Published online Feb 4, 2017. doi: 10.5492/wjccm.v6.i1.65
Peer-review started: August 25, 2016
First decision: October 20, 2016
Revised: November 6, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 4, 2017
Processing time: 151 Days and 4.4 Hours
To characterize the prescribing patterns for hydrocortisone for patients with septic shock and perform an exploratory analysis in order to identify the variables associated with better outcomes.
This prospective cohort study included 59 patients with septic shock who received stress-dose hydrocortisone. It was performed at 2 critical care units in academic hospitals from June 1st, 2015, to July 31st, 2016. Demographic data, comorbidities, medical management details, adverse effects related to corticosteroids, and outcomes were collected after the critical care physician indicated initiation of hydrocortisone. Univariate comparison between continuous and bolus administration of hydrocortisone was performed, including multivariate analysis, as well as Kaplan-Meier analysis to compare the proportion of shock reversal at 7 d after presentation. Receiver operating characteristic (ROC) curves determined the best cut-off criteria for initiation of hydrocortisone associated with the highest probability of shock reversal. We addressed the effects of the taper strategy for discontinuation of hydrocortisone, noting risk of shock relapse and adverse effects.
All-cause 30-d mortality was 42%. Hydrocortisone was administered as a continuous infusion in 54.2% of patients; time to reversal of shock was 49 h longer in patients who were given a bolus administration [59 h (range, 47.5-90.5) vs 108 h (range, 63.2-189); P = 0.001]. The maximal dose of norepinephrine after initiation of hydrocortisone was lower in patients on continuous infusion [0.19 μg/kg per minute (range, 0.11-0.28 μg)] compared with patients who were given bolus [0.34 μg/kg per minute (range, 0.16-0.49); P = 0.004]. Kaplan-Meier analysis revealed a higher proportion of shock reversal at 7 d in patients with continuous infusion compared to those given bolus (83% vs 63%; P = 0.004). There was a good correlation between time to initiation of hydrocortisone and time to reversal of shock (r = 0.80; P < 0.0001); ROC curve analysis revealed that the best criteria for prediction of shock reversal was a time to initiation of hydrocortisone of ≤ 13 h after administration of norepinephrine, with an area under the curve of 0.81 (P < 0.001). The maximal dose of norepinephrine at initiation of hydrocortisone with the highest association with shock reversal was ≤ 0.28 μg/kg per minute, with an area under the curve of 0.75 (P = 0.0002). On a logistic regression model, hydrocortisone taper was not associated with a lower risk of shock relapse (RR = 1.29; P = 0.17) but was related to a higher probability of hyperglycemia [odds ratio (OR), 5.3; P = 0.04] and hypokalemia (OR = 10.6; P = 0.01).
Continuous infusion of hydrocortisone could hasten the resolution of septic shock compared to bolus administration. Earlier initiation corresponds with a higher probability of shock reversal. Tapering strategy is unnecessary.
Core tip: Until now, the indications, timing, administration, and discontinuation of hydrocortisone for septic shock patients have been widely variable. Our study found that continuous infusion was the most effective method compared to bolus administration; we also identified a time from vasopressor administration of ≤ 13 h and/or a norepinephrine dose of ≤ 0.28 μg/kg per minute as the best clinical criteria for initiation of hydrocortisone. We found no benefit from the tapering strategy, which was only associated with a higher incidence of hyperglycemia and hypokalemia.
- Citation: Ibarra-Estrada MA, Chávez-Peña Q, Reynoso-Estrella CI, Rios-Zermeño J, Aguilera-González PE, García-Soto MA, Aguirre-Avalos G. Timing, method and discontinuation of hydrocortisone administration for septic shock patients. World J Crit Care Med 2017; 6(1): 65-73
- URL: https://www.wjgnet.com/2220-3141/full/v6/i1/65.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v6.i1.65
Since William Schumer stated in the mid-1970s that early administration of adjunctive steroids could be helpful in the management of patients with septic shock[1], investigators have developed experimental animal and human trials to study the role of corticosteroid therapy; however, this benefit remains controversial[2,3]. The controversy may exist because the studies have varied in their design, steroid preparation, dose, strategy of administration (intermittent bolus or continuous infusion), length of therapy, time of initiation, and patterns of discontinuation[4].
