Published online Jul 31, 2019. doi: 10.5492/wjccm.v8.i4.49
Peer-review started: March 15, 2019
First decision: June 6, 2019
Revised: June 21, 2019
Accepted: July 17, 2019
Article in press: July 17, 2019
Published online: July 31, 2019
Processing time: 138 Days and 16 Hours
Independent lung ventilation, though infrequently used in the critical care setting, has been reported as a rescue strategy for patients in respiratory failure resulting from severe unilateral lung pathology. This involves isolating and ventilating the right and left lung differently, using separate ventilators. Here, we describe our experience with independent lung ventilation in a patient with unilateral diffuse alveolar hemorrhage, who presented with severe hypoxemic respiratory failure despite maximal ventilatory support. Conventional ventilation in this scenario leads to preferential distribution of tidal volume to the non-diseased lung causing over distension and inadvertent volume trauma. Since each lung has a different compliance and respiratory mechanics, instituting separate ventilation strategies to each lung could potentially minimize lung injury. Based on review of literature, we provide a detailed description of indications and procedures for establishing independent lung ventilation, and also provide an algorithm for management and weaning a patient from independent lung ventilation.
Core tip: Severe unilateral lung disease presents a unique scenario where the diseased lung has very poor compliance, while the non-diseased lung remains normally compliant. In these patients, conventional positive pressure ventilation causes preferential distribution of tidal volume to the non-diseased lung causing its overdistension and inadvertent volutrauma. Placement of a double lumen endotracheal tube and providing independent lung ventilation, with a ventilator for each lung, can potentially minimize lung injury. This will allow institution of lung protective ventilation strategies to each lung, individualized based on their respective compliances.
- Citation: Berg S, Bittner EA, Berra L, Kacmarek RM, Sonny A. Independent lung ventilation: Implementation strategies and review of literature. World J Crit Care Med 2019; 8(4): 49-58
- URL: https://www.wjgnet.com/2220-3141/full/v8/i4/49.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v8.i4.49
Independent lung ventilation (ILV), though infrequently used in the critical care setting, has been reported by various authors as a rescue strategy for patients with unilateral lung pathology. These are mostly confined to case reports or small case series, but span a variety of patient populations, including medical[1-3], surgical[4-6], pediatric[7-10], and trauma[3,11]. ILV involves anatomical as well as physiological separation of each lung into separate units, and the success of implementation depends on the experience of the critical care team with ILV. Outside of a critical care setting anatomical separation of the lung is routinely performed in thoracic surgical operating rooms to either facilitate lung surgeries or to improve surgical exposure during other intrathoracic procedures. The complexity and lack of experience of many providers with ILV makes it an underutilized ventilation strategy in the intensive care unit (ICU). Here, we describe the use of ILV for management of respiratory failure in a patient with unilateral diffuse alveolar hemorrhage. We then critically review available literature on the use of ILV and provide a detailed description of indications and procedures for establishing ILV and provide an algorithm for management and weaning a patient from ILV.
Recently, we cared for a 63-year-old man who presented to our surgical ICU with hypoxemic respiratory failure. His medical history was notable for hepatitis C, atrial fibrillation, myelodysplasia treated with allogenic stem cell transplantation complicated by graft vs host disease and persistent thrombocytopenia. His chest X-ray showing complete white out of the right lung. Though aspiration, and unilateral pneumonia were important differentials, unilateral diffuse alveolar hemorrhage was the most likely etiology in the setting of his severe thrombocytopenia. Severe hypoxemia persisted (P/F about 60) despite tracheal intubation and mechanical ventilation. X-ray continued to show complete white out of right lung, and suggested over inflation of the left lung. With continued worsening of hypoxemia, we decided to place a double lumen tube, and independent lung ventilation was initiated as a rescue measure. Independent lung ventilation lead to improvement in oxygenation, and allowed titration of ventilation parameters independently for each lung based on their best compliance. Once his unilateral lung pathology improved substantially, he was transitioned back to a single lumen endotracheal tube and conventional ventilation was resumed. He was eventually weaned and extubated after 10 d of mechanical ventilation.
Independent lung ventilation requires anatomical and physiological separation of the lungs. Anatomical separation involves physical isolation of one lung from the other, while physiological separation refers to ventilating the two lungs independently as separate units. The focus of this article is on physiological separation of lungs, specifically, indications as well as ventilation and weaning strategies in patients receiving ILV. Techniques for anatomical separation is well described elsewhere[12-14].
The indications for ILV in a critical care setting may be broadly classified into two types based on the need for anatomical separation alone vs need for physiological separation of the lungs (Table 1). Anatomical separation is typically sought for conditions which require lung isolation to prevent cross contamination of the healthy lung by harmful material contained within the diseased lung. Physiological separation of lung is instituted for refractory respiratory failure resulting from unilateral lung disease, causing marked differences in pulmonary mechanics between right and left lung. For instance, in the presence of a poorly compliant diseased lung, such as in our case, conventional positive pressure ventilation would result in preferential over distension of the non-diseased lung potentially causing volutrauma to the non-diseased lung[15]. In addition, over distention of the non-diseased lung could result in diversion of pulmonary blood flow to the diseased lung thereby worsening shunt and hypoxemia[16]. Institution of an independent ventilation strategy for each lung may prevent volume trauma to the non-diseased lung, reduce shunting and allow for alveolar recruitment in the diseased lung.
The most commonly reported indications for ILV include differential lung injury due to unilateral pneumonia[1,3,7,17], large air leak from bronchopleural fistula[6,18], pulmonary hemorrhage[6,19], and pulmonary contusion[3,11,20]. ILV has been reported to be useful in patients who develop primary graft dysfunction following single lung transplantation, resulting in a poorly compliant graft lung and a native lung with markedly different lung mechanics[5,21]. However, the data on single lung transplantation is from one center, and additional factors such as role of early extracorporeal membrane oxygenation (ECMO), and effect of double lumen tube (DLT) on bronchial anastomotic healing needs to be considered.
The severity of unilateral lung disease where one should consider ILV is unclear. Most reports have instituted ILV as a rescue strategy after conventional ventilation failed to maintain adequate oxygenation or ventilation. It can be argued that early institution of ILV may be more beneficial in reducing ventilator induced lung injury superimposed on the existing lung injury especially in the non-diseased injured lung. This is especially important with accumulating evidence favoring use of low tidal volumes during positive pressure ventilation of normal healthy lungs[22]. It is conceivable that by reducing lung injury and decreasing shunt, the use of ILV might decrease the need for utilizing more invasive strategies like ECMO, associated with a higher risk of complications. Moreover, ECMO is contraindicated in presence of thrombocytopenia (as in our patient), disseminated intravascular coagulation, or recent tPA use. In addition, ECMO requires a dedicated team and advanced institutional capabilities, which might not be available in resource poor locations. Thus, ILV is likely underutilized and there maybe potential benefit from earlier institution of ILV than typically reported.
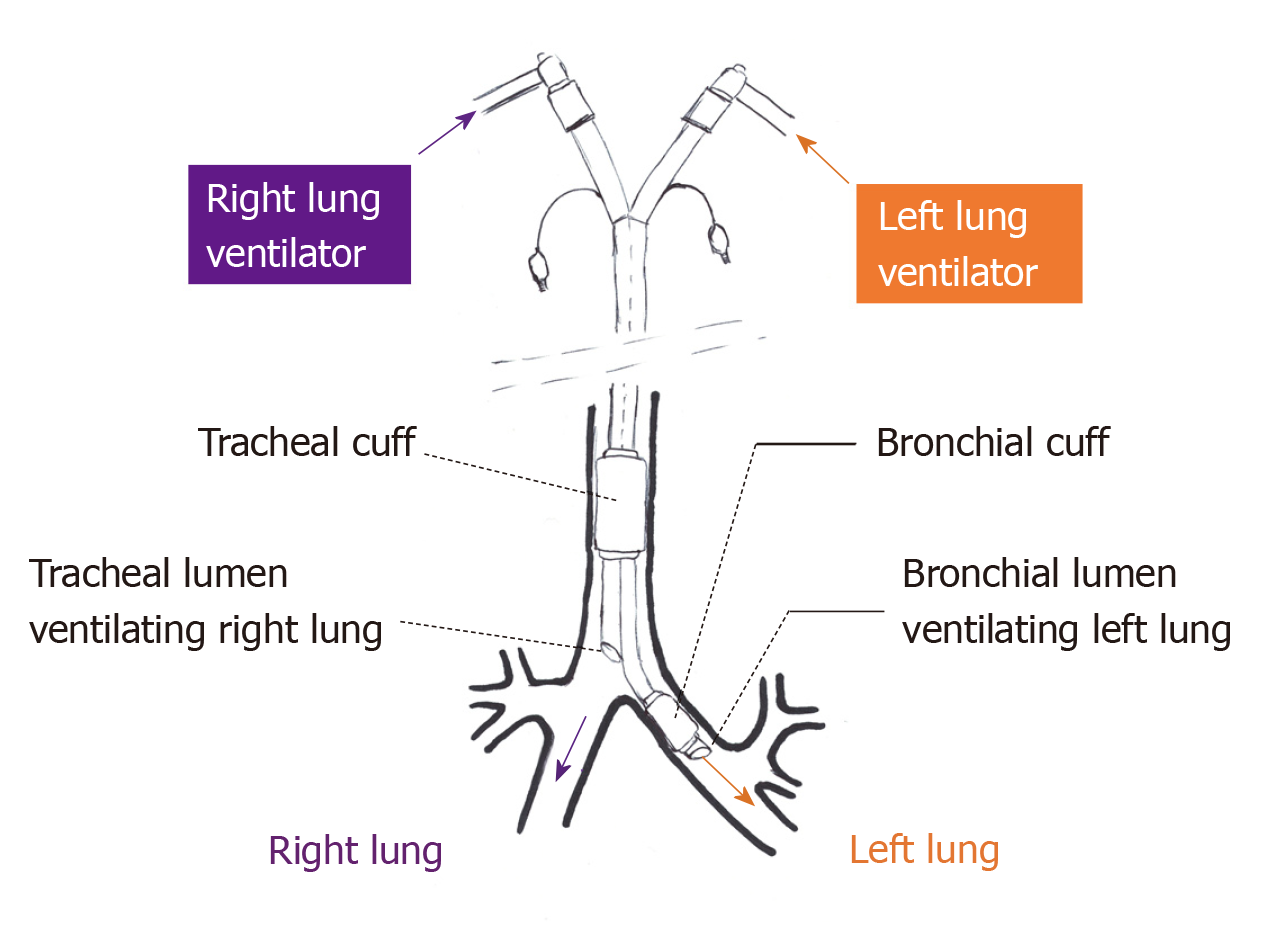
A DLT is most commonly used for lung isolation during thoracic surgery. Similarly, DLT is the most commonly reported method for instituting ILV. DLTs are endotracheal tubes with two lumens and two cuffs (tracheal and bronchial), the tracheal lumen terminating in trachea and the bronchial lumen in either the right or left main stem bronchus (Figure 1). Some others have described using two endotracheal tubes, one for each lung, placed via a tracheostomy[2]. Since the smallest available DLT (26F, outer diameter- 8.7 mm) is recommended for patients between 8 and 10 years of age[23], endotracheal intubation with two single lumen tubes is the only way to achieve ILV in younger pediatric patients[9].
Interruption of ventilation, though momentary, during placement of DLT has potential for significant hypoxemia, especially in a critically ill patient with limited reserve. This risk is especially significant in patients with high levels of ventilator support, or in patients with a difficult airway. Thus, these need to be performed by individuals experienced with airway management, with difficult airway equipment and bronchoscope at the bedside.
Though anatomical separation is confirmed with bronchoscopy, adequate functional separation needs to be established as well. In the past, investigators have assessed functional lung separation by either water bubble or balloon inflation techniques. However, these require temporary interruption of ventilation and might not be a feasible strategy for an ICU patient with limited reserve. Functional separation can be assessed with most modern ventilators by measuring the inspired and expired tidal volumes from each lung. Loss or gain of tidal volume would suggest a leak. However, interpretation may be more difficult in the presence of a bronchopleural fistula.
Management of patients on ILV, outside of ventilation strategies, should be guided by the patient’s needs and not influenced by institution of ILV. Though paralysis was thought to be necessary for institution of ILV, use of ILV without paralysis is reported[4]. However, DLT is more stimulating to the airway than a single lumen tube and might require more sedation for patient tolerance and comfort.
Lung isolation is maintained in the operating room under the constant surveillance of an anesthesia provider experienced in airway and lung isolation. ILV may be safely performed in the ICU with nurses and respiratory therapists properly trained in the care of patients receiving ILV. They should be able to identify and notify a clinician when endotracheal tube dislodgement is suspected. Tube malposition may inadvertently occur during patient movement or during routine change of patient’s position[24]. Malposition should be suspected with sudden change in tidal volumes, or an increase in airway pressure. When dislodgement is suspected bronchoscopic assessment should be performed quickly to re-establish appropriate tube position.
DLTs have low volume high pressure cuffs. If not monitored, bronchial cuff pressure may be as high a 50 mmHg with as little as 2 cc of air[25]. The effects of prolonged use of a bronchial cuff on bronchial mucosal blood flow is unknown, since most data is from intraoperative literature where lung isolation only lasts for a few hours. In addition, a critically ill patient might already have a compromised mucosal blood flow, increasing the risk of mucosal ischemia. Ideally, cuff pressure should be maintained at 25 to 30 cm H2O by an automated continuous pressure cuff controller preventing tracheal mucosa injury and air leak at peak inspiratory pressure. Complications reported to be associated with DLT use include bronchial ischemia and stenosis, bronchial rupture resulting in pneumothorax, pneumo-mediastinum and subcutaneous emphysema[7]. Though the typical duration of ILV reported in literature ranges from 2 to 4 d, some have used it for over two weeks without complications[3,7].
Physiological separation of lungs requires ability to independently alter ventilator parameters for each lung. This is best achieved using two separate ventilators one for each lung. Historically, a single ventilator had been used to ventilate two lungs, however in most cases each lung requires a different PEEP level. This was accomplished by connecting one ventilator to both limbs of the DLT through a Y-connector. This strategy allows for independent titration of PEEP between the two lungs, by adding a PEEP valve between the Y-connector and the limb of DLT ventilating the lung requiring additional PEEP. This approach is suboptimal as the presence of a PEEP valve in the circuit may impede accurate measurement of airway pressure by the ventilator, and generation of high levels of auto-PEEP might not be detected by the ventilator. In addition, other parameters such as tidal volume, respiratory rate and inspired oxygen concentration cannot be independently altered with this approach. Using a separate ventilator for each lung allows for independent adjustment of ventilator parameters, an essential feature for optimization of patients with ILV.
Synchronous vs asynchronous ventilation results from the presence or absence of coordination between ventilated breaths provided to each lung. A single ventilator strategy evidently delivers synchronous ventilation. While using two ventilators, the most common strategy for ILV, synchronous ventilation can be accomplished by electronically linking the two ventilators using an external cable. Initiation of ventilation by one ventilator would transmit a signal through the external cable triggering the second ventilator resulting in near simultaneous delivery of a breath by that ventilator. It was thought that asynchronous ILV might result in cardiovascular compromise, from decreased (systemic and pulmonary) venous return as inflation of each lung at different times would result in elevated intrathoracic pressure for a longer duration of time. Subsequently, it has been shown that asynchronous ventilation strategies can be instituted without these concerns and is equally well tolerated by patients[17]. Asynchronous ventilation strategy with two ventilators is much less complicated, offer greater flexibility allowing for individual titration of ventilation parameters, and thus is the preferred strategy for ILV.
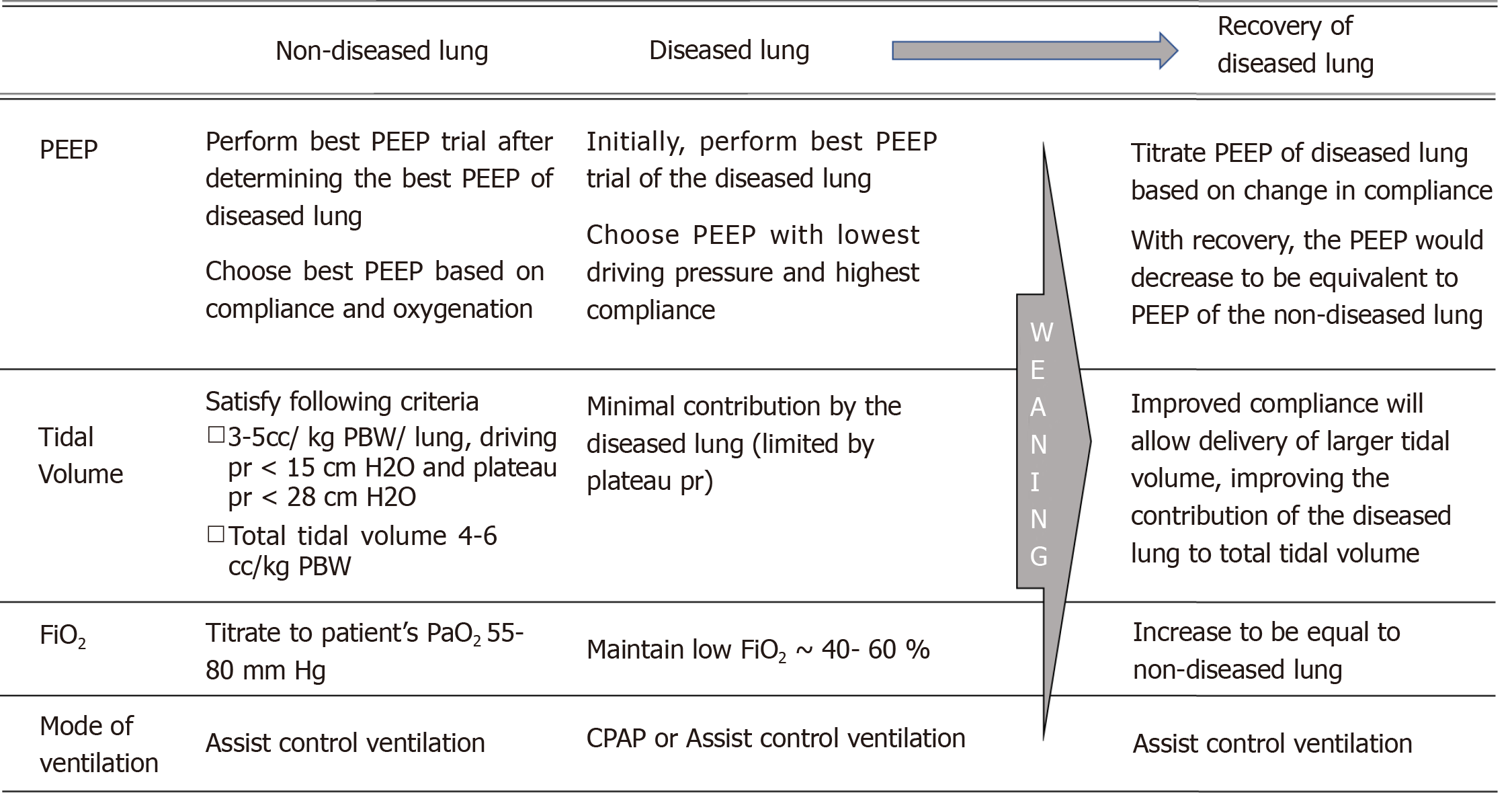
The selection of ventilator strategy for ILV is guided by the underlying pathology of each lung based and on principles of lung protective ventilation. Institution of ILV in patients with different lung compliances can ensure delivery of an appropriate tidal volume to each lung. Most of the literature on ventilation strategies during single lung ventilation comes from thoracic anesthesia literature, but may be extrapolated to ILV. Below we describe some principles for determining optimal ventilator parameters during ILV (Figure 2).
Positive end expiratory pressure: As in conventional ventilation, positive end expiratory pressure (PEEP) in ILV should be determined based on a PEEP titration trial (‘best PEEP’ trial) to identify the optimal PEEP providing highest lung compliance and adequate oxygenation. Since compliance of the diseased and non-diseased lung are markedly different, the best PEEP for each lung should be determined separately and instituted independently. Certain factors unique to ILV, must be considered while performing a best PEEP trial for each lung. Due to the impairment in gas exchange associated with severe unilateral lung disease, the diseased lung largely functions as a shunt, contributing to hypoxemia. A high PEEP applied to the normal lung may further worsen shunting through the diseased lung, and thereby worsen oxygenation.
The best strategy would be to initially perform a best PEEP trial of the diseased lung. The PEEP trial in the diseased lung should be primarily driven by compliance, since the diseased lung has minimal contribution to gas exchange. The PEEP resulting in the lowest driving pressure or the highest compliance might be chosen as the optimal PEEP in the diseased lung. Subsequently, a best PEEP trial for the non-diseased lung may follow. Determination of best PEEP of the non-diseased lung should also consider chronic underlying pathology such as asthma, emphysema or pulmonary fibrosis. Since increasing PEEP on the non-diseased lung may worsen shunting and hypoxemia, titration of optimal PEEP in the non-diseased should be based on oxygenation and compliance, rather than compliance alone.
Tidal volume, driving pressure and minute ventilation: In patients with lung injury or adult respiratory distress syndrome (ARDS) receiving conventional ventilation, protective lung ventilation involves limiting tidal volume to 4 to 8 cc/kg of predicted body weight (kg PBW), plateau pressures < 28 cmH20 and driving pressure < 15 cmH2O. Maintaining a tidal volume lower than 5 cc/ kg PBW and a plateau pressure lower than 28 cmH2O during one lung ventilation has consistently been associated with decreased lung injury in patients undergoing lung surgeries[26]. These estimates are based on ventilation for a few hours during surgery, as opposed to ILV in ICU which may last days. Also, there is strong evidence on the benefits of low tidal volume ventilation, even when used intraoperatively for a few hours, in patients with normal lungs[22]. Thus a low tidal volume strategy (3 to 5 cc/kg PBW) should be adhered to separately for each lung, including the non-diseased lung, during ILV. The tidal volume delivered to the diseased lung may be further limited by need to keep plateau pressure less than 28 cmH2O and driving pressure < 15 cmH2O. Since lower driving pressures is known to independently determine survival in ARDS, ability to keep driving pressure below 15 cmH2O in the diseased lung should primarily drive the delivered tidal volume[27]. This might be best achieved by using a pressure control ventilation strategy in the diseased lung. Overall, it should be ensured that the additive tidal volume delivered to both lungs should not exceed 6-8 cc/kg PBW and that the plateau pressure and driving pressure for each lung is below 28 and 15 cmH2O, respectively.
During ILV, each lung may have different minute ventilations, tidal volumes and respiratory rates. In the initial period, more benefit would be obtained by titrating the minute ventilation of the non-diseased lung to pCO2, since it contributes most to ventilation. The ventilation strategy to be instituted for the diseased lung when it is not contributing to ventilation is unclear. There exists some evidence for providing lung rest (very low frequency positive pressure ventilation) and thus decreasing volutrauma, while instituting extracorporeal CO2 removal in patients with hypercarbic respiratory failure[28,29]. Extrapolating that data to ILV, one may advocate for just providing continuous positive airway pressure to the diseased lung, especially in the presence of a severely diseased lung where the plateau and driving pressure are high. This may especially be considered when the diseased lung is not contributing much to oxygenation or CO2 clearance. With improvement in compliance of the diseased lung and radiological improvement, ventilation can be resumed in a stepwise manner. One should favor permissive hypercapnia than to choose ventilator settings that contributes to lung injury.
Fractional concentration of inspired oxygen: Inspired oxygen concentration (FiO2) of the non-diseased lung should be determined based on the systemic oxygenation. The FiO2 of the non-diseased lung should be titrated to maintain the partial pressure of arterial oxygen between 55 and 80 mmHg and SpO2 between 88% and 95%. Various considerations exist while choosing FiO2 for the diseased lung. A lower FiO2 in the diseased lung may result in poorer oxygenation of the blood circulating through the diseased lung, thereby worsening the impact of shunt. On the other hand, a higher FiO2 may result in an increased risk for hyperoxic injury to the diseased lung. Also, the higher FiO2 in the diseased lung might mitigate the hypoxic pulmonary vasoconstriction, thereby worsen shunt through the diseased lung. FiO2 for the diseased lung should be titrated based on these competing factors. Thus, when the disease severity results in minimal contribution to oxygenation by the diseased lung, an FiO2 between 40% and 60% might be favorable. This could be further titrated based on its impact on systemic oxygenation. Once the disease severity improves and the diseased lung contributes to oxygenation, the FiO2 in that lung may be titrated similarly and equally with that of the non-diseased lung, to optimize systemic oxygenation.
Mode of ventilation: Various modes of ventilation have been reported with ILV, based on the underlying pathology and the comfort of the critical care team instituting ILV. These include assist control volume or pressure ventilation, pressure support ventilation, or high frequency oscillatory ventilation. Assist control is the most commonly utilized mode for ILV reported in literature. In a severely diseased low compliant lung which is not contributing significantly to oxygenation or ventilation, continuous positive airway pressure may be utilized initially. Though various studies have shown no mortality benefit with using high frequency oscillatory ventilation in severe ARDS[30], its role when preferentially applied to the diseased lung in ILV is uncertain. As the diseased lung begins to recover, an assist control pressure ventilation targeting driving pressures < 15 cmH2O might be a useful strategy.
Evaluation of the readiness to wean the ventilator requirements should happen regularly and independently for each individual lung. However, ventilator parameters of the diseased lung can only be weaned when its pathological process begins to resolve. An important goal of weaning ventilator support in ILV is continual assessment of lung mechanics of each lung independently, to evaluate feasibility of transitioning to conventional ventilation using a single lumen endotracheal tube and one ventilator.
Though weaning happens separately for each lung during ILV, changing support on one lung may affect the other. The following considerations and principles should be borne in mind while weaning from ILV (Figure 2).
FiO2: When the diseased lung is not contributing to gas exchange, the FiO2 of the non-diseased lung may be weaned based on systemic oxygenation. However, as the diseased lung starts recovering and contributes to gas exchange, its FiO2 may be titrated similarly (and made equal) to that of the non-diseased lung.
PEEP: Weaning PEEP may occur separately for each lung based on the ‘best PEEP’ calculated for each lung, and principles previously discussed. The goal of PEEP titration is to maintain maximum compliance in each lung and thereby minimizing driving pressures. As the diseased lung recovers, its compliance improves resulting in a reduced level of PEEP, bringing it closer to that of the non-diseased lung.
Tidal volume: If delivery of adequate tidal volume was initially limited in the diseased lung to maintain a lung protective driving pressure (< 15 cmH2O), improvement in disease process will allow delivery of adequate tidal volume (3- 5 cc/ kg PBW/ lung).
Mode of ventilation: If separate modes of ventilation were used for each lung during ILV, recovery of the diseased lung should allow use of same mode. Assist control ventilation is the preferred mode of ventilation for both lungs, before transitioning to conventional ventilation.
Various measures have been described in the literature to determine the readiness to transition back from ILV to conventional single ventilator ventilation (Table 2). These are primarily based on assessment of improvement in the underlying unilateral lung pathology. The goal is to ensure that restoration of standard single ventilator ventilation would not result in markedly unequal distribution of tidal volumes resulting in volutrauma, or exacerbation of leak in bronchopleual fistula. With resolution of the unilateral lung pathology, lung mechanics, which were initially markedly different between the lungs, will progressively converge. Perhaps the most important parameter to follow would be individual lung compliances. Similar compliance between the two lungs would ensure that tidal volume delivered during conventional ventilation would be comparably distributed to each lung. Some authors have successfully discontinued ILV when the tidal volume and compliance differed between the lungs by less than 100 mL and 20%, respectively[11,31]. Use of capnography for each lung has shown that the diseased lung often has a much lower end tidal carbon dioxide concentration, likely from its minimal contribution to ventilation. Equivalence of end tidal carbon dioxide concentration between the two lungs during ILV could point towards comparable contribution to ventilation by each lung[31]. Other indicators would be radiological improvement and decrease in air leak from the chest tube in patients with unilateral bronchopleural fistula.
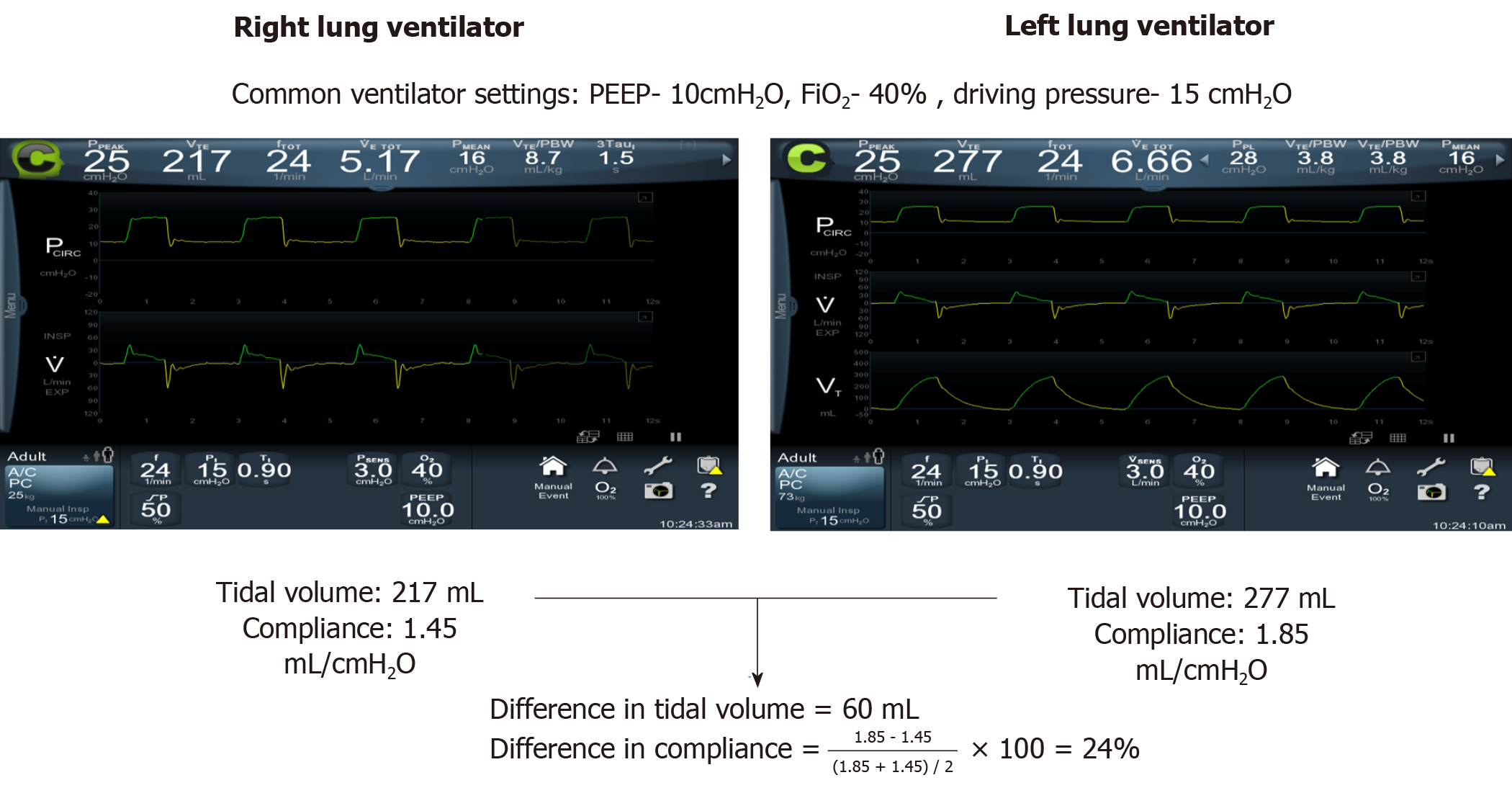
| Near complete or complete resolution of the disease process- clinically or radiologically |
| Difference in tidal volume between the two lungs < 100 cc |
| Difference in compliance between the two lungs < 20% |
| Difference in end tidal carbon dioxide concentration between the two lungs < 20% |
Before institution of single ventilator ventilation, its feasibility should be measured by temporarily ventilating each lung with the exact same settings. It is best achieved by ventilating both lungs using assist control pressure ventilation. This allows one to use the same settings (FiO2, PEEP, driving pressure, and minute ventilation) when transitioning to conventional single ventilator ventilation. Maintaining oxygenation should not be the sole criteria for determining feasibility. Presence of markedly different compliances may result in adequate oxygenation, but could result in volutrauma to the healthy lung. Thus, comparable compliance and tidal volume (Table 2) in each lung on the same ventilator settings establishes feasibility for switching to single ventilator ventilation. Figure 3 compares tidal volumes and compliance for each lung in our patient, before conventional ventilation was instituted. Continuation of ILV also needs to be weighed against the risks associated with the duration of ILV. The deeper sedation necessary with ILV prevents patient participation in physical therapy, and minimizes patient effort in ventilation causing respiratory muscle atrophy. Longer duration of ILV may also increase the risk of airway mucosal injury from DLT. Moreover, with resolution of underlying pathology, mucus plugging and secretion clearance could become important considerations. Suctioning or bronchoscopic clearance of secretions are difficult through a DLT due to its narrow lumen, but may be more easily accomplished through a single lumen tube. Once single ventilator ventilation is tolerated, the DLT can be exchanged to a single lumen tube and conventional ventilation instituted.
Unilateral lung injury presents a markedly different scenario from the heterogeneous lung injury seen with ARDS. ILV is likely the most optimal way to provide lung protective ventilation in patients with severe unilateral lung pathology, thereby avoiding ECMO, which is more invasive and unavailable in resource poor locations. Safe utilization of ILV requires education and a collaborative effort by critical care nurses, respiratory therapists and physicians. With the stepwise clinical flow-chart proposed here, we hope to encourage more utilization of ILV. However, optimal strategies for ventilating the diseased lung and weaning from ILV needs further characterization.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nacak M, Nardo MD S-Editor: Ma YJ L-Editor: A E-Editor: Wu YXJ
| 1. | Fujita M, Tsuruta R, Oda Y, Kaneda K, Miyauchi T, Kasaoka S, Maekawa T. Severe Legionella pneumonia successfully treated by independent lung ventilation with intrapulmonary percussive ventilation. Respirology. 2008;13:475-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Skjeflo GW, Dybwik K. A new method of securing the airway for differential lung ventilation in intensive care. Acta Anaesthesiol Scand. 2014;58:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Yamakawa K, Nakamori Y, Fujimi S, Ogura H, Kuwagata Y, Shimazu T. A novel technique of differential lung ventilation in the critical care setting. BMC Res Notes. 2011;4:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Achar SK, Chaudhuri S, Krishna H, Sagar M. Re-expansion pulmonary oedema - differential lung ventilation comes to the rescue. Indian J Anaesth. 2014;58:330-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Badesch DB, Zamora MR, Jones S, Campbell DW, Fullerton DA. Independent ventilation and ECMO for severe unilateral pulmonary edema after SLT for primary pulmonary hypertension. Chest. 1995;107:1766-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Shekar K, Foot CL, Fraser JF. Independent lung ventilation in the intensive care unit: desperate measure or viable treatment option? Crit Care Resusc. 2008;10:144-148. [PubMed] |
| 7. | Graciano AL, Barton P, Luckett PM, Morriss F, Sommerauer JF, Toro-Figueroa LO. Feasibility of asynchronous independent lung high-frequency oscillatory ventilation in the management of acute hypoxemic respiratory failure: a case report. Crit Care Med. 2000;28:3075-3077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Murkute A, Angadi U, Jain P, Sharique T, Hegde R. Paediatric pulmonary haemorrhage: Independent lung ventilation as effective strategy in management. Indian J Crit Care Med. 2014;18:694-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Di Nardo M, Perrotta D, Stoppa F, Cecchetti C, Marano M, Pirozzi N. Independent lung ventilation in a newborn with asymmetric acute lung injury due to respiratory syncytial virus: a case report. J Med Case Rep. 2008;2:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Plötz FB, Hassing MB, Sibarani-Ponsen RD, Markhorst DG. Differentiated HFO and CMV for independent lung ventilation in a pediatric patient. Intensive Care Med. 2003;29:1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Cinnella G, Dambrosio M, Brienza N, Bruno F, Brienza A. Compliance and capnography monitoring during independent lung ventilation: report of two cases. Anesthesiology. 2000;93:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Campos JH. Lung isolation techniques. Anesthesiol Clin North Am. 2001;19:455-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Campos JH. Progress in lung separation. Thorac Surg Clin. 2005;15:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Narayanaswamy M, McRae K, Slinger P, Dugas G, Kanellakos GW, Roscoe A, Lacroix M. Choosing a lung isolation device for thoracic surgery: a randomized trial of three bronchial blockers versus double-lumen tubes. Anesth Analg. 2009;108:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Siegel JH, Stoklosa JC, Borg U, Wiles CE, Sganga G, Geisler FH, Belzberg H, Wedel S, Blevins S, Goh KC. Quantification of asymmetric lung pathophysiology as a guide to the use of simultaneous independent lung ventilation in posttraumatic and septic adult respiratory distress syndrome. Ann Surg. 1985;202:425-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Parish JM, Gracey DR, Southorn PA, Pairolero PA, Wheeler JT. Differential mechanical ventilation in respiratory failure due to severe unilateral lung disease. Mayo Clin Proc. 1984;59:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Stow PJ, Grant I. Asynchronous independent lung ventilation. Its use in the treatment of acute unilateral lung disease. Anaesthesia. 1985;40:163-166. [PubMed] |
| 18. | Minhas JS, Halligan K, Dargin JM. Independent lung ventilation in the management of ARDS and bronchopleural fistula. Heart Lung. 2016;45:258-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Sarnaik A. The use of independent lung ventilation for unilateral pulmonary hemorrhage. Int J Respir Pulm Med. 2015;2:13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Rico FR, Cheng JD, Gestring ML, Piotrowski ES. Mechanical ventilation strategies in massive chest trauma. Crit Care Clin. 2007;23:299-315, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Pilcher DV, Auzinger GM, Mitra B, Tuxen DV, Salamonsen RF, Davies AR, Williams TJ, Snell GI. Predictors of independent lung ventilation: an analysis of 170 single-lung transplantations. J Thorac Cardiovasc Surg. 2007;133:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ladha K, Vidal Melo MF, McLean DJ, Wanderer JP, Grabitz SD, Kurth T, Eikermann M. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 23. | Seefelder C. Use of the 26-French double-lumen tube for lung isolation in children. J Cardiothorac Vasc Anesth. 2014;28:e19-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Inoue S, Nishimine N, Kitaguchi K, Furuya H, Taniguchi S. Double lumen tube location predicts tube malposition and hypoxaemia during one lung ventilation. Br J Anaesth. 2004;92:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Brodsky JB, Adkins MO, Gaba DM. Bronchial cuff pressures of double-lumen tubes. Anesth Analg. 1989;69:608-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg. 2015;121:302-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 27. | Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1595] [Article Influence: 159.5] [Reference Citation Analysis (2)] |
| 28. | Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P, Dell'Amore A, Urbino R, Mascia L, Evangelista A, Antro C, D'Amato R, Sucre MJ, Simonetti U, Persico P, Nava S, Ranieri VM. Extracorporeal Co2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med. 2015;43:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 29. | Gattinoni L, Agostoni A, Pesenti A, Pelizzola A, Rossi GP, Langer M, Vesconi S, Uziel L, Fox U, Longoni F, Kolobow T, Damia G. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2:292-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 160] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Ng J, Ferguson ND. High-frequency oscillatory ventilation: still a role? Curr Opin Crit Care. 2017;23:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Cinnella G, Dambrosio M, Brienza N, Giuliani R, Bruno F, Fiore T, Brienza A. Independent lung ventilation in patients with unilateral pulmonary contusion. Monitoring with compliance and EtCO(2). Intensive Care Med. 2001;27:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 32. | Anantham D, Jagadesan R, Tiew PE. Clinical review: Independent lung ventilation in critical care. Crit Care. 2005;9:594-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |