immunoreactive products of Kir6.1 were observed in the cytoplasm of hepatocytes (Figure 3B). Compared with the control group, the expression of Kir6.1 was enhanced in the liver 3 h after exposure (Figure 4A), which almost recovered 24 h after exposure (Figure 4B) to 0.2 Gy. In the livers exposed to the medium (1 Gy) and high (5 Gy) doses of γ-ray, the expression level of Kir6.1 was decreased at 3 h (Figure 4C and D) and significantly reduced at 24 h after irradiation (Figure 4E and F).
Figure 3 Immunostaining for Kir6.1 in the liver of mice in the control group.
A: Low magnification; B: High magnification. Bars: 100 μm (A); 20 μm (B).
Figure 4 Immunostaining for Kir6.1 in the liver of mice after γ-ray irradiation with different doses.
A: 3 h after exposure to low dose (0.2 Gy); B: 24 h after exposure to low dose (0.2 Gy); C: 3 h after exposure to medium dose (1 Gy); D: 3 h after exposure to high dose (5 Gy); E: 24 h after exposure to medium dose (1 Gy); F: 24 h after exposure to high dose (5 Gy). Bars: 20 μm.
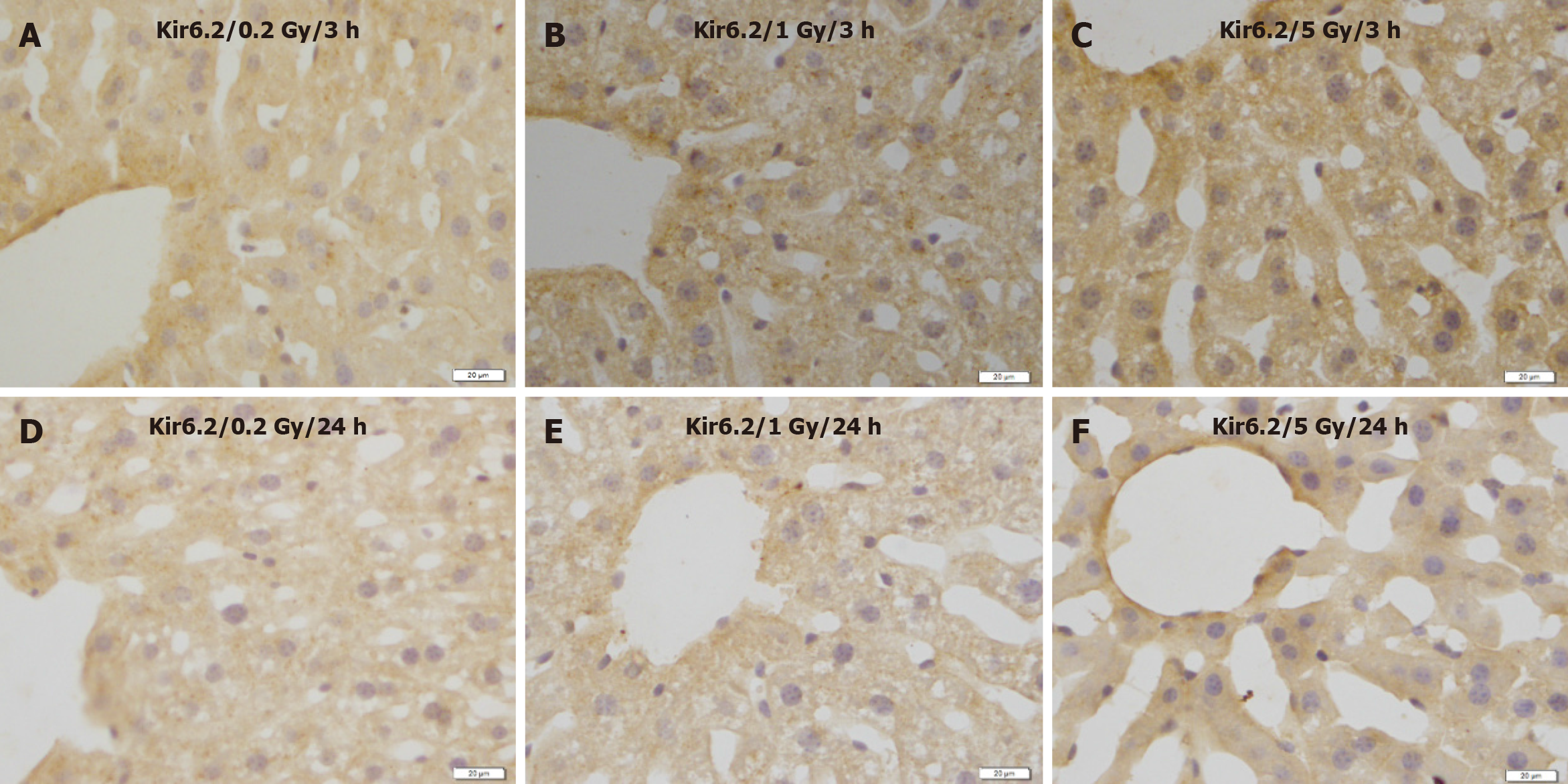
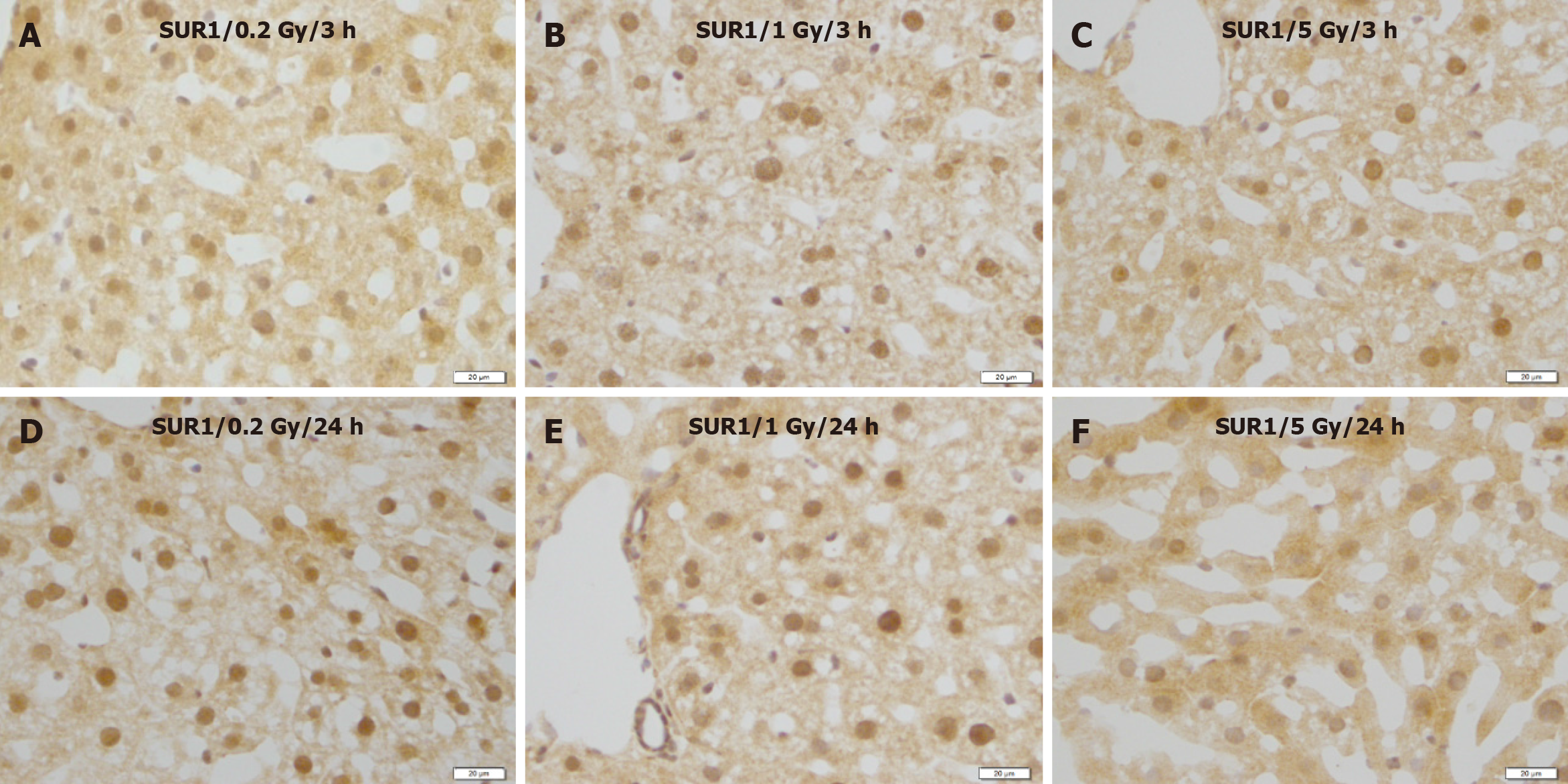
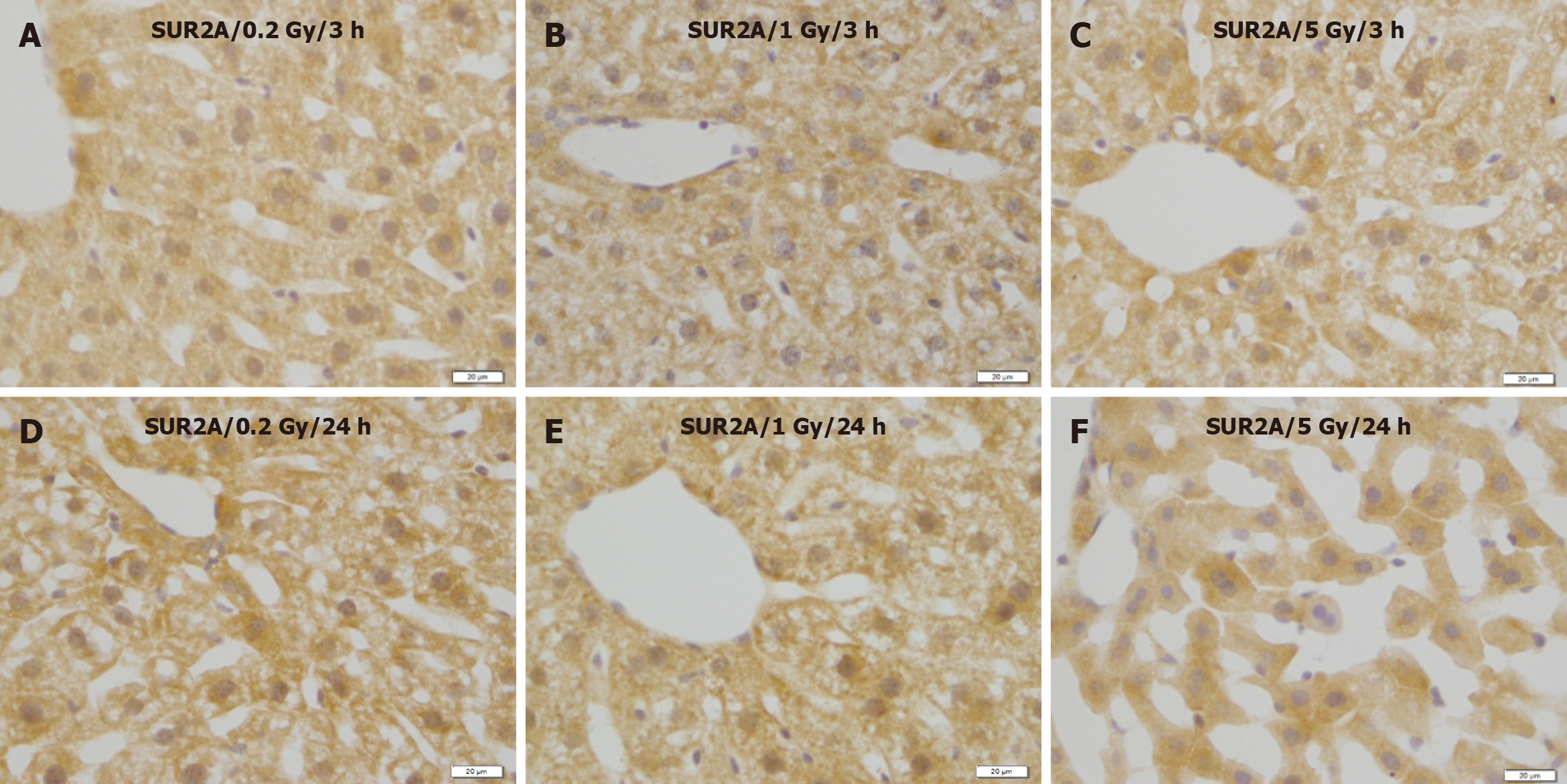
No remarkable changes were observed in the expression levels of Kir6.2 (Figure 5), SUR1 (Figure 6), or SUR2A (Figure 7) in hepatocytes at 3 and 24 h after irradiation at all doses.
Figure 5 Immunostaining for Kir6.2 in the liver of mice after γ-ray irradiation with different doses.
Representative images show no remarkable change induced by irradiation. A: 3 h after exposure to low dose (0.2 Gy); B: 3 h after exposure to medium dose (1 Gy) ; C: 3 h after exposure to high dose (5 Gy); D: 24 h after exposure to low dose (0.2 Gy); E: 24 h after exposure to medium dose (1 Gy); F: 24 h after exposure to high dose (5 Gy). Bars: 20 μm.
Figure 6 Immunostaining for SUR1 in the liver of mice after γ-ray irradiation with different doses.
These images show no remarkable changes in different time courses. A: 3 h after exposure to low dose (0.2 Gy); B: 3 h after exposure to medium dose (1 Gy); C: 3 h after exposure to high dose (5 Gy); D: 24 h after exposure to low dose (0.2 Gy); E: 24 h after exposure to medium dose (1 Gy); F: 24 h after exposure to high dose (5 Gy). Bars: 20 μm.
Figure 7 Immunostaining for SUR2A in the liver of mice after γ-ray irradiation with different doses.
A: 3 h after exposure to low dose (0.2 Gy); B: 3 h after exposure to medium dose (1 Gy); C: 3 h after exposure to high dose (5 Gy); D: 24 h after exposure to low dose (0.2 Gy); E: 24 h after exposure to medium dose (1 Gy); F: 24 h after exposure to high dose (5 Gy). Bars: 20 μm.
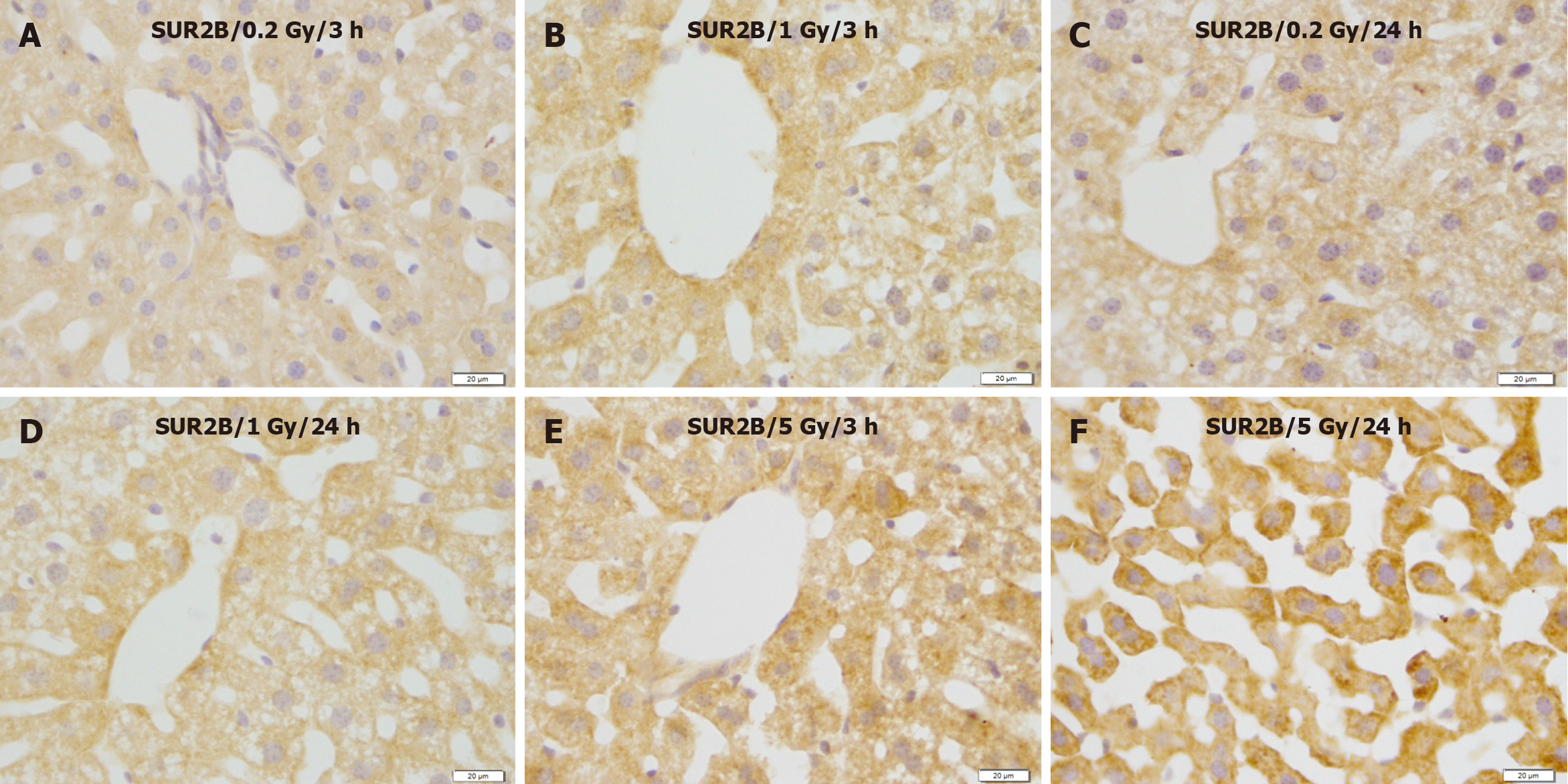
In contrast, compared with the control group (Figure 8A and B), SUR2B expression showed no apparent changes at 3 and/or 24 h after exposure to 0.2 and 1 Gy (Figure 9A-D), whereas SUR2B expression clearly increased 3 h after exposure to 5.0 Gy (Figure 9C), and more highly increased after exposure to 5.0 Gy (Figure 9E), and more highly increased 24 h after exposure to 5.0 Gy (Figure 9F).
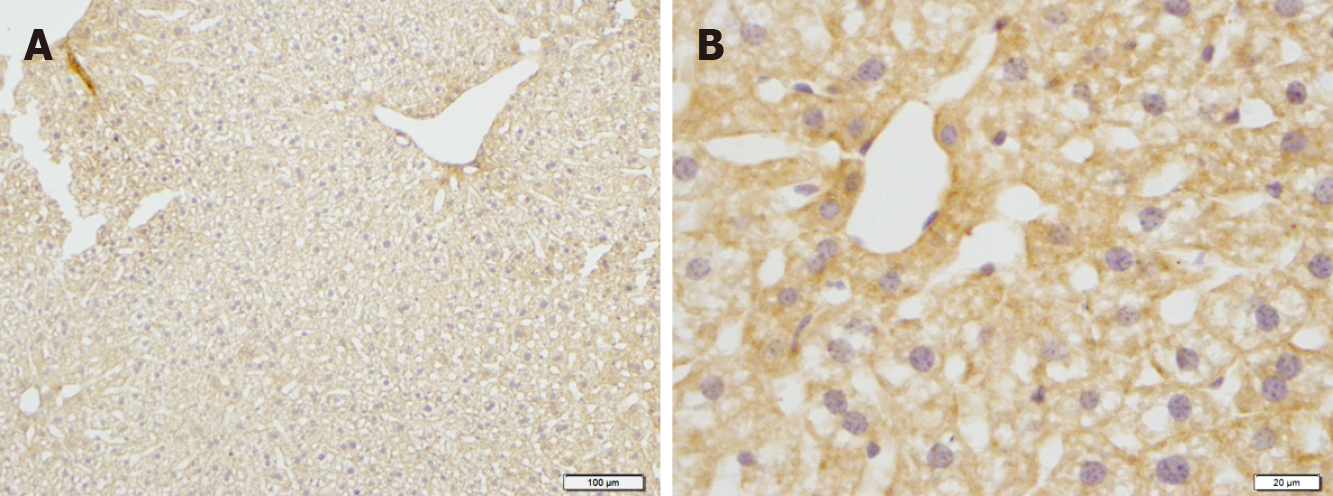
Figure 8 Immunostaining for SUR2B in the liver of mice in the control groups.
A: Low magnification image; B: High magnification image. Bars: 100 μm (A); 20 μm (B).
Figure 9 Immunostaining for SUR2B in the liver of mice after γ-ray irradiation with different doses.
SUR2B expression showed no obvious change 3 and 24 h after exposure to 0.2 and 1.0 Gy, but increased remarkably 24 h after exposure to 5 Gy. A: 3 h after exposure to low dose (0.2 Gy); B: 3 h after exposure to medium dose (1 Gy); C: 24 h after exposure to low dose (0.2 Gy); D: 24 h after exposure to medium dose (1 Gy); E: 3 h after exposure to high dose (5 Gy); F: 24 h after exposure to high dose (5 Gy). Bars: 20 μm.
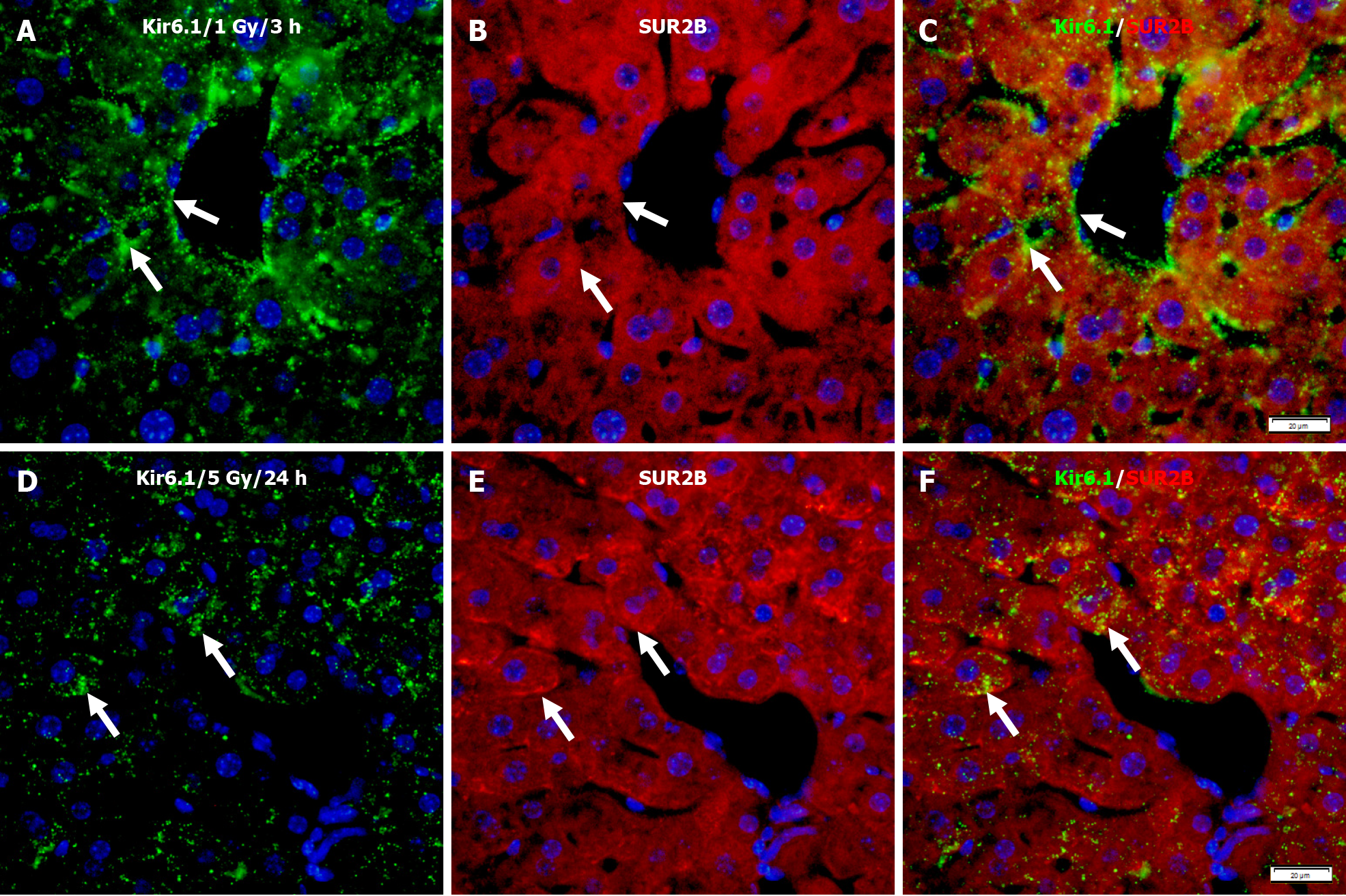
Immunofluorescence double staining for Kir6.1 and SUR2B after 1 Gy γ-ray irradiation showed only partial co-localizion of Kir6.1 with SUR2B (Figure 10A-C). However, at 24 h after mice were exposed to 5 Gy, despite a lower expression level of Kir6.1 compared to the lower dose group, almost all Kir6.1 was co-localized with SUR2B, described as punctate immunoreactivity (Figure 10D-F).
Figure 10 Immunofluorescence double staining for Kir6.1 and SUR2B in the liver of mice after irradiation with different doses.
A-C: Representative images show the expression of Kir6.1 alone (green, A), SUR2B alone (red, B), and their co-localization (white arrows, C) 3 h after 1 Gy exposure; D-F: Representative images show the expression of Kir6.1 alone (green, D), SUR2B alone (red, E), and their co-localization (F) 24 h after 5 Gy exposure (white arrows, F). Bars: 20 μm.
DISCUSSION
Radiation effects were considered mainly due to nuclear DNA damage and their management by repair mechanisms[32]. The liver has numerous functions including bile production, ingested nutrient metabolism, waste product elimination, glycogen storage, and protein synthesis[4]. Additionally, it is radiosensitive, particularly in young animals[33]. Liver cancer, with high mortality, is one of the most common solid tumors in the world[34]. Although surgical resection is the first choice of treatment for hepatocellular carcinoma, radiation therapy is an actively used and essential treatment modality for locally advanced hepatocellular carcinoma or tumors in the upper abdomen, right lower lung, distal section of the esophagus, or whole body[4,5,35]. Liver injury induced by radiation is a significant complication of radiotherapy for liver cancer or other upper abdominal malignant tumors that have poor pharmacological therapeutic options because normal tissues exposed to radiation during radiotherapy or radioscopy will suffer injury and metabolic alterations[36]. A significant challenge in radiotherapy is to promote the tolerance of normal cells by protecting their transformation into malignant cells, thus increasing patients’ quality of life[37].
Ionizing radiation induces oxidative stress and is pivotal in the pathogenesis of cellular damage induced by radiation; dietary antioxidants were suggested to protect against irradiation-induced subsequent tissue damage[37].
Oxidative stress from generating reactive oxygen species (ROS) results in an imbalance in cells’ pro-oxidant/antioxidant status[38]. Cell survival and proliferation capacities are highly dependent on the activation and regulation through molecular components of organelles, especially mitochondria, which are pivotal in maintaining cellular homeostasis and genomic stability after irradiation[32].
Mitochondria ensure general cellular metabolism and high-energy provision via ATP and oxidative phosphorylation to maintain cell survival and homeostasis. During oxidative phosphorylation, a few electrons may react with oxygen, forming ROS and oxidative stress, thus constituting non-negligible targets for irradiation[32]. Excessive production of ROS results in mitochondria dysfunction; a series of pathological changes can be induced by radiation via direct energy deposition or reactive free radical generation[36]. Mitochondria are involved in oxidative stress-induced apoptotic cell death[39].
Upregulation of antioxidant enzymes was mimicked by treatment with the sulfonylureas tolbutamide and gliclazide (KATP channel blockers and inhibitors); the loss of KATP channel activity conferred resistance to radiation[39], suggesting a probable role for KATP channels in radiation-induced injury.
KATP channels are composed of two subunits: Kir6.x and SURs. Different combinations of these subunits are expressed in different cells and tissues and exhibit different physical characteristics[40]. KATP channels are involved in various physiological conditions, including hyperglycemia, hypoglycemia, ischemia, and hypoxia[19,40]. Kir6.1 and SUR2B are important for cellular energy and stability in the cell membrane and the mitochondrion. Mitochondria generate energy for the cell to maintaining cellular homeostasis, genomic stability, and sensitivity to irradiation[32]. Cellular oxidative stress can affect the function of potassium channels, influencing the vasomotor function in multiple disease states[41]. We have previously reported that the mitochondrial KATP channel subunits Kir6.1 and SUR2B protect heart tissues damaged by ischemia, hypoxia, or other conditions [10,11]. A recent study demonstrated that Kir6.1 and SUR2B are essential for functional brain hyperemic responses and vascular smooth muscle cell differentiation[42]. In this study, high levels of γ-ray irradiation in mice induced liver injury (Figure 2) and modulated KATP channel subunits expression, resulting in decreased Kir6.1 (Figure 4) and increased SUR2B (Figure 9) levels. These phenomena were documented using double fluorescence immunostaining (Figure 10). Although Kir6.1 had a lower expression level, the two subunits were more co-localized. High expression levels of SUR2B regulate the KATP channel in the mouse liver during high-dose irradiation. Thus, redox modulation of potassium channel activity induced by radiation is a crucial mechanism for regulating smooth muscle membrane potential, ultimately influencing hepatocyte injury.
Resent research has revealed that opening the KATP channels may increase liver tolerance to ischemia/reperfusion injury and reduce the systemic inflammatory responses[43]. The KATP channel enhances liver regeneration after partial hepatectomy[17]. However, another study showed that glibenclamide, a KATP channel blocker, prevents acute radiation-induced liver injury[44]. The effects of KATP channels in different organs and tissues are not always evident; for example, the KATP channel blocker glibenclamide ameliorated the ischemia-reperfusion injury in the rat testis, whereas 5-hydroxydecanoate does not; KATP openers mediate pharmacological post-conditioning in the heart or brain, but cannot reduce ischemia-reperfusion injury in the kidneys, intestine, and lungs[45].
Thus, the expression levels of the KATP channel subunits in the liver and their potential roles in radiation-induced liver injury still need to be investigated.
This study observed that high levels of γ-ray exposure induced liver injury in mice and modulated KATP channel subunit expression, resulting in decreased Kir6.1 and increased SUR2B levels. These findings suggest that KATP channels affect radiation-induced liver injury, and may serve as parameters for the evaluation of radiation injury. Further investigation is required to elucidate the precise roles of KATP channels in radiation-induced liver injury.