Corticosteroids had been shown to be associated with a faster reversal of shock compared to placebo[5-9]. For that reason, the 2012 Surviving Sepsis Campaign Guidelines recommended administration of hydrocortisone (200 mg/d) if hemodynamic stability is not achievable after fluid resuscitation and vasopressor therapy[2]. Nevertheless, the patterns in clinical practice remain widely heterogeneous because of differing interpretations of the definition of poor responsiveness of shock to fluid and vasopressor therapy, discrepancy between clinicians’ interpretation of guidelines, discrepancy in clinical practice, and unfamiliarity with existing evidence[10].
The aim of this observational study was to characterize the use of hydrocortisone in septic shock patients in order to identify the most effective method of administration and withdrawal, and to find the best clinical criteria for initiation of corticosteroid therapy to increase the probability of shock reversal.
This was a prospective cohort study conducted in 2 medical/surgical intensive care units at tertiary academic hospitals from June 1st, 2015, through July 31st, 2016. All patients recruited in Instituto Jalisciense de Cancerología had oncologic disease; there were no oncologic patients recruited at Hospital Civil Fray Antonio Alcalde. Inclusion criteria for patients were a diagnosis of septic shock, defined as sepsis induced hypotension persisting despite adequate fluid resuscitation[2], for which the attending intensivist determined the need for adjunct hydrocortisone therapy at a stress dose (no more than 200 mg/d), regardless of the timing and method of administration. Shock reversal was considered when the arterial pressure remained stable (SAP > 90 mmHg or MAP > 70 mmHg without requirement of new vasopressor infusion) for more than 24 h. Relapse was defined as recurrence of septic shock, requiring norepinephrine resumption within first 7-d after reversal. Patients with a previous diagnosis of adrenal insufficiency, who received hydrocortisone at a dose more than 200 mg/d, and who died within the first 48 h after intensive care unit (ICU) admission were excluded. The scientific and ethics committees at Instituto Jalisciense de Cancerología (INV-01/16) and Hospital Civil Fray Antonio Alcalde (HCG/CEI-0321/16) approved this investigation.
After patients with septic shock were deemed candidates for initiation of hydrocortisone, informed consent was obtained from patient or their next of kin, and data were prospectively collected. Recorded information included demographic data, comorbidities, maximal dose (calculated to ideal body weight) and length of vasopressor requirement, timing, method of administration and discontinuation of hydrocortisone, adverse effects of corticosteroids, ICU length of stay, time to death, and 30-d mortality. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score and the Sequential Organ Failure Assessment score were calculated within the first day of ICU admission.
Continuous variables were reported as the mean [standard deviation (SD)] if they were normally distributed, or the median [interquartile range (IQR)] if they were not normally distributed, according to the Shapiro-Wilk test. A Mann-Whitney or t-test was used for comparison between groups as appropriate. We used a two-way mixed ANOVA test for comparison between pre- and post- hydrocortisone maximal doses of norepinephrine. Categorical variables were expressed as the number of measurements (%) and were compared by χ2 test. We constructed receiver-operating characteristic (ROC) curves for time to initiation of hydrocortisone and dosage of norepinephrine at initiation of hydrocortisone to determine the ability for prediction of shock reversal; optimal cut-off values were obtained with the greatest sum of sensitivity and specificity using the Youden index[11]. The relationship between time to initiation of hydrocortisone and total duration of shock was estimated with the Spearman correlation coefficient test. We performed a Kaplan-Meier analysis to compare the shock reversal rate at 7 d between continuous and bolus administration. Multivariate logistic regression was performed to identify factors associated with shock reversal and adverse effects. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test, considered as adequate if P > 0.05[12]. Based on vasopressor dosages in a previous study of septic shock patients with our same settings[13], we determined that 26 patients with continuous infusion and 26 with bolus administration of hydrocortisone would be needed to detect a difference of 0.10 μg/kg per minute in norepinephrine maximal dosage from 12 h after initiation of corticosteroid with a 90% statistical power and type I error of 5%. For all tests, P-values were two-sided, and a value lower than 0.05 was considered statistically significant. We used MedCalc (Ver 16.4.3, Ostend, Belgium) for calculating sample size and for the statistical analysis.
The statistical methods of this study were reviewed by Miguel A. Ibarra-Estrada, clinical investigator and biomedical statistics analysis expert from Critical Care Unit, Instituto Jalisciense de Cancerología, Guadalajara Jalisco 44280, Mexico; Critical Care Unit, Hospital General Regional #180, Instituto Mexicano del Seguro Social, Tlajomulco de Zúñiga Jalisco 45655, Mexico.
Throughout the study period, 826 patients were admitted to both ICUs, of which 66 (7.9%) had a diagnosis of septic shock; 59 patients met the inclusion criteria because 7 subjects died within the first 48 h (Figure S1, supplementary material). The median age was 57 years (IQR, 38-65), 26 patients (44.1%) were men, 25 patients (42.4%) were oncologic, and 42 patients (71.2%) were surgical patients. The most common source of infection was pneumonia, which presented in 26 patients (44.1%). The mean APACHE II score was 21.5 (SD ± 5.8). Hydrocortisone was administered as a continuous infusion in 54.2% of patients; the median dose of norepinephrine at initiation of hydrocortisone was 0.3 μg/kg per minute (IQR, 0.18-0.39), there were no systemic steroids administered other than hydrocortisone, time from norepinephrine to initiation of hydrocortisone was 12 h (IQR, 6-27), and length of vasopressor requirement was 83 h (IQR, 49-120). Among survivors, hydrocortisone was tapered in 23 patients (53.5%). Overall 30-d mortality was 42.4%.
There were no significant differences in demographic and baseline clinical characteristics between patients in the continuous infusion and bolus groups (Table 1). We found no differences in these characteristics between recruitment centers (Table S1, supplementary material). Patients in the bolus group received hydrocortisone 6 h later than patients with continuous infusion (P = 0.01), and time to shock reversal was 49 h longer (P = 0.001). Concerning adverse affects, bolus administration was significantly associated with higher incidence of new onset hyperglycemia, with a relative risk (RR) of 2.7 (P = 0.03); hypokalemia was also more common with bolus administration, with a RR of 1.8 (P = 0.03). There was a trend to higher mortality in the bolus group, but this was not statistically significant (RR = 1.5; P = 0.06).
| Characteristics | Continuous infusion (n = 32) | IV bolus (n = 32) | P value |
| Age, median (IQR) | 50 (37-64) | 61 (39-70) | 0.19 |
| Male gender, n (%) | 12 (37.5) | 14 (51.9) | 0.27 |
| Oncologic disease, n (%) | 15 (46.9) | 10 (37) | 0.45 |
| Surgical patients, n (%) | 25 (78.1) | 17 (63) | 0.2 |
| Infection source, n (%) | |||
| Pneumonia | 13 (40.6) | 13 (48.1) | 0.56 |
| Ventilator associated | 7 (21.8) | 6 (22.2) | 0.87 |
| Health care associated | 3 (9.3) | 4 (12.5) | 0.66 |
| Community acquired | 3 (9.3) | 3 (11.1) | 0.52 |
| Abdomen | 14 (43.7) | 10 (37) | 0.6 |
| Soft tissue | 4 (12.5) | 1 (3.7) | 0.23 |
| Urinary tract | 1 (3.1) | 2 (7.4) | 0.45 |
| Other | 0 (0) | 1 (3.7) | 0.27 |
| Diabetes, n (%) | 8 (25) | 4 (14.8) | 0.33 |
| Acute kidney injury, n (%) | 14 (43.7) | 17 (63) | 0.14 |
| Baseline creatinine, mg/dL, median (IQR) | 0.8 (0.7-1.4) | 1.1 (0.7-1.5) | 0.32 |
| ARDS, n (%) | 10 (31.2) | 11 (40.7) | 0.45 |
| APACHE II score (SD) | 21 ± 6 | 21.7 ± 5.6 | 0.76 |
| SOFA score (SD) | 10 ± 2.9 | 11 ± 2.7 | 0.16 |
| Vasopressin use, n (%) | 12 (37.5) | 4 (14.8) | 0.5 |
| Maximum NE dose (mcg/kg per minute), median (IQR) | 0.25 (0.17-0.36) | 0.33 (0.20-0.39) | 0.55 |
| Hydrocortisone dose (mg/kg per day), median (IQR) | 2.63 ± 0.27 | 2.75 ± 0.31 | 0.13 |
| NE to hydrocortisone (h), median (IQR) | 8 (4-19.5) | 14 (8-31.5) | 0.01 |
| Time to shock reversal (h), median (IQR) | 59 (47.5-90.5) | 108 (63.2-189) | 0.001 |
| Shock relapse, n (%) | 4 (18.2) | 7 (38.9) | 0.14 |
| Hydrocortisone tapered, n (%) | 10 (41.7) | 13 (68.4) | 0.08 |
| Diuretic use, n (%) | 19 (59.4) | 11 (40.7) | 0.15 |
| New onset hypernatremia, n (%) | 17 (53.1) | 18 (66.7) | 0.29 |
| New onset hypokalemia, n (%) | 12 (37.5) | 18 (66.7) | 0.02 |
| New onset hyperglycemia, n (%) | 19 (59.4) | 23 (85.2) | 0.03 |
| Superinfection, n (%) | 3 (9.4) | 5 (18.5) | 0.31 |
| Wound dehiscence, n (%) | 3 (9.4) | 2 (7.4) | 0.78 |
| UGIB, n (%) | 1 (3.1) | 0 (0) | 0.35 |
| ICU-AW, n (%) | 8 (25) | 9 (33.3) | 0.48 |
| Vasopressor-free days, median (IQR) | 3 (2-5) | 2 (0-3.7) | 0.12 |
| ICU LOS, median (IQR) | 8.5 (6-13) | 9 (5-13) | 0.81 |
| 30-d mortality, n (%) | 10 (31.2) | 15 (55.6) | 0.06 |
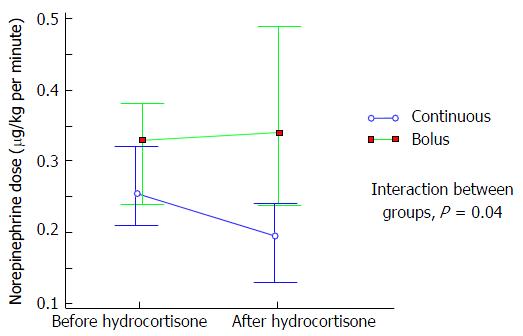
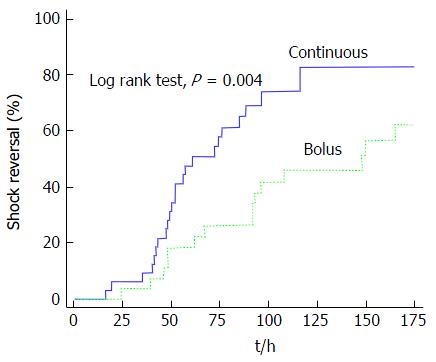
Regarding efficacy, continuous infusion was associated with a lower norepinephrine maximal dose requirement, from 12 h after hydrocortisone initiation. At two-way mixed ANOVA test, the maximal dose of norepinephrine for patients with continuous infusion after initiation of hydrocortisone was 0.19 μg/kg per minute, compared to 0.34 μg/kg per minute for patients on bolus administration, with a significant interaction between groups (P = 0.04; Figure 1). At Kaplan-Meier analysis, continuous infusion was also significantly associated with a higher proportion of shock reversal at 7 d after presentation of shock (83% vs 63%; P = 0.004; Figure 2); this difference remained significant after adjustment for vasopressin use with Cox proportional hazards regression (P = 0.02).
At survival analysis, there was a trend to higher survival in patients on continuous infusion, with a hazard ratio for death of 0.47; however, this was not significant after adjustment for time to initiation of hydrocortisone (P = 0.06).
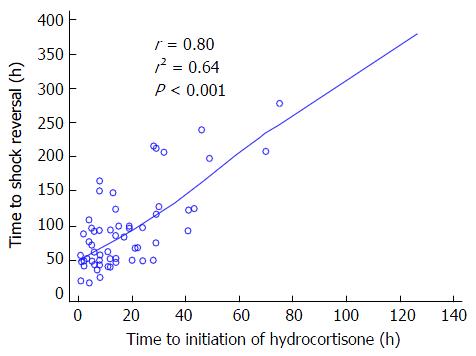
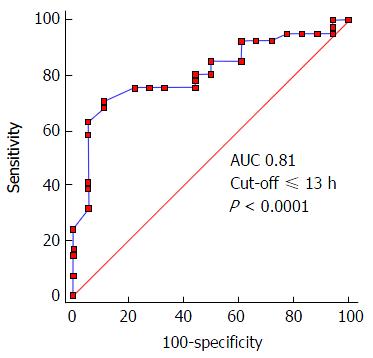
We found a significant correlation between time to initiation of hydrocortisone and time to shock reversal, with a Spearman correlation coefficient of 0.80 (P≤ 0.001; Figure 3). Moreover, we built a ROC curve for this variable and obtained the best cut-off at ≤ 13 h, with a significant area under the curve (AUC) at 0.81 (P≤ 0.0001), obtaining a sensitivity of 70% and specificity of 88% for prediction of shock reversal (Figure 4). Taking the dose of norepinephrine as a potential criterion for prompting initiation of hydrocortisone, ROC curve analysis revealed the best cut-off at ≤ 0.28 μg/kg per minute, with an AUC of 0.75 (P = 0.0002), a sensitivity of 65%, and a specificity of 88% for shock reversal.
As expected for patients with shock reversal, length of hydrocortisone administration was significantly longer for patients with the tapering strategy than for patients with sudden discontinuation [121 h (IQR, 81-245) vs 50 h (IQR, 44-101); P = 0.001]. Taper strategy was independently associated with a higher risk of hyperglycemia (RR = 3.2; P = 0.042), and hypokalemia (RR = 2.8; P = 0.005). Because bolus administration was also independently associated with higher risk of hyperglycemia and hypokalemia at univariate analysis, we performed logistic regression models adjusting for potential confounding variables; only the taper strategy maintained statistical significance for higher risk of hyperglycemia (Table 2) and had the highest OR for hypokalemia (Table 3).
| Variable | Univariate | P value | Multivariate | P value | |
| NO-H (n = 42) | No NO-H (n = 17) | Adjusted OR (95%CI) | |||
| Bolus hydrocortisone, n (%) | 19 (45.2) | 13 (76.5) | 0.04 | 3.2 (0.5-26.5) | 0.99 |
| Hydrocortisone taper, n (%) | 20 (64.5) | 3 (27.3) | 0.03 | 5.3 (1.8-34.5) | 0.04 |
| Diabetes, n (%) | 11 (26.2) | 1 (5.9) | 0.08 | 6.2 (0.4-79.0) | 0.95 |
| Variable | Univariate | P value | Multivariate | P value | |
| NO-HK (n = 30) | No NO-HK (n = 29) | Adjusted OR (95%CI) | |||
| Bolus hydrocortisone, n (%) | 12 (40) | 20 (69) | 0.02 | 8.5 (1.2-59.9) | 0.03 |
| Hydrocortisone taper, n (%) | 17 (77.3) | 6 (30) | 0.002 | 10.6 (1.5-73.3) | 0.01 |
| AKI, n (%) | 13 (43.3) | 18 (62.1) | 0.08 | 0.1 (0.01-0.8) | 0.03 |
| Diuretic use, n (%) | 20 (66.7) | 10 (34.5) | 0.01 | 6.3 (0.95-42.0) | 0.05 |
Hydrocortisone taper was not associated with a lower risk of shock relapse (RR = 1.29; P = 0.17).
In order to identify the variables associated with a higher probability of shock reversal, we performed a logistic regression model, adjusting for relevant covariables. As shown in Table 4, only the initiation of hydrocortisone ≤ 13 h from vasopressor administration and initiation of norepinephrine at a dose ≤ 28 μg/kg per minute were statistically significant.
| Variable | Univariate | P value | Multivariate | P value | |
| Shock reversal (n = 30) | No-reversal (n = 29) | Adjusted OR (95%CI) | |||
| Age (yr), SD | 53 ± 16.3 | 50 ± 16.3 | 0.46 | ||
| Male gender, n (%) | 15 (36.6) | 11 (61.1) | 0.08 | 1.4 (0.21-10.1) | 0.68 |
| Medical disease, n (%) | 11 (26.8) | 6 (33.3) | 0.61 | ||
| Oncologic disease, n (%) | 20 (48.8) | 5 (27.8) | 0.13 | 1.0 (0.18-6.3) | 0.92 |
| AKI, n (%) | 17 (41.5) | 14 (77.8) | 0.01 | 0.3 (0.05-2.0) | 0.23 |
| ARDS, n (%) | 12 (29.3) | 9 (50) | 0.12 | 2.7 (0.4-16.9) | 0.27 |
| Superinfection, n (%) | 5 (12.2) | 3 (16.7) | 0.68 | ||
| APACHE II score (SD) | 20 ± 5.4 | 23 ± 6.4 | 0.16 | 1.1 (0.9-1.3) | 0.18 |
| SOFA score (SD) | 10 ± 3.0 | 10 ± 2.4 | 0.69 | ||
| Vasopressin use, n (%) | 10 (24.4) | 6 (33.3) | 0.48 | 2.5 (0.4-15.4) | 0.31 |
| Early hydrocortisone ( ≤ 13 h from NE), n (%) | 28 (68.3) | 2 (11.1) | 0.0001 | 13.8 (1.4-129) | 0.02 |
| NE dose at hydrocortisone initiation ≤ 0.28 μg/kg per minute, n (%) | 28 (68.3) | 2 (11.1) | 0.0001 | 32.4 (2.7-382) | 0.005 |
The main finding in our study is that, compared to bolus strategy, the administration of hydrocortisone by continuous infusion may lead to a faster reversal of shock and is associated with a higher proportion of vasopressor-free patients at 7 d. Furthermore, we identified optimal cut-off criteria for initiation of hydrocortisone, either based on the time from initiation of vasopressor, or the current maximal dose of norepinephrine. This study also suggests there is no benefit of the tapering strategy because it does not lower the risk of shock relapse but is only associated with a higher incidence of adverse effects.
The current Surviving Sepsis Campaign Guidelines[2] suggest using continuous infusion, rather than a repetitive bolus of hydrocortisone. This recommendation is only based on the results of an observational study in which bolus hydrocortisone was associated with increased blood glucose levels and more variable peak values compared to continuous infusion[14]. This assumption was confirmed by univariate analysis in our study, and the findings strengthen this recommendation’s effectiveness because the vasopressor requirement was 2 d shorter for patients on continuous infusion.
Most current studies, including meta-analyses, only focus on the association between corticosteroids and mortality in septic shock patients[6-10,15-20]; therefore, information related to the hemodynamic effects of both methods of administration is limited. In a recent Chinese study of septic shock patients, the continuous infusion strategy was correlated with a slight improvement in mean arterial pressure but only at 6 h after corticosteroid treatment, and the response was not sustained[21]. To our knowledge, this is the first study comparing both methods in which continuous infusion was found to hasten shock reversal. A possible explanation is the noted high variability in glucocorticoid sensitivity among septic shock patients with severe disease, as measured by suppression of inflammatory cytokine production[22]. Moreover, it has been found that a common genetic variation in the promoter of NF-KB1 (insertion-deletion polymorphism - 94ins/delATTG) is, in fact, associated with nonresponse and a 3-fold increase in risk of death in patients receiving hydrocortisone[23]. As these factors were not included in our study, the distribution of this potential bias in our population is unknown.
The primary change in practice reported after the publication of the Corticosteroid Therapy of Septic Shock study[9] and the updated Surviving Sepsis Campaign Guidelines was that physicians no longer used the cosyntropin stimulation test to identify which patients would benefit from corticosteroids[24]. However, since there are no specific criteria for definition of poorly responsive shock, a major discrepancy between clinicians’ interpretation guidelines and clinical practice is the trigger for initiation of hydrocortisone. In a recent study[10], the most common clinical threshold for prescribing corticosteroids was the presence of 2 or more vasopressors in 64% of patients. We believe that there should be a global agreement according to variables associated with the higher probability of shock reversal. Based on our results at ROC curve analysis and correlation with time to shock reversal, we suggest initiation of hydrocortisone at ≤ 13 h after vasopressor administration. This conclusion agrees with a recent study of severely shocked patients in which the early administration of hydrocortisone (< 9 h) was associated with a significantly lower total time of vasopressors and mortality[25].
In a retrospective review addressing norepinephrine as a trigger for initiation of corticosteroids, the dose was arbitrarily defined as non-weight-based low, moderate, or high[10]. Through a more objective approach, we found a dose of ≤ 0.28 μg/kg per minute to be the best predictor for shock reversal, a lower threshold than what has been used in most studies[9,25,26], reinforcing the recommendation of an earlier initiation of hydrocortisone also based on vasopressor dose[27,28].
Another interesting finding of this study is the apparent lack of benefits to the tapering strategy for discontinuation of hydrocortisone. The Surviving Sepsis Campaign Guidelines suggest tapering from steroids when they are no longer required; therefore, this strategy is the most commonly used (in up to 74% of patients), depending on the duration of hydrocortisone therapy[10]. Unfortunately, there has been no comparative study between tapering and abrupt cessation, and the main argument for this suggestion is a small crossover study in which shock relapse occurred in 30% of patients with sudden discontinuation[29]; however, the study was underpowered to reach that conclusion, because the data arose from a subgroup analysis of 20 patients. Therefore, tapering has a 2D recommendation (the weakest possible) on the GRADE system. Furthermore, there have been other randomized controlled studies in which corticosteroids were abruptly discontinued without reported increased risk of shock relapse[7,8]. In the current study, tapering was only associated with adverse effects; therefore, we suggest this should be avoided, especially for patients with ongoing hyperglycemia and/or hypokalemia.
The main strength of this study is that it was specifically designed to compare the efficacy between both methods of administration of hydrocortisone, according to vasopressor requirement, indirectly assessing their effects on immunomodulation and vasomotor tone improvement.
This study has some limitations; we did not address the effects of the use of some drugs known to affect adrenal function (e.g., etomidate, antifungals, benzodiazepines, and opioids)[30]. Medical management for septic shock patients is always based on the current Surviving Sepsis Campaign Guidelines and are very similar at both hospitals; however, due to the observational and nonrandomized design of the study, we cannot ensure completely homogeneous treatment regarding other relevant variables associated with improving outcomes (e.g., appropriateness and type of fluid resuscitation and correct and timely use of antibiotics). This study was powered to detect differences in short-term vasopressor requirements and to find the best cut-offs for initiation of hydrocortisone only; therefore, results concerning analysis between groups should be interpreted cautiously, and should be taken as hypothesis-generating data for the design of future clinical randomized controlled trials.
In conclusion, we found that continuous infusion of hydrocortisone could hasten resolution of septic shock compared with bolus administration, and that earlier initiation based on time and/or norepinephrine dose is related with a higher probability of shock reversal. The tapering strategy appears unnecessary and may be only related to additional adverse effects.
We acknowledge Hilario Coronado Magaña, Department Chair of the Intensive Care Unit at Hospital Civil Fray Antonio Alcalde, for his general support of this work. We would also like to thank Victoria L. Clifton, MLIS, ELS, for her assistance with language editing and the editorial preparation of this manuscript.
The latest Surviving Sepsis Campaign Guidelines recommend administration of low-dose hydrocortisone (200 mg/d) when hemodynamic stability is not achievable after fluid resuscitation and vasopressor therapy. Although the benefits of hydrocortisone are increasingly being recognized, several issues concerning its specific indications, clinical criteria for initiation, adequate method of administration, and strategy of discontinuation are unresolved, which leads to widely heterogeneous prescribing practices among critical care physicians. Studies addressing those issues are lacking.
The reported prescribing patterns of hydrocortisone for patients with septic shock come from studies performed only in the United States and describe a wide variability of clinical practices. In Mexico, this kind of information is scarce.
Continuous infusion of hydrocortisone leads to faster resolution of shock than bolus administration. Initiation of hydrocortisone at ≤ 13 h after starting norepinephrine and/or a maximal dose of ≤ 28 μg/kg per minute is associated with a higher probability of shock reversal. There is no benefit from a tapering strategy because this was only shown to lead to additional adverse effects.
The results of the study add valuable information, which could contribute to the initiation of a widespread agreement regarding specific indications on the correct use of hydrocortisone in septic shock patients.
It is a well design study, though performed in a small group of patients.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Katsenos CS, Lazzeri C, Piacentini EA S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 346] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 3979] [Article Influence: 331.6] [Reference Citation Analysis (0)] |
| 3. | Dellinger RP. Steroid therapy of septic shock: the decision is in the eye of the beholder. Crit Care Med. 2008;36:1987-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am J Respir Crit Care Med. 2012;185:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 541] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 548] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Yildiz O, Doganay M, Aygen B, Güven M, Keleştimur F, Tutuû A. Physiological-dose steroid therapy in sepsis [ISRCTN36253388]. Crit Care. 2002;6:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2198] [Cited by in RCA: 1968] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 9. | Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1696] [Cited by in RCA: 1356] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 10. | Contrael KM, Killian AJ, Gregg SR, Buchman TG, Coopersmith CM. Prescribing patterns of hydrocortisone in septic shock: a single-center experience of how surviving sepsis guidelines are interpreted and translated into bedside practice. Crit Care Med. 2013;41:2310-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 12. | Hosmer DW, Taber S, Lemeshow S. The importance of assessing the fit of logistic regression models: a case study. Am J Public Health. 1991;81:1630-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 265] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Ibarra-Estrada MÁ, López-Pulgarín JA, Mijangos-Méndez JC, Díaz-Gómez JL, Aguirre-Avalos G. Respiratory variation in carotid peak systolic velocity predicts volume responsiveness in mechanically ventilated patients with septic shock: a prospective cohort study. Crit Ultrasound J. 2015;7:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Weber-Carstens S, Deja M, Bercker S, Dimroth A, Ahlers O, Kaisers U, Keh D. Impact of bolus application of low-dose hydrocortisone on glycemic control in septic shock patients. Intensive Care Med. 2007;33:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 16. | Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database Syst Rev. 2015;CD002243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Huh JW, Choi HS, Lim CM, Koh Y, Oh YM, Shim TS, Lee SD, Kim WS, Kim DS, Hong SB. Low-dose hydrocortisone treatment for patients with septic shock: a pilot study comparing 3days with 7days. Respirology. 2011;16:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Tagami T, Matsui H, Fushimi K, Yasunaga H. Low-dose corticosteroid treatment and mortality in refractory abdominal septic shock after emergency laparotomy. Ann Intensive Care. 2015;5:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Wang C, Sun J, Zheng J, Guo L, Ma H, Zhang Y, Zhang F, Li E. Low-dose hydrocortisone therapy attenuates septic shock in adult patients but does not reduce 28-day mortality: a meta-analysis of randomized controlled trials. Anesth Analg. 2014;118:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Chen Z, Yang C, He H, He Z. [The impacts of low-dose corticosteroids infusion given in different manners on refractory septic shock patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Cohen J, Pretorius CJ, Ungerer JP, Cardinal J, Blumenthal A, Presneill J, Gatica-Andrades M, Jarrett P, Lassig-Smith M, Stuart J. Glucocorticoid Sensitivity Is Highly Variable in Critically Ill Patients With Septic Shock and Is Associated With Disease Severity. Crit Care Med. 2016;44:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Schäfer ST, Gessner S, Scherag A, Rump K, Frey UH, Siffert W, Westendorf AM, Steinmann J, Peters J, Adamzik M. Hydrocortisone fails to abolish NF-κB1 protein nuclear translocation in deletion allele carriers of the NFKB1 promoter polymorphism (-94ins/delATTG) and is associated with increased 30-day mortality in septic shock. PLoS One. 2014;9:e104953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Bruno JJ, Dee BM, Anderegg BA, Hernandez M, Pravinkumar SE. US practitioner opinions and prescribing practices regarding corticosteroid therapy for severe sepsis and septic shock. J Crit Care. 2012;27:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Katsenos CS, Antonopoulou AN, Apostolidou EN, Ioakeimidou A, Kalpakou GT, Papanikolaou MN, Pistiki AC, Mpalla MC, Paraschos MD, Patrani MA. Early administration of hydrocortisone replacement after the advent of septic shock: impact on survival and immune response*. Crit Care Med. 2014;42:1651-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Torgersen C, Luckner G, Schröder DC, Schmittinger CA, Rex C, Ulmer H, Dünser MW. Concomitant arginine-vasopressin and hydrocortisone therapy in severe septic shock: association with mortality. Intensive Care Med. 2011;37:1432-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Bentzer P, Fjell C, Walley KR, Boyd J, Russell JA. Plasma cytokine levels predict response to corticosteroids in septic shock. Intensive Care Med. 2016;42:1970-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, Brett SJ. The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Crit Care Med. 2014;42:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk HD, Doecke WD, Falke KJ, Gerlach H. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 405] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 30. | Greenberg SB, Coursin DB. Timing of corticosteroids in refractory septic shock: a key or wishful thinking?*. Crit Care Med. 2014;42:1733-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |