Published online Dec 20, 2023. doi: 10.5493/wjem.v13.i5.115
Peer-review started: August 31, 2023
First decision: October 9, 2023
Revised: October 19, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: December 20, 2023
Processing time: 109 Days and 15 Hours
Compared with patients with other causes of acute pancreatitis, those with hypertriglyceridemia-induced acute pancreatitis (HTG-AP) are more likely to develop persistent organ failure (POF). Therefore, recognizing the individuals at risk of developing POF early in the HTG-AP process is a vital for improving outcomes. Bedside index for severity in acute pancreatitis (BISAP), a simple parameter that is obtained 24 h after admission, is an ideal index to predict HTG-AP severity; however, the suboptimal sensitivity limits its clinical application. Hence, current clinical scoring systems and biochemical parameters are not sufficient for predicting HTG-AP severity.
To elucidate the early predictive value of red cell distribution width (RDW) for POF in HTG-AP.
In total, 102 patients with HTG-AP were retrospectively enrolled. Demographic and clinical data, including RDW, were collected from all patients on admission.
Based on the Revised Atlanta Classification, 37 (33%) of 102 patients with HTG-AP were diagnosed with POF. On admission, RDW was significantly higher in patients with HTG-AP and POF than in those without POF (14.4% vs 12.5%, P < 0.001). The receiver operating characteristic curve demonstrated a good discriminative power of RDW for POF with a cutoff of 13.1%, where the area under the curve (AUC), sensitivity, and specificity were 0.85, 82.4%, and 77.9%, respectively. When the RDW was ≥ 13.1% and one point was added to the original BISAP to obtain a new BISAP score, we achieved a higher AUC, sensitivity, and specificity of 0.89, 91.2%, and 67.6%, respectively.
RDW is a promising predictor of POF in patients with HTG-AP, and the addition of RDW can promote the sensitivity of BISAP.
Core Tip: Red cell distribution width (RDW) reflects systemic inflammation, which is significantly associated with the severity of acute pancreatitis. However, the relationship between RDW and hypertriglyceridemia-induced acute pancreatitis (HTG-AP) remains unclear. Herein, RDW exhibited a potent discriminatory power for predicting persistent organ failure in patients with HTG-AP. Furthermore, the addition of RDW is able to promote the sensitivity of bedside index for severity in acute pancreatitis.
- Citation: Lv YC, Yao YH, Zhang J, Wang YJ, Lei JJ. Red cell distribution width: A predictor of the severity of hypertriglyceridemia-induced acute pancreatitis. World J Exp Med 2023; 13(5): 115-122
- URL: https://www.wjgnet.com/2220-315X/full/v13/i5/115.htm
- DOI: https://dx.doi.org/10.5493/wjem.v13.i5.115
Hypertriglyceridemia is the most common etiology of acute pancreatitis (AP)[1-3]. Compared with patients with other causes of AP, those with hypertriglyceridemia-induced AP (HTG-AP) are more likely to suffer from persistent organ failure (POF)[4,5]. POF is a major determinant of mortality in early HTG-AP and correlates with the magnitude of inflammatory responses[1], thereby indicating the prognosis of patients. Given this, early recognition of HTG-AP patients at risk of POF is necessary for improving patients’ outcomes.
Red cell distribution width (RDW) reflects the size of circulating erythrocytes[6]. Studies[7,8] have reported that high RDW is significantly associated with AP severity. The mechanism underlying this association may be attributed to the following: (1) Inflammation blocks anti-apoptosis or promotes erythrocyte death[9]; (2) inflammatory cytokines can desensitize bone marrow erythroid progenitors to erythropoiesis, thereby inhibiting erythrocyte maturation[10]; and (3) during AP, erythrocytes cannot absorb vital materials, including vitamin B12, iron, and folic acid[11].
However, the relationship between RDW and HTG-AP was not well elucidated in previous studies; therefore, our study aimed to evaluate the predictive value of RDW for POF in patients with HTG-AP.
In this retrospective study, consecutive patients with HTG-AP who were hospitalized at the Affiliated Baiyun Hospital of Guizhou Medical University from January 2017 to February 2021 were enrolled. AP and POF (lasting > 48 h) were diagnosed based on the Revised Atlanta Classification[12]. This study was reviewed and approved by the Science and Research Office of the Affiliated Baiyun Hospital of Guizhou Medical University. Patients’ consent for inclusion was waived owing to the retrospective nature of the study.
HTG-AP was diagnosed if serum triglyceride levels were > 11.3 mmol/L or between 5.56 and 11.30 mmol/L accompanied by chylous effusion, with the exclusion of other etiologies[13,14].
The exclusion criteria were as follows: (1) Patients who were admitted > 24 h after AP onset, (2) those who were < 18 years of age; and (3) those with other etiology-induced AP, recurrent AP, gestational AP, AP with anemia (woman < 110 g/L, man < 120 g/L), chronic pancreatitis, and cancer history. The primary endpoint was in-hospital POF.
All data were collected from our hospital’s electronic medical database. For each patient, age, sex, and medical history were collected as baseline demographic data. Furthermore, information on vital signs was collected from all patients on admission, and the results of laboratory tests, radiological imaging, and clinical outcomes were collected within 24 h of admission. The bedside index for severity in acute pancreatitis (BISAP)[15] and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score[16] were calculated within 24 h of admission.
On admission, 2-3 mL of blood was collected, placed in EDTA-K2 anticoagulation tubes, and stored at 4-8℃. The whole blood samples were directly loaded into a hematology analyzer (XN-2000, Japan Sysmex) for detection. RDW (%) = SD of red blood cell volume/mean corpuscular volume x 100. The reference range of RDW was 11.0%-16.0%.
Data were presented as median (interquartile range) or mean ± SD. Categorical data were presented as percentages (%). Univariate analysis was performed using the Student’s t-test, Mann-Whitney U test, and chi-squared test, as appropriate. Admission variables that were significant in univariate analysis were subjected to multivariate regression analysis.
A receiver-operating characteristic (ROC) curve was used to estimate predictive accuracy. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) were calculated. Pearson’s analysis was performed to determine the correlations between two variables. GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, United States) and SPSS 25.0 (IBM Corp., Armonk, NY, United States) were used to perform statistical analyses. A P value < 0.05 was considered statistically significant.
Table 1 summarizes the demographic, laboratory, and clinical characteristics of patients with and without POF. In total, 102 patients were included in this retrospective study. The age range was 20-58 years and 34 (33%) of 102 patients developed POF; no patients died.
| Variable | Non-POF (n = 68) | POF (n = 34) | Univariate P | Multivariate P |
| Age (yr) | 40 ± 8 | 40 ± 9 | 0.967 | |
| Sex (M/F) | 56/12 | 29/5 | 0.785 | |
| Hypertension | 16 (24) | 7 (21) | 0.876 | |
| Diabetes | 33 (49) | 22 (65) | 0.144 | |
| Fatty liver disease | 39 (57) | 16 (47) | 0.679 | |
| Alcohol | 28 (41) | 15 (44) | 0.833 | |
| Smoking | 45 (66) | 21 (62) | 0.667 | |
| WBC (x109/L) | 12.4 (10.9, 15.9) | 14.9 (13.2, 17.1) | 0.009 | 0.172 |
| MPV (fL) | 10.42 ± 1.76 | 11.54 ± 2.06 | 0.012 | 0.085 |
| RDW (%) | 12.5 (12.0, 13.0) | 14.4 (13.4, 14.9) | < 0.001 | 0.002 |
| Amylase (U/L) | 124 (66, 258) | 239 (83, 917) | 0.036 | 0.383 |
| Calcium (mmol/L) | 2.35 (2.25, 2.42) | 2.29 (2.10, 2.95) | 0.064 | |
| Triglyceride (mmol/L) | 16.42 (11.58, 20.31) | 19.57 (15.23, 24.70) | 0.010 | 0.101 |
| ALT (U/L) | 36.0 (25.0, 47.4) | 35.2 (20.8, 46.6) | 0.412 | |
| AST (U/L) | 25.9 (19.1, 31.7) | 25.4 (17.9, 33.6) | 0.879 | |
| Albumin (g/L) | 46.1 (44.1, 48.4) | 44.8 (42.1, 48.3) | 0.078 | |
| BUN (mmol/L) | 4.30 (3.60, 5.18) | 3.85 (2.90, 5.00) | 0.057 | |
| Creatinine (μmol/l) | 60.50 (47.50, 76.55) | 54.90 (41.70, 71.05) | 0.158 | |
| C-reaction protein (mg/L) | 24.89 (11.14, 57.54) | 63.10 (23.57, 99.91) | 0.008 | 0.185 |
| BISAP | 1 (0, 1) | 2 (1, 2) | < 0.001 | 0.001 |
| APACHE II | 4 (2, 5) | 5 (4, 8) | < 0.001 | 0.119 |
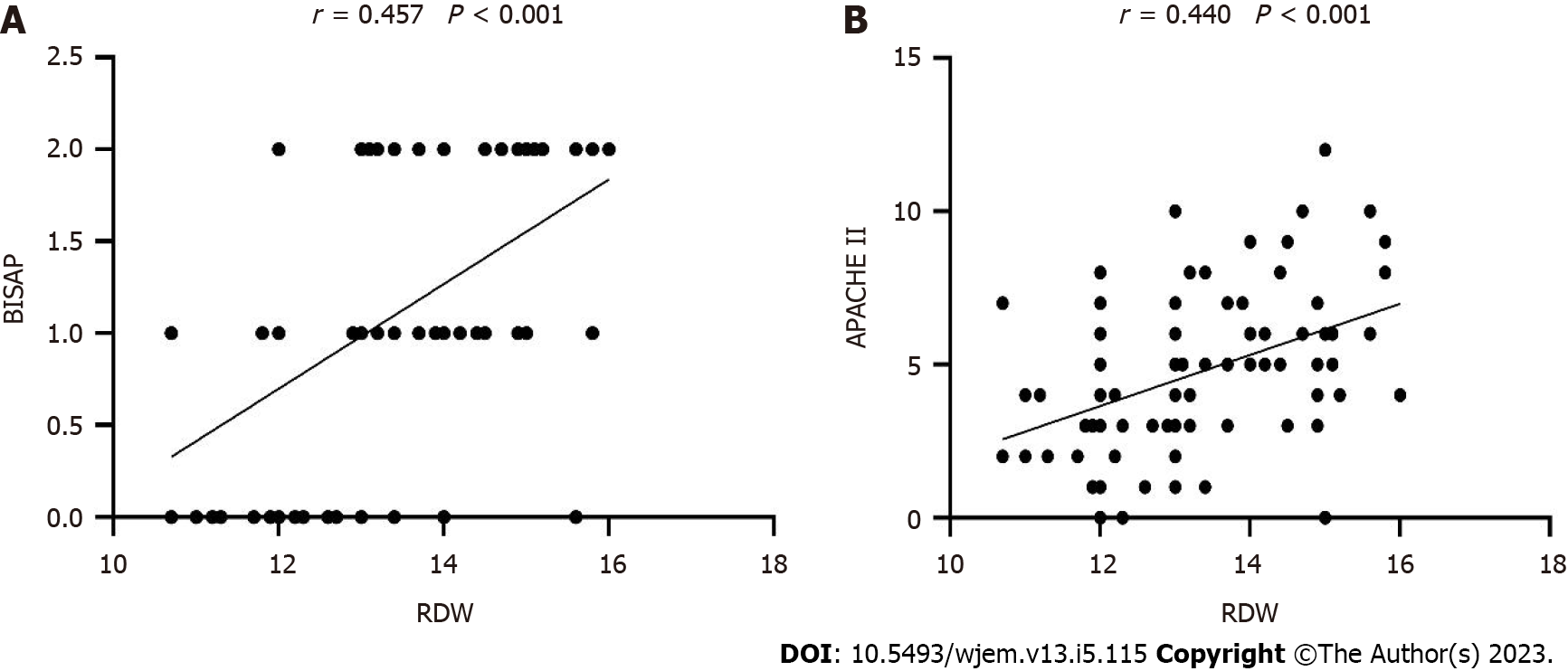
Univariate analysis identified several risk factors of POF on admission (P < 0.05). Multivariate analysis revealed that RDW (P = 0.002) and BISAP (P = 0.001) were associated with POF (Table 1). RDW was positively correlated with BISAP (r = 0.457, P < 0.001) and APACHE II (r = 0.440, P < 0.001) (Figure 1).
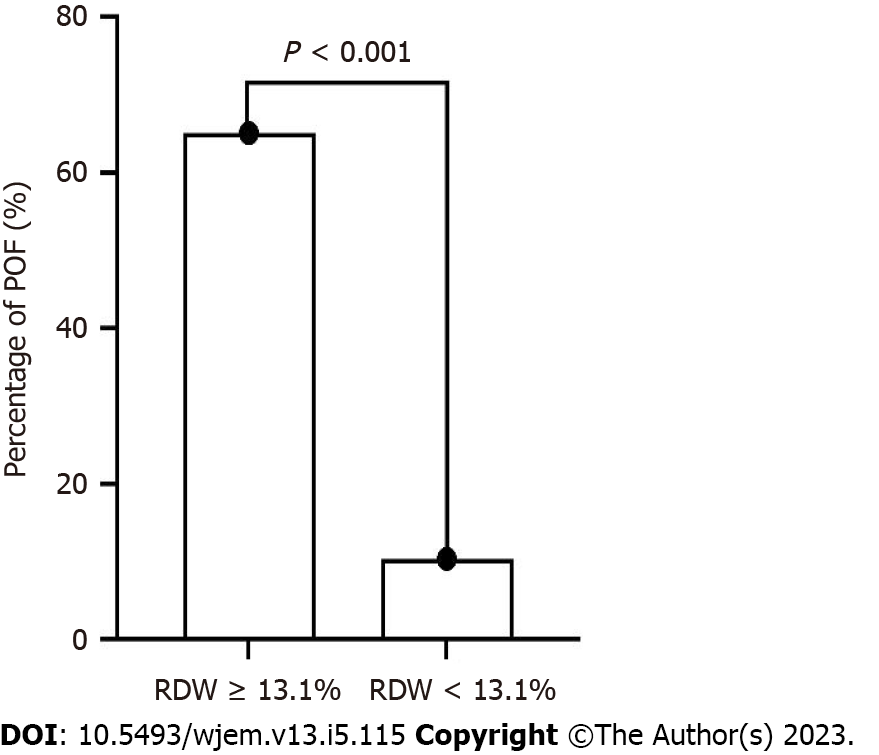
ROC analysis indicated that the optimal cutoff value for RDW was 13.1%. The sensitivity, specificity, PPV, and NPV were 82.4%, 77.9%, 65.1%, and 89.8%, respectively. Using the cutoff for RDW, the 102 patients with HTG-AP were divided into two groups: RDW < 1 3.1% and RDW ≥ 13.1% groups. More POF patients were presents in the RDW ≥ 13.1% group (P < 0.001) (Figure 2 and Table 2).
| Variable | AUC | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| RDW | 0.85 | 13.1 | 82.4 | 77.9 | 65.1 | 89.8 |
| BISAP | 0.82 | 2 | 64.7 | 82.4 | 64.7 | 82.4 |
| APACHE II | 0.74 | 5 | 73.5 | 60.3 | 48.1 | 82.0 |
| BR | 0.89 | 2 | 91.2 | 67.6 | 58.5 | 93.9 |
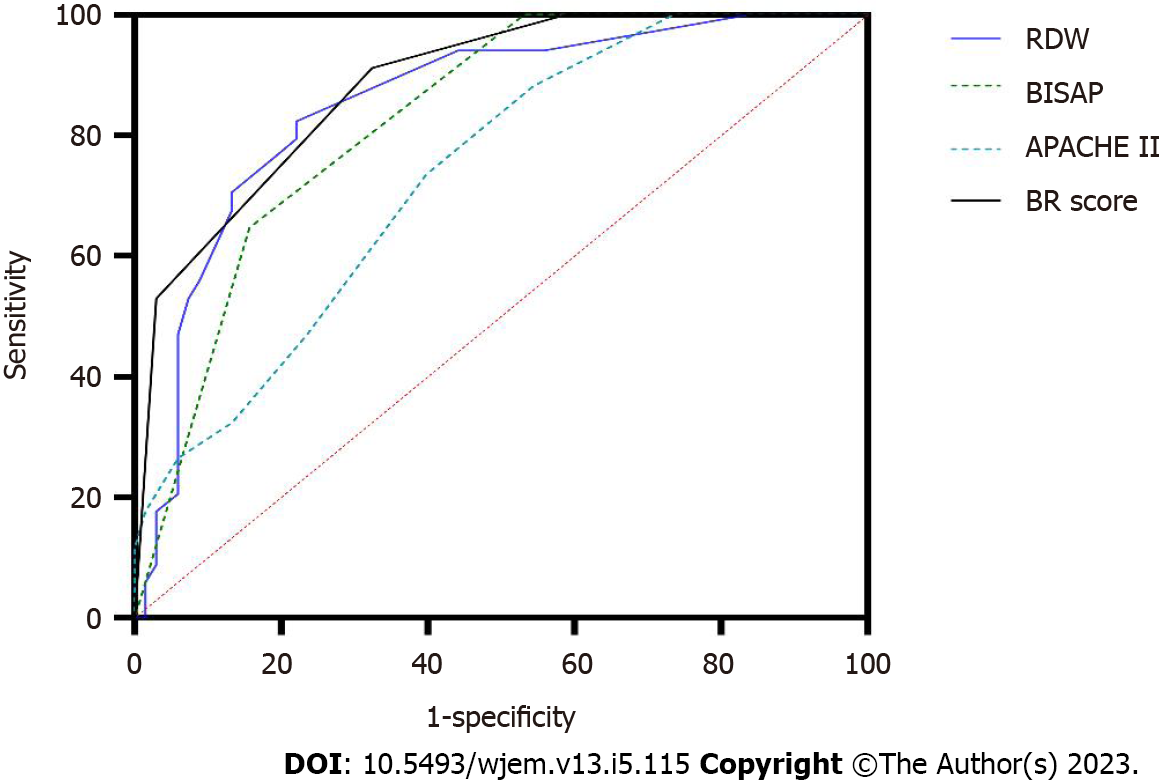
When RDW was ≥ 13.1%, one point was added to the BISAP score to obtain a new BISAP score (BISAP plus RDW: BR score). When the cutoff value of BR score was set as 2 points, the AUC, sensitivity, specificity, PPV, and NPV for predicting POF was 0.89, 91.2%, 67.6%, 58.5%, and 93.9%, respectively (Figure 3 and Table 2). Compared with BISAP alone (AUC = 0.82, sensitivity = 64.7%, specificity = 82.4%, PPV = 64.7%, and NPV = 82.4%) and APACHE II (AUC = 0.74, sensitivity = 73.5%, specificity = 60.3%, PPV = 48.1%, and NPV = 82.0%), BR score exhibited a higher predictive accuracy (Figure 3 and Table 2).
Herein, our study suggested that RDW on admission was a promising indicator for POF and that the BR score could efficiently predict POF in patients with HTG-AP. This is the first study to uncover the association between RDW and POF in patients with HTG-AP.
Compared with other AP etiologies, HTG-AP is more likely to causes POF, which results in an unfavorable prognosis for patients[12,17,18]. Therefore, the predictive value of RDW is of significance because the application of this biomarker can facilitate the discrimination of patients who have a high risk of developing POF and the design of treatment regimens. Moreover, RDW examination is reproducible, inexpensive, and convenient, and thus can be widely implemented in almost every hospital.
The production of inflammatory cytokines and corresponding inflammatory cascade have long been considered a critical pathogenic mechanism of POF in AP patients[1,8]. RDW reflects systemic inflammation, and several studies[6-8] have proposed the RDW can predict in-hospital mortality and severity in AP. However, these studies only included gallstones- and alcohol-induced AP but not HTG-AP. Consequently, our study focused on the association between RDW and POF in patients with HTG-AP. The results indicated that RDW was positively correlated with BISAP and APACHE II scores, which were commonly used indicators of POF. Meanwhile, RDW exhibited a promising value in predicting POF.
Although the mechanism underlying increased RDW in HTG-AP patients developing POF, it may be attributed to factors as follows: inflammatory cascade triggers triglyceride-mediated lipotoxicity; in turn, increased triglyceride levels augment the intensity of the inflammatory cascade, which contributes to the elevation of RDW[17,18]. Notably, we excluded patients with anemia, which would affect RDW; this might promote the predictive value of RDW[6,9,19].
Several multiparameter predictors, including Ranson criteria, BISAP, and APACHE II, have been adopted to evaluate POF in HTG-AP patients[20-22]. However, Ranson criteria can only be used after 48 h of hospitalization and the calculation of APACHE II is complicated[16]. Although BISAP is widely used because of its simplicity of calculation, its low sensitivity limits its clinical application[16]. Li et al[21] compared the differences in scoring systems for predicting the prognosis of HTG-AP patients. They suggested that BISAP was the best indicator for predicting the HTG-AP severity. However, BISAP had a suboptimal sensitivity (66.7%) for predicting POF. Similarly, Yang et al[20] reported that BISAP exhibited low sensitivity (54%) for predicting HTG-AP severity. Consistent with previous findings, our results revealed that BISAP also exhibited a low sensitivity (64.7%) for predicting POF.
Several studies have demonstrated the improved predictive value by combining BISAP and other biomarkers. Zheng et al[22] found that BISAP plus C-reactive protein levels for predicting POF in AP had increased AUC, sensitivity, and specificity compared with using BISAP alone (0.873, 81.6%, and 85.2% vs 0.856, 80.8%, and 84.1%, respectively). Zhou et al[19] (BISAP plus RDW) also suggested that while the addition of RDW promoted the predictive value of BISAP, it decreased the sensitivity. In the present study, the results indicated that the BR score exhibited higher AUC (0.89 vs 0.82) and sensitivity (91.2% vs 64.7%) than BISAP alone for predicting POF in HTG-AP patients, whereas the specificity (67.6% vs 82.4%) dropped.
Fluid therapy is a long-established cornerstone to prevent organ hypoperfusion within 24 h of AP onset[16,23]. Recent studies[17,23] have indicated that aggressive fluid therapy in AP may result in poor outcomes, particularly in older patients. Since HTG-AP patients tend to have a younger age, aggressive fluid therapy is a relatively safe treatment. However, our results suggested that more attention should be paid to patients with RDW ≥ 13.1%.
Our study has some limitations. First, no patient died in our cohort; therefore, the ability of RDW to predict mortality could not be investigated. However, considering the intimate association between POF development and unfavorable prognosis at the early stage of AP[12], we inferred that the mortality prediction performance of RDW might be superior in HTG-AP than in POF. Second, this study was limited by its retrospective design and small sample size, making it impossible to draw definitive conclusions regarding the diagnostic value of evaluated biomarkers for predicting POF in HTG-AP. Third, the definition of HTG-AP differed between countries[17], which limited the extension of our findings to other countries. Moreover, we did not perform a subgroup analysis to exclude the influence of metabolic disorders on RDW. Furthermore, BR score had a high false positive rate, indicating that the BR score was not an optimal indicator for predicting POF. Nevertheless, the BR score had a relatively high sensitivity, so it could be a screening indicator for POF in HTG-AP patients. Additional new biomarkers and scoring systems should be developed to better predict POF.
RDW on admission is an independent predictor of POF in patients with HTG-AP. The addition of RDW improves the low sensitivity of BISAP. Large-scale, multicenter prospective studies are required to verify the results.
In clinical settings, compared with patients with other causes of acute pancreatitis (AP), those with hypertriglyceridemia-induced acute pancreatitis (HTG-AP) commonly suffer from severe acute pancreatitis; therefore, it is critical to identify severe HTG-AP early. However, current clinical scoring systems and biochemical parameters are not efficient for predicting HTG-AP severity.
Red cell distribution width (RDW) may be closely associated with the mortality of patients with AP. Meanwhile, clinical application of the bedside index for severity in acute pancreatitis (BISAP) is limited by its low sensitivity. Therefore, new parameters or scoring systems are warranted for determining HTG-AP severity early.
To determine whether RDW can be used as a potential biomarker for predicting POF in HTG-AP.
We explored the relationship between RDW and POF in patients with HTG-AP and determined the cutoff value of RDW using ROC analysis. BISAP plus RDW improved the suboptimal sensitivity of BISAP when RDW was ≥ 13.1% and one point was added to the BISAP.
On admission, RDW was significantly higher in patients with HTG-AP and POF. Compared with BISAP and Ranson criteria, BISAP plus RDW had a higher accuracy for predicting POF in HTG-AP patients.
BISAP plus RDW exhibited a promising predictive value for POF in HTG-AP patients, despite its low specificity.
The combination of BISAP and other indicators may better predict HTG-AP severity. However, additional large-scale, multicenter prospective studies are required to verify the results.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isaji S, Japan S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 470] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Guo YY, Li HX, Zhang Y, He WH. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med. 2019;27:101-109. [PubMed] |
| 4. | Huang YX, Jia L, Jiang SM, Wang SB, Li MX, Yang BH. Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong, China: a retrospective multicenter study. Pancreas. 2014;43:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Deng LH, Xue P, Xia Q, Yang XN, Wan MH. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558-4561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Cetinkaya E, Senol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol. 2014;20:14450-14454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Goyal H, Awad H, Hu ZD. Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review. Ann Transl Med. 2017;5:342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Şenol K, Saylam B, Kocaay F, Tez M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med. 2013;31:687-689. [PubMed] |
| 9. | Krichenko A, Bueno RR, Andrade PM, Cunha CP, Souza AM, Ribeiro EJ, Pereira MA, Rossi PR, Carvalho RG, Brofman PR. Aortic coarctation in an infant treated with balloon catheter. A case report. Arq Bras Cardiol. 1986;46:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 479] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 11. | Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 705] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 12. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4306] [Article Influence: 358.8] [Reference Citation Analysis (45)] |
| 13. | Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 14. | de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 15. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 16. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 476] [Article Influence: 119.0] [Reference Citation Analysis (1)] |
| 17. | Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology. 2016;16:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Nawaz H, Koutroumpakis E, Easler J, Slivka A, Whitcomb DC, Singh VP, Yadav D, Papachristou GI. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study. Medicine (Baltimore). 2019;98:e15275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Yang L, Liu J, Xing Y, Du L, Chen J, Liu X, Hao J. Comparison of BISAP, Ranson, MCTSI, and APACHE II in Predicting Severity and Prognoses of Hyperlipidemic Acute Pancreatitis in Chinese Patients. Gastroenterol Res Pract. 2016;2016:1834256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Li M, Xing XK, Lu ZH, Guo F, Su W, Lin YJ, Wang DH. Comparison of Scoring Systems in Predicting Severity and Prognosis of Hypertriglyceridemia-Induced Acute Pancreatitis. Dig Dis Sci. 2020;65:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Zheng J, Zhang J, Gao J. Early evaluations of BISAP plus C-reactive protein in predicting the severity of acute pancreatitis. Zhonghua Yi Xue Za Zhi. 2015;95:925-928. [PubMed] [DOI] [Full Text] |
| 23. | de-Madaria E, Soler-Sala G, Sánchez-Payá J, Lopez-Font I, Martínez J, Gómez-Escolar L, Sempere L, Sánchez-Fortún C, Pérez-Mateo M. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |