Published online Jan 31, 2020. doi: 10.5492/wjccm.v9.i1.1
Peer-review started: October 11, 2019
First decision: November 1, 2019
Revised: December 23, 2019
Accepted: January 13, 2020
Article in press: January 13, 2020
Published online: January 31, 2020
Processing time: 121 Days and 20 Hours
Cytokines and inflammatory mediators are the hallmarks of sepsis. Extracorporeal cytokine hemoadsorption devices are the newer clinical support system to overcome the cytokine storm during sepsis.
To retrospectively evaluate the clinical outcomes of patients admitted in intensive care unit with septic shock with different etiologies.
The laboratory parameters including biomarkers such as procalcitonin, serum lactate and C-reactive protein; and the hemodynamic parameters; mean arterial pressure, vasopressor doses, sepsis scores, cytokine levels and other vital parameters were evaluated. We evaluated these outcomes among survivors and non-survivors.
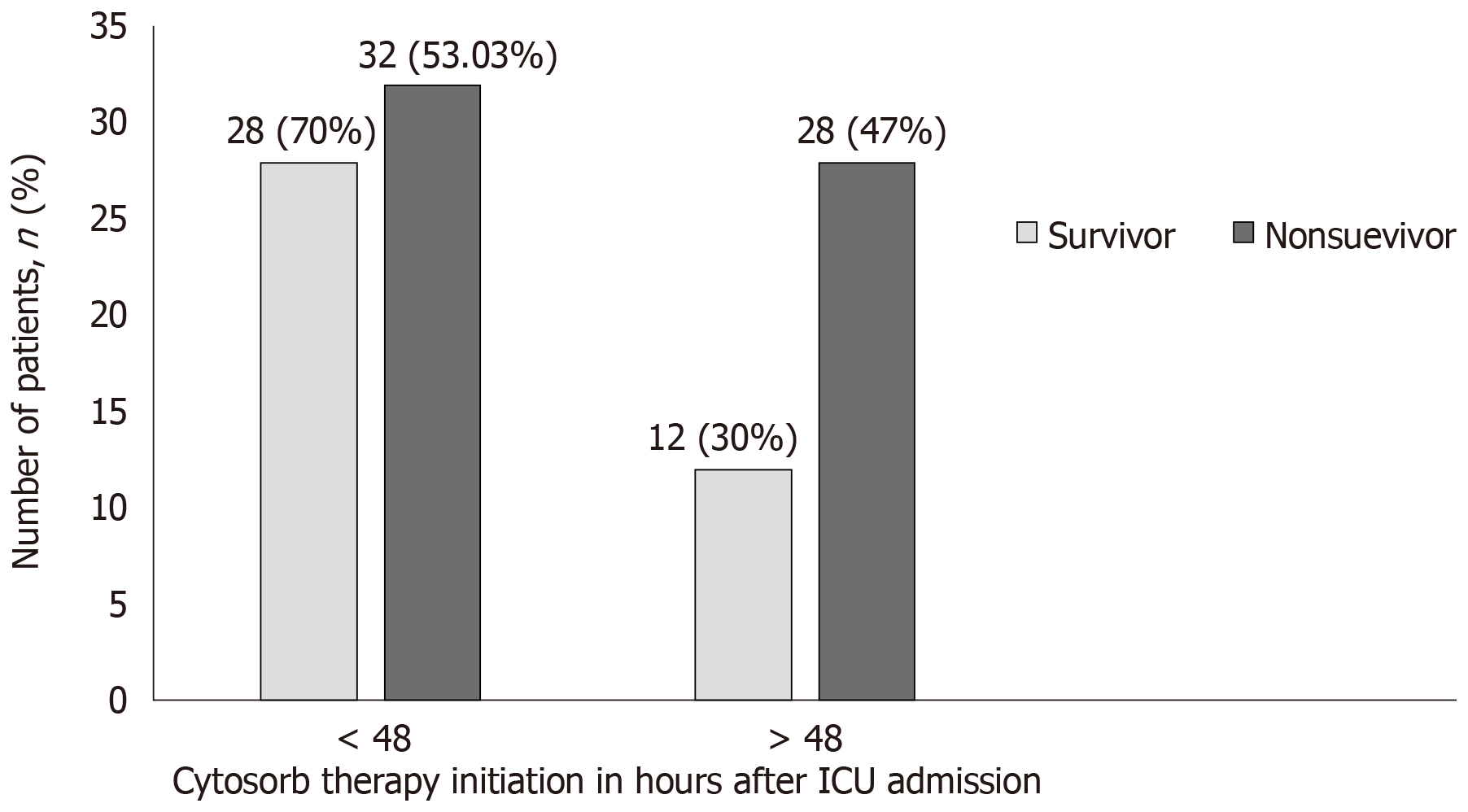
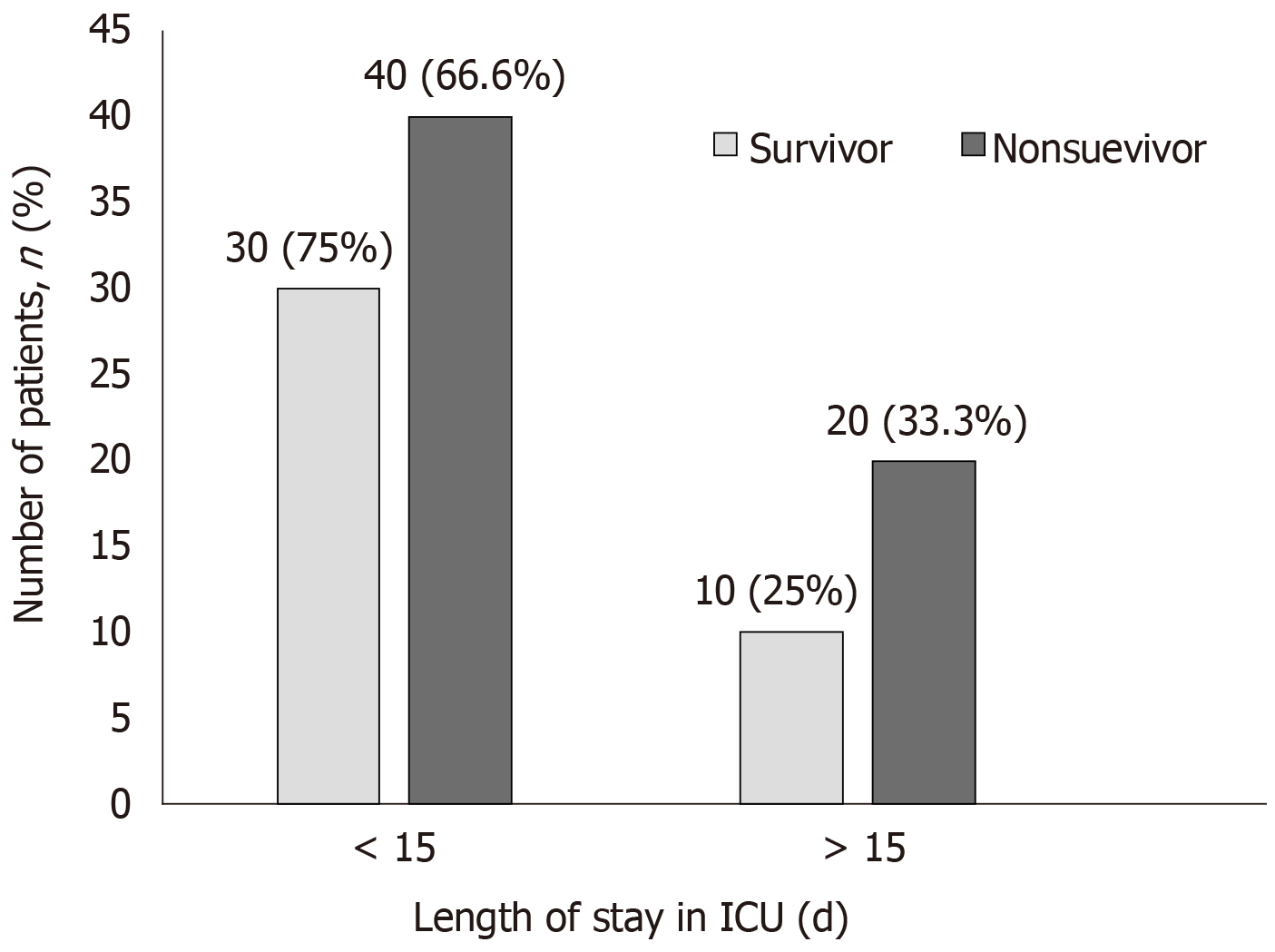
Of 100 patients evaluated, 40 patients survived. Post treatment, the vasopressors dosage remarkably decreased though it was not statistically different; 34.15% (P = 0.0816) for epinephrine, 20.5 % for norepinephrine (P = 0.3099) and 51% (P = 0.0678) for vasopressin. In the survivor group, a remarkable reduction of biomarkers levels; procalcitonin (65%, P = 0.5859), C-reactive protein (27%, P = 0.659), serum lactate (27%, P = 0.0159) and bilirubin (43.11%; P = 0.0565) were observed from baseline after CytoSorb® therapy. A significant reduction in inflammatory markers; interleukin 6 and interleukin 10; (87% and 92%, P < 0.0001) and in tumour necrosis factor (24%, P = 0.0003) was also seen. Overall, 28 (28%) patients who were given CytoSorb® therapy less than 48 h after onset of septic shock survived and the maximum duration of stay for 70% of these patients in intensive care unit was less than 15 d.
CytoSorb® is a safe and well tolerated rescue therapy option in patients with septic shock. However, early (preferably within < 48 h after onset of septic shock) initiation could result in better clinical outcomes. Further randomized trials are needed to define the potential benefits of this new treatment modality.
Core tip: CytoSorb® is a promising new extracorporeal cytokine hemoadsorption device that can modulate the cytokine storm during sepsis. This retrospective study evaluated clinical outcomes after CytoSorb® therapy of 100 patients admitted to intensive care unit with sepsis. We observed a significant reduction in vasopressors dosage in 40 patients who survived. The survivors also had a reduction in all the biomarker levels (procalcitonin, C-reactive protein, serum lactate and bilirubin) and inflammatory markers (interleukin 6, interleukin 10 and tumour necrosis factor) after CytoSorb® therapy. Notably, 28% of patients who were given CytoSorb® therapy < 48 h after onset of septic shock survived.
- Citation: Mehta Y, Mehta C, Kumar A, George JV, Gupta A, Nanda S, Kochhar G, Raizada A. Experience with hemoadsorption (CytoSorb®) in the management of septic shock patients. World J Crit Care Med 2020; 9(1): 1-12
- URL: https://www.wjgnet.com/2220-3141/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v9.i1.1
Sepsis results due to complex interactive reactions between infecting microbe and the immune system of host. In patients admitted to intensive care units (ICU), sepsis is a major health problem worldwide and is associated with high mortality rates. Approximately, 30% of patients admitted to ICU have sepsis[1]. If not managed properly, sepsis can result in septic shock, systemic hyper inflammation and multiple organ failure[2]. Use of inappropriate antibiotics, virulence of bacteria and host response aggravates the activation of the inflammatory response which leads to dysregulation of inflammatory homeostasis with increased levels of both pro-inflammatory [interleukin (IL)-1β and tumour necrosis factor (TNF) α] and anti-inflammatory (IL-6, IL-8, IL-10) plasma mediators[3]. It results in major complications such as hypotension, reduced organ perfusion, need of organ support system like dialysis and mechanical ventilation[4,5].
Various extracorporeal blood purification therapies have been used to remove excess of inflammatory mediators or microbial toxins to improve health outcomes of patients with severe sepsis. Assuring results are obtained by various techniques including hemoperfusion, immunoglobulin therapy, endotoxin- binding polymyxin B hemoperfusion, high-volume hemofiltration, high cut-off membrane hemofiltration /hemodialysis, plasma exchange, and coupled plasma filtration adsorption dialysis and plasma filtration etc. However, the mortality rate still remains high with these techniques as observed in the recent EUPHRATES trial[6-9].
CytoSorb® is an European CE mark approved and ISO certified hemoadsorption device which helps in reduction of excess inflammatory cytokines in the blood[10,11]. This unique therapy can eliminate bacterial exotoxins, myoglobin, free hemoglobin, bilirubin, activated complement and hosts of other inflammatory agents which can lead to fatal systemic inflammatory response syndrome (SIRS)[11]. Its clinical utility is also observed in various other clinical conditions including cardiac surgeries, liver failure, respiratory failures and various autoimmune diseases and infections[11-13]
In previous studies, CytoSorb® therapy has shown clinical benefits if used early (< 24 h) in patients with septic shock[14,15]. The aim of the present study was to evaluate the clinical outcomes after CytoSorb® therapy in patients admitted to ICU with septic shock due to different clinical conditions.
This retrospective and observational study was conducted at Medanta medicity, Gurgaon, India for duration of 2 years (2016-2018). The study was approved by an institutional ethics committee and conducted in compliance with the current International Council for Harmonization, good clinical practice, Schedule Y, and Indian Council of Medical Research guidelines. A written informed consent was obtained from all patients’ relatives before initiating the therapy. The patients/caretakers were briefed about the usage, advantages and disadvantages of treatment. CytoSorb® is a whole blood perfusion cartridge meant for single use. It is made up of biocompatible, polystyrene and divinylbenzene polymer beads with a large surface area. It can be easily used in conjunction with various renal replacement therapies and as a standalone therapy as well. The cartridge is attached in a close loop circuit with a pump. Venous blood of the patient enters at one end of the hemadsorption cartridge, and reinfused from the other port of dialysis catheter. It can be used maximum for 24 h. It removes hydrophobic molecules between 5-60 Kda by adsorption. Molecules beyond this range remain unaffected. Its use may be challenging in patients with contraindication to systemic anticoagulation. It is associated with decrease in platelet levels, though this has not been found to be clinically significant[12].
Inclusion/exclusion criteria: The medical records of the patients who had received CytoSorb® therapy following diagnosis of sepsis or septic shock (as per the surviving sepsis guidelines) and hospitalized in ICU between 2016 and 2018 were included. We selected the patients with acute kidney injury and sepsis for dialysis and CytoSorb® combination therapy.
Evaluation of application of CytoSorb® scoring system for patient selection to start the therapy: We retrospectively evaluated the application of the CytoSorb® scoring (CS) system developed by a group of clinicians for initiating CytoSorb® therapy on the basis of their practical experience. The scoring system was derived from five parameters (hemodynamic, renal, respiratory, laboratory and sepsis scores), representing five different organ system which get affected in sepsis patients. At the end, final scores were calculated by adding all the individual organ system scores. Supplementary Table 1 presents the CS system. Scores of 8-13 were considered ideal for recommending CytoSorb® therapy. Scores > 13 implied that the patient condition was critical and required aggressive therapy.
| Baseline characteristics | Survivors (n = 40) | Non-survivors (n = 60) | P value (survivors vs non-survivors) |
| Age (yr) | 51.3 ± 16.66 | 53.66 ± 16.47 | 0.4864 |
| Urine output (mL/d) | 551.13 ± 524.60 | 666.48 ± 595.25 | 0.3224 |
| MAP (mmHg) | 62.82 ± 9.73 | 66.31 ± 9.48 | 0.0774 |
| GCS | 6.26 ± 3.67 | 6.12 ± 4.56 | 0.8715 |
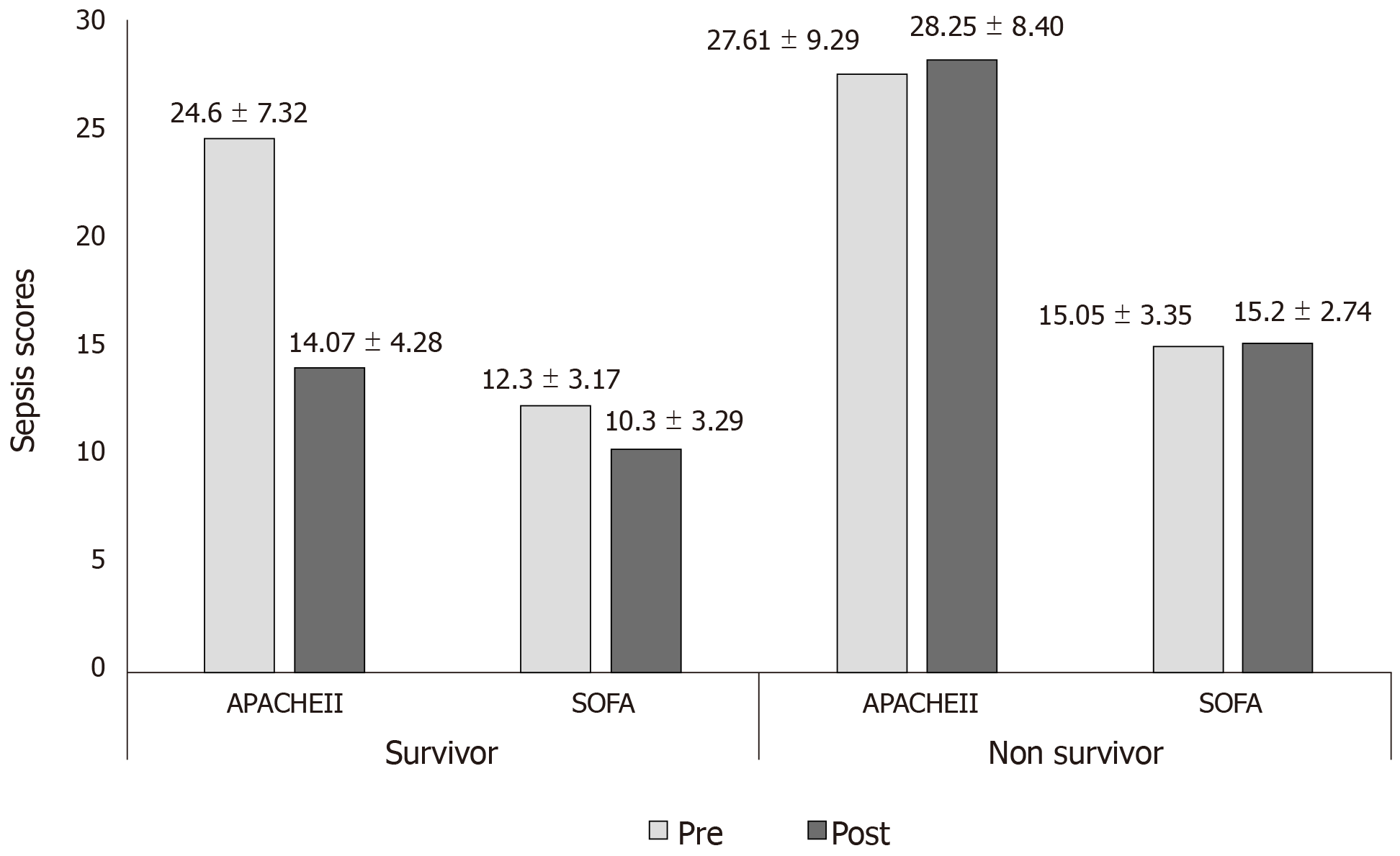
| APACHE-II | 24.6 ± 7.32 | 27.61 ± 9.29 | 0.0881 |
| SOFA | 12.3 ± 3.17 | 15.05 ± 3.35 | 0.0001 |
| Leucocytes (cells/mm3) | 15.60 ± 8.56 | 21.40 ± 26.17 | 0.1794 |
| Platelets (cells/mm3) | 123.95 ± 51.42 | 110.53 ± 50.18 | 0.1976 |
| BUN | 58.45 ± 36.94 | 108.55 ± 92.10 | 0.0015 |
| SGOT(U/L) | 1135.74 ± 2206.67 | 616.25 ± 1353.71 | 0.1477 |
| SGPT(U/L) | 504.63 ± 876.89 | 540.93 ± 1216.70 | 0.8712 |
| S. Creatinine (mg/dL) | 2.73 ± 1.86 | 7.01 ± 23.41 | 0.2521 |
| S. Lactate (mg/dL) | 3.71 ± 2.30 | 4.18 ± 3.23 | 0.3812 |
| PaCO2 | 38.46 ± 14.51 | 40.89 ± 12.20 | 0.3682 |
| PaO2 | 96.78 ± 41.42 | 84.50 ± 48.56 | 0.1920 |
| FiO2 | 49.32 ± 18.71 | 69.15 ± 67.74 | 0.0744 |
Evaluation of laboratory and vital parameters: Baseline patient data including relevant demographic details, vitals, clinical diagnosis were recorded in the case record form. The related laboratory tests for renal, liver and metabolic parameters were evaluated in both pre and post CytoSorb® therapy and a comparison was done between the survivor and the non-survivor group. All the laboratory parameters’ limits (values) were categorized as per the scoring system (Supplementary Table 1). Routine ICU monitoring parameters were also noted like routine biochemical investigations, and clinical parameters like Glasgow Coma Scale (GCS). GCS is a neurological scale used as a part of several ICU scoring systems for assessment of central nervous system.
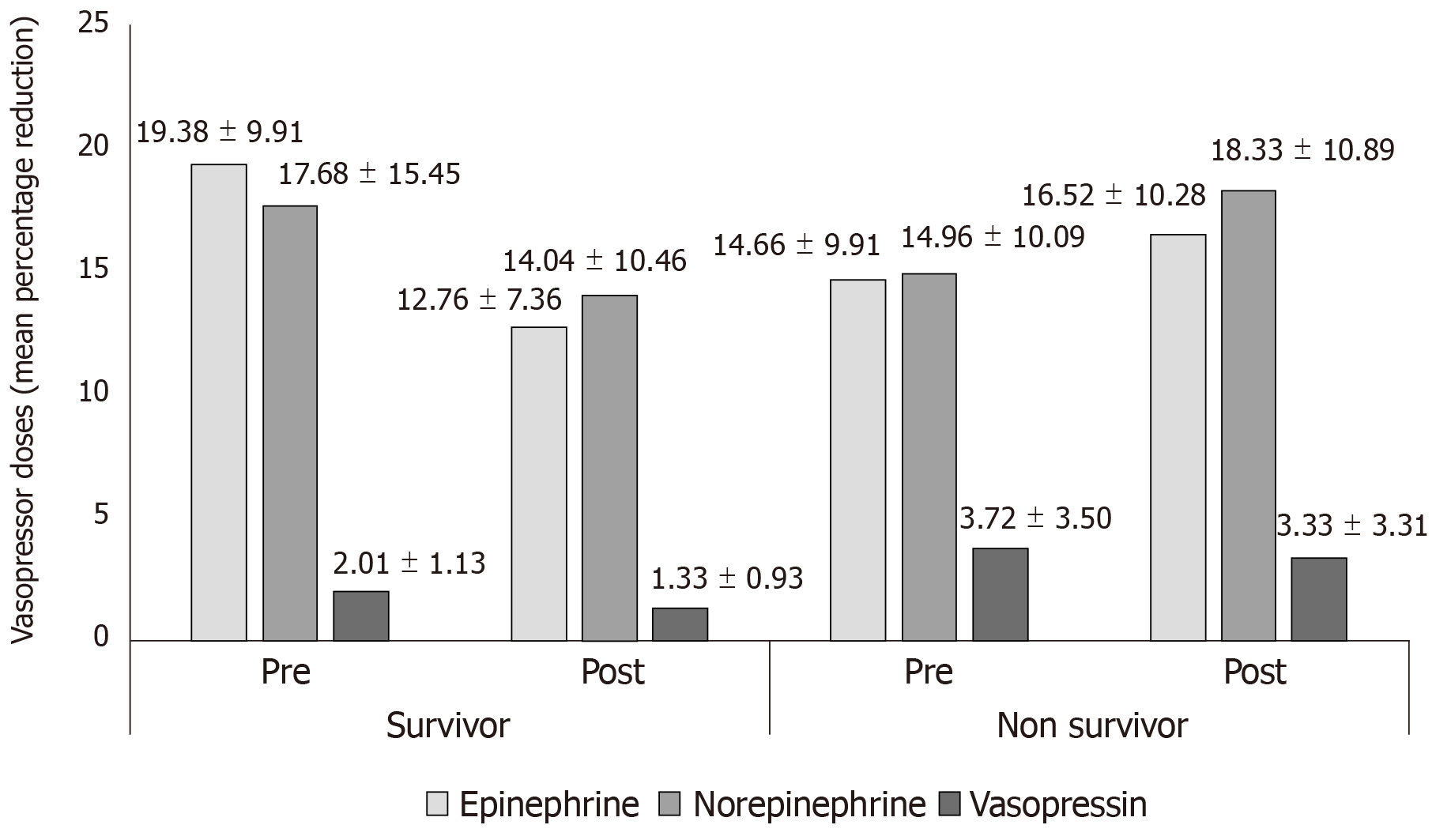
Vasopressors dose and hemodynamic parameters: We compared the mean arterial pressure (MAP) improvement and vasopressor dose (percentage reduction) between pre and post CytoSorb® therapy among survivors and non-survivors. Post therapy, the percentage decrease in number of patients needing both reduced number and doses of vasopressor drugs, i.e., norepinephrine (NE), epinephrine (E), and vasopressin (V) was evaluated.
Evaluation of other outcomes: Inflammatory parameters including interleukins; IL1, IL6, IL10, TNF and sequential organ failure assessment (SOFA) score were recorded pre and post therapy. Acute physiology and chronic health evaluation (APACHE II) were also recorded. Survival outcomes were determined on the basis of time taken (< 48 h or > 48 h) to initiate CytoSorb® therapy after admitting in ICU. Length of patients’ stay in ICU (total number of days spent by the patient in ICU before, during and post CytoSorb® therapy) was also recorded. Predicted percentage mortality calculated using APACHE-II calculator was used as a severity score and mortality estimation tool[16].
The continuous data were presented as mean ± SD and categorical as frequency and percentage (%). The analysis was performed using paired t test. P < 0.05 was considered to be statistically significant.
A total of 100 patients were included in the study. The mean age of all the patients was 52.53 ± 16.46 years. Majority of the patients were men (77.0%) with mean age of 51.33 ± 17.11 years. The mean age of women patients was 56.52 ± 13.62 years. Of these 100 patients, 40 (40%) patients survived (survivor group). The baseline characteristics of all the patients in both the groups are presented in Table 1.
Effect of CytoSorb® therapy on vasopressor dose and MAP levels: In the survivor group, an improvement in post CytoSorb® therapy MAP (68.23 ± 7.50 mmHg) as compared to pre CytoSorb® MAP (62.82 ± 9.73 mmHg; P = 0.1805) was observed.
We also observed a reduction in doses of E (post CytoSorb® therapy: 12.76 ± 7.36 mcg/ min vs pre CytoSorb® therapy: 19.38 ± 9.91 mcg/ min; P = 0.0816), NE (post CytoSorb® therapy: 14.04 ± 10.46 mcg/ min vs pre CytoSorb® therapy: 17.68 ± 15.45 mcg/min; P = 0.3099) and V (post CytoSorb® therapy: 1.33 ± 0.93 mcg/min vs pre CytoSorb® therapy: 2.01 ± 1.13 mcg/min; P = 0.0678).
In the non-survivor group, there was no improvement in MAP (64.31 ± 10.88 mmHg vs 66.31 ± 9.48 mmHg) post CytoSorb® therapy vs pre post CytoSorb® therapy. Further, no reduction in vasopressor dose was reported post CytoSorb® therapy. Figure 1 shows the comparison of mean percentage reduction in doses of vasopressor drugs for the patients in the survivor and non-survivor groups.
Evaluation of CytoSorb scores and number of CytoSorb® devices required: Prior to CytoSorb® therapy, majority of the patients were on dialysis and continued to be on dialysis post therapy. In the survivor group, patients were on different types of dialysis treatment CRTT (n = 42), HD (n = 24) and SLED (n = 34). The number of CytoSorb® devices used per patient varied between 1-3.
Using the clinicians’ designed scoring system for initiation of CytoSorb® therapy, we tried to retrospectively validate this scoring system in our patients. Through this scoring system, we observed that the patients in the survivor group had mean score of 12 as compared to those in the non-survivor group with mean score of 14. Patients with CytoSorb (CS) scores of 10 and 11 had mostly received one CytoSorb® device. Overall, there were 79 patients (32 from survivor and 47 from non-survivor group) with high CS score (12-14) and were recommended more than one CytoSorb® device. Only one patient each with CS score 13 and CS score 14 were recommended 3 CytoSorb® devices. The correlation of CS scores with number of devices recommended for both the groups is shown in Table 2.
| CS score | Survivor (n) | CS score (n, number of devices used) | Non-survivor (n) | CS score (n, number of devices used) |
| < 8 | 0 | - | 0 | - |
| 8-13 | 35 | 10 (n = 1, 1) | 21 | 11 (n = 1, 1) |
| 11 (n = 7, 1) | 12 (n = 6, 1) | |||
| 12 (n = 19; 18 = 1, 1 = 2) | 13 (n = 14; 13 = 1, 1 = 3) | |||
| 13 (n = 8; 1 = 1, 3 = 2, 1 = 3) | ||||
| > 13 | 5 | 14 (n = 5; 1 = 1, 3 = 2, 1 = 3) | 39 | 14 (n = 27; 21 = 1, 6 = 2) |
| 15 (n = 12; 11 = 1, 4 = 2) |
Effect of CytoSorb® therapy on laboratory and vital parameters: In the survivor group, 16% decrease (from 15.60 ± 8.56 cells/mm3 to 13.09 ± 6.71 cells/mm3, P = 0.1484) in total leucocyte count was reported post CytoSorb® therapy. The platelet count decreased slightly by 4.2% (from 123.95 ± 51.42 cells/mm3 to 118.75 ± 48.33 cells/mm3, P = 0.6425). Serum creatinine and Serum lactate reduced by 17% (from 2.73 ± 1.86 mg/dL to 2.27 ± 1.31 mg/dL, P = 0.2048) and 27% (from 3.71 ± 2.30 mg/dL to 2.28 ± 0.89 mg/dL, P = 0.0159), respectively. Procalcitonin (PCT) levels reduced by 65% (from 121.56 ± 421.20 ng/dL to 42.80 ± 69.89 ng/dL), C-reactive protein (CRP) levels reduced by 27% (from 165.68 ± 169.26 mg/dL to 120.33 ± 63.72 mg/dL) and bilirubin levels dropped by 43% (from 3.27 ± 2.67 mg/dL to 1.86 ± 1.51 mg/dL, P = 0.05).
Improvement was also reported in GCS in the patients in survivor group as compared with patients in the non-survivor group. One patient showed an improvement of more than 50% (from score 5 to 10) and one patient showed an improvement of 75% (from score 3 to 12). There was an overall 22.3% (8.05 ± 3.91) improvement in GCS. Slight improvement in other vital parameters like heart rate, respiratory rate, BP and body temperature was also reported.
Among non-survivors, a significant reduction in serum creatinine (25%, P = 0.008) was observed. Other laboratory and vital parameters except GCS (30%, P = 0.0129) did not show any significant improvement. Change in the laboratory and vital parameters pre and post therapy among survivors and non survivors is shown in Tables 3 and 4.
| Parameters | Pre CytoSorb® therapy | Post CytoSorb® therapy | Percentage change | P value |
| Urine output (mL/d) | 551.13 ± 524.60 | 862.88 ± 682.46 | 56.56 | 0.0247a |
| CRP (mg/dL) | 165.68 ± 169.26 | 120.33 ± 63.72 | -27.4 | 0.6590 |
| PCT (ng/dL) | 121.56 ± 421.20 | 42.81 ± 69.89 | -65 | 0.5859 |
| MAP (mm/Hg) | 62.82 ± 9.73 | 68.23 ± 7.50 | 8.6 | 0.1805 |
| GCS | 6.26 ± 3.67 | 8.05 ± 3.92 | 22.36 | 0.0417c |
| Leucocytes (cells/mm3) | 15.60 ± 8.56 | 13.09 ± 6.71 | -16.02 | 0.1484 |
| Platelets (cells/mm3) | 123.95 ± 51.42 | 118.75 ± 48.33 | -4.2 | 0.6425 |
| S. Creatinine (mg/dL) | 2.73 ± 1.86 | 2.27 ± 1.31 | -16.84 | 0.2048 |
| S. Lactate (mmol/L) | 3.71 ± 2.30 | 2.28 ± 0.89 | -26.66 | 0.0159a |
| SGOT (U/L) | 1135.74 ± 2206.67 | 1078.92 ± 1890.45 | -5.00 | 0.9222 |
| SGPT (U/L) | 504.63 ± 876.89 | 316.59 ± 645.41 | -37.26 | 0.3796 |
| BUN | 58.45 ± 36.94 | 56.67 ± 28.24 | -3.05 | 0.8266 |
| Bilirubin (mg/dL) | 3.27 ± 2.67 | 1.86 ± 1.51 | -43.11 | 0.0565a |
| Sodium (mmol/L) | 136.59 ± 24.49 | 136.31 ± 24.22 | -0.20 | 0.9615 |
| Potassium (mmol/L) | 4.22 ± 0.65 | 3.75 ± 0.56 | -11.14 | < 0.0001a |
| Albumin (g/L) | 2.64 ± 0.58 | 2.65 ± 0.62 | 0.38 | 0.9412 |
| Arterial pH | 7.33 ± 0.13 | 7.37 ± 0.13 | 0.55 | 0.1727 |
| Bicarbonate | 20.32 ± 4.05 | 22.825 ± 3.86 | 12.35 | 0.0060a |
| PaO2 | 96.78 ± 41.42 | 85.88 ± 27.89 | -11.26 | 0.1714 |
| PaCO2 | 38.46 ± 14.51 | 38.36 ± 14.53 | -0.26 | 0.9755 |
| FiO2 | 49.32 ± 18.71 | 41.95 ± 13.71 | -14.94 | 0.0550 |
| Parameters | Pre CytoSorb® therapy | Post CytoSorb® therapy | Percentage change | P value |
| Urine output (mL/d) | 666.48 ± 595.25 | 493.85 ± 433.11 | -25.90 | 0.0718 |
| CRP (mg/dL) | 1175.22 ± 126.60 | - | - | - |
| PCT (ng/dL) | 24.91 ± 24.51 | 48.97 ± 57.57 | 96.58 | 0.0766 |
| MAP (mm/Hg) | 66.13 ± 9.485 | 64.31 ± 10.87 | -2.75 | 0.3304 |
| GCS | 6.12 ± 4.56 | 4.27 ± 2.91 | -30.23 | 0.0129a |
| Leucocytes (cells/mm3) | 21.40 ± 26.17 | 20.25 ± 18.25 | -5.34 | 0.7327 |
| Platelets (cells/mm3) | 110.53 ± 50.18 | 99.67 ± 47.81 | -9.83 | 0.2273 |
| S. Creatinine (mg/dL) | 7.01 ± 23.41 | 5.27 ± 23.19 | -24.82 | 0.0088a |
| S. Lactate (mmol/L) | 4.18 ± 3.23 | 5.05 ± 3.75 | 17.2 | 0.1759 |
| SGOT (U/L) | 616.25 ± 1353.71 | 1418.14 ± 2068 | 130.12 | 0.0693 |
| SGPT (U/L) | 540.93 ± 1216.70 | 577.38 ± 945.94 | 6.74 | 0.9048 |
| BUN | 108.55 ± 92.10 | 95.02 ± 84.83 | -12.46 | 0.4362 |
| Bilirubin (mg/dL) | 5.15 ± 14.19 | 3.84 ± 4.09 | -25.44 | 0.6543 |
| Sodium (mmol/L) | 133.79 ± 26.22 | 139.51 ± 7.32 | 4.28 | 0.1244 |
| Potassium (mmol/L) | 4.43 ± 1.03 | 4.15 ± 1.03 | -6.32 | 0.1392 |
| Albumin (g/L) | 3.03 ± 1.07 | 2.85 ± 0.80 | -5.94 | 0.2988 |
| Arterial pH | 7.28 ± 0.14 | 7.22 ± 0.18 | -0.82 | 0.0438 |
| Bicarbonate | 24.52 ± 24.21 | 22.16 ± 22.19 | -9.62 | 0.6560 |
| PaO2 | 84.50 ± 48.56 | 90.42 ± 51.14 | 7.01 | 0.5256 |
| PaCO2 | 40.89 ± 12.20 | 45.05 ± 33.71 | 10.17 | 0.3760 |
| FiO2 | 69.15 ± 67.74 | 62.6 ± 28.61 | -9.47 | 0.5016 |
There was a significant reduction in levels of inflammatory markers IL6, IL10 and TNF in the survivor group. A high percentage reduction in IL6 and IL10; (87% and 92%, P < 0.0001) and in TNF (24%, P = 0.0003) was observed. Among non-survivors, there was no improvement in any of the cytokine levels. Tables 5 and 6 show the cytokine assay data for patients in survivor and non-survivor group
| Cytokine (pg) | Pre CytoSorb® therapy | Post CytoSorb® therapy | Percentage change | P value |
| IL-1 | 5.52 ± 2.59 | 5.79 ± 2.55 | 4.89 | 0.7364 |
| IL-6 | 2273.51 ± 1212.82 | 2638.24 ± 1518.26 | 16.04 | 0.1486 |
| IL-10 | 296.00 ± 146.4 | 295.67 ± 112.00 | -0.111 | 0.9894 |
| TNF | 19.43 ± 6.07 | 20.40 ± 6.26 | 5.00 | 0.3914 |
Post therapy 16.2% (P = 0.0070) fall in SOFA scores was observed in the survivor group. Among non-survivors1% rise in SOFA score, was observed after therapy. Figure 2 shows the change in APACHE II and SOFA scores in both groups for pre and post CytoSorb® therapy.
As per APACHEII calculator[16], the mean predicted percentage mortality was 54% (53.68 ± 28.84) for the survivor group and 62% (62.32 ± 29.44) for the non-survivor group.
CytoSorb® therapy was started as per the severity of septic shock and clinical parameters of patients. From an overall pool of patients (n = 100), 60 patients were started with CytoSorb® therapy within 48 h of ICU admission and 40 patients more than 48h of ICU admission. We observed that in the survivor group (n = 40), 70% (n = 28) of patients received CytoSorb® therapy within 48 h of ICU admission as compared to 72% of non survivors (n = 43/60) in whom CytoSorb® was initiated after 48 h after ICU admission. Figure 3 and Figure 4 show the patient survival data for both the groups.
In the management of sepsis, it is necessary to stabilize the hemodynamic levels in patients undergoing treatment for septic shock. Resuscitation in septic shock can be rapidly achieved by restoration of perfusion by administration of intravenous fluids, inotropic supports, and vasopressor drugs. It is of utmost importance to maintain the appropriate MAP levels[17]. Some studies have also shown successful and effective results in the treatment of hemodynamics accompanied by a decrease in vasopressor doses with CytoSorb® therapy[14]. For evaluating the hemodynamic parameters, we used multiple vasopressor drugs; NE, E and V in patients with septic shock > 48 h having MAP > 65 mmHg. Post therapy, in the survivor group, we observed hemodynamic stability with improvement in MAP as compared to pre CytoSorb® therapy. We also observed significant reduction in mean percentage doses of all vasopressors. Post CytoSorb® therapy, the survival rate was 40%. Patients in the survivor group showed better clinical outcomes than non-survivor group in all aspects of laboratory, vital parameters, sepsis scores, cytokine levels and vasopressor needs. A crucial aspect of this study was to look for the patients’ suitability for this therapy and to determine the extent of improvement in laboratory and hemodynamic parameters post therapy. Therefore, our clinical team designed a scoring system based on patients’ baseline characteristics including five parameters which directly affect the body’s main organ system that are prone to undergo dysfunction during sepsis (Supplementary Table 1). As per the CS scoring system, CytoSorb® therapy should be recommended to the patients with scores between 8-13. For patients with CS between 10-14, dialysis in combination with one or more CytoSorb® device depending on their clinical outcomes should be followed.
Laboratory parameters such as PCT, CRP and serum lactate are well known biomarkers that indicate cytokine storm[18-20]. We evaluated these parameters considering the target cut off for maximum severity score 3 as PCT (> 3 ng/mL), serum lactate (> 4 mmol) and CRP (> 200 mg/dL). In our study results, we reported remarkable reduction in patients’ PCT (65%), CRP (27%), bilirubin (43%, P = 0.05), and serum lactate (27%, P = 0.0159) levels post CytoSorb® therapy. CytoSorb® device is capable of removing more than 90% bilirubin (0.7 kDa), PCT (13 kDa), and IL-6 (26 kDa)[21,22]. Our study reports were consistent with the study conducted by Hawchar et al[23], in 20 patients (CytoSorb® and control group; n = 10 each) on mechanical ventilation with baseline PCT > 3 ng/mL and serum lactate > 2.0 mmol/L. CytoSorb® therapy was initiated within 24 h of septic shock and resulted in significant improvement in patients for PCT levels; T0 = 20.6 [QR: 6.5-144.5] ng/mL, T48 = 5.6 [QR: 1.9-54.4] ng/mL, P = 0.004. In the control group, PCT levels improved as; T0 = 13.2 [QR: 7.6-47.8] ng/mL, T48 = 9.2 [QR: 3.8-44.2] ng/mL. Serum lactate was reduced by 33% in CytoSorb® group and 53.3% in control group. However, no significant difference was observed in both the groups for CRP concentration. This could be due to high molecular weight of CRP around 25 kDa that might not be absorbed by CytoSorb® as efficiently as PCT. Both groups showed a decrease of CRP by T48[23].
Elevation of proinflammatory cytokines such as TNF and interleukins is a potential marker of the hyper-inflammatory phase of sepsis[20]. In this study, IL6 and IL10 showed significant reduction (P < 0.0001) after the therapy in survivor group. Our results were supported by Mitzner and coworkers’ study who reported that the use of CytoSorb® within 24 h in a patient with septic shock and chronic kidney failure decreased the levels of IL-6, CRP, serum creatinine, PCT, and leukocytes during the treatment and in the following days. CytoSorb® hemoadsorber treatment appeared to be safe and was well tolerated by the patient as reported by them[24].
Our study showed a significant improvement in SOFA (P = 0.0070) score in survivor group. Improvement in SOFA scores indicated improvement in clinical condition including laboratory and hemodynamic parameters.
We also studied the correlation between early use of CytoSorb® therapy (< 48 h and > above) with better outcome and evaluated the survival outcomes on the basis of number of days spent in ICU by patients. Two patients were discharged within a day of treatment. Our results were well supported by other studies which reported that use of this therapy within 24h of sepsis diagnosis could lead to decreased mortality in both medical and post-surgical patients[14,15,25].
Overall, the study showed a reduction in the vasopressor dose, a significant reduction in cytokine levels, remarkable reduction in diagnostic markers such as PCT, CRP, bilirubin in and serum lactate after using CytoSorb® therapy. However, the current study has some limitations. First, the present study was a small, single-center retrospective study and underpowered for any significant outcome analysis. Further studies with a larger patient group are needed to deal with this question. Second, the lack of a control group precludes conclusions about effectiveness and cause of the therapy applied. Furthermore appropriate time of initiation of therapy needs to be well defined.
In conclusion, the study showed that the CytoSorb® is a safe and well tolerated rescue therapy option in patients with severe septic shock. However, early (preferably within < 48 h after onset of septic shock) initiation might result in better clinical outcomes. These results may provide important support and guidance to future protocol designs and can help to define the appropriate study end points. Further, prospective randomized controlled trials should be performed to substantiate this hypothesis.
Sepsis is one of the world’s leading cause of death in the intensive care unit (ICU) and yet remains the most significant unmet medical need. Sepsis results due to complex interactive reactions between infecting microbe and the immune system of host. CytoSorb® is an European CE mark approved and ISO certified hemoadsorption device which helps in reducing cytokine storm in the blood. In this study, clinical outcomes were evaluated after the use CytoSorb® device as an adjuvant therapy in patients who were admitted in ICU with sepsis between 2016 and 2018.
Most of the patients with septic shock end up dying even though control of inflammation has been attempted through various means. CytoSorb® is an emerging extracorporeal hemadsorption device but there is a paucity of clinical evidence supporting its benefits and clinical outcomes after use. Previous individual studies have shown promising results after use of CytoSorb® therapy in patients with sepsis and septic shock. We used CytoSorb® in 100 patients admitted to ICU with sepsis a rescue therapy but had not analyzed the data to evaluate clinical outcomes in these patients. This study will serve as an important link to guide doctors about the usage of CytoSorb® and possible clinical outcomes. Further, this study will help answer an important question of when to start the CytoSorb® therapy after the onset of septic shock and how many devices are optimums for patients.
The objective of this study was to evaluate the clinical benefits of CytoSorb® therapy in critically ill patients admitted in ICU. We looked for the patients’ suitability for this therapy and determined the extent of improvement in laboratory and hemodynamic parameters post therapy with CytoSorb®. Future research should have the objective of a comparative study with a control group and a prospective randomized controlled trial should be performed to provide more evidence.
A retrospective observational study was carried out over a period of 2 years. We used the CytoSorb® scoring (CS) system that was developed by group of clinicians for initiating CytoSorb® therapy on the basis of their practical experience for the evaluation of patients. The scoring system was derived from five parameters (hemodynamic, renal, respiratory, lab and sepsis scores), representing five different organ system which get affected in sepsis patients. At the end, final scores were calculated by adding all the individual organ system scores. We evaluated the vitals, laboratory and other parameters by observing the data pre and post CytoSorb® administration.
The survivor group had a decrease in total leucocyte count, serum creatinine, serum lactate and platelet count. In the non-survivor group, serum creatinine levels and other parameters did not improve. We also observed that there was a significant decrease in inflammatory markers in the survivor group. Another major observation is that 70% of those who received the CytoSorb® therapy within 48 h had better chances of survival.
CytoSorb® score used in this study is a newly devised scoring system that can guide doctors about usage of CytoSorb® therapy. This study proposes that the CytoSorb® therapy should be recommended to the patients with scores between 8-13. For patients with CS between 10-14; dialysis in combination with one or more CytoSorb® device depending on their clinical outcomes should be followed. In summary, this study showed a reduction in the vasopressor dose, a significant reduction in cytokine levels, remarkable reduction in diagnostic markers such as PCT, CRP, bilirubin and serum lactate after the usage of CytoSorb® therapy. The new hypothesis that this study proposed is there is an improvement in MAP levels, vasopressor dose and other laboratory and clinical parameters when the CytoSorb® therapy is initiated early after onset of septic shock. We used a newly devised scoring system called CytoSorb® score that was derived from five parameters (hemodynamic, renal, respiratory, laboratory and sepsis scores), representing five different organ system which get affected in sepsis patients. Through this study, we reinforced that the CytoSorb® is a safe and well tolerated rescue therapy option in patients with severe septic shock.
The lesson learnt from this study is that CytoSorb® therapy should be initiated early in critically ill patients with sepsis and septic shock. In the future, we should design randomized clinical studies that can compare the results with control population. The best method would be to use CS score to decide the usage of CytoSorb® therapy.
The authors acknowledge Mr. Pradeep Yanamala for end to end coordination and extended support in formatting of this paper and in that line improved the manuscript significantly and Knowledge Isotopes Pvt. Ltd (https://www. knowledgeisotopes.com) for the medical writing assistance.
Manuscript source: Unsolicited Manuscript
Specialty type: Critical care medicine
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yeh YC, Spasojevic SD, Okumura K, Xavier-Elsas P S-Editor: Wang YQ L-Editor: A E-Editor: Qi LL
| 1. | Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Ñamendys-Silva SA, Martin-Loeches I, Leone M, Lupu MN, Vincent JL; ICON Investigators. Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit. Open Forum Infect Dis. 2018;5:ofy313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 2. | Genga KR, Russell JA. Update of Sepsis in the Intensive Care Unit. J Innate Immun. 2017;9:441-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm. 2013;2013:165974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 504] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 4. | Sander M, von Heymann C, von Dossow V, Spaethe C, Konertz WF, Jain U, Spies CD. Increased interleukin-6 after cardiac surgery predicts infection. Anesth Analg. 2006;102:1623-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Donadello K, Polati E. Hemadsorption in cardiac surgery: myth against reality. Minerva Anestesiol. 2019;85:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 7. | Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. 2007;35:2677-2685. [PubMed] |
| 8. | Bellomo R, Baldwin I, Ronco C. Extracorporeal blood purification therapy for sepsis and systemic inflammation: its biological rationale. Contrib Nephrol. 2001;367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Klein DJ, Foster D, Schorr CA, Kazempour K, Walker PM, Dellinger RP. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock): study protocol for a randomized controlled trial. Trials. 2014;15:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Shum HP, Yan WW, Chan TM. Extracorporeal blood purification for sepsis. Hong Kong Med J. 2016;22:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Hyde RA, Ishikawa MY, Jung EKY, Langer R, Leuthardt EC, Myhrvold NP, Sweeney EA, Wood JR LL. Device, system, and method for controllably reducing inflammatory mediators in a subject. America: TVPP, 2009. |
| 12. | Bonavia A, Groff A, Karamchandani K, Singbartl K. Clinical Utility of Extracorporeal Cytokine Hemoadsorption Therapy: A Literature Review. Blood Purif. 2018;46:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Hassan K, Kannmacher J, Wohlmuth P, Budde U, Schmoeckel M, Geidel S. Cytosorb Adsorption During Emergency Cardiac Operations in Patients at High Risk of Bleeding. Ann Thorac Surg. 2019;108:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Friesecke S, Stecher SS, Gross S, Felix SB, Nierhaus A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs. 2017;20:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 16. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] |
| 17. | Manohar V, Raj S, Sreekrishnan T, Kumar GK. Cytokine hemoadsorption therapy-An adjuvant in the management of septic shock with multi-organ dysfunction: A case report. Nat J Physiol Pharm Pharmacol. 2018;8:297-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Riedel S. Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn Microbiol Infect Dis. 2012;73:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Trásy D, Molnár Z. Procalcitonin - Assisted Antibiotic Strategy in Sepsis. EJIFCC. 2017;28:104-113. [PubMed] |
| 20. | Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50:23-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 21. | Faltlhauser A, Kullmann F. Use of Hemoadsorption in a Case of Severe Hepatic Failure and Hyperbilirubinemia. Blood Purif. 2017;44:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Singh A, Mehta Y, Trehan N. Bilirubin Removal Using CytoSorb Filter in a Cardiac Surgical Patient. J Cardiothorac Vasc Anesth. 2019;33:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 24. | Mitzner SR, Gloger M, Henschel J, Koball S. Improvement of hemodynamic and inflammatory parameters by combined hemoadsorption and hemodiafiltration in septic shock: a case report. Blood Purif. 2013;35:314-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Träger K, Fritzler D, Fischer G, Schröder J, Skrabal C, Liebold A, Reinelt H. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs. 2016;39:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |