Published online Dec 20, 2011. doi: 10.5493/wjem.v1.i1.10
Revised: December 12, 2011
Accepted: December 16, 2011
Published online: December 20, 2011
More than two decades have passed since the first gene therapy clinical trial was conducted. During this time, we have gained much knowledge regarding gene therapy in general, but also learned to understand the fear that persists in society. We have experienced drawbacks and successes. More than 1700 clinical trials have been conducted where gene therapy is used as a means for therapy. In the very first trial, patients with advanced melanoma were treated with tumor infiltrating lymphocytes genetically modified ex-vivo to express tumor necrosis factor. Around the same time the first gene therapy trial was conducted, the ethical aspects of performing gene therapy on humans was intensively discussed. What are the risks involved with gene therapy? Can we control the technology? What is ethically acceptable and what are the indications gene therapy can be used for? Initially, gene therapy was thought to be implemented mainly for the treatment of monogenetic diseases, such as adenosine deaminase deficiency. However, other therapeutic areas have become of interest and currently cancer is the most studied therapeutic area for gene therapy based medicines. In this review I will be giving a short introduction into gene therapy and will direct the discussion to where we should go from here. Furthermore, I will focus on the use of the Herpes simplex virus-thymidine kinase for gene therapy of malignant gliomas and highlight the efficacy of gene therapy for the treatment of malignant gliomas, but other strategies will also be mentioned.
- Citation: Wirth T. A short perspective on gene therapy: Clinical experience on gene therapy of gliomablastoma multiforme. World J Exp Med 2011; 1(1): 10-16
- URL: https://www.wjgnet.com/2220-315X/full/v1/i1/10.htm
- DOI: https://dx.doi.org/10.5493/wjem.v1.i1.10
Gene therapy has experienced several ups and downs during the last few decades. The last one was particularly troublesome as many promising therapies have failed in clinical trials. However, with the increasing knowledge of epigenetics and their impact in many diseases, such as cancer and atherosclerosis, the potential of gene therapy has regained attention. Also, with the introduction of safer and more specific gene transfer vectors, the fear of gene therapy has been released, at least to some extent. According to the European Medicines Agency (EMA), a gene therapy medicinal product means “a biological medicinal product that contains an active substance which contains or consists of a recombinant nucleic acid used in or administered to human beings with a view of regulating, replacing, adding or deleting a genetic sequence, as well as if its therapeutic, prophylactic or diagnostic effect relates to the recombinant nucleic acid sequence it contains, or to the products of genetic expression of this sequence”. Gene therapy can be categorized into (1) germ line gene therapy and (2) somatic gene therapy. In somatic gene therapy, the genetic material is not passed along to the next generation, whereas in germ line gene therapy it is. This difference is of importance as to date, gene therapy is only allowed on somatic cells. Gene therapy to somatic cells is prohibited.
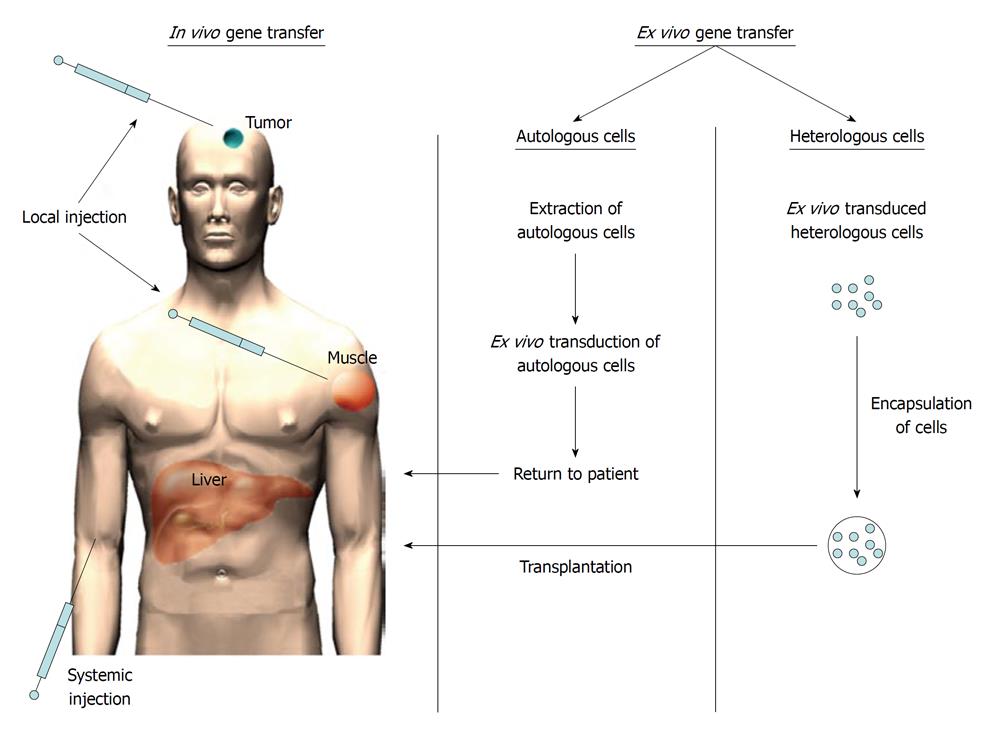
There are several strategies how foreign genetic material (i.e. a transgene) can be introduced to the patient. Firstly, the transgene can be introduced directly to patients (i.e. in vivo gene therapy) (Figure 1)[1-3] or alternatively, the genetic material can be introduced ex vivo[4-6]. In this case, either autologous or heterologous cells are transduced outside the patient (i.e. ex vivo) and then administered to the patient (Figure 1). When heterologous cells are used, the cells need to be protected from the recipient’s immune system. This might be achieved by cell encapsulation of transduced cells into microparticles[7-9].
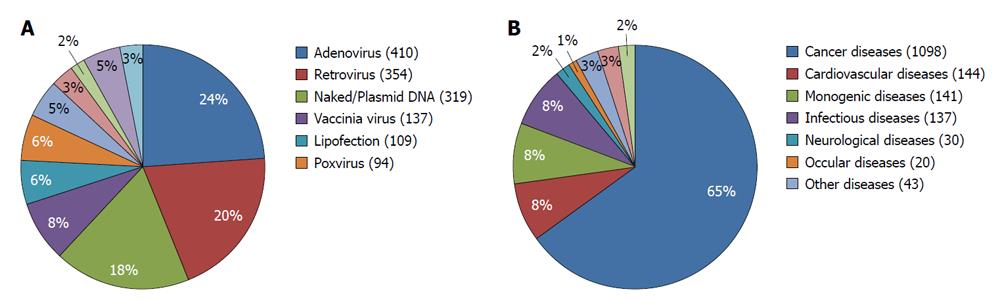
Different gene delivery methods have been used for introducing the genetic material into the cells. These methods can be categorized into physical, viral and non-viral methods. Examples of physical methods are electroporation, ultrasound and gene gun methods. In case of viral or non-viral gene delivery, a biological (a virus) or a synthetic (liposomes or nanoparticles) are used as gene carriers to deliver the genetic material into the cells. Of the viral vectors, adenoviruses are currently the most dominant gene delivery vectors used in gene therapy, followed by retroviral vectors (including lentiviral vectors) and plasmid DNA. Adeno associated viral vectors have also gained momentum recently and represent an interesting alternative to retroviral vectors. Other gene transfer vectors that are more or less commonly used are vaccinia viruses and poxviruses (Figure 2A). The use of bacteria as gene transfer vectors is not new but only recently has gained more attention.
CURRENT STATUS
So far, neither the Food and Drug Administration nor the EMA has approved any human gene therapy product for commercial use. Initially, the main targets for gene therapy were monogenic disorders, such as adenosine deaminase deficiency and adrenoleukodystrophy. Even though drawbacks have been reported, which is exemplified by the work of Hacei-Bey-Abina for example, there have also been successes[5,10-13]. Eventually gene therapy has shifted from these monogenetic diseases into other disease areas such as cancer and cardiovascular diseases[1,14-19]. Currently, cancer is the most common disease area that gene therapy is applied to. More than 60% of all ongoing gene therapy clinical trials are developed for the treatment of cancer (Figure 2B). Many cancer types have been targeted with gene therapy including tumors of the brain, lung, breast, pancreatic, liver, colorectal, prostate, bladder, head and neck, ovarian and renal cancer. The first clinical trial on cancer started in 1990, where patients with advanced melanoma were treated with tumor infiltrating lymphocytes genetically modified ex-vivo to express tumor necrosis factor[20].
In October 2003, China became the first country to approve the commercial production of a gene therapy product[21-23]. Shenzhen SiBiono GenTech (Shenzhen, China) obtained a drug license from the State Food and Drug Administration of China for its recombinant adenovirus-p53 gene therapy (Gendicine). Gendicine was approved for the treatment of head and neck squamous cell carcinoma (HNSCC). Two years later, in 2005, the conditionally replicating adenovirus H-101 (i.e. an adenovirus wherein the E1B-55 kDa gene has been deleted, allowing the virus to selectively replicate in and lyse p53-deficient cancer cells) gained marketing approval for HNSCC[24]. More recently, Rexin-G, a pathotropic targeted retroviral vector designed to interfere with cyclin GI gene by integrating into the host DNA, has recently been approved in the Philippines for the treatment of all solid tumors that are refractory to standard chemotherapy[25]. In addition, it is currently in clinical trials in the U.S. and has been granted Orphan Drug Status by the FDA for three cancer indications: (1) pancreatic cancer; (2) soft tissue sarcoma; and (3) osteosarcoma[25-30].
GENE THERAPY FOR MALIGNANT GLIOMAS
Brain tumors bear several features that make them amenable to gene therapy, particularly for suicide gene therapy. First of all, brain tumors are, in most cases, single, localized lesions of dividing cells in a background of non-dividing cells. Secondly, primary brain tumors rarely metastasize outside the central nervous system and if recurrence occurs, it typically happens in the close vicinity of the original lesion[31-33]. Different approaches have been utilized for the treatment of malignant gliomas using different gene transfer vectors. Among these, pro-drug activation/suicide gene therapy, anti-angiogenic gene therapy, oncolytic virotherapy and immune modulation are the most commonly used strategies[1,2,34-44]. The very first clinical gene therapy trial against brain cancer was registered in 1992[45]. In that trial, autologous tumor cells were modified ex vivo with retrovirus to express interleukin-2 gene in neuroblastoma. In the following year, brain cancer patients were treated with herpes simplex virus thymidine kinase suicide gene therapy using retrovirus vectors producing cells and concomitant administration of ganciclovir. However, transduction efficiency was a major problem in these trials, resulting in poor therapeutic efficacy. In 1996, Eck et al[46] published the first phase I clinical trial where adenovirus Herpes simplex virus-thymidine kinase was used with intention in patients with recurrent gliomas. In 2000, Sandmair et al[34] published a study wherein 21 patients were enrolled to compare the efficacy of retrovirus-packaging cells to adenovirus mediated gene transfer. In this study, the therapeutic efficacy of the Herpes simplex virus-thymidine kinase, using these two approaches, was compared in context of the treatment of primary or recurrent gliomas. The mean survival time in the adenovirus Herpes simplex virus-thymidine kinase group was 15 mo and significantly longer, when compared to the survival time of the retrovirus-packaging-cells group, which was 7.4 mo. The control group, which received adenovirus LacZ had a mean survival time of 8.3 mo. Although the retrovirus-packaging-cells approaches were found safe, no efficacy was observed in malignant glioma patients. The low gene transfer efficacy with retrovirus and the lack of the treatment response indicated that retroviral Herpes simplex virus-thymidine kinase gene therapy may not be efficient enough in human clinical settings. This was further confirmed by the results from the first randomized, open-label, parallel group phase III clinical trial of 248 patients, where Herpes simplex virus-thymidine kinase produced by retroviral producing cells did not result in an improvement of survival[2]. Some years later, in 2003, a phase I clinical trial described the use of an adenoviral vector encoding for the tumor suppressor gene TP53 for the treatment of patients with recurrent malignant gliomas[47]. In that study, 15 patients underwent intratumoral stereotactic injection of the adenoviral vector via an implanted catheter, followed by en bloc resection of the tumor and treatment of the post-resection cavity. Due to the design of the study, tumor response could not be assessed but it proved to be safe, demonstrating minimal toxicity. No systemic viral dissemination was observed and a maximum tolerated dose was not reached in this study. Analysis of tumor specimens demonstrated restricted transgene expression close to the injection site. Chiocca et al[38] published a phase I dose-escalation trial of the oncolytic adenovirus ONYX-015, which preferentially replicates and thereby lyses p53-deficient cells (a common feature in tumor cells). In that trial, 24 patients with recurrent malignant glioma were injected with ONYX-015 with doses ranging from 107 to 1010 pfu (plaque forming units) in a total of 10 injections into 10 different sites of the cavity of resected tumors. None of the patients experienced serious adverse events related to the virus. However, in that trial, the maximum tolerated dose was not reached. All patients showed tumor progression with a median time of 46 d and a median survival time of 6.2 mo. One patient with anaplastic astrocytoma had stable disease and two patients who underwent a second resection had lymphocytic and plasmacytoid cell infiltration at the site of injection. Nevertheless, despite a good safety profile, the overall therapeutic efficacy was poor. In another study performed by Chiocca et al[48], 11 patients were injected with different doses of interferon-β-expressing adenoviruses ranging from 2 × 1010 to 2 × 1011 viral particles stereotactically into the tumor. This was followed by surgical removal of the tumor 4-8 d later with additional injections of the adenovirus into the tumor bed. Generally, the treatment was well tolerated with only one patient experiencing a dose-limiting side effect after post-operative injection with the highest dose. However, all patients had disease progression and/or recurrence within 4 mo after the treatment. The median time to tumor progression was 9.3 wk and the median overall survival was 17.9 wk.
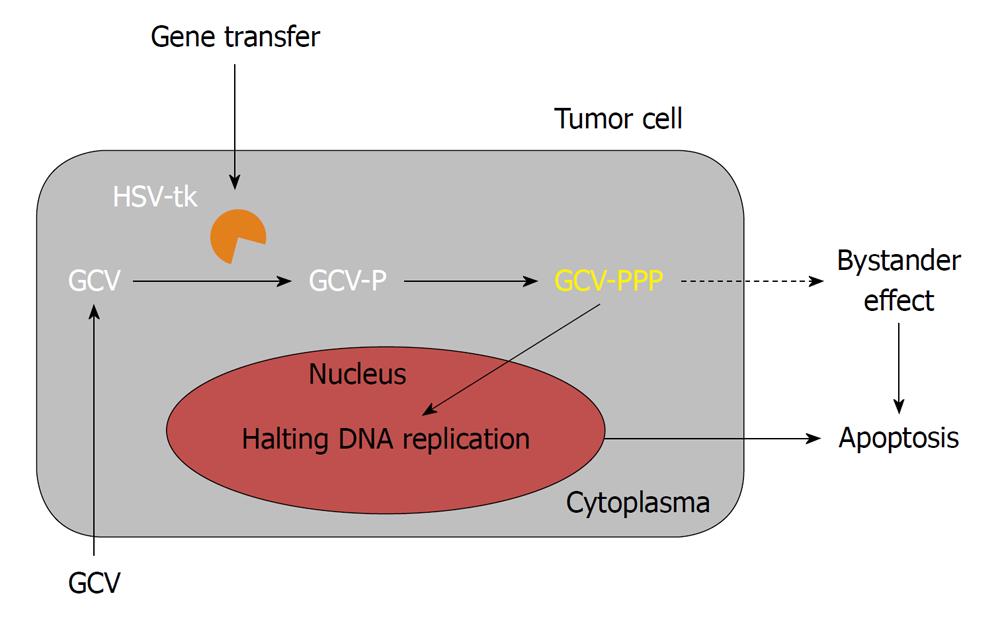
The clinical efficacy of sitimagene ceradenovec was evaluated first in two separate phase II clinical trials; a phase IIa trial and a phase IIb trial[1,34,49]. Sitimagene ceradenovec is an adenoviral vector encoding for the Herpes simplex virus-thymidine kinase, which is injected into the tumor cavity of resected gliomas, followed by the administration of the pro-drug ganciclovir (Figure 3). In the randomized and controlled phase IIb trial published by Immonen, carried out in 36 patients, seventeen patients with operable or recurrent malignant gliomas receiving sitimagene ceradenovec implicated a survival advantage over control patients who did not receive gene therapy. The mean survival of the patients in the sitimagene ceradenovec group (70.6 wk) was significantly longer (P < 0.0095) when compared to the standard care group (39.0 wk) or a historical control group (P < 0.0017). This study was also historically the first randomized, controlled trial with sitimagene ceradenovec where increased survival of the patients was shown when compared to standard therapy. The results from the study were very encouraging and it was concluded that sitimagene ceradenovec could provide an effective adjuvant treatment for patients with operable primary or recurrent malignant glioma. Therefore a multicenter, standard care controlled, randomized clinical phase III trial was commenced. However, the results from that trial were not as significant as those from the previous IIb trial. As a result, suggestions by the EMA were given for further clinical evaluation as they concluded that the data did not provide sufficient evidence of significant clinical benefit compared to current standard treatment.
IMPROVING GENE DELIVERY
One of the major hurdles has been how to get the relevant genetic material into a sufficient number of target cells and how to avoid the transduction of non-target cells (i.e. how to target the gene transfer vector to cells of interest). Obviously, as gene therapy has matured from clinical trials to the first commercial products, understanding of the mechanisms of gene delivery has increased notably. This is also reflected in the progress we have made in the development of viral vectors[50-53]. A number of improvements have been achieved in order to tackle issues of transduction efficiency, biodistribution and safety[51,52,54,55]. Ideally, a gene transfer vector should be able to efficiently and specifically transduce the target cells (dividing and non-dividing) and result in the expression of the transgene for a sufficient duration of time. The vector should not have any limitations in the transgene insertion capacity and it should be able to be manufactured easily and cost effectively in high concentrations. Furthermore, the vector should not induce any immune responses within the host, enabling repeated, safe vector administrations without adverse effects. In order to fulfill these criteria, different strategies have been exploited. However, none of the strategies are without limitations. For example, to improve gene transfer efficiency, specificity and thereby patient safety, target cells may be removed from the patient, transduced with viral vectors and re-introduced back into the patient[5,56,57]. This approach has been shown to be effective, but is limited to the cells which are available (i.e. either by extraction or by growing from the stem cells in vitro)[58]. When the vector is delivered directly to the patient (in vivo), either locally (for example intratumoral) or systemically (i.e. into the blood circulation), a limiting factor can be the size and shape of the gene transfer vector, resulting in poor distribution within the tissue (when administered locally into the tissue) or the lack of specificity when administered systemically. To improve specificity and hence also safety of systemically administered gene delivery vectors, the surface of these vectors has been modified[52,59-62]. For example, retroviruses and lentiviruses have been frequently pseudotyped to widen their tropism, increase their yield in production and improve their safety, most often with the Vesicular Stomatitis virus G-protein[63-65].
CONCLUSION
Gene therapy is an intriguing therapeutic modality and sooner or later will be part of the standard care for a variety of different diseases. However, at the same time, when the first patients were treated utilizing gene therapy based technology, debates about the ethical aspects of gene therapy started[66-69]. It is important that we acknowledge and understand the differences in human beings and in what they believe in. Obviously, there are concerns when it comes to the use of gene therapy. We have to ask ourselves several questions before we can justify gene therapy in humans: What is our current understanding regarding gene therapy? For example, what are the technical details of the DNA and vector to be used? The technical aspects involved, risks endeavored by the patient, and the fear of human genetic engineering are some of the major reasons why human gene therapy trials have long been difficult to conduct. Are we able to control this technology? In which diseases is gene therapy is ethically acceptable and what are the costs for this type of therapy? Why is gene therapy more tolerated for life-threatening diseases (e.g. diseases like cancer or AIDS) than in the correction of learning disorders? Also, somatic gene therapy appears to be more tolerated than germline gene therapy. Where do we draw a line when dealing with genetic or chromosomal disorders? Would it be ethically acceptable to practice gene therapy on people with Dawn syndrome? What would be the justification of using gene therapy in the enhancement of some individual physical or mental properties? The use of viral vectors, such as lentiviruses and adeno-associated viruses, raises skepticism because of their ability to integrate into the genome[11,65,70,71]. Understandably, this raises concerns about the safety of these vectors. This is furthermore supported by the somewhat contradictory data available in the literature[72,73]. Non-viral vectors are not efficient enough yet but have gained better acceptance in the society. Despite the progress in gene therapy research, we are still at the beginning of the era of gene therapy based medicines. Emphasis should be put on the development of targeted and regulated gene transfer vectors. Especially in case of integrating vectors (such as retroviral vectors), emphasis should be laid on the development of vectors, where the integration of the transgene can be controlled, in order to avoid insertional mutagenesis. In either case, it is of utmost importance that the normal principles of good clinical research apply in the conduct of the ethical evaluation of gene therapy protocols. The safety of an individual must be the first concern of the treatment protocols and, last but not least, the integrity and free will of a patient should be respected.
Peer reviewers: Magali Cucchiarini, PhD, Assistant Professor, Molecular Biology, Experimental Orthopaedics and OA Research, Saarland University Medical Center, Kirrbergerstr. Bldg 37, D-66421 Homburg, Germany; Umberto Galderisi, PhD, Associate Professor of Molecular Biology, Department Experimental Medicine, Second University of Naples, Via L. De Crecchio 7, 80138 Napoli, Italy
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Immonen A, Vapalahti M, Tyynelä K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Ylä-Herttuala S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 479] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Candolfi M, Kroeger KM, Pluhar GE, Bergeron J, Puntel M, Curtin JF, McNiel EA, Freese AB, Ohlfest JR, Moore P. Adenoviral-mediated gene transfer into the canine brain in vivo. Neurosurgery. 2007;60:167-77; discussion 178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Naldini L. Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet. 2011;12:301-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1112] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 6. | Ngo MC, Rooney CM, Howard JM, Heslop HE. Ex vivo gene transfer for improved adoptive immunotherapy of cancer. Hum Mol Genet. 2011;20:R93-R99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Lim GJ, Zare S, Van Dyke M, Atala A. Cell microencapsulation. Adv Exp Med Biol. 2010;670:126-136. [PubMed] |
| 8. | Santos E, Zarate J, Orive G, Hernández RM, Pedraz JL. Biomaterials in cell microencapsulation. Adv Exp Med Biol. 2010;670:5-21. [PubMed] |
| 9. | Wikström J, Elomaa M, Syväjärvi H, Kuokkanen J, Yliperttula M, Honkakoski P, Urtti A. Alginate-based microencapsulation of retinal pigment epithelial cell line for cell therapy. Biomaterials. 2008;29:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2671] [Cited by in RCA: 2619] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 11. | Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1291] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 12. | Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669-672. [PubMed] |
| 13. | Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 14. | Hedman M, Muona K, Hedman A, Kivelä A, Syvänne M, Eränen J, Rantala A, Stjernvall J, Nieminen MS, Hartikainen J. Eight-year safety follow-up of coronary artery disease patients after local intracoronary VEGF gene transfer. Gene Ther. 2009;16:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Hedman M, Hartikainen J, Syvänne M, Stjernvall J, Hedman A, Kivelä A, Vanninen E, Mussalo H, Kauppila E, Simula S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation. 2003;107:2677-2683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 16. | Kastrup J, Jørgensen E, Fuchs S, Nikol S, Bøtker HE, Gyöngyösi M, Glogar D, Kornowski R. A randomised, double-blind, placebo-controlled, multicentre study of the safety and efficacy of BIOBYPASS (AdGVVEGF121.10NH) gene therapy in patients with refractory advanced coronary artery disease: the NOVA trial. EuroIntervention. 2011;6:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Taniyama Y, Sawa Y, Kaneda Y, Ogihara T. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011;31:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Kalil RA, Salles FB, Giusti II, Rodrigues CG, Han SW, Sant'Anna RT, Ludwig E, Grossman G, Prates PR, Sant'anna JR. VEGF gene therapy for angiogenesis in refractory angina: phase I/II clinical trial. Rev Bras Cir Cardiovasc. 2010;25:311-321. [PubMed] |
| 19. | Horowitz JD, Rosenson RS, McMurray JJ, Marx N, Remme WJ. Clinical Trials Update AHA Congress 2010. Cardiovasc Drugs Ther. 2011;25:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 815] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 21. | Pearson S, Jia H, Kandachi K. China approves first gene therapy. Nat Biotechnol. 2004;22:3-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Wilson JM. Gendicine: the first commercial gene therapy product. Hum Gene Ther. 2005;16:1014-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Räty JK, Pikkarainen JT, Wirth T, Ylä-Herttuala S. Gene therapy: the first approved gene-based medicines, molecular mechanisms and clinical indications. Curr Mol Pharmacol. 2008;1:13-23. |
| 24. | Shi J, Zheng D. An update on gene therapy in China. Curr Opin Mol Ther. 2009;11:547-553. [PubMed] |
| 25. | Hall FL, Levy JP, Reed RA, Petchpud WN, Chua VS, Chawla SP, Gordon EM. Pathotropic targeting advances clinical oncology: tumor-targeted localization of therapeutic gene delivery. Oncol Rep. 2010;24:829-833. [PubMed] |
| 26. | Subbiah V, Kurzrock R. Phase 1 clinical trials for sarcomas: the cutting edge. Curr Opin Oncol. 2011;23:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Chawla SP, Chua VS, Fernandez L, Quon D, Blackwelder WC, Gordon EM, Hall FL. Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol Ther. 2010;18:435-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer. Expert Opin Biol Ther. 2010;10:819-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Chawla SP, Chua VS, Fernandez L, Quon D, Saralou A, Blackwelder WC, Hall FL, Gordon EM. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol Ther. 2009;17:1651-1657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Galanis E, Carlson SK, Foster NR, Lowe V, Quevedo F, McWilliams RR, Grothey A, Jatoi A, Alberts SR, Rubin J. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16:979-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 33. | Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 34. | Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, Hurskainen H, Tyynelä K, Turunen M, Vanninen R. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, Hamilton WJ, Rojas-Martinez A, Chen SH, Woo SL. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Aghi MK, Chiocca EA. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol Ther. 2009;17:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, Rozenman-Yair S, Panet A, Libson E, Irving CS. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 38. | Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Kanai R, Tomita H, Shinoda A, Takahashi M, Goldman S, Okano H, Kawase T, Yazaki T. Enhanced therapeutic efficacy of G207 for the treatment of glioma through Musashi1 promoter retargeting of gamma34.5-mediated virulence. Gene Ther. 2006;13:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Myers R, Harvey M, Kaufmann TJ, Greiner SM, Krempski JW, Raffel C, Shelton SE, Soeffker D, Zollman P, Federspiel MJ. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19:690-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 42. | Xiong W, Candolfi M, Liu C, Muhammad AK, Yagiz K, Puntel M, Moore PF, Avalos J, Young JD, Khan D. Human Flt3L generates dendritic cells from canine peripheral blood precursors: implications for a dog glioma clinical trial. PLoS One. 2010;5:e11074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, Hashimoto N, Maruno M, Elisseeva OA, Shirakata T. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Phase I study of cytokine-gene modified autologous neuroblastoma cells for treatment of relapsed/refractory neuroblastoma. Hum Gene Ther. 1992;3:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Eck SL, Alavi JB, Alavi A, Davis A, Hackney D, Judy K, Mollman J, Phillips PC, Wheeldon EB, Wilson JM. Treatment of advanced CNS malignancies with the recombinant adenovirus H5.010RSVTK: a phase I trial. Hum Gene Ther. 1996;7:1465-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508-2518. [PubMed] |
| 48. | Chiocca EA, Smith KM, McKinney B, Palmer CA, Rosenfeld S, Lillehei K, Hamilton A, DeMasters BK, Judy K, Kirn D. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | van Putten EH, Dirven CM, van den Bent MJ, Lamfers ML. Sitimagene ceradenovec: a gene-based drug for the treatment of operable high-grade glioma. Future Oncol. 2010;6:1691-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Thaci B, Ulasov IV, Wainwright DA, Lesniak MS. The challenge for gene therapy: innate immune response to adenoviruses. Oncotarget. 2011;2:113-121. [PubMed] |
| 51. | Wang J, Faust SM, Rabinowitz JE. The next step in gene delivery: molecular engineering of adeno-associated virus serotypes. J Mol Cell Cardiol. 2011;50:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, Baker AH. Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses. 2010;2:2290-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Räty JK, Lesch HP, Wirth T, Ylä-Herttuala S. Improving safety of gene therapy. Curr Drug Saf. 2008;3:46-53. [PubMed] |
| 54. | Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Lesch HP, Pikkarainen JT, Kaikkonen MU, Taavitsainen M, Samaranayake H, Lehtolainen-Dalkilic P, Vuorio T, Määttä AM, Wirth T, Airenne KJ. Avidin fusion protein-expressing lentiviral vector for targeted drug delivery. Hum Gene Ther. 2009;20:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Nguyen TH, Mainot S, Lainas P, Groyer-Picard MT, Franco D, Dagher I, Weber A. Ex vivo liver-directed gene therapy for the treatment of metabolic diseases: advances in hepatocyte transplantation and retroviral vectors. Curr Gene Ther. 2009;9:136-149. [PubMed] |
| 57. | Wehling P, Reinecke J, Baltzer AW, Granrath M, Schulitz KP, Schultz C, Krauspe R, Whiteside TW, Elder E, Ghivizzani SC. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Hum Gene Ther. 2009;20:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Heyde M, Partridge KA, Oreffo RO, Howdle SM, Shakesheff KM, Garnett MC. Gene therapy used for tissue engineering applications. J Pharm Pharmacol. 2007;59:329-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Morizono K, Ku A, Xie Y, Harui A, Kung SK, Roth MD, Lee B, Chen IS. Redirecting lentiviral vectors pseudotyped with Sindbis virus-derived envelope proteins to DC-SIGN by modification of N-linked glycans of envelope proteins. J Virol. 2010;84:6923-6934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Markusic DM, van Til NP, Hiralall JK, Elferink RP, Seppen J. Reduction of liver macrophage transduction by pseudotyping lentiviral vectors with a fusion envelope from Autographa californica GP64 and Sendai virus F2 domain. BMC Biotechnol. 2009;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Funke S, Schneider IC, Glaser S, Mühlebach MD, Moritz T, Cattaneo R, Cichutek K, Buchholz CJ. Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther. 2009;16:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, Brouillet E, Hantraye P, Bonvento G, Déglon N. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57:667-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Mátrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 64. | Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387-398. [PubMed] |
| 65. | Dropulić B. Lentiviral vectors: their molecular design, safety, and use in laboratory and preclinical research. Hum Gene Ther. 2011;22:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Fletcher JC. Controversies in research ethics affecting the future of human gene therapy. Hum Gene Ther. 1990;1:307-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Fletcher JC. Evolution of ethical debate about human gene therapy. Hum Gene Ther. 1990;1:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Beutler E. The Cline affair. Mol Ther. 2001;4:396-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Deakin CT, Alexander IE, Kerridge I. The ethics of gene therapy: balancing the risks. Curr Opin Mol Ther. 2010;12:578-585. [PubMed] |
| 70. | Biffi A, Bartolomae CC, Cesana D, Cartier N, Aubourg P, Ranzani M, Cesani M, Benedicenti F, Plati T, Rubagotti E. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood. 2011;117:5332-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 71. | Deyle DR, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009;11:442-447. [PubMed] |
| 72. | Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 73. | Li H, Malani N, Hamilton SR, Schlachterman A, Bussadori G, Edmonson SE, Shah R, Arruda VR, Mingozzi F, Wright JF. Assessing the potential for AAV vector genotoxicity in a murine model. Blood. 2011;117:3311-3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |