Copyright
©The Author(s) 2023.
World J Exp Med. Sep 20, 2023; 13(4): 59-74
Published online Sep 20, 2023. doi: 10.5493/wjem.v13.i4.59
Published online Sep 20, 2023. doi: 10.5493/wjem.v13.i4.59
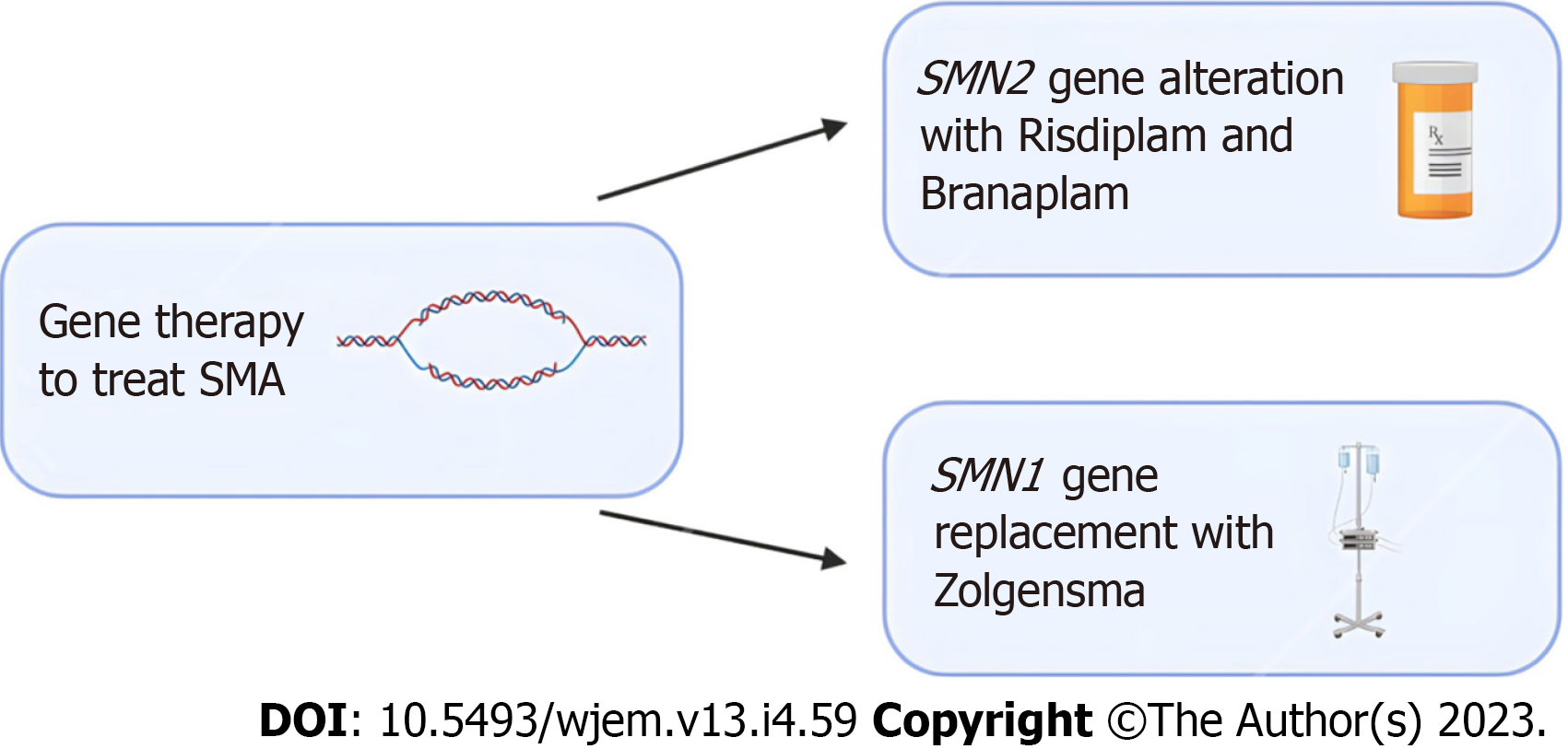
Figure 1 Emerging gene editing treatment options for spinal muscular atrophy.
Risdiplam and Branaplam are oral medications which can cross the blood-brain barrier and increase the number of spinal muscular atrophy (SMA) full length proteins by targeting the SMN2 gene. For patients that require replacement of the SMA1 gene, Zolgensma is an intravenous medication that uses an adeno-associated viral vector to deliver a functional copy of the gene. Clinical trials in patients treated with Zolgensma have shown positive outcomes. SMA: Spinal muscular atrophy.
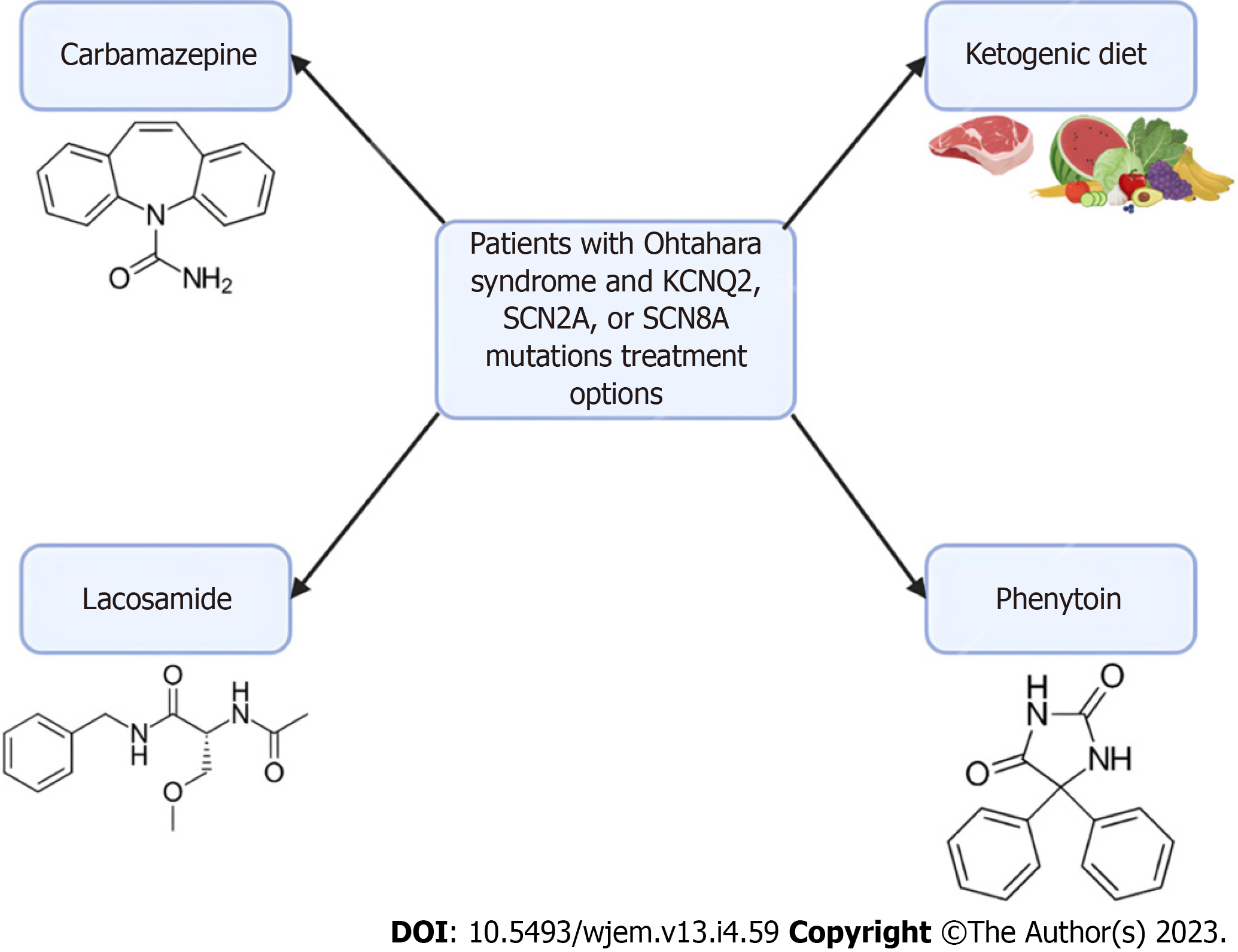
Figure 2 Treatment options for patients with Ohtahara syndrome.
In patients with Ohtahara Syndrome with KCNQ2, SCN2A, or SCN8A mutations, there are several treatment options available. Carbamazepine, lacosamide, and phenytoin are sodium channel anti-seizure medications. These medications don’t offer a cure and are currently only used to manage the frequency of seizures. Additionally, implementation of a ketogenic diet has been shown to reduce seizure frequency in infants
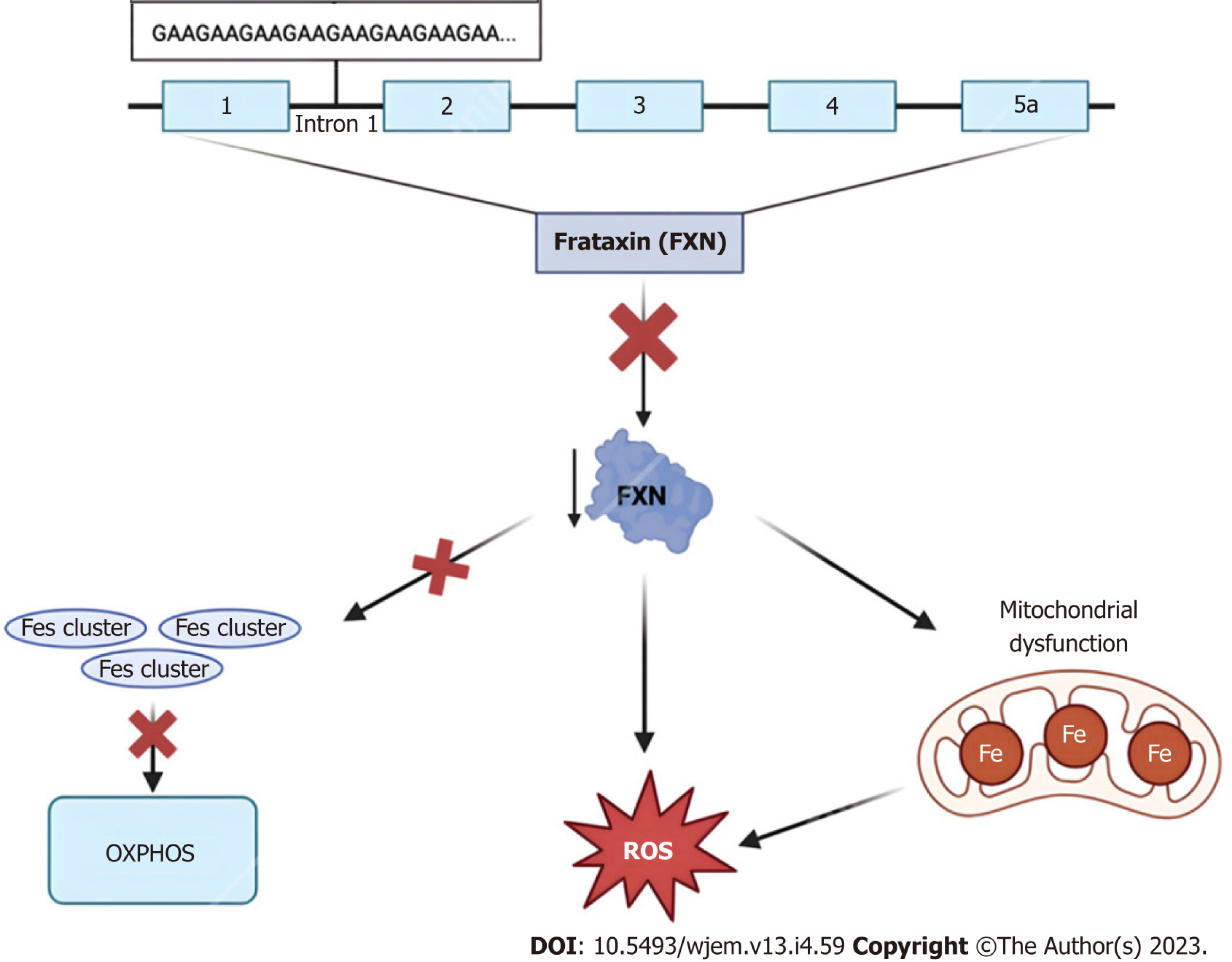
Figure 3 Frataxin protein dysregulation.
The 2100 amino acid protein frataxin is encoded within the first intron of the FXN gene on chromosome 9q13. Due to trinucleotide repeat expansions ranging from approximately 44–1700 “GAA” triplet sequences, affected individuals experience numerous characteristic signs and symptoms of Friedreich Ataxia. At the cellular level, dysregulation of this small mitochondrial protein results in the overproduction of iron-sulfur clusters, free radical oxidative stress, and mitochondrial iron overload[75]. FXN: Frataxin; ROS: Reactive oxygen species.
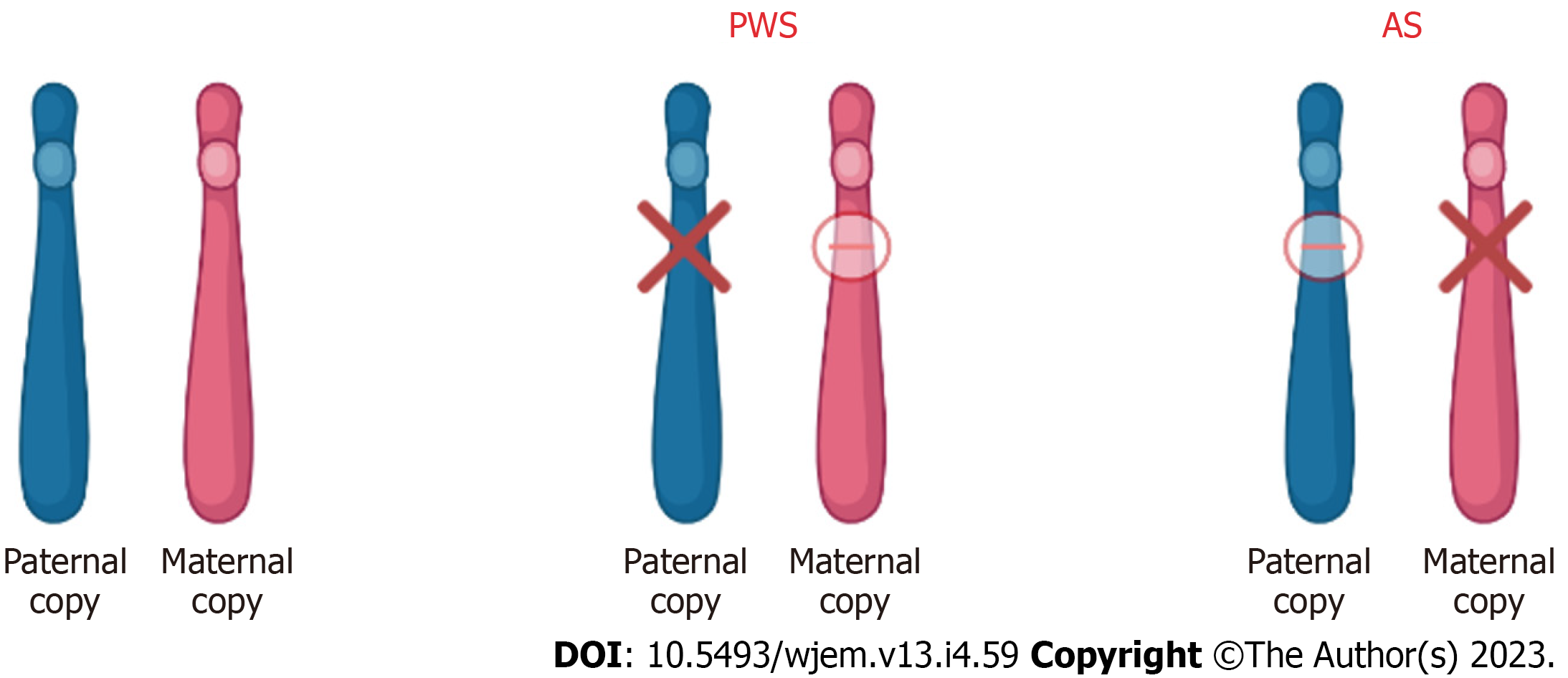
Figure 4 Chromosome 15 (15q11-13) abnormality in patients with Prader-Willi syndrome and Angelman syndrome.
In patients with Prader-Willi syndrome (PWS) and Angelman syndrome (AS), the imprinted gene abnormality is the 15q11-q13 region of chromosome 15. Although the abnormality is in the same region, the difference in disease and phenotype depends on the parental contribution. In PWS, the disease results due to loss of paternal gene expression. In AS, the disease results due to loss of maternal gene expression. PWS: Prader-Willi syndrome; AS: Angelman syndrome.
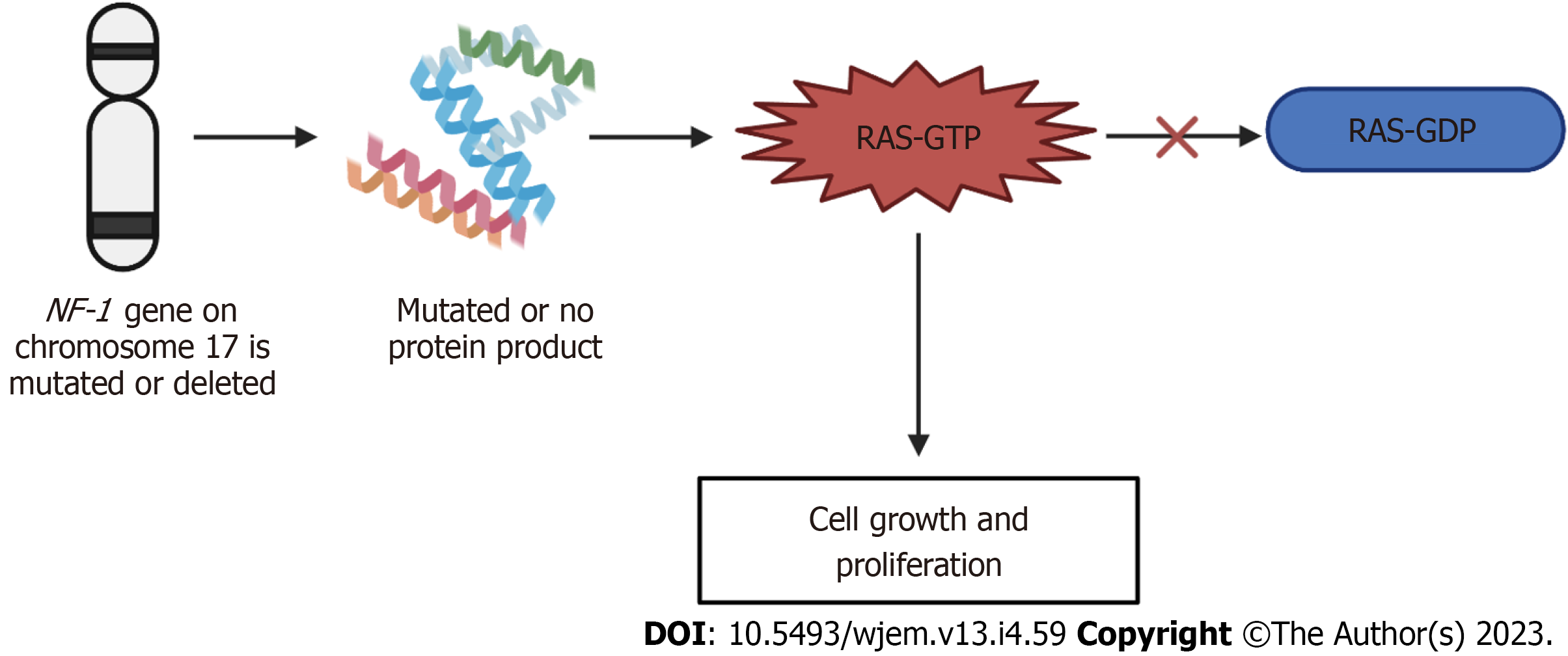
Figure 5 Mechanism of neurofibromatosis-1.
If the neurofibromatosis-1 gene on chromosome 17 is either mutated or deleted, either no protein product is formed, or a mutated protein is made. This mutation or deletion then results in the inhibition of the conversion of Ras-GTP to Ras-GDP, ultimately resulting in the increased signaling of Ras-GTP and leading to cell growth and proliferation, producing the neurofibromatosis phenotype. GTP: Guanosine triphosphate; GDP: Guanosine 5'-diphosphate; NF-1: Neurofibromatosis-1.
- Citation: Kioutchoukova IP, Foster DT, Thakkar RN, Foreman MA, Burgess BJ, Toms RM, Molina Valero EE, Lucke-Wold B. Neurologic orphan diseases: Emerging innovations and role for genetic treatments. World J Exp Med 2023; 13(4): 59-74
- URL: https://www.wjgnet.com/2220-315X/full/v13/i4/59.htm
- DOI: https://dx.doi.org/10.5493/wjem.v13.i4.59