Published online Feb 4, 2017. doi: 10.5492/wjccm.v6.i1.21
Peer-review started: July 1, 2016
First decision: September 5, 2016
Revised: October 21, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: February 4, 2017
Processing time: 207 Days and 20.4 Hours
In view of the enormous popularity of mass sporting events such as half-marathons, the number of patients with exertional rhabdomyolysis or exercise-induced heat stroke admitted to intensive care units (ICUs) has increased over the last decade. Because these patients have been reported to be at risk for malignant hyperthermia during general anesthesia, the intensive care community should bear in mind that the same risk of life-threatening rhabdomyolysis is present when these patients are admitted to an ICU, and volatile anesthetic sedation is chosen as the sedative technique. As illustrated by the three case studies we elaborate upon, a thorough diagnostic work-up is needed to clarify the subsequent risk of strenuous exercise, and the anesthetic exposure to volatile agents in these patients and their families. Other contraindications for the use of volatile intensive care sedation consist of known malignant hyperthermia susceptibility, congenital myopathies, Duchenne muscular dystrophy, and intracranial hypertension.
Core tip: Recent research has shown that a substantial proportion of patients with exercise-induced heatstroke harbor mutations in the ryanodine-receptor one gene on Chromosome 19 (RYR1), encoding for the principal calcium-release channel in striated muscle. These same mutations are known to result in a massively increased calcium-conductivity and life-threatening rhabdomyolysis when malignant hyperthermia (MH) susceptible patients are exposed to volatile anesthetics during general anesthesia. In view of this, exposure to volatile anesthetic sedation - an emerging trend in intensive care units - is contraindicated, not only in patients with known MH susceptibility and other congenital myopathies, but also in patients admitted because of exertional rhabdomyolysis and heatstroke.
- Citation: Heytens K, De Bleecker J, Verbrugghe W, Baets J, Heytens L. Exertional rhabdomyolysis and heat stroke: Beware of volatile anesthetic sedation. World J Crit Care Med 2017; 6(1): 21-27
- URL: https://www.wjgnet.com/2220-3141/full/v6/i1/21.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v6.i1.21
Exercise-induced rhabdomyolysis (EIR), also known as exertional rhabdomyolysis, is a clinical emergency characterized by extensive post-exercise muscle necrosis and the release of intracellular muscle components into the circulation. The diagnosis is confirmed by a significantly elevated serum creatine kinase (CK) level and/or the presence of myoglobinemia and myoglobinuria. There is no definitive pathological CK cut-off, and therefore, clinical symptoms such as muscle swelling, myalgia, and tenderness occurring with low-intensity exercise, or lasting several days longer than expected, are important clinical signs.
The most severe end of the clinical spectrum-and one of the leading causes of death in young athletes-is known as exertional heat stroke (EHS), a syndrome clinically defined as a body temperature over 40 °C, with severe rhabdomyolysis and signs of encephalopathy ranging from confusion to convulsions or coma. It is often complicated by multiorgan failure, including acute renal and hepatic failure, and it may result in death if appropriate treatment in an intensive care setting is delayed.
It has been reported for over two decades that a significant part of patients with a history of EIR and/or heat stroke is at increased risk of developing malignant hyperthermia (MH) during general anesthesia[1,2].
MH is well known among anesthetists. It is inherited as an autosomal dominant, genetically heterogeneous trait that manifests as an acute rhabdomyolysis during general anesthesia when susceptible individuals are exposed to volatile anesthetics and/or succinylcholine. The incidence is generally estimated to be approximately 1 in 50000 general anesthesias. In the 1970-1980s, mortality was over 80%, but now, it is fortunately less than 5%[3].
A fulminant MH-crisis is characterized by a combination of clinical and biochemical events: Inappropriate hypercapnia and respiratory acidosis, polypnea, tachyarrhythmia, rapidly increasing body temperature, sweating, generalized muscle rigidity, hemodynamic instability, dark urine due to myoglobinuria, significant CK-increase (often > 10.000 IU/L), and postoperative stiffness and myalgia. Death may result from severe hyperkalemia in combination with respiratory and metabolic acidosis, acute renal failure, hyperthermia > 42 °C, disseminated intravascular coagulation and fatal cardiac arrhythmias.
Mortality has decreased significantly over the last 20 years because of our better understanding of the syndrome, the use of end-tidal CO2 (ETCO2) monitoring resulting in earlier diagnosis, and the availability of the antidote dantrolene.
Treatment consists of the immediate discontinuation of volatile anesthetic agents and the administration of dantrolene. The surgical procedure should be terminated as quickly as possible and if the procedure cannot be aborted, the anesthesia technique has to be converted to total intravenous anesthesia. Dantrolene should be administered as a loading bolus of 2.5 mg/kg IV. Subsequent doses of 1 mg/kg IV can be repeated up to a maximum dose of 10 mg/kg, and are administered until the clinical signs (hypercapnia, hyperthermia, rigidity) abate. Concomitantly minute volume should be maximized to reduce respiratory acidosis.
Despite adequate treatment, complications still occur in 20% of patients. The most common complication is renal dysfunction and acute renal failure. The complication rate increases to ≥ 30% when 20 or more minutes elapse between the first clinical sign and dantrolene treatment[4].
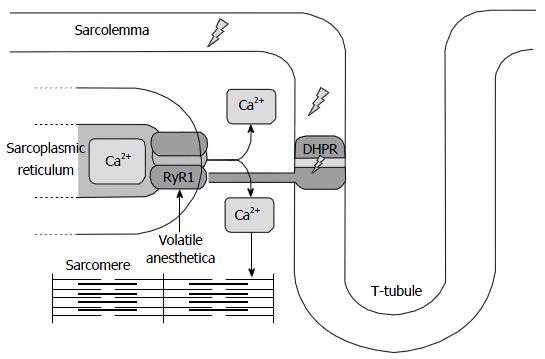
This life-threatening anesthesia-related complication is due to the occurrence of point mutations in the genes coding for the calcium-release channel of the sarcoplasmic reticulum, e.g., the didyropyridine - ryanodine receptor complex, resulting in a disturbed skeletal muscle calcium homeostasis. The ryanodine receptor (RYR1 gene on Chromosome 19q) contains the actual “calcium pore”. The NH2-terminal of this protein forms cytosolic protrusions that extend toward, and make contact with, the voltage-gated dihydropyridine receptor located in the T-tubular wall. The corresponding gene for this protein is CACNA1S on Chromosome 1. Depolarization of the sarcolemma and T-tubule first activates the dihydropyridine receptor which in turn activates the ryanodine receptor and opens the calcium-channel as such.
As illustrated in Figure 1, the muscle from patients harboring RYR1 and/or DHPR mutations upon exposure to volatile anesthetics has been shown to exhibit an increased calcium-conductivity and massive calcium release from the SR, in turn resulting in sustained muscle contraction, a catabolic state, and hyperacute muscle breakdown[3].
Susceptibility to MH is diagnosed by in in vitro contracture testing (IVCT) in which skeletal muscle fascicles, obtained by biopsy, are exposed to incremental concentrations of caffeine and halothane in a test bath. The muscle contracture obtained in response to the drugs is measured, and is, when above a certain threshold, indicative of MH susceptibility. The test is considered positive [malignant hyperthermia susceptible (MHS)] if a contracture of at least 2 mN is obtained at halothane concentrations of 2 Vol% or less, and/or a caffeine concentration of 2 mmol/L or less. Malignant Hyperthermia negative (MHN) patients do not develop a significant contracture at these concentrations. The European Malignant Hyperthermia Group has standardized this test across Europe. The degree of sensitivity and specificity is 99% and 93.5% respectively[5]. The invasive character of the test however hampers its widespread use as a screening instrument.
Genetically, MH is dominantly inherited. Its estimated prevalence is 1 in 3000[3]. Over the years both chromosomal and allelic heterogeneity have been demonstrated but fortunately, RYR1 mutations can be found in 50% to 70% of families[3]. Therefore, in an emergency setting, if general anesthesia is required in MHS patients, a trigger free anesthetic technique - avoiding volatile anesthetic agents and/or succinylcholine - is indicated to avoid this potentially lethal complication.
By extension, patients with a history of EIR/EHS are at increased risk of developing an MH event, e.g., severe life-threatening rhabdomyolysis when they are exposed to volatile anesthetic sedation, which is an emerging trend in intensive care units (ICUs). Because this fact may not be well known enough among non-anesthetist intensive care specialists, we report three patients with recurrent exertional rhabdomyolysis and/or heat stroke to illustrate the complex relationship with MHS, and we review the literature on this topic.
Male, 43 years of age. Reason for investigation: Recurrent episodes of EIR when participating in half-marathons. During a 2007 half-marathon, he presented with an exercise-induced heat stroke (hyperthermia > 40 °C). No further clinical details are available. A second episode of ER was noticed 1 year later during a 20-km run. He developed an epileptic insult before finishing the race. At admission, his temperature was 39.8 °C. More convulsions occurred and his Glasgow coma scale score dropped to 8/15. Sedation with propofol was started, and he was intubated and artificially ventilated. Rhabdomyolysis was diagnosed on the basis of a CK level of 92.000 IU/L, and acute kidney failure occurred (max creatinine level of 3.2 mg/dL), yet with spontaneous recovery and without the need for hemodialyis. The patient could be weaned from mechanical ventilation after 7 d. Further recovery was uneventful. Personal and medical history: Wrist fracture reduction under general anesthesia, active sportsman (handball and squash for years, recent focus on jogging). In between the two episodes of hyperthermia, there were no signs of any myopathy: No myalgias, no cramps, no weakness, and no episodes of cola-colored urines. The family history was negative for myopathies, sudden death, or anesthesia-related complications. His physical examination was normal, as well as Holter monitoring and echocardiography. The resting CK level was 90 IU/L (N < 193 IU/L), lactate 0.68 mmol/L (N < 2 mmol/L). The ischemic exercise test forearm was normal, ruling out glycogen storage disease and myoadenylate deaminase deficiency. The EMG only showed non-specific minor myogenic changes. A 2009 muscle biopsy of the left M. quadriceps revealed a significantly increased number of centrally located nuclei. His IVCT was normal, and not indicative of MHS. A molecular genetic analysis could not reveal any mutation in the carnitine palmitoyl transferase 2 gene (CPTII), or RYR1.
Male, 34 years of age. Reason for investigation: Recurrent episodes of EIR during cycling. The first major episode occurred during preparation for a cycling event in 2010 when he complained of severe cramping pain in both thighs, and his CK level was found to be 49.536 IU/L and myoglobinuria. Renal failure did not develop. In 2013, after cycle training for 5-6 h, he complained of pronounced muscle weakness with difficulty to walk for a few days. This was accompanied by a CK increase to 9.222 IU/L. He is an avid amateur cyclist, practicing several days a week, most often totaling 400 km/wk, and quite regularly participates in a semi-professional tour of up to 220 km in one day. Retrospective analysis showed similar complaints to have started at the age of 18, mainly consisting of myalgia with feeling of stiffness, and intermittent myoglobinuria and CK increase. In between the crises, there is normal mobility and power, and no CK increase. He had not yet been exposed to volatile anesthetics. The family history is negative for anesthesia-related events. His father ran marathons, reporting no clinical events. His neurological examination was normal, and functional and biochemical tests showed a basal CK serum level of 293 U/L, a lactate of 0.8 mmol/L, a normal ischemic forearm exercise test and EMG. Muscle biopsy of the left M. quadriceps femoris in 2011 showed an increased number of fibers with internal nuclei and a minor increase of neutral fat drops in some type 1 fibers. These were interpreted as unspecific findings. A repeat muscle biopsy of the left M. quadriceps femoris in 2014 again demonstrated findings of mild myopathic disease: Increased number of fibers with internal nuclei (25%) but also a small number of “cores”, which are zones devoid of enzymatic staining. IVCT results: 8 mN contracture at 2 Vol% Halothane, 6 mN contracture with 2 mmol/L Caffeine, indicative of MHS. Molecular genetic analysis did not reveal any mutation in the CPTIIgene or RYR1.
Male, 56 years of age. Reason for investigation: Recurrent myalgia confined to the thigh muscles, described as a sensation of severe tension or cramps, sometimes unilateral, sometimes bilateral. The type of effort appeared not to be related to the complaints: At times a prolonged effort did not provoke any myalgia, whereas at other times, the pain/cramps started after as short an effort of 5-10 min such as descending a staircase. CK values measured during an episode of myalgia rose up to 14.000 IE/L. He is also an amateur cyclist, but complaints were only noticed after the age of 40. There was no previous exposure to volatile anesthetics, and the family history was also negative for anesthesia-related events. His son is not active in sports (his daughter is), but until now has not mentioned any complaints. His clinical examination was normal, but he showed an increase in basal CK value of 269-1771 IU/L at diverse occasions, lactate was 1.2 mmol/L, and the ischemic forearm test was normal. A 2015 muscle biopsy only showed atypical myogenic anomalies. The IVCT was abnormal (5 mN contracture with 2 mmol/L Caffeine, 14 mN contracture with 2 Vol% Halothane), demonstrating MHS. Molecular genetic analysis did not reveal a mutation in the CPTII gene, but RYR1- mutation analysis demonstrated a base-pair change in exon 43 (p.N2342S; c.7025A > G).
There is an emerging trend of using inhalational anesthetics for ICU sedation[6]. The principle motivation is that inhalational anesthetics have an interesting pharmacokinetic profile compared with the usual combination of propofol or benzodiazepine with an analgesic drug. Several practical advantages have been proposed, such as rapid onset and offset of action, low potential for accumulation in fat tissue, drug clearance through the lungs and therefore independent of liver and/or renal function, no tachyphylaxis, an opium sparing effect, bronchodilatory properties, and end-organ protective properties, such as ischemic preconditioning of the heart, although clinical data remain limited on this.
Until about a decade ago, the technical prerequisites for ICU use of volatile anesthetics were not fulfilled, and therefore, their use remained confined to the OR. Technological advances, however, have greatly simplified the application of inhalational anesthetics for ICU sedation. An important milestone was reached in 2005 with the introduction of a volatile anesthetic reflection filter, the anesthetic conserving device (AnaConDaTM, Sedana Medical, Uppsala, Sweden), retaining 90% of the volatile anesthetic and thereby enormously reducing the consumption of volatile anesthetic. Since then, other AnaConDaTM competing devices have been produced and tested such as the MirusTM (Pall Medical, Dreieich, Germany)[7]. Suitable volatile agents are isoflurane, sevoflurane, and desflurane. From a drug delivery point of view, these technical developments have indeed resulted in easy titration to the clinical end-point and the possibility of breath-by-breath bedside monitoring. A 0.3-0.6 minimal alveolar concentration of sevoflurane is most often sufficient in the ICU setting. In view of the increased CO2 through the systems’ enlarged dead space (100 mL), minute volume usually has to be increased by being guided by ETCO2 or blood gas analysis. Desflurane is not commonly used in the ICU in view of its boiling point of 22.8 °C, requiring a vaporizer heated to 39 °C, and the higher cost. It can be administered by the MirusTM device, not the AnaConDaTM.
Recent studies have indicated that the currently available scavenger devices that are based on a silica zeolite matrix, adequately adsorb the volatile agents and thereby minimize environmental pollution by volatile anesthetic agents guaranteeing workplace safety[8].
The question still remains to be answered whether volatile anesthetics will really become and stay a player in critical care sedation[6]; however, the technique is being increasingly used in Europe and North-America.
A pilot randomized controlled trial is currently being set up to evaluate the practicability and dangers related to volatile anesthetic agents when used for long-term critical care sedation[9]. This prospective multicenter trial is blinded to the data analyst and aims to recruit 60 adult ICU patients requiring mechanical ventilation and sedation for at least 48 h, in which 40 patients will receive inhaled isoflurane and 20 patients will receive intravenous midazolam and/or propofol, titrated to a targeted Sedation Analgesia Score. Primary outcomes will assess adherence to the particular sedation protocol and atmospheric volatile concentration levels. Secondary outcomes that will be investigated include the quality of sedation, delirium, vasoactive drug support, time to extubation, serum fluoride levels, and mortality.
With an ever-rising concern for adverse events involved in our handling of patients, for any technique to stand a chance of proving itself, all side-effects have to be clearly identified and communicated to the end users.
In this sense, Purrucker et al[10] has issued a warning concerning the use of volatile anesthetic sedation in patients with a high risk of intracranial hypertension. Switching from IV sedation to sevoflurane decreased MAP and CPP in one-third of the patients studied to such an extent that the early termination of sevoflurane administration was required. The mechanism felt to be responsible was vasodilation in response to a decreasing MAP and a slightly raised partial-pressure carbon dioxide in patients with an already low baseline cerebral compliance.
In this paper, we want to warn about the use of volatile anesthetic sedation in a second subset of ICU patients, in particular those with EIR/EHS in need of intensive care.
Although EIR/EHS is most often encountered in a military setting, the recent worldwide trend to organize mass (semi)marathons has resulted in a significant increase in the frequency of this problem in ICUs worldwide. Indeed, the Centers for Disease Control and Prevention of the United States reported that EHI occurs both during practice and competition, with a disturbing trend of increasing incidence, and mentioning EIR/EHS as one of the leading causes of death in young athletes each year[11].
General anesthesia with volatile anesthetic agents, such as the currently used sevoflurane and desflurane, is known to potentially induce acute rhabdomyolysis in patients with a genetic predisposition to MH. Logically, the same risk is present when genetically MHS patients are exposed to volatile anesthetic sedation in the intensive care setting. A recent publication reported on the development of MH in an ICU patient sedated with sevoflurane[12].
Other groups of patients are also at risk. A link between MHS and EIR has been suggested in the anesthesia literature for over two decades. The earlier reports linked MHS with EIR by demonstrating a positive in vitro contracture test. One of the larger series published[2] reports on the IVCT results in 12 unrelated patients with EIR. Ten of the 12 patients had IVCT results indicative of MHS. In the ensuing years, reports occurred providing evidence that “MH-mutations” in RYR1 were being associated with EIR. In 2002, Davis et al[13] reported two patients with EIR in which MHS was confirmed through in-vitro contracture testing and the presence of a RYR1 mutation.
Several similar cases were reported, and in 2013, Dlamini et al[14] published a large series of 39 unrelated families with rhabdomyolysis and/or exertional myalgia in which nine heterozygous mutations were found in 14 families, several of them recurrent. Five of these mutations had previously been associated with MH. They conclude that RYR1 mutations account for a substantial proportion of patients presenting with unexplained rhabdomyolysis and/or exertional myalgia, but also that various stressors (e.g., pain, environmental heat, viral infections, drugs) may need to be present to elicit acute rhabdomyolysis. They also suggest that “additional family studies are paramount in order to identify potentially MH susceptible relatives”.
In a 2014 paper, Zhao et al[15] actually raised the question on whether the two disorders represent one and the same disorder, which they called the Human Stress Syndrome. This hypothesis has gained support, and MH and EIR/EHS are increasingly believed to be different presentations of the “expanding spectrum of RYR1-related myopathies”[16].
In view of the growing evidence that EIR in a substantial portion of the patients admitted to the ICU is a “non-anesthetic RYR1-related rhabdomyolysis”[16], it is of great importance for the patients and families involved to undergo a thorough investigation on the cause of the life-threatening rhabdomyolysis, certainly if the problem has been found to be recurrent. The following reasons substantiate this statement: (1) several “common” underlying causes with significant impact on patients’ lives have to be ruled out, such as sickle cell trait, CPTII deficiency, McArdle’s disease (glycogen storage disease V), myoadenylate deaminase deficiency, and others; (2) to identify potentially MHS individuals as implications for future general anesthesia are important. If a patient is found to be MHS, preventive measures concerning the anesthetic technique have to be taken. Over the last decade, several experts have stated that individuals who have a history of EHS should be screened for MHS[2,16-18]. Even though the IVCT is an invasive test, it is still considered to be the most sensitive and specific test to determine a patient’s predisposition to MHS. The estimation of the MH risk in a particular patient is certainly not straightforward, as illustrated by the case presentations. Patient 1 had a negative IVCT and a negative genetic analysis, and is considered to be non-MHS. Therefore, volatile anesthetics can safely be administered in the operating room/ICU to this patient. Patient 2 had a positive IVCT, but no mutation was found upon RYR1 sequencing. However, it is well known that a RYR1 mutation is found in only 50%-70% of patients with a positive IVCT[19]. CACNA1S cDNA sequencing was not performed in our patients in view of the cost and the large number of sequence changes reported, most of which are of unclear significance[20]. In this patient, volatile anesthesia has to be avoided in the future, because he is considered MHS by IVCT. Patient 3 had a positive IVCT and a positive RYR1 mutation (p.N2342S; c.7025A>G - exon 43) that was previously linked to MH, and therefore, this patient is clearly at risk for MH upon exposure to volatile anesthetic agents; and (3) If RYR1 mutations are found, the condition should be considered to be hereditary, and additional family studies are indicated.
The patient should be seen by a neurologist, because a large number of diverse etiologies have been implicated in acute EIR/EHS, and certain additional clinical features can aid to define the most appropriate investigations. A guideline for a diagnostic EIR/HS workup has been suggested by Capacchione et al[21]. Because of the rarity and heterogeneity of these conditions, however, this EIR/HS workup remains a real diagnostic challenge.
A third group of patients at risk when given volatile anesthetic sedation are patients with congenital myopathies. This is a group of rare genetic muscle disorders (6 in 100000 live births) characterized by different structural abnormalities in skeletal muscle fibers either observable by light- or EM microscopy, and symptoms of hypotonia and muscle weakness present at birth (although the clinical expression may be delayed until childhood or even adult life). These myopathies are genetically heterogeneous, but a substantial subgroup is linked with “gain-of-function” mutations in the RYR1-gene, resulting in increased calcium conductance of the calcium-release channel and the potential for acute rhabdomyolysis when exposed to volatile anesthetics. This has been shown to be the case for central core disease, multiminicore disease, centronuclear myopathy, congenital fiber type disproportion, late-onset axial myopathy, and King-Denborough syndrome[22].
The propensity of patients with muscular dystrophies (especially Duchenne muscular dystrophy) to react adversely to volatile anesthetic sedation is well known; however, this does not occur through the presence of RYR1 mutations but rather is the result of “toxic effects” of these agents as well as succinylcholine on the fragile sarcolemma. Prolonged exposure to volatile anesthetic agents, and certainly the use of succinylcholine, has to be avoided in this group of patients.
If intensive care sedation is needed in these patients, a combination of propofol, benzodiazepines, morphinomimetics, neuraxial block, and, as last resort, non-depolarizing neuromuscular blockers can be used. Dexmedetomidine use for procedural sedation is safe.
Dominantly inherited MHS is rare. Therefore, in the general population, life-threatening acute rhabdomyolysis following exposure to volatile anesthetics either in the operating room or the ICU is seldom encountered. However, because a substantial proportion of patients with EIR/EHS and congenital myopathies harbor RYR1 mutations resulting in an increased calcium-conductivity of the Ca-release channel of the SR, volatile anesthetic sedation should not be used in these high-risk patients.
Contraindications for volatile anesthetic sedation in intensive care consist of: (1) known susceptibility for MH; (2) congenital myopathies (central core disease, multiminicore disease, centronuclear myopathy, congenital fiber type disproportion, late-onset axial myopathy, and King-Denborough syndrome); (3) duchenne muscular dystrophy; (4) exertional rhabdomyolysis; and (5) intracranial hypertension.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B,B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Beltowski J, Lin JA, Oji C, Stocco G S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Hopkins PM, Ellis FR, Halsall PJ. Evidence for related myopathies in exertional heat stroke and malignant hyperthermia. Lancet. 1991;338:1491-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Wappler F, Fiege M, Steinfath M, Agarwal K, Scholz J, Singh S, Matschke J, Schulte Am Esch J. Evidence for susceptibility to malignant hyperthermia in patients with exercise-induced rhabdomyolysis. Anesthesiology. 2001;94:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 331] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 4. | Riazi S, Larach MG, Hu C, Wijeysundera D, Massey C, Kraeva N. Malignant hyperthermia in Canada: characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg. 2014;118:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Ording H, Brancadoro V, Cozzolino S, Ellis FR, Glauber V, Gonano EF, Halsall PJ, Hartung E, Heffron JJ, Heytens L. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand. 1997;41:955-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Jerath A, Parotto M, Wasowicz M, Ferguson ND. Volatile Anesthetics. Is a New Player Emerging in Critical Care Sedation? Am J Respir Crit Care Med. 2016;193:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane--the Mirus™. Anaesthesia. 2014;69:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Wong K, Wasowicz M, Grewal D, Fowler T, Ng M, Ferguson ND, Steel A, Jerath A. Efficacy of a simple scavenging system for long-term critical care sedation using volatile agent-based anesthesia. Can J Anaesth. 2016;63:630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Jerath A, Ferguson ND, Steel A, Wijeysundera D, Macdonald J, Wasowicz M. The use of volatile anesthetic agents for long-term critical care sedation (VALTS): study protocol for a pilot randomized controlled trial. Trials. 2015;16:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Purrucker JC, Renzland J, Uhlmann L, Bruckner T, Hacke W, Steiner T, Bösel J. Volatile sedation with sevoflurane in intensive care patients with acute stroke or subarachnoid haemorrhage using AnaConDa®: an observational study. Br J Anaesth. 2015;114:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Centers for Disease Control and Prevention. Heat illness among high school athletes --- United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1009-1013. [PubMed] |
| 12. | Rosenberg H, Schuster F, Johannsen S. The introduction of a lightweight mini vaporizer and malignant hyperthermia. Can J Anaesth. 2015;62:319. [PubMed] |
| 13. | Davis M, Brown R, Dickson A, Horton H, James D, Laing N, Marston R, Norgate M, Perlman D, Pollock N. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br J Anaesth. 2002;88:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Dlamini N, Voermans NC, Lillis S, Stewart K, Kamsteeg EJ, Drost G, Quinlivan R, Snoeck M, Norwood F, Radunovic A. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscul Disord. 2013;23:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Zhao X, Song Q, Gao Y. Hypothesis: exertional heat stroke-induced myopathy and genetically inherited malignant hyperthermia represent the same disorder, the human stress syndrome. Cell Biochem Biophys. 2014;70:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Snoeck M, Treves S, Molenaar JP, Kamsteeg EJ, Jungbluth H, Voermans NC. “Human Stress Syndrome” and the Expanding Spectrum of RYR1-Related Myopathies. Cell Biochem Biophys. 2016;74:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Grogan H, Hopkins PM. Heat stroke: implications for critical care and anaesthesia. Br J Anaesth. 2002;88:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Sambuughin N, Capacchione J, Blokhin A, Bayarsaikhan M, Bina S, Muldoon S. The ryanodine receptor type 1 gene variants in African American men with exertional rhabdomyolysis and malignant hyperthermia susceptibility. Clin Genet. 2009;76:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Carpenter D, Robinson RL, Quinnell RJ, Ringrose C, Hogg M, Casson F, Booms P, Iles DE, Halsall PJ, Steele DS. Genetic variation in RYR1 and malignant hyperthermia phenotypes. Br J Anaesth. 2009;103:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Carpenter D, Ringrose C, Leo V, Morris A, Robinson RL, Halsall PJ, Hopkins PM, Shaw MA. The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med Genet. 2009;10:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Capacchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Snoeck M, van Engelen BG, Küsters B, Lammens M, Meijer R, Molenaar JP, Raaphorst J, Verschuuren-Bemelmans CC, Straathof CS, Sie LT. RYR1-related myopathies: a wide spectrum of phenotypes throughout life. Eur J Neurol. 2015;22:1094-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |