Copyright
©The Author(s) 2015.
World J Exp Med. Nov 20, 2015; 5(4): 206-217
Published online Nov 20, 2015. doi: 10.5493/wjem.v5.i4.206
Published online Nov 20, 2015. doi: 10.5493/wjem.v5.i4.206
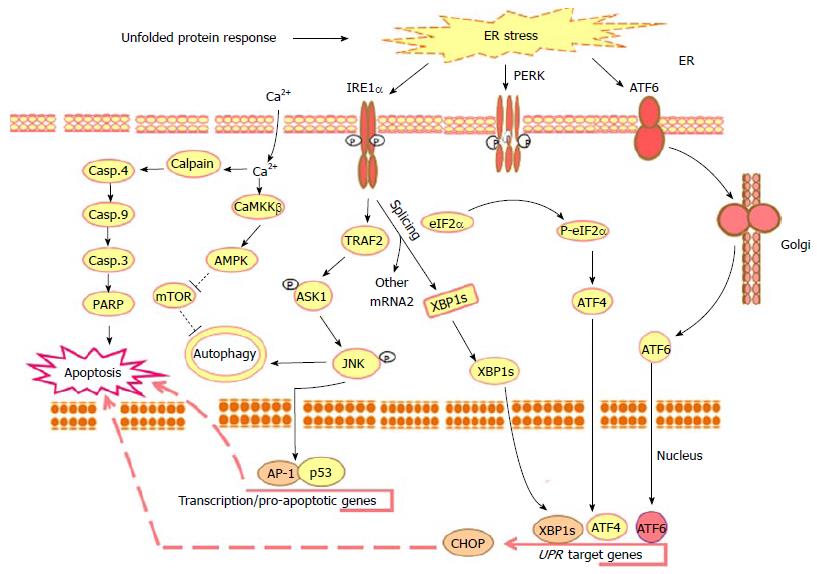
Figure 1 Outline of the main unfolded protein response-mediated mechanisms in response to the molecular action of anti-cancer agents.
Upon the accumulation of misfolded proteins in the ER lumen, chaperones, the ER stress sensors PERK, IRE1α and ATF6 become active. The phosphorylation of PERK allows it to assemble in a homo-dimer to form an active form that, in turn, results in the phosphorylation of eukaryotic initiation factor2α (eIF2α) to initiate UPR downstream response leading to reduction of the protein overload to ER by the suppression of the translation and activation of ATF4 together with ER stress associated transcription factors such as, CHOP, PERK, IRE1 and ATF6. Activated PERK phosphorylates the translation initiation factor eIF2α to decrease the protein synthesis and enhance stress-inducible messages, such as ATF4. During ER stress the ATF6 traffics to the Golgi apparatus, where it is cleaved by S1P/S2P proteases. The cleavage of ATF6 from the Golgi membrane facilitates its localization to the nucleus, where it enhances the transcriptional up-regulation of UPR target genes leading to apoptosis. Whereas, activated IRE1α is implicated into several functions: One of these functions is essential to drive the splice mechanism of the XBP-1 mRNA to allow the translation of mature XBP-1 protein that, in turn, functions as a transcription factor to promote the transcription of UPR target genes such as CHOP leading to apoptosis; the other function of the activated IRE1α is to recruit TRAF2 that subsequently mediates the phosphorylation of ASK1 and the activation of its downstream JNK leading to the activation of the transcription factors AP-1 and p53 that are essential for the transcription of the pro-apoptotic genes and genes implicated in the processes of autophagsome formation and autophagy. UPR results also in intracellular calcium release leading to cell death via Calpain/Caspase-4, Caspase-9, caspase-3 and PARP axis or autophagy via CaMKKβ/AMPK axis leading to the inhibition of mTOR and subsequently autophagy. ER: Endoplasmic reticulum; PERK: Protein kinase RNA-like endoplasmic reticulum kinase; IRE1α: Inositol-requiring protein 1; ATF6: Activating transcription factor 6; UPR: Unfolded protein response; XBP-1: X-box binding protein 1; CHOP: CCAAT/enhancer-binding protein (C/EBP) homologous protein; TRAF2: TNF receptor-associated factor 2; ASK1: Apoptosis signal regulating kinase 1; JNK: C-jun-N-terminal kinase; PARP: Poly (ADP-ribose) polymerase; CaMKKβ: Calmodulin-dependent protein kinase kinase-β; AMPK: AMP-activated protein kinase; mTOR: Mammalian target of rapamycin.
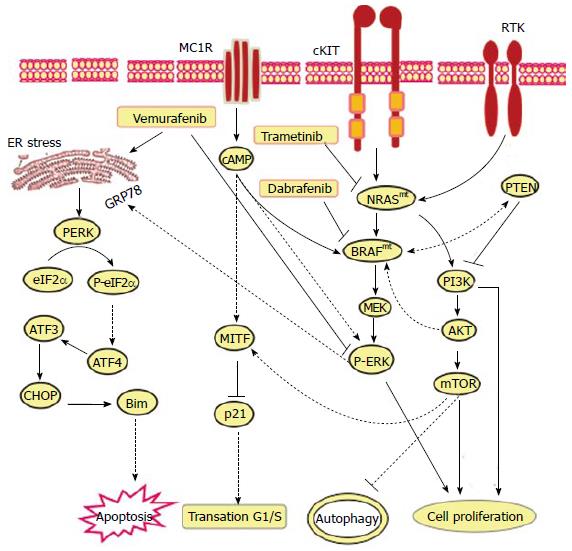
Figure 2 Proposed models for the mechanistic role of endoplasmic reticulum stress in the modulation of the anti-tumor efficiency of vemurafenib, dabrafenib and trametenib in melanoma patients harboring activating neuroblastoma RAS viral (v-ras) oncogene homolog or rapidly accelerated fibrosarcoma murine sarcoma viral (v-raf) oncogene homolog B mutations.
The main function of MC1R, TRK on melanoma cell is to transmit extracellular signaling that is essential for the activation of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways to mediate various cellular functions including melanoma initiation, progression and resistance. Thus, the exposure of melanoma (BRAFmt and NRASmt) cells to either Vemurafenib, Dabrafenib or Trametenib, respectively, results in inhibition of MC1R, cKIT and TRK-mediated activation of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways. As consequence the inhibition of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways results in the execution melanoma cell death via mechanism-mediated by ER stress-dependent pathways including PERK-eIF2α-ATF4-ATF3-CHOP-Bim pathway. NRAS: Neuroblastoma Rat Sarcoma viral (v-ras) oncogene homolog; BRAF: Rapidly Accelerated Fibrosarcoma murine sarcoma viral (v-raf) oncogene homolog B; MC1R: Melanocortin 1 receptor; TRK: CKIT and receptor tyrosine kinase; PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT: Protein kinase B.
- Citation: Hassan M, Selimovic D, Hannig M, Haikel Y, Brodell RT, Megahed M. Endoplasmic reticulum stress-mediated pathways to both apoptosis and autophagy: Significance for melanoma treatment. World J Exp Med 2015; 5(4): 206-217
- URL: https://www.wjgnet.com/2220-315X/full/v5/i4/206.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i4.206