Published online Sep 20, 2023. doi: 10.5493/wjem.v13.i4.75
Peer-review started: January 20, 2023
First decision: April 13, 2023
Revised: May 22, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: September 20, 2023
Processing time: 238 Days and 4.2 Hours
Since an initial diagnosis of Alzheimer disease (AD) in 1907, early detection, was unavailable through 116 years. Up-regulation of V-Ets erythroblastosis virus E26 oncogene homolog 2 (Ets2) is capable to enhance neuronal susceptibility and degeneration. Protein expression (PE) of Ets2 has functional impact on AD and Down’s syndrome, with diverse intensity. PE of Ets2 has an influential pathogenic impact on AD. Clinical aspects of neurological disorders directly interact with psychological maladies. However, deterioration requires an early management including programmed based protection.
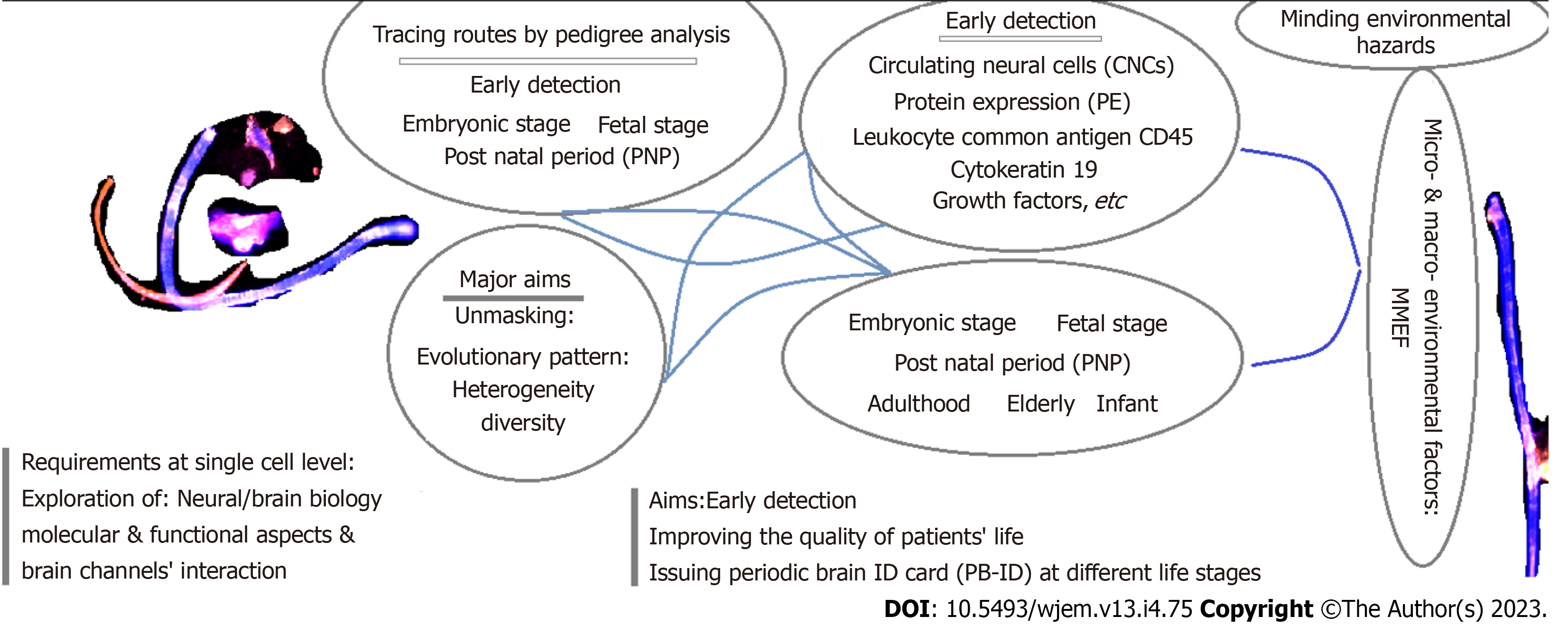
To include cell biology in neuro-genetics; personalized, prognostics, predictive, preventive, predisposing (5xP) platform, accompanied by stratifying brain chan
Include exploration of PE assay and electroencephalography of brain channels. The processes are applied according to: (1) Triangle style, by application of cellular network; and (2) PE assay of Ets2 in the peripheral blood of the patients with AD, by Manual single cell based analysis, and Flow-cytometry. (1) Applying the Genetic counselling and pedigree analysis; (2) considering the psychological status of the referral cases; (3) considering the macro-and/or micro-environmental factors; (4) performing the required Genetics’ analysis; and (5) applying the required complementary test(s).
PE of Ets2 has pathogenic role in AD. PE unmasked the nature of heterogeneity/diversity/course of evolution by exploring Ets2, D1853N polymorphism in Ataxia Telangiectasia mutated gene (ATM), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and course of evolution at the single cell level of the brain. Ets2 revealed different cellular behavior in the blood and suggested the strategy as ‘Gene Product-Based Therapy’ and the personalized managements for the patients. PE reflected weak expression of ATM, mosaic pattern of Ets2; remarkable expression of VEGF and EGF by highlighting an early detective platform, considering circulating neural cells (CNCs) and the required molecular investigation, for the target individual(s) predisposed to AD or other neural disease including brain neoplasia. Brain channels-cooperation with diverse/interactive-ratios lead to strategic balancing for improving the life-quality in AD.
We highlighted application of the single CNCs and correlated Ratio based between Brain channels by providing the 5xP personalized clinical management model for an early detection and therapy of the patients with AD and their targeted/predisposed relatives. Novel-evolutionary/hypothetic/heterogenic-results in brain-channels offer personalizd/constructive markers with unlimited cooperation in health and disease.
Core Tip: The provided outcomes emphasize on the personalized/classified/functional/single cell based/complementary insights, and systematic strategy in Neuro-Science. The successful bridging approach between Neuro-Science and Medicine requires: (1) The combination of the molecular and functional insights at single cell level; (2) by emphasizing on the course of evolution; and (3) to expedite towards unmasking the functional modifications in the blood stream of the patients with neurological disorders including Alzheimer disease (AD). However, exploration of the circulating neural cells accelerate to unmask the course of evolution by providing the personalized and translatable model to the target based therapy. Let’s improve the life quality of the patients with neurological disorders including AD with simply the light music which corresponds with 40 Hz in gamma sensory stimulation therapy. It is essential to differentiate between neurological- and neuro-psychiatric diseases. Surprisingly, we offer an early detection of the stem cells, including the neural CD133 at fetal period, i.e., as early as 8-9 wk, or later through the circulating fetal cells in the maternal blood stream.
- Citation: Mehdipour P, Fathi N, Nosratabadi M. Personalized clinical managements through exploring circulating neural cells and electroencephalography. World J Exp Med 2023; 13(4): 75-94
- URL: https://www.wjgnet.com/2220-315X/full/v13/i4/75.htm
- DOI: https://dx.doi.org/10.5493/wjem.v13.i4.75
Present introduction provides the multi-directional insights towards the Alzheimer disease (AD), including the function of involved protein expression (PE) at single cell level; and electroencephalography (EEG), to observe the role of light music (LM) on the brain channels, and the interaction between different brain channels. It was also aimed to provide the personalized periodic chart for the predisposed individual and/or patients affected with AD. In addition, an initial description of the histopathology of AD has been provided by Alois Alzheimer[1] in 1907, which is categorized by brain atrophy, an extracellular, cumulated of Aβ peptide (amyloid plaques), loss of neuron and synapse, composition of tau protein, as neurofibrillary ingredient[1]. Regulation of the Human Presenilin-1 Gene by V-Ets erythroblastosis virus E26 oncogene homolog 2 (Ets2) Transcription Factors and p53 play key role in AD-pathogenesis[2]. The decisive role of DNA by controlling 90% of the expression of presenilin 1 gene located in chromosome 21 (-35 to +6) is highlighted. This region harbors an Ets transcription factor-binding motif, and a 2-base pair alteration within the sequence of (GGAA to TTAA) of the Ets2 territory leading to the 90% transcriptional reduction. It was also reported that Ets1/2 transcription factors activate PS1 transcription.
The pathological diagnosis in AD, and accumulation of amyloid, occur almost decades before an initial sign of clinical symptoms[3]. Besides, functional aspect of brain network could be unmasked by magnetoencephalography. Most importantly, application of the non-invasive strategy, as an early detection of the symptom(s) of neuropathology at pre-developmental period of the AD is essential.
Initially, the micro- and macro-environmental factors, including nutrition play, partly, the influential roles in initiation and progression of AD. The most challenging panel in this topic is the late diagnosis and the risky procedures through the exploration of AD and therapy. In addition, EEG is the non-invasive and trustable method to detect synaptic dysfunction and the course of the disease. However, performance of EEG is helpful, but very late.
In addition, the combined strategy to classify the EEG-data, by applying an early functional assay of the target protein(s), at single cell level, and as the personalized analytical procedures in AD is, currently, our ongoing process.
Brain function could be, routinely, examined by EEG in AD with significant resolution[4]. EEG is revealed to be: (1) Non-invasive; (2) unmasks the alphabetic informative codes of the neural territory; (3) recording many required targets; (4) unmasking the health conditions related to the patients’ age; and (5) deals with the pharmacological modeling[5,6]. Actually, EEG is capable to unmask the basic prognostic mechanisms in AD. Furthermore, it is proposed that the progression in AD is supposed to be associated to the “functional disconnection”[7].
In the resting phase, the increased and decreased functional connection is indicative of activity for the prefrontal and posterior regions through the alpha band[8,9]. Besides, by considering the decreased function in EEG exploration, its consequences on the connectivity is remarkable which depends on the individuals and technology.
Regarding the genetic test, the Ets2 up-regulation may lead to an increased neuronal degeneration, apoptosis and susceptibility[10]. The Ets2 gene is involved in two different group of patients, affected at totally diverse age of onset including AD and Down’s syndrome (DS)[11].
The primary predisposing/predictive/early detective strategy is the pedigree-based analysis of the proband affected with nervous system/brain disorder(s) including AD. Such approach will cover the candidate relatives of the proband(s) for the screening program. This strategy will unmask: (1) The predisposing factor through the pedigree; and (2) the target and candidate relatives for an early and non-invasive screening.
Circulating neural cells (CNCs) offer: (1) The non-invasive screening; (2) at single cell level; and (3) unmasking the heterogeneity/diversity and evolution.
Ethics play fundamental and supportive role in Genetics and Psychiatry. It facilitates, care and guarantee the benefit of patients in different stages including sampling, research, and clinical managements. Through such platforms, the chain of events including predisposition, prognosis, prediction, prevention (as 4xP aim), diagnosis and personalized therapy are required to be considered. In cell biology, the single cell level play the key role in unmasking the brain’s behavior and have complimentary role and functional impact on the neurological system.
The personalized, prognostics, predictive, preventive, predisposing (5xP) strategy would be achievable through the systematic exploration with an early-detection and personalized therapeutic management. Besides, the pedigree-based analysis provides the predictive stage for the relatives of proband, affected with AD at any age.
Personalized screening, at single cell level, with adequate cell analysis, as early as possible, is scientifically trustable. Therefore, it is an urgent aim for both Scientific and Medical platforms to consider an early detection of the neural disorders.
Brain is a sophisticated and transmittable organ. The pathology of the brain is classified and available[3]. By considering Genetics and cell biology, the specific factors in the neural cells play key role in the neural territory. As a result, the manner of current systems have the influential and functional roles on the specific neurological mechanisms at single cell level. Therefore, to unmask the alterations, it requires the most comprehensive exploration at somatic- and genomics level[11].
Brain harbors the multi-channels, which is characterized with the miscellaneous and essential proteins.
Conclusively, neural cells are derived from the pool of homogeneous progenitor cells. Furthermore, environmental factors, affect the genetic variations and predisposing factors. Concerning the required techniques and the essential therapeutic aspects of the neurological disorders, the informative panel including the histo-pathologic, PE at single cell level and the molecular techniques are required.
As the routine manner, the most puzzling information achieved at the global level, which does not provide the essential Information on the diversity and heterogeneity. Furthermore, the single cell based analysis of PE, is capable to unmask the architecture of the expression and co-expression at the final cellular procedure and production.
The routine and global molecular based analysis are, rapidly, used in the neuroscience research and diagnosis, but are not informative to unmask the heterogeneity and diversity of the functional single cell based which has key role on the occurrence of evolution.
As the matter of fact, the roots of different diseases including cancer and non-cancerous are either related to their ancestral lines and sides through the pedigrees, or the rules and natural events at embryonic, and/or fetal, adulthood and elderly durations (Figure 1). However, micro- and macro-environmental factors play the influential role to initiate and developing disorders. Finally, the complicated periodic chart is required to be considered in the management of health and diseases for the index case and their targeted relatives through their pedigrees.
Cancer and Alzheimer are the historical diseases and have, their roots, hidden at the single cells and brain territory.
The physicians and scientists need to take their valuable action as the experienced archeologists, to trace the roots of both diseases, one by one at single cell level and not at global dimension.
Figure 1 presents the flow-chart which may assist the medical team, including clinicians, pharmacologists, nurses, and scientists to achieve the required complementary data, as early as possible. The prompt therapy requires an early diagnosis. At a glance, the matter of fact includes the occurrence and diagnosis of diversity, heterogeneity and evolution. Such a triangle, will direct the medical, pharmaceutics and scientific teams to: (1) The translatable platform; and (2) to plan the personalized clinical management including an early-therapy (Figure 1).
Ten patients, including six females and four males, affected with AD; and ten healthy individuals are participated in our previous study[11]. The lymphocytes were cultured in RPMI media (Sigma Aldrich, St Louis, MO, United States) for 35 min at 37 ºC. Then the cells were treated with the hypertonic solution, and were fixed. The lymphocytes were stained with the polyclonal Ets2 antibody (Avivasysbio, CA, United States), washed by Phosphate buffer solution, and then stained with secondary antibody (FITC-conjugated goat-anti-rabbit). Flow-cytometry (FC) assay was performed by BD FACS Calibur FC (BD FACSCALIBURTM FC-System, United States) and the results were analyzed by Flowjo-7.6. software.
Data was analyzed by SPSS 18 (SPSS Inc, IL, United States) The Spearman and Pearson Correlation Coefficients were also performed. The, P value less than 0.05 was considered as significant[11].
The single cell based assay is an essential channel to unmask heterogeneity, diversity, and evolution. Besides, the high cell enumeration is required. There are apparatus; capable to automate, cell analysis, but very few cells is enumerated. Besides, through the manual analysis, broad exploration of multi thousand single cells are analyzable to unmask the new cellular function and their interaction.
Manual single cell based analysis by immunofluorescence (IF), also based on high enumeration, with different magni
The applied processes are performed according to the triangle style, by highlighting the power of cellular network, which reflects the association between the PE of Ets2 is also performed in the peripheral blood of the patients with AD, according to the standard flow cytometry technique[11]. The PE assay was capable to unveil the nature of heterogeneity/diversity/course of evolution by exploring D1853N polymorphism in ATM gene, and PE assay of vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) at the single cell level of the brain and through the course of evolution[12,13].
However, there are challenges regarding the CNCs in neurological disorders[14]. Furthermore, an innovative and complementary insight is required for an intensive exploring of the brain channels as well. Buzsáki et al[14] have focused on “The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes”[14]. Briefly, the challenges and achieved data in circulating tumor cells, and EEG highlights the involvement of two diverse territories and the related machinery in AD[14,15].
By considering the presence of family history of AD in the related pedigree; CNCs is offered as an applicable biomarker based test: (1) At any age, by only 2 mL of the pregnant maternal peripheral blood to screen the status of target embryo and/or fetus for Ets2 gene and the CNCs; and (2) screening the same test by buccal mucosa or buccal smear of the born child at different stages of his or her life.
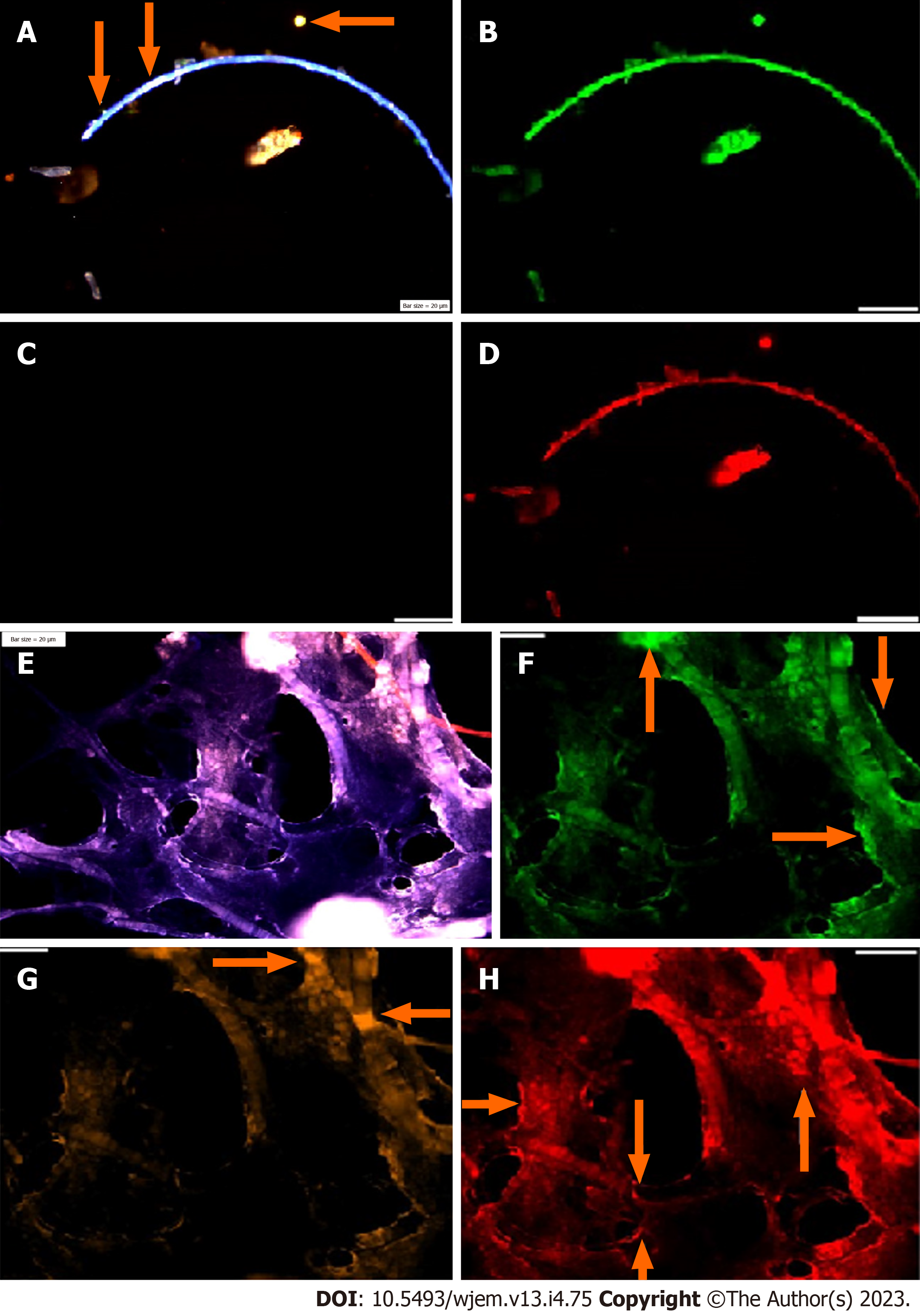
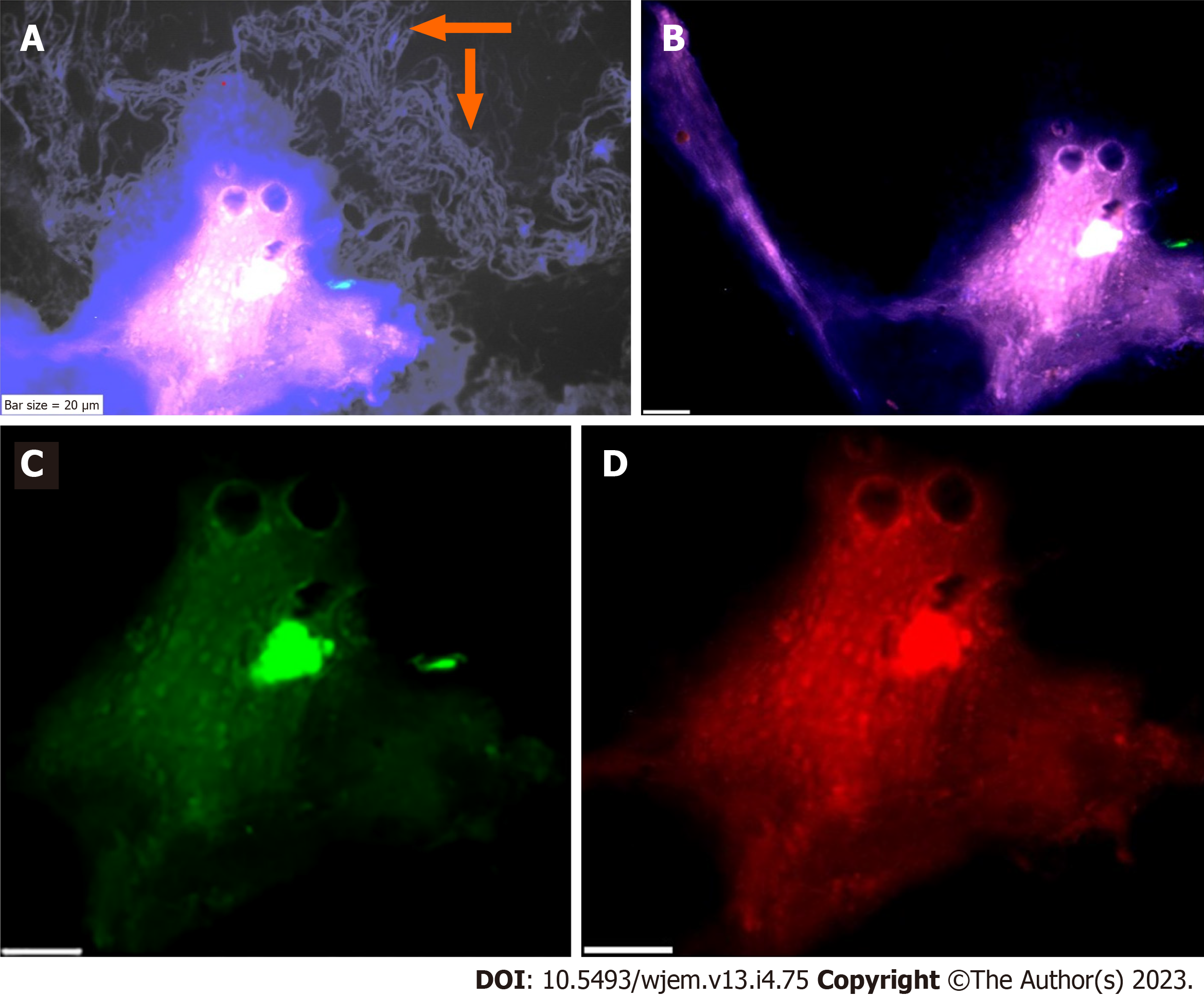
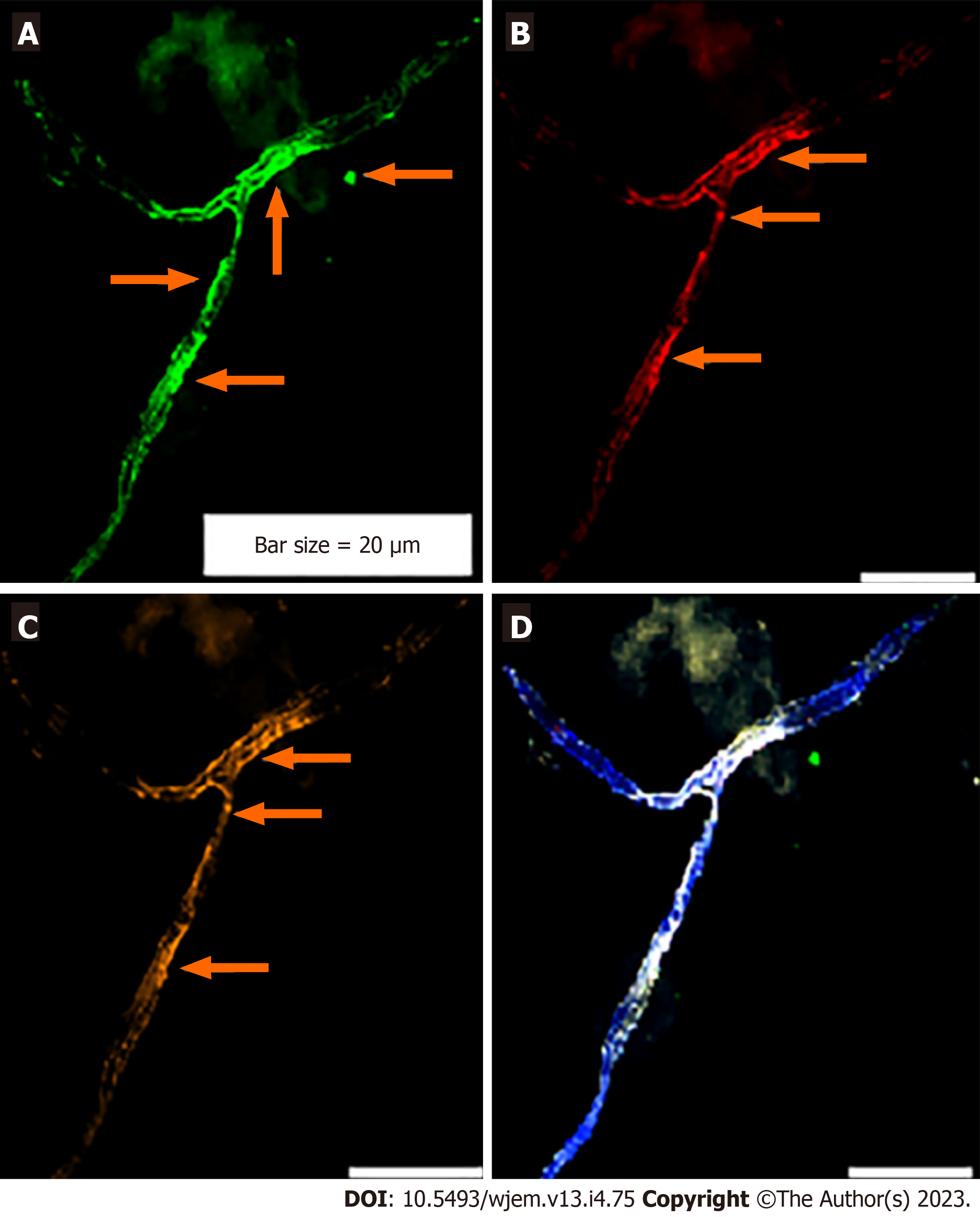
Analysis of PE was performed in the embryonic and chorionic villus samples; and patients affected with AD. PE is assayed by IF in the peripheral blood samples and 1000-3000 cells were analyzed at single cell level, by the manual exploration. An aborted embryonic sample at the early gestational week has been explored to assay the mode of PE, including neural marker (NE), neural stem cell (CD133) and VEGF (Figure 2A-C). PE at early stage of embryonic period revealed to be high for NE (Figure 2A) and VEGF (Figure 2C). But, the Neural stem cell marker (CD133) is characterized with an absolutely lack of PE, which reflects no sign of stem cell function at early stage of embryo in this aborted sample (Figure 2A-D).
Regarding the chorionic villus, sample, the behavior of PE seems to be diverse, the highest PE is traced for VEGF with high angiogenesis in the whole sample (Figure 2E-H); mosaic pattern of PE for the NE is more remarkable (Figure 2B) than VEGF, the stem cell CD133, reveals a notable, low expression, accompanied by few cells with high expression (Figure 2E-H) which reflect the initiative step for activation of CD133 stem cell, and significantly differs from the embryonic sample, lacking any expression of CD133. This is the course of evolution, which could be influenced by micro- and macro environmental factor during the fetal growth. Such behavior could occur for any other involved marker(s) for cancer initiation and/or progression. By projecting the impact of early detection, the initial target to unmask any sign of functional alteration related to the neurological disorders including AD by circulating fetal cells (CFCs), is available. In this regard, we offer an extremely early detection of neural stem cell (CD133), Ets2, and any related key proteins, involved in the initiation and progression of neurological disorders including AD at 8-9 gestational week (Figure 2E-H). Furthermore, screening the evolutionary course of the molecular and functional alteration(s) is possible by the follow-up strategy and only 2-3 mL maternal peripheral blood as often as, is essential. In addition, we provide an early detective platform including the stem cells (CD133) at fetal period, i.e., as early as 8-9 wk through the CFCs/chorionic villus in the maternal blood stream. Besides the key proteins including cyclins B, D, E, EGFs and VEGF could be also analysed. The benefit of such an extremely early screening is to deliberate the preliminary and preventive strategy including micro- and macro-environmental factors, counting nutrition. Besides, planning the essential and non-invasive screening, such as EEG at the right time through the offspring’s life, under the guide and care of physician could be planned to detect any sign of AD manifestation(s) for applying any essential 5xP aims.
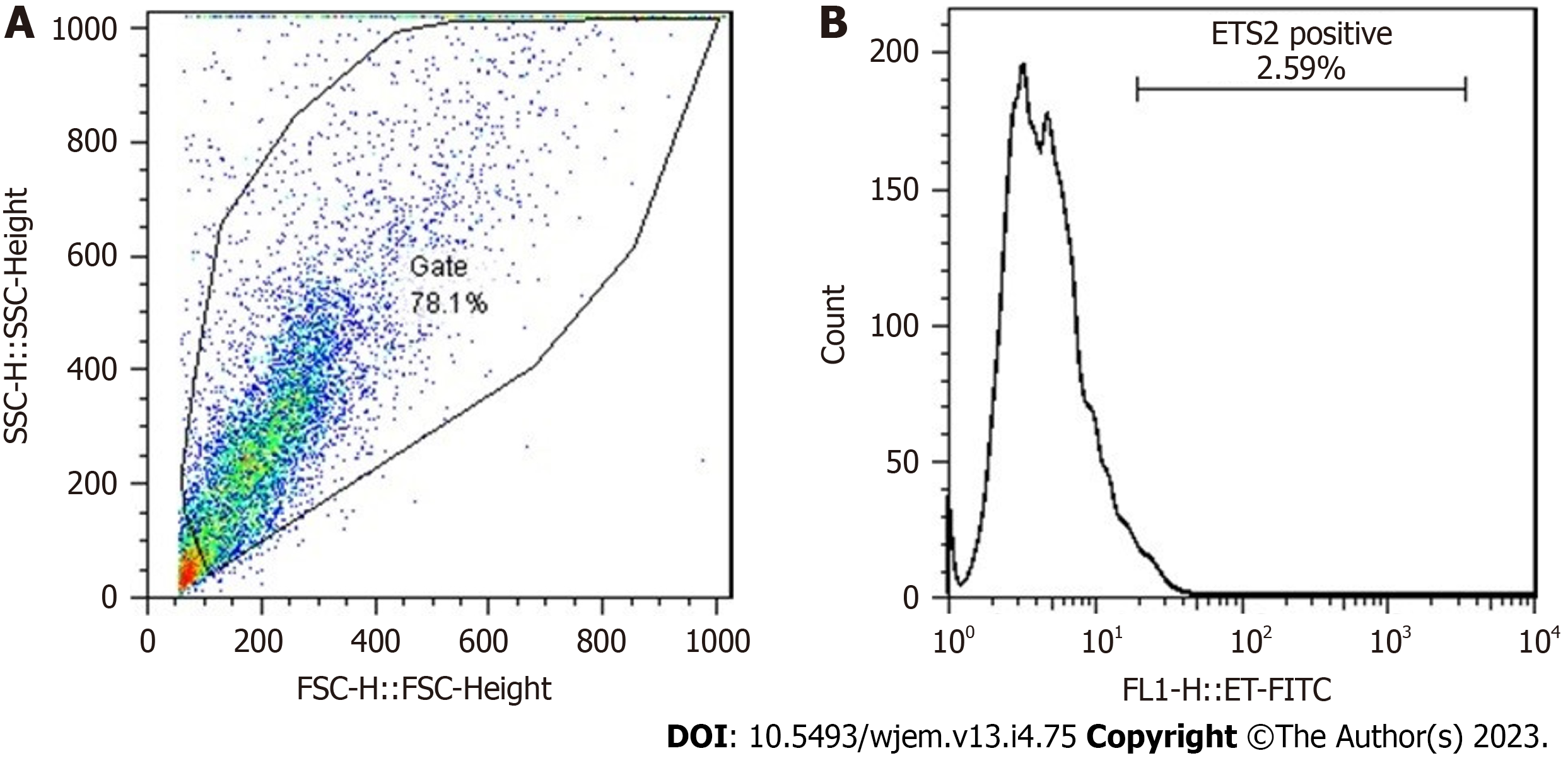
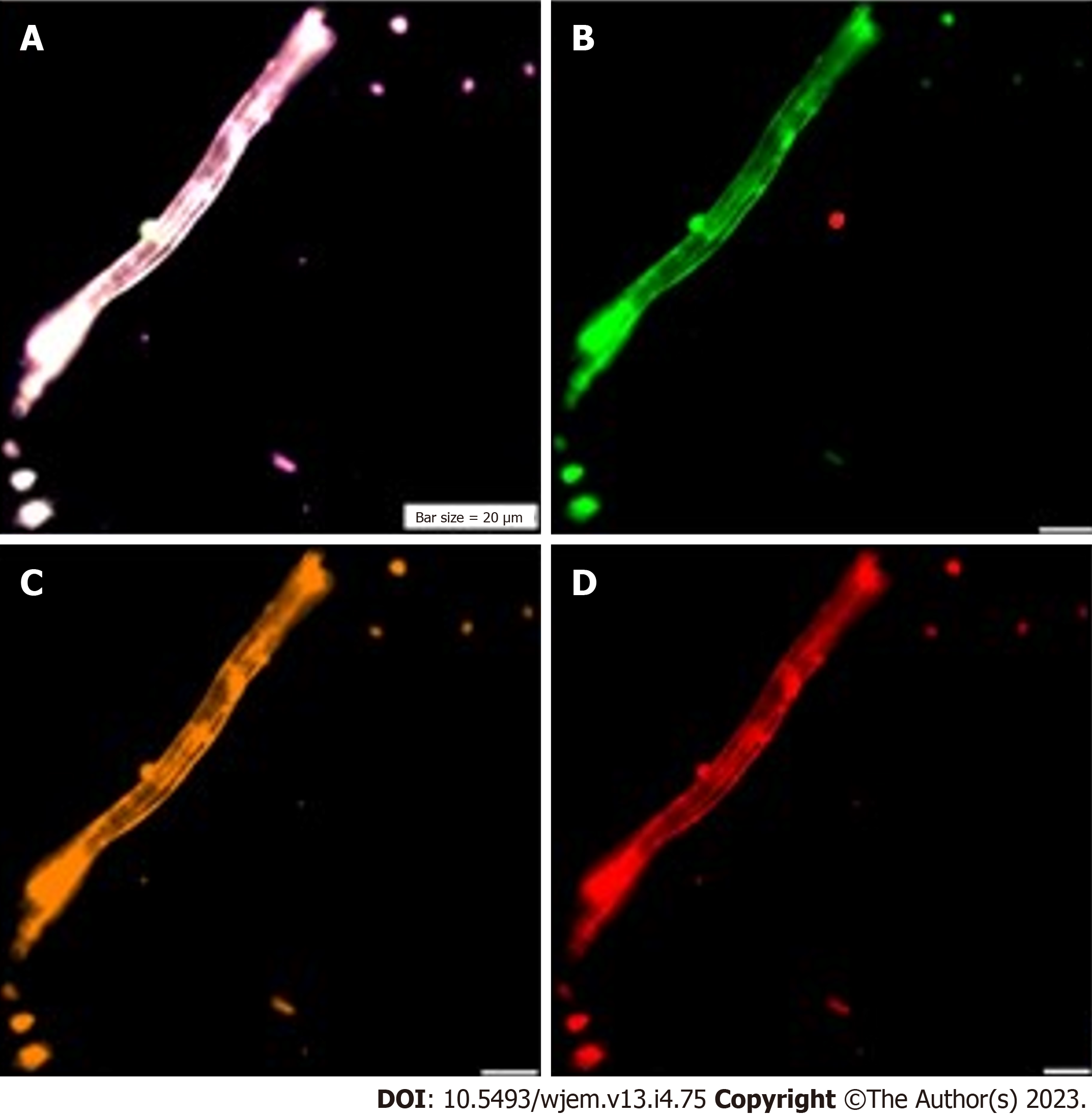
Besides the manual detection by immunofluorescence, automated analysis of PE by FC, is performed for Ets2 protein (Figure 3).
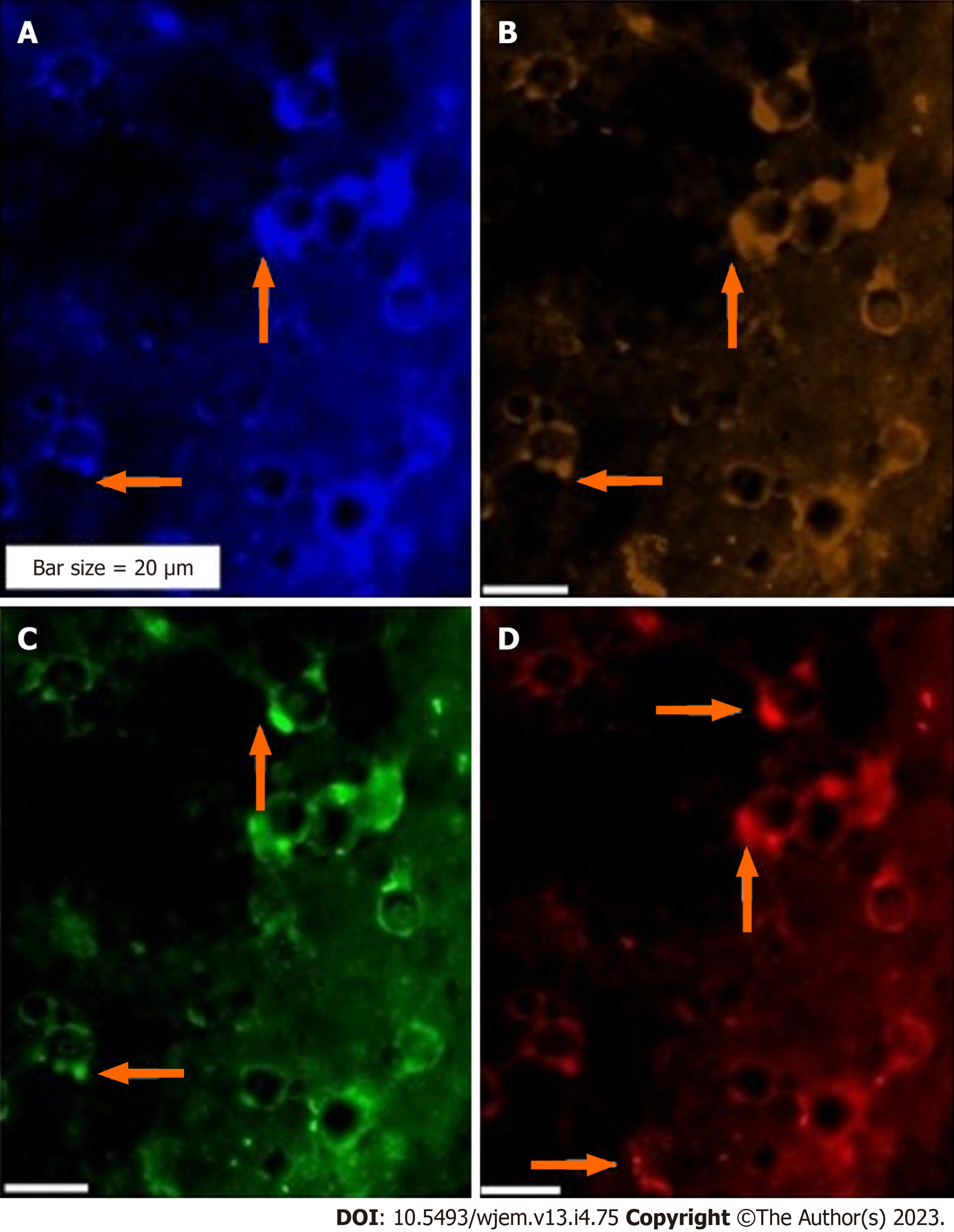
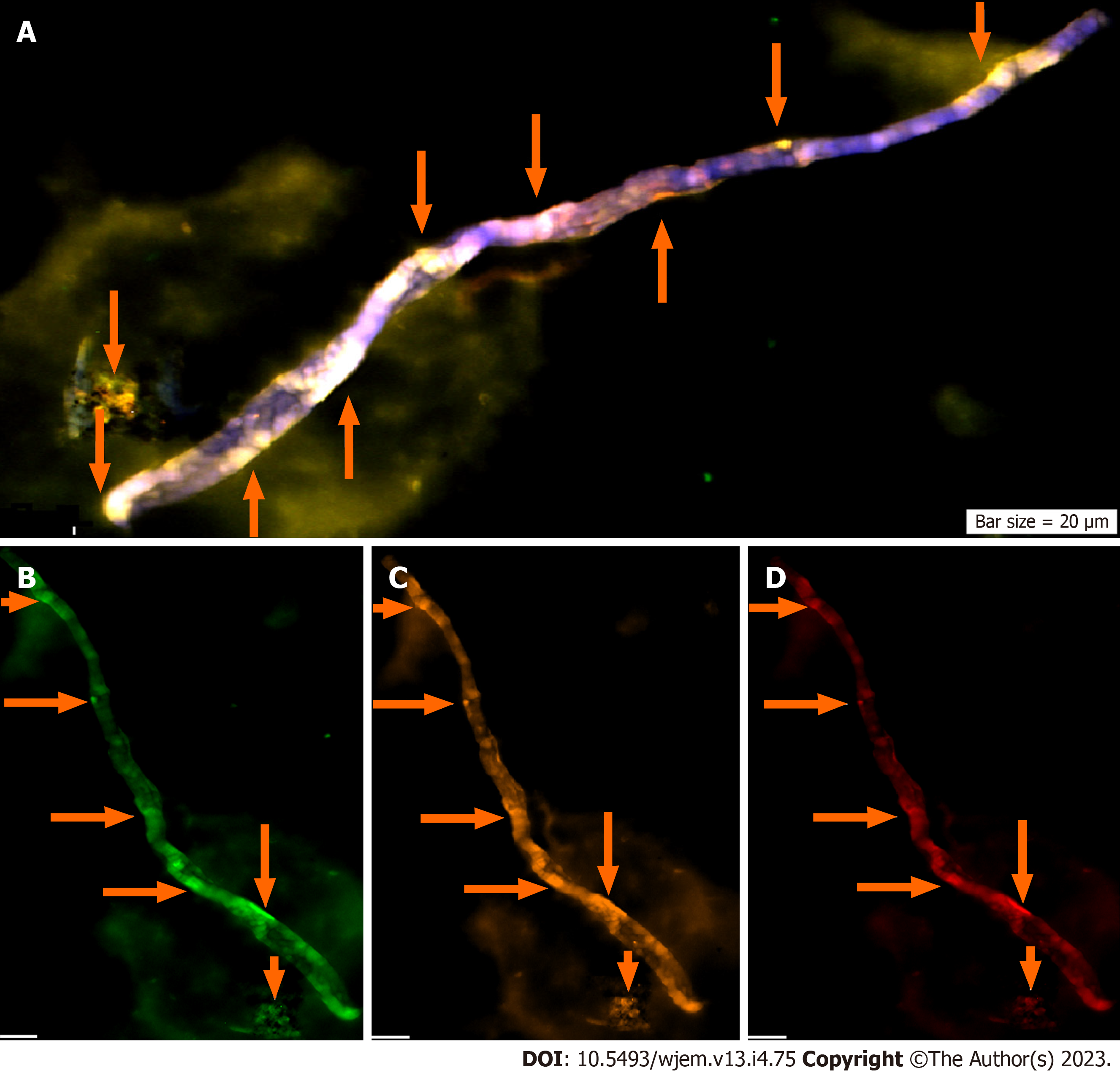
Three proteins including ATM, Ets2 and VEGF are reflective of: (1) Weak expression of ATM as a tumor suppressor gene; (2) mosaic pattern of PE for Ets2; and (3) remarkable expression of VEGF which is indicative of angiogenesis in the neural cells (Figure 4).
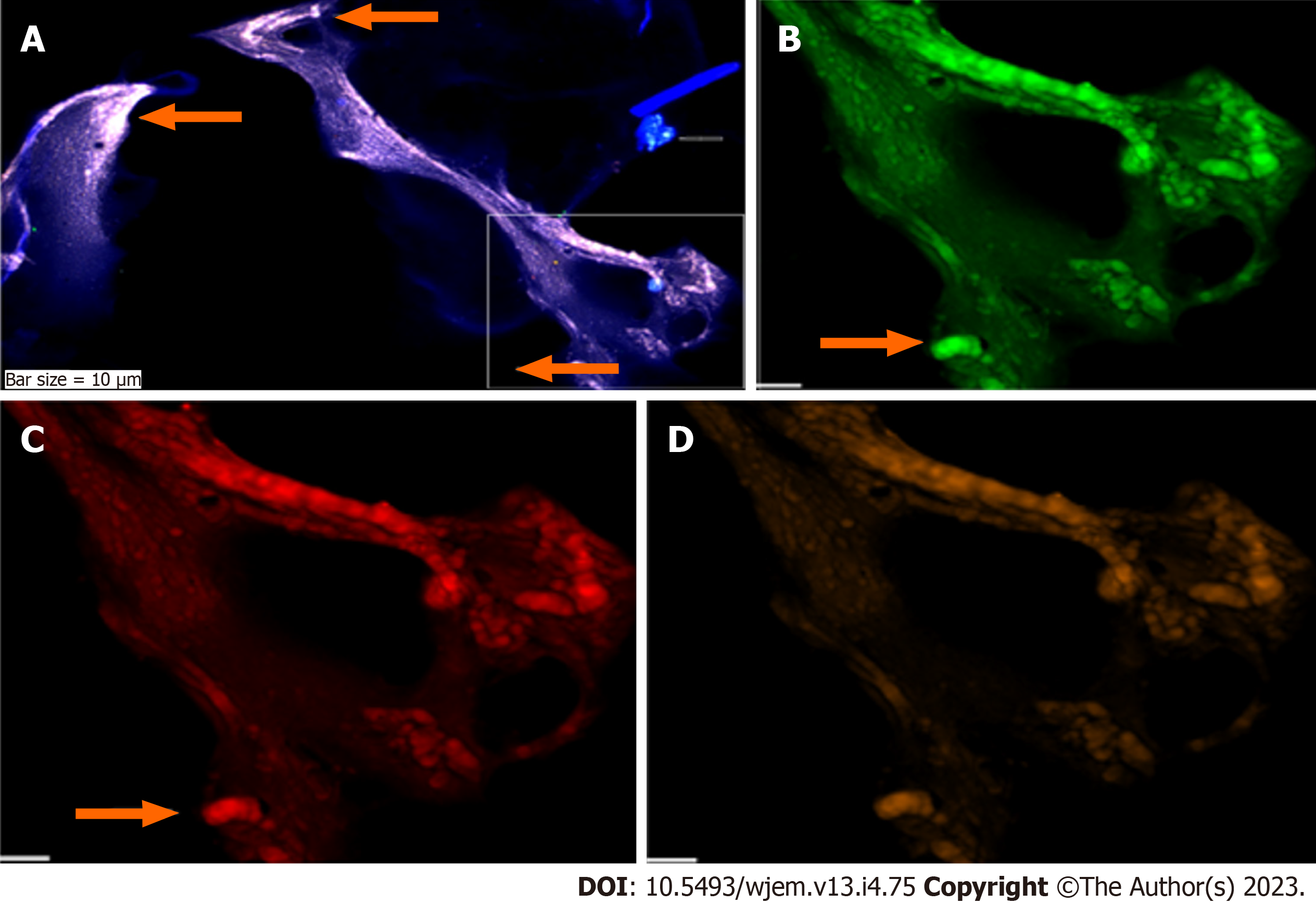
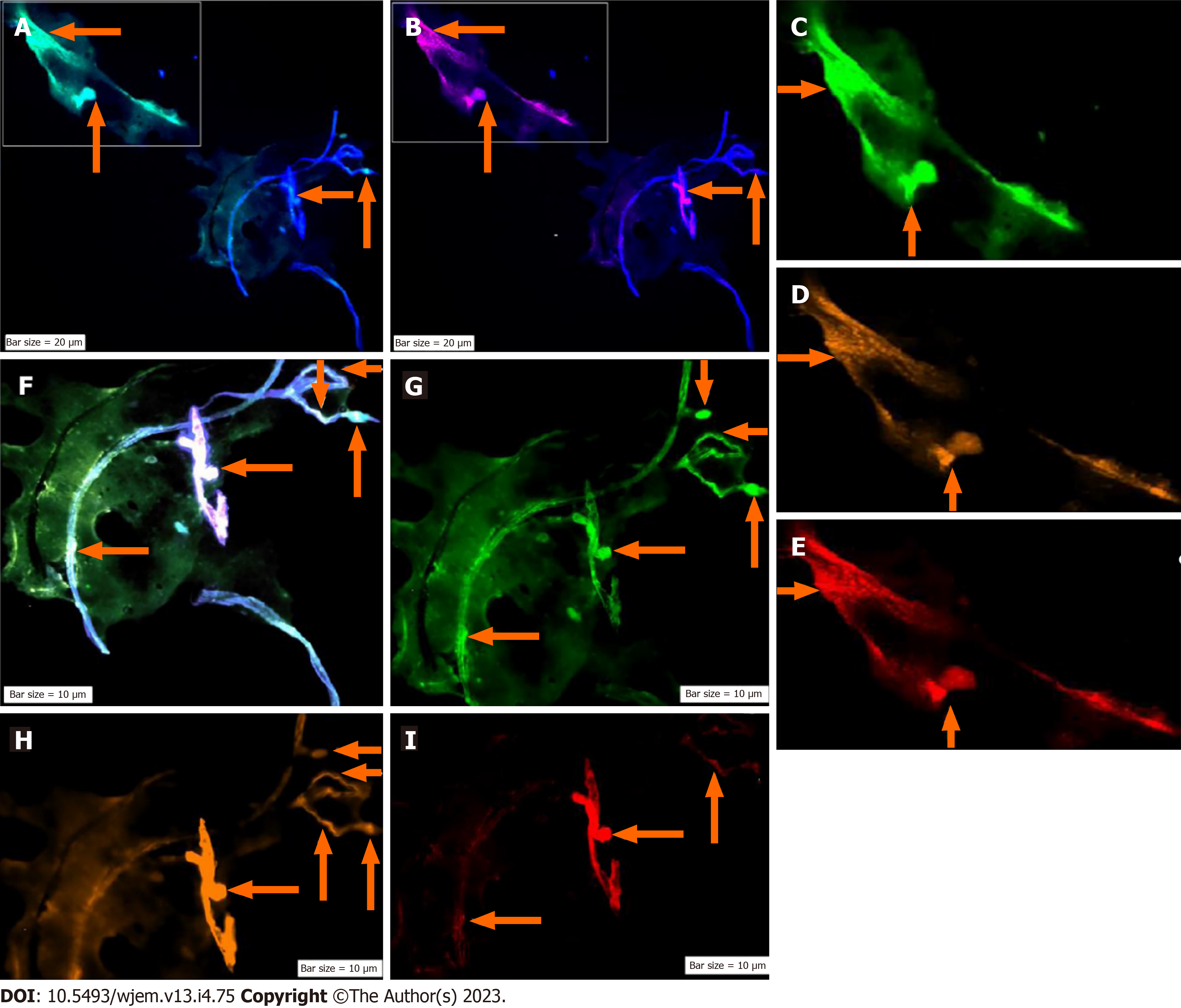
The CNCs were explored by IF method for combination of NE, neural stem cell and angiogenesis (NE/CD133/VEGF (Figures 5 and 6); and for Ne/CD133 (Figure 7). The PE of NE/VEGF/Ets2 is also provided (Figure 8). The migrated neural cells with high or low expression are shown with arrows. The micro-vesicle and the vascular section harboring the migrated neural cells are detectable. The CNCs were explored by IF method for combination of NE and neural stem cell (NE/CD133) (Figure 7). The PE of NE/VEGF/Ets2 is also provided (Figure 8). Lower expression of this triangle (NE/VEGF/Ets2) is a reliable predictive/early and non-invasive platform for exploring the status of AD at any stage of life. The CNCs’ ratio, between pre- and post- intervention were analyzed based on the PE-screening of cytokeratin 19 (CK19), and leukocyte common antigen (CD45) and EGF. The cells conjugated with VEGF (Rpe), having lower expression (Figure 8), the CNCs’ ratio, between pre- and post- intervention were analyzed based on the PE-screening of CK19, and leukocyte common antigen (CD45) and EGF. The personalized insight is considered/and the ratio value is estimated for the cases. The Ratios are characterized as < 1 and > 1 between different channels within the horizontal and vertical axes. Finally, the diverse patterns were classified according to the distribution including clusters or sporadically forms. The CNCs’ ratio, between pre- and post- intervention were analyzed based on the PE- screening of CK19, leukocyte common antigen (CD45) and EGF (Figure 9). The confirmation of CNCs was based on the mode of positive CK19+ and negative CD45. The personalized insight is considered. The ratio value is estimated in cases. The Ratios are characterized as < 1 and > 1 between different channels within the horizontal and vertical axes. Finally, the diverse patterns were classified according to the distribution including clusters or sporadically forms.
Conclusively, by aiming an early clinical management, including diagnosis and therapy, the application of an available multi-panels based on: (1) Non-Invasive; (2) repeatable; (3) cost benefit; and (4) safe CNCs sample, in only 2 mL peripheral blood or buccal smear, for an early detection of the target proteins expression in the brain cells could be considered. Let’s Revise Alzheimer disease, based on single cell based vocabulary (Figure 10).
The combination of neurology/genetics/cell biology/psychology/ethics is required for the research and clinical management for the neurological disorders. Moreover, the following items harmonize the 5xP by: (1) Applying the Genetic counselling by documenting the patients’ pedigree, including the family history of the related diseases, up to 3-4 or more generations; (2) organization of the accustomed plan according to the psychological status of the referral cases; (3) considering the macro-and/or micro-environmental factors in the patients’ pedigrees; (4) performing the required Genetics’ analysis according to the information, based on the pedigree study; (5) focusing on the apparently normal relatives, having the family history of the relatives affected with the diseases through generations in the pedigree; (6) applying the required complementary test(s); and (7) application of the predictive and early detective strategy for the target suspicious patients with AD and their target relatives.
Genetics/cell biology organize and play the most fundamental roles, by considering a triangle model to unmask the heterogeneity/diversity/evolution. Through such manner, the personalized diagnosis and therapy could be developed. AD as a major aim is selected to explore Ets2. This gene has been explored in two different group of patients affected with neuro-degenerative disorder including AD and DS[11]. Ets2 with its pathogenic role, revealed different cellular behavior in two different diseases. The results have suggested the strategy as ‘Gene Product-Based Therapy’ and the personalized managements for both diseases.
The information of patients’ and controls are provided in Tables 1 and 2. Total cells of 10000 in AD patients were analyzed by FC, but without providing any heterogeneity. The FC-results of Ets2, is provided (Figure 3). PE assay unmasked the nature of heterogeneity/diversity/course of evolution by exploring Ets2, D1853N polymorphism in ATM gene, VEGF, EGF and course of evolution at the single cell level of the brain. The arrangement of Genetics/Cell biology/Psychology/Ethics is essential quadrat-radial format for programming the research and clinical management. The required steps in the Genetics and cell biological programs include: (1) Genetic counselling/pedigree documentation; (2) considering the macro-and/or micro-environmental factors; (3) performing the required Genetics’ analysis, based on the pedigree-information; (4) considering the target proband’s relatives for the required screening; and (5) applying the required Genetic test(s). Besides, harmonizing the 5xP insights are the essential target in the clinics.
| Case No | Gender | Age, yr | Clinical feature | Family History of AD (years old) | Ets2 protein expression (%) | Total cells evaluated by flow-cytometry |
| 1 | F | 69 | ML | - | 146 (3.47) | 4207 |
| 2 | F | 57 | ML, tremble (hands) | Cousin of paternal father (65) | 155 (2.13) | 7276 |
| 3 | F | 92 | ML, MD | - | 121 (1.11) | 10900 |
| 4 | M | 76 | ML | Half brother (70) | 607 (15.8) | 3841 |
| 5 | M | 79 | ML | - | 810 (11) | 7363 |
| 6 | M | 67 | ML (severe) | - | 166 (2.43) | 6831 |
| 7 | M | 42 | ML (severe) | - | 1100 (6.75) | 16296 |
| 8 | F | 54 | ML | - | 228 (3) | 7600 |
| 9 | F | 81 | ML, HC, BP | Maternal aunt (95) | 98 (5.12) | 1914 |
| 10 | F | 79 | ML | - | 541 (18.9) | 2862 |
| ID | Age, yr | Ets2 protein expression (%) | Total cells analyzed |
| C-1 | 65 | 2 (0.066) | 3025 |
| C-2 | 56 | 10 (0.19) | 5214 |
| C-3 | 79 | 0 (0) | 1546 |
| C-4 | 74 | 3 (0.065) | 4587 |
| C-5 | 79 | 6 (0.48) | 1232 |
| C-6 | 62 | 3 (0.38) | 783 |
| C-7 | 42 | 0 (0) | 923 |
| C-8 | 54 | 4 (0.10) | 9823 |
| C-9 | 81 | 7 (004) | 874 |
| C-10 | 79 | 3 (0.19) | 1542 |
| Total | 38 (0.12) | 29549 | |
Complementary and comparative insights between different panels of PE assay is the key aim in the patients with AD. PE of Ets2 by IF at analyzable single cell level of the AD patients are provided (Figures 4 and 9). However, PE is a challenging assay and the cells with high magnification are required to: (1) Reflect the clear panorama of the PE; and (2) most importantly, to echo the cellular heterogeneity clearly. The PE of Ets2 is also assayed for ten control individuals (Table 2). Two different single cell- based platforms are indicative of diverse conclusions. In fact, the most diagnostic and visibility of cellular diversity is achievable by IF through which the territory of the migrated cells from the brain domain is detectable (Figures 3 and 8). The provided figure by IF, clearly, reflect heterogeneity at single cell level.
An extra copy of Ets2 gene in the DNA of brain cells is reported by quantitative densitometry[16] and the provided guidlines[17,18]. Based on this valuable finding, the possible correlation of an extra chromosome 21 in DS as the cause of AD was also reported. In this regard, the expression of Ets2 was also high in DS by: (1) A segmental trisomy model for transcriptome during postnatal development of DS in a mouse model[19]; (2) leading to induction of neural apoptosis[20]; and (3) high-expression of Ets2 led to amplification of the APP gene expression and raising β-amyloid proteins, with consequential brain anomaly in AD and DS[21]. The Most interesting report was related to the beta amyloid gene duplication in both Alzheimer's disease and the case of DS with normal karyotype[21]. It has been, also, emphasized that through the mechanism of an imbalance process for gene dosage, the mental disorder could be evolved through the molecular/cellular machinery in DS[22]. Conclusively, the PE of Ets2, as a remarkable pathogenic factor, has the fundamental role at single cell level of two different disease including AD and DS.
It is reported that application of “Sensory-Evoked 40-Hz Gamma” facilitates and improves sleeping process and the quality of life in the patients affected with Alzheimer's Disease[23]. Similarly, the light music has also the same impact on the individuals affected with AD as well.
As a major component of the cerebral cortex, the frontal lobe serves a variety of functions. It is believed that the orbitofrontal cortex (PFC) is involved in the cognitive process of decision-making and other ventromedial prefrontal cortex regions of frontal lobe; it plays a significant role in the regulation of motivation and emotion as well[24,25]. The ventromedial PFC plays a more specialized function in pleasure, happiness, and reward conditioning[26]. Executive function, however, is one of the most significant frontal lobe-related functional groups. Executive functions are typically cognitive processes that help people tackle challenging, unique, and complicated tasks by choosing and fusing actions or thoughts with internal goals and mediating activities over time[27-29]. Working memory, cognitive flexibility, and inhibition are all components of executive function, which is reliant on top-down (i.e., goal-driven) control of distributed processes taking place across the brain. The nature of the processes being controlled determines the precise behavioral output[30]. Key cognitive processes relating to social, emotional, and motivational elements of conduct are carried out by prefrontal cortical areas. Working memory, goal-driven attention, task switching, planning, problem-solving, and the need for novelty are all functions of the dorsal lateral prefrontal cortex. The medial prefrontal cortex is involved in self-awareness, motivation, emotional regulation, and updating goal-directed behavior; the orbitofrontal cortex is involved in personality, inhibition, and emotional and social reasoning. The ventral lateral prefrontal cortex is involved in inhibition, response selection, and monitoring[27]. Dysexecutive syndromes have historically been linked to dorsolateral prefrontal cortex damage, but it is now understood that they can also be caused by a parietal-temporal-frontal system impairment, which is the focus of a specific type of atypical AD. Simple daily tasks requiring executive control and a variety of neuropsychological tests are among the tasks that this dysexecutive Alzheimer phenotype performs poorly on. Disrupted executive control over social, emotional, and motivational elements of behavior characterizes dysexecutive syndromes, which are more closely associated with the frontal lobe[31].
It is crucial to take into account both the rhythms' location in the brain during EEG analysis in addition to the rhythms themselves. For instance, because alpha rhythms represent the activity of several neuronal populations, they have different neurological correlations when they are observed in the occipital, temporal, or frontal cortexes[32].
The majority of EEG studies used to diagnose AD have been based on spectral decomposition of scalp signals in both the resting state with closed eyes and open eyes. We now understand that healthy aging causes changes in brain activity, which are consequently recorded in EEG recordings. They include an increase in delta (1-4 Hz) and theta (4-8 Hz) power, a decrease in background activity, and a slowing of alpha activity (8-13 Hz)[33]. During physiological aging, the magnitude of the alpha rhythm reduces in posterior cortical regions, and this is related to the level of general cognitive function[34]. However, numerous studies indicate that compared to age-matched controls, both mild cognitive impairment (MCI) and AD suffer from changes in the pattern of their EEG recordings: Alpha and beta rhythms typically decline, whereas delta and theta oscillations generally increase[35-37]. Such evidences highlight the key application of EEG through the course of disease.
At the AD-initiation, there is an increase in slow waves, notably in the theta rhythm. The earliest indicators of cognitive deterioration and the theta power rise are related[38]. Theta relative power, (which is expressed as the percentage of theta band power to all other bands), is higher in AD than in MCI and higher in MCI than in healthy controls and is associated with worsening performance across all cognitive domains[39]. alpha power is lower in AD than in MCI, and in MCI than in normal people[40]. There is a correlation between the decline in alpha activity and the severity of the illness and the cognitive abnormalities[40]. In addition to spectrum features, EEG also has synchronization features. EEG synchronization also describes how various brain oscillations are adjusted. When two distinct signals lock in phase, become phase-amplitude coupling, or modulate their amplitudes simultaneously (amplitude-amplitude coupling), they are said to be coupled[41]. One of these is spectral coherence measurement, which measures the spectral covariance of activity between two electrode locations. When compared to healthy controls, EEG coherence in AD participants exhibited statistically significant changes[42-44]. Alpha wave coherence in the temporo-parieto-occipital regions decreased in AD patients, although delta wave coherence between the frontal and posterior regions increased[45]. Considering the previously mentioned theory that basal forebrain neurons are severely impaired, all EEG characteristics associated with AD, such as changes in frequency patterns and synchrony measurements, may be caused by the loss of neurons, altered anatomical structure of the neuronal tracts, as well as altered release of neurotransmitters, all of which lead to impairments in neural activity[44].
Neurological disorders are extremely complicated with an influential impact on the whole society including the patients’ family and close friends. Therefore, the educational packages are required to create the cooperative surrounded living atmosphere between the patients, family and the friend.
Regarding the muti-dimentional approach of the neurological disorders, including AD, the following facts and directive points of view in the recent review article provided by Tanaka and Vécsei[46]: Differentiating the “neuroprotective and neurodegenerative modules of neurological and neuropsychiatric diseases”, highlighting the etiology, available techniques, diagnostic and therapeutic approaches of neurodegenerative diseases.
As the whole, neural engagements can be stabilized by the” neuroplasticity” which is characterized by: (1) The nervous system capabilities to convert other components; (2) based on the status of functions, structural format, and constructive ability; and (3) being malleable, tolerated and adaptive against the non-desirable event and challenges[46].
Regarding the traditional brain technology for AD- patients, and by focusing on the psychological disorders, the neuro-developmental aspects of the brain, have not been much unmasked. Indeed, EEG, as a globally trusted screening system, applied to measure the brain motion for a century. EEG affords the brain’s electrical motion and measure the electro cortical rhythms[6,7,9,14].
The EEG reports are skillful in: (1) Resting status; and (2) closed eyes using a Delta-med digital EEG acquisition system for 20 min. Scalp electrodes were adjusted according to “the modified International 10 ± 20 system plus 11 additional electrodes by 256 Hz sampling rate”. Thirty electrodes were positioned on the scalp include Fp1, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FC7, FC4, FT8, T3, C3, Cz, C4, T4, TP7, CP3, CPz, CP4, TP8, T5, P3, Pz, P4, T6, O1, Oz, and O2.
Furthermore, by considering especial care about the patients affected with AD at preclinical zone, information including classification up to the diagnostic principles requires the complementary attention[17].
Ratio was calculated between different brain- channels to achieve the manner of motions between different channels at pre- and post-mediation with the LM. Therefore, the LM as a balancing and relaxing the neural targets mediated the Volunteers.
The history and the benefit of EEG in research and clinics are, approximately, related to the 120 years ago (since 1930s). However, the scientific knowledge lacks the course of evolution through the brain channels. Whatever the Scientists unmask, is required to reflect: (1) The initial/fundamental event(s) through the brain channels; (2) priority of the events; (3) the cascade manner of the alterations; (4) interaction between the channels; (5) stability of the alteration(s); (6) unmasking the resting state of EEG; and (7) unveiling the course of evolution in the human brain channels.
The function and role of Broadman’s areas including 64 channels of EEG are directive to unmask the complementary/paralleled function of the brain territory. However, the interaction between the channels requires to be personalized and classified. Any kind of mediation may increase or decrease the activity of alpha and gamma waves, respectively, and affect the brain metabolism and anxiety.
Mediation by LM led us to provide a brain channel based and personalized model, and to unmask the nature of process through the brain channels, including a significant increase in Alpha and Beta bands at frontal, central and occipital lobes of the brain.
Ratio between different brain channels unveil the diverse signal pattern at the pre- and post-mediation with the LM.
As a question, whether any mechanism relies on an interactive system between the brain’s channels or not. So, 63 channels were investigated in 5 cases, at pre- and post-mediation with LM.
Based on the t-test, a significant increased pattern in alpha wave- and decreased in gamma wave activity, and reduction in the brain metabolism and anxiety were observed in the candidates’ EEG (P < 0.05). As the result, a significant increase in Alpha and Beta bands of the frontal, central and occipital lobes of the brain were observed. The waves including Alpha 1,2; Beta 1-3, in both eye conditions, either open or close, the power is found between 1 to 1.6. Diversity is remarkable with close eyes than in open eyes condition.
By considering the power ratio of brain signals, the post- vs pre-mediation of LM was explored in the candidates. The outcomes of mediation with LM commanded to significant escalation of the power of alpha band wave at Fz, F4, FC2 sites (frontal lobe), Cz (central lobe) and O1 (occipital lobe). The increased activity of Alpha waves in Centro-frontal regions represents edited brain activity, metabolism, and reduced anxiety. Conclusively, the brain requires fewer properties for managing its cognitive task. Data of EEG revealed that the mediation, has significantly led to: (1) Increasing the Alpha and Beta bands of the frontal, central and occipital lobes of the brain; (2) reduction of the brain metabolism and anxiety; and (3) declining the activity of gamma wave in the EEG.
Remarkable diverse pattern was observable between absolute pattern for Gamma 1 and 2 at open eye- (characterized with < 1 absolute power) than close eye-condition (characterized with > 1, ranging between 1-7).
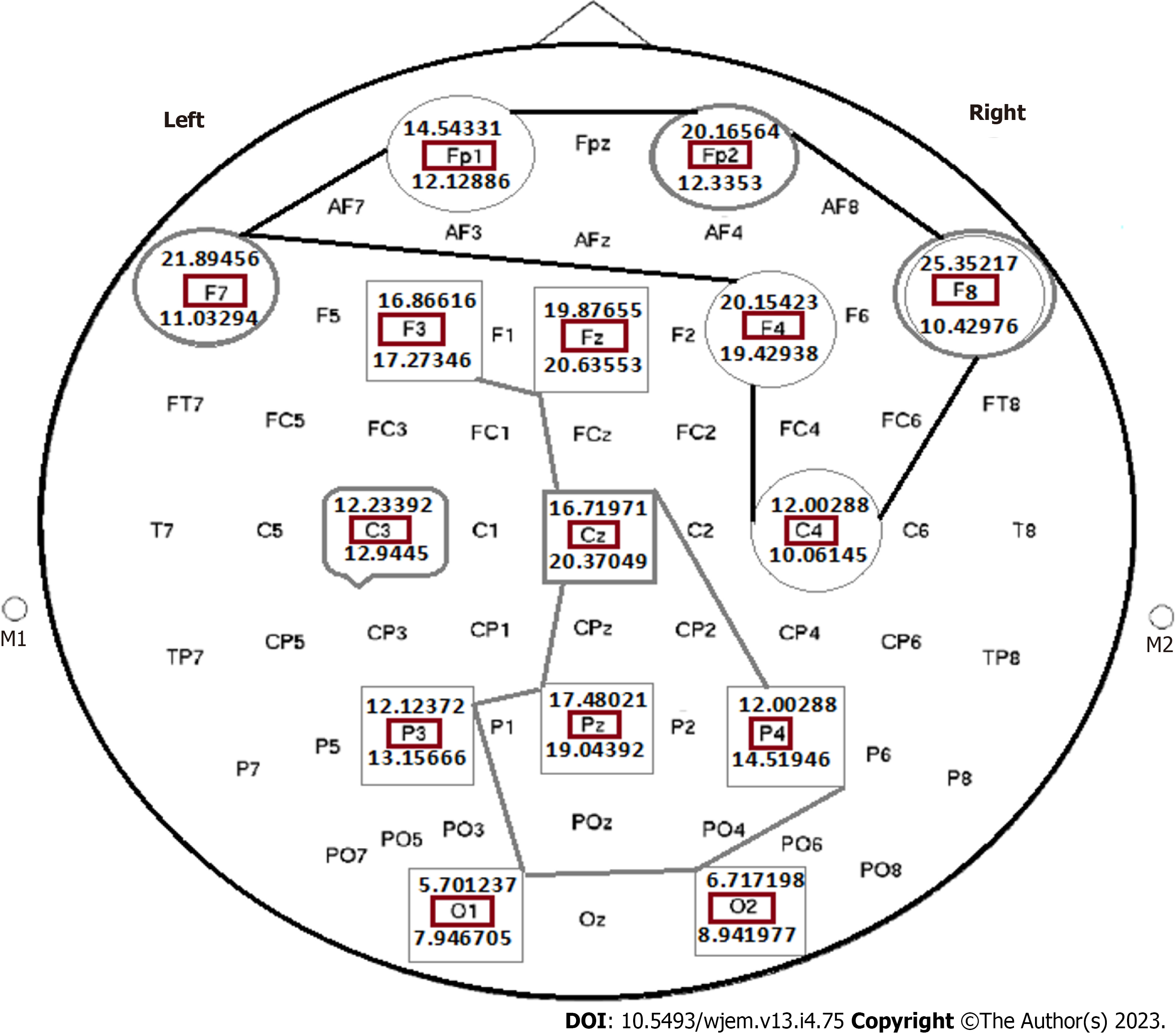
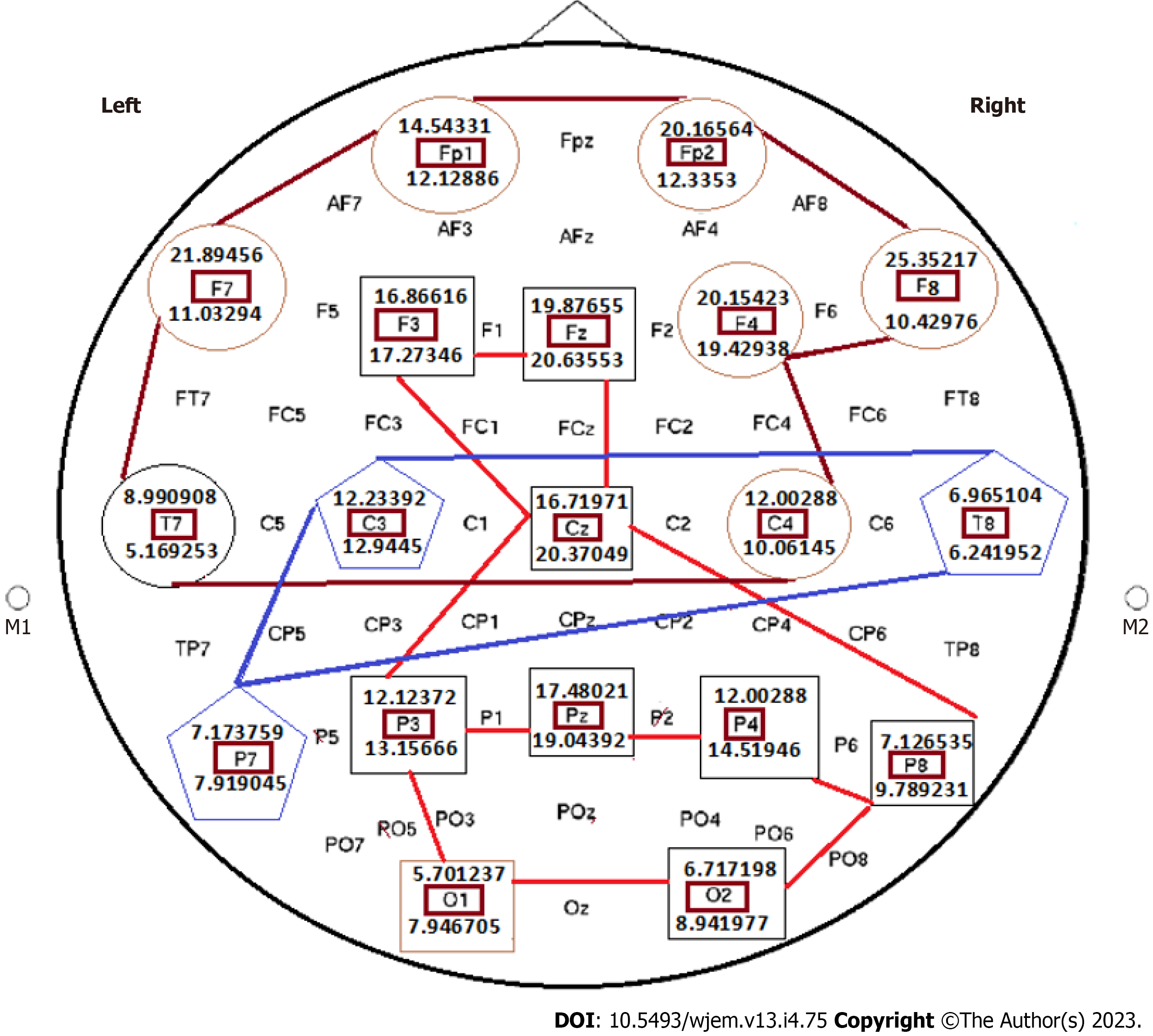
Status of the absolute power ratio in all brain channels sounds as an Informative panel in the brain research and with possible translational impact on personalized classification in Medical Neurology. Exploring of the absolute power ratio in all brain channels could be performed upon the physician’s authorization, at different periods of life, including childhood, adulthood and elderly, by issuing the ‘Brain ID card’. Such preliminary and three complementary ID-card pave the way towards tracing the course of evolution at brain channels, and the probable connective pathways to the certain disorders. Such Information provides the 5xP package for any preventive, and/or the clinical recommendations to the target patients (Figures 10, 11, 12, and 13).
The provided Ratios are considered as the reliable indices for organizing the behavioral status of brain channels (Figures 10, 11, 12, and 13) to: (1) Guarantee an early detection; (2) being accompanied by performance of PE in the non-invasive tissues, including 2-3 mL peripheral blood, buccal smear and/or mucosa, nail, urine, stool, and/or through performing circulating cells from the target organs/tissues; (3) considering as the personalized, predictive, preventive, and prognostic panel for the patient’s relatives within the pedigree; (4) cooperation between different channels with diverse interactive ratios lead to the balancing strategy for improving the life-quality and quantity of the patients with neurological disorders and an apparently normal individuals, by light nusic and any other safe, non-invasive and beneficial intervention(s); and (5) by considering the provided hypothetic based data, heterogenic/diverse results presented the occurrence of evolutionary course within the brain channels. Multi-interaction between different channels, are suggested as the personalized and constructive markers with unlimited cooperation in health and disease.
The influence of the light music on the brain channels reflected the diverse patterns in the absolute power ratio at pre- to post-intervention (Figures 10, 11, 12, and 13).
Mean Power of Delta (HZ) before and 20 min after mediation with LM revealed the significant differences, in the following brain channels: Mean Power of the Delta waves frequency (Hz) at before and after mediation with LM is found to be significant with p-value ranging between 0.013-0.031 for the channels including Fz-, F44, FC2-,Cz- and O1-LE. Furthermore, the mean power of Beta- gamma before and after mediation ranged between 0.001-and 0.048 for the channels Fz-Le; F4-Le; FC2-Le; Cz-Le; and O1-Le of Alpha wave.
It is not documented that whether status of the delta-wave frequency, between the closed and opened eyes in different brain channels is diverse or not. Therefore, we aimed to explore the ratio-pattern between absolute powers in different channels (Figures 10, 11, 12, and 13).
Interaction between different brain channels, either balanced or imbalanced, is a personalized pattern, leading to the new classification in the brain. Exploration of EEG according to the following results and discussion reflect the remarkable diversity between different channels, which could be classified as the unique/personalized classification for the brain behavioral Science (Figures 10, 11, 12, and 13).
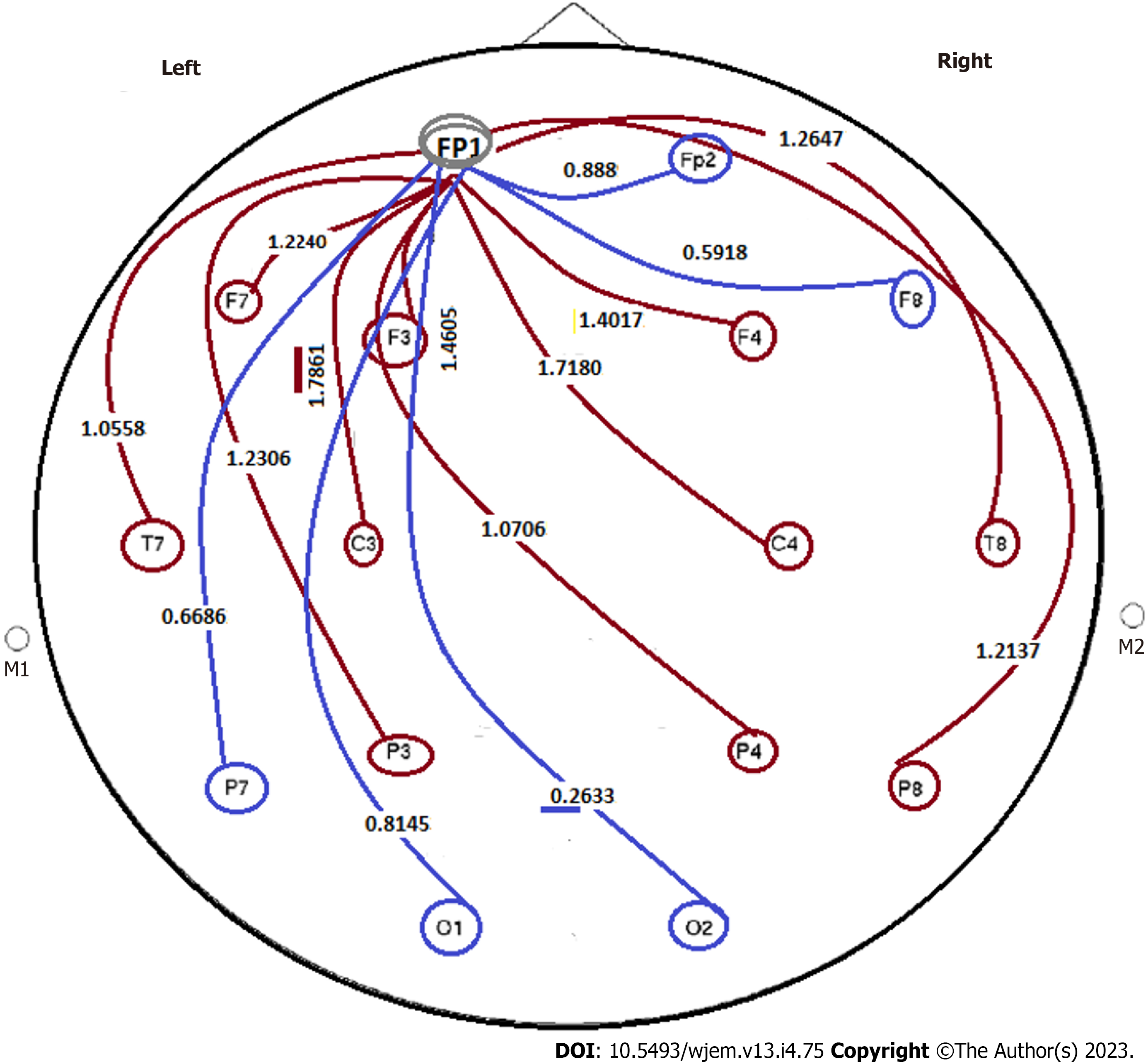
Amongst the current diverse status of the delta-wave frequency, 8/9 ratios are characterized with the higher frequency for the closed eyes than in the opened eyes’ condition including F8, F7, FP1, T7, C4, F3, and F4. Besides, the diverse status includes the ratio with higher values in the opened eyes including Cz, F3, Fz, Pz, P4, P8, P3, O1 and O2 with less remarkable diversity, but still diversity is notable.
By considering the wave frequency of Delta, the ratio of closed eyes to the open eyes is reflective of: The most stable targets are traced in the C3 (12.23392/12.9445), T8 (CE: 6.965104/6.241952); and P7 (CE: 7.173759/OE: 7.919045) (Figure 11).
The indices as discordance based grouping with < 1 ratio, at post mediation by LM: The F3, Fz, Cz, P3, Pz, P4, P8, O1, O2 are classified as discordance grouping with ratio < 1. With the current diverse status of the Delta-wave frequencies, 8/9 ratios are characterized with the higher frequency for the closed eyes than in the opened eyes’ condition including F8, F7, FP1, T7, C4, F3, and F4. Besides, the diverse status includes the ratio with higher values in the opened eyes including Cz, F3, Fz, Pz, P4, P8, P3, O1, and O2 with less remarkable diversity (Figure 11).
By considering the wave frequency of Delta, the ratio of closed eyes to the open eyes is reflective of: The most stable targets are traced in the C3 (12.23392/12.9445), T8 (CE: 6.965104/6.241952), and P7 (CE: 7.3759/OE: 7.919045) (Figures 11 and 12).
Diverse grouping with discordance index: The indices as discordance based grouping with > 1 ratio, at post mediation by LM: F7, Fp2, F8, FP1, T7 in > 1 category, of those F8, and F7, reflect remarkable high ratio than others in this classified group (Figure 12).
Conclusively, remarkable diversity is detectable for the Ratio between closed eye/open eyes after the post mediation by LM.
Descriptive data includes before and after mediation with the LM, at the significant level of the alpha band. Mediation by LM, significantly decreases the power of Beta-Gamma band wave at points Fz, F4, FC2 (forehead areas), Cz (central region) and O1 (occipital region of the brain). Such a decreased power of beta-gamma reflects the reduced activity and connectivity of brain between frontal/central regions.
Concisely, an increased alpha power and decreased beta-gamma power after mediation of the LM is remarkable. This link between neural oscillations in diverse frequency bands proposed to harmonize neural processing.
Furthermore, concordance and discordance between different channels of brain is the 5xP model after mediation with LM or any selected mediated factor, upon the specialists/physician’s recommendation for further planned clinical management(s).
Application of CNCs and correlated Ratio based between brain channels by providing the 5xP personalized clinical management model for early detection and therapy of the patients with AD and their targeted/predisposed relatives. Highlights offer an early detective platform by considering neuro-genetics/cell biology, CNCs; molecular investigation, for the predisposed individuals to AD- or other neural disease. The initiation and functional stage of triangle PE including neural-marker, stem cell (CD133), and VEGF is unmasked not only at the embryonic phase, but through the beginning of chorionic villus period around 8th-10th of gestational weeks. The mediation with LM resulted to remarkable escalation of the power of alpha band wave at frontal lobe including Fz, F4, FC2 sites, Cz in central lobe and O1 in occipital lobe and the increased activity of Alpha waves in Centro-frontal regions.
Circulating neural cells (CNCs) is an essential strategy which offers the pentagonal application including the Personalized, Prognostics, Predictive, Preventive, predisposing (5xP) panel in the clinical neurology and Neuro-Science. Therefore, it was aimed to explore the developing picture of CNCs’ behavior of the brain cells in the blood stream. CNCs are an available approach for the preventive Medicine and the personalized/target-based therapy.
This strategy includes and offers: (1) Routinizing the CNCs assay through the high and reliable enumeration of the CNCs; (2) providing the required information’s of CNCs to the neurological clinics for the patients affected with neurological disorders including Alzheimer disease (AD); (3) providing adequate Information on the application of CNCs for the protocol to the target clinical centers for either their referral patients affected with, or predisposed to AD as promptly as possible; (4) highlighting the safety, rapidness of the process, non-invasiveness of the CNCs exploration; and an early/preventive strategic detection of the AD in the probands and the relatives through their pedigree; and (5) considering the pedigree-based analysis for tracing of any micro- and macro-environmental predisposing factors, including nutrition and the history of the neurological based diseases including AD.
In the Alzheimer-prone families, the history of any related clinical sign of the neurological symptoms including light and/or progressive through different generations of the referral suspicious case, could be candidate for exploring CNCs. If there is any persuasive micro-and/or macro-environmental risks, the target of the proband’s offspring may be candidate for the CNCs.
The originality of the provided research is based on the Manuel performance and exploration of the single cell based analysis with high enumeration. This strategy is capable to unmask the heterogeneity, diversity and highlights the specific role of the target proteins, and identification of the novel involvement of additional protein through the developmental stages of AD. Furthermore, by respecting the bridging system between Medicine and Science, and according to the primary results of Electroencephalography, as a golden performance, an innovative, complementary and predictive model is provided in this paper. Finally, based on an early detection, and the developmental process through the passed progressive period of AD, the personalized detection, hopefully, leads to the personalized therapy.
The novel results of the provided manuscript are characterized with: Personalized data; Single cell based strategy; and translatable data for the index case and their relatives; an early detection of the protein expression (PE) and brain channels bridging systems, the personalized therapy, could be translated, either for Alzheimer or the related psychological related to the brain disorders. Hypothetic/heterogenic/diverse results highlight evolutionary course within the brain channels.
Unmasking the diverse pattern of PE of the migrated cells from brain to the blood stream, by an adequate enumeration of the CNCs based on the Manuel analytical approach. Unveil the heterogenic pattern of the analyzed target proteins’ expression. Multi-interaction between brain channels, are considered as the personalizd/constructive markers with unlimited cooperation in health and disease.
Unmasking the role of other candidate protein(s). Considering the informative publication(s) as the complementary educational package. Bridging between Science and Medicine by considering the pedigree based translational system for neuro-model, as an early detective platform. Suggesting for stablishing the Neuro-clinic with the aims including ‘Personalized-Predictive/Early detection/Preventive clinic for the families with ‘Alzheimer disease’ and in future with neuro-genetics disorders’.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Masaru T, Hungary S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH
| 1. | Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clin Anat. 1995;8:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 814] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 2. | Pastorcic M, Das HK. Regulation of transcription of the human presenilin-1 gene by Ets transcription factors and the p53 protooncogene. J Biol Chem. 2000;275:34938-34945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Zeng H, Sanes JR. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci. 2017;18:530-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 545] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 4. | Stam CJ. Use of magnetoencephalography (MEG) to study functional brain networks in neurodegenerative disorders. J Neurol Sci. 2010;289:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Fernández A, Zuluaga P, Abásolo D, Gómez C, Serra A, Méndez MA, Hornero R. Brain oscillatory complexity across the life span. Clin Neurophysiol. 2012;123:2154-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Porcaro C, Balsters JH, Mantini D, Robertson IH, Wenderoth N. P3b amplitude as a signature of cognitive decline in the older population: An EEG study enhanced by Functional Source Separation. Neuroimage. 2019;184:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Stam CJ, de Haan W, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer's disease. Brain. 2009;132:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 8. | Maestú F, Campo P, Del Río D, Moratti S, Gil-Gregorio P, Fernández A, Capilla A, Ortiz T. Increased biomagnetic activity in the ventral pathway in mild cognitive impairment. Clin Neurophysiol. 2008;119:1320-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | López-Sanz D, Bruña R, Garcés P, Martín-Buro MC, Walter S, Delgado ML, Montenegro M, López Higes R, Marcos A, Maestú F. Functional Connectivity Disruption in Subjective Cognitive Decline and Mild Cognitive Impairment: A Common Pattern of Alterations. Front Aging Neurosci. 2017;9:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Wolvetang EJ, Bradfield OM, Hatzistavrou T, Crack PJ, Busciglio J, Kola I, Hertzog PJ. Overexpression of the chromosome 21 transcription factor Ets2 induces neuronal apoptosis. Neurobiol Dis. 2003;14:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Mehdipour P, Javan J, Estiar MA, Khaleghian M, Novin N, Zaeim G, Kohan H, Akhondzadeh S. Protein Expression of ETS2 in Alzheimer’s Disease and Down’s syndrome as a Personalized Insight: One Gene, One Feature in Common, Diverse-Function-and Diseases. SRL Neuro Neurosur. 2017;3:001-007. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mehdipour P, Javan F, Jouibari MF, Khaleghi M, Mehrazin M. Evolutionary model of brain tumor circulating cells: Cellular galaxy. World J Clin Oncol. 2021;12:13-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Mehdipour P, Javan F, Faghih-Jouibari M, Khaleghi M. Diversity in tumor territory of meningioma: Protein expression in vascular endothelial growth factor and epidermal growth factor. Iran J Neurol. 2019;18:43-49. [PubMed] |
| 14. | Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2363] [Cited by in RCA: 2541] [Article Influence: 195.5] [Reference Citation Analysis (0)] |
| 15. | Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1012] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 16. | Jelski W, Mroczko B. Molecular and Circulating Biomarkers of Brain Tumors. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O'Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR Jr; Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer's Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1310] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 18. | World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer's disease International. 2015. Available from: https://www.alzint.org/resource/world-alzheimer-report-2015/. |
| 19. | Dauphinot L, Lyle R, Rivals I, Dang MT, Moldrich RX, Golfier G, Ettwiller L, Toyama K, Rossier J, Personnaz L, Antonarakis SE, Epstein CJ, Sinet PM, Potier MC. The cerebellar transcriptome during postnatal development of the Ts1Cje mouse, a segmental trisomy model for Down syndrome. Hum Mol Genet. 2005;14:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Lyle R, Gehrig C, Neergaard-Henrichsen C, Deutsch S, Antonarakis SE. Gene expression from the aneuploid chromosome in a trisomy mouse model of down syndrome. Genome Res. 2004;14:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Saran NG, Pletcher MT, Natale JE, Cheng Y, Reeves RH. Global disruption of the cerebellar transcriptome in a Down syndrome mouse model. Hum Mol Genet. 2003;12:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Rachidi M, Lopes C. Mental retardation in Down syndrome: from gene dosage imbalance to molecular and cellular mechanisms. Neurosci Res. 2007;59:349-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Cimenser A, Hempel E, Travers T, Strozewski N, Martin K, Malchano Z, Hajós M. Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer's Disease Patients. Front Syst Neurosci. 2021;15:746859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Damasio AR. Descartes' error and the future of human life. Sci Am. 1994;271:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 137] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc. 2006;12:224-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2517] [Cited by in RCA: 2633] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 28. | Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1135] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 29. | Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7688] [Cited by in RCA: 7909] [Article Influence: 329.5] [Reference Citation Analysis (0)] |
| 30. | Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1166] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 31. | Jones DT, Graff-Radford J. Executive Dysfunction and the Prefrontal Cortex. Continuum (Minneap Minn). 2021;27:1586-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | Asadzadeh S, Yousefi Rezaii T, Beheshti S, Delpak A, Meshgini S. A systematic review of EEG source localization techniques and their applications on diagnosis of brain abnormalities. J Neurosci Methods. 2020;339:108740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 33. | Ishii R, Canuet L, Aoki Y, Hata M, Iwase M, Ikeda S, Nishida K, Ikeda M. Healthy and Pathological Brain Aging: From the Perspective of Oscillations, Functional Connectivity, and Signal Complexity. Neuropsychobiology. 2017;75:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 34. | Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Galderisi S, Hirata K, Lanuzza B, Miniussi C, Mucci A, Nobili F, Rodriguez G, Luca Romani G, Rossini PM. Sources of cortical rhythms in adults during physiological aging: a multicentric EEG study. Hum Brain Mapp. 2006;27:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 35. | Kowalski JW, Gawel M, Pfeffer A, Barcikowska M. The diagnostic value of EEG in Alzheimer disease: correlation with the severity of mental impairment. J Clin Neurophysiol. 2001;18:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | van der Hiele K, Vein AA, Reijntjes RH, Westendorp RG, Bollen EL, van Buchem MA, van Dijk JG, Middelkoop HA. EEG correlates in the spectrum of cognitive decline. Clin Neurophysiol. 2007;118:1931-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Lizio R, Del Percio C, Marzano N, Soricelli A, Yener GG, Başar E, Mundi C, De Rosa S, Triggiani AI, Ferri R, Arnaldi D, Nobili FM, Cordone S, Lopez S, Carducci F, Santi G, Gesualdo L, Rossini PM, Cavedo E, Mauri M, Frisoni GB, Babiloni C. Neurophysiological assessment of Alzheimer's disease individuals by a single electroencephalographic marker. J Alzheimers Dis. 2016;49:159-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Prichep LS, John ER, Ferris SH, Reisberg B, Almas M, Alper K, Cancro R. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging. 1994;15:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol. 2004;115:1490-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 804] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 40. | Lejko N, Larabi DI, Herrmann CS, Aleman A, Ćurčić-Blake B. Alpha Power and Functional Connectivity in Cognitive Decline: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2020;78:1047-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Pikovsky A, Rosenblum M, Kurths J. Synchronization: a universal concept in nonlinear science. American Association of Physics Teachers; 2002. Available from: assEts.cambridge.org/97805215/92857/frontmatter/9780521592857_frontmatter.pdf. |
| 42. | Wang R, Wang J, Yu H, Wei X, Yang C, Deng B. Power spectral density and coherence analysis of Alzheimer's EEG. Cogn Neurodyn. 2015;9:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Handayani S, Diansari Y, Bahar E, Novantina W. EEG changes in patients with intracranial tumors and seizures symptom at Mohammad Hoesin Hospital Palembang. J Phys Conf Ser. 2019;1246:012014. [DOI] [Full Text] |
| 44. | Monllor P, Cervera-Ferri A, Lloret MA, Esteve D, Lopez B, Leon JL, Lloret A. Electroencephalography as a Non-Invasive Biomarker of Alzheimer's Disease: A Forgotten Candidate to Substitute CSF Molecules? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Locatelli T, Cursi M, Liberati D, Franceschi M, Comi G. EEG coherence in Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1998;106:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Tanaka M, Vécsei L. Editorial of Special Issue 'Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection'. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |