Published online Aug 4, 2016. doi: 10.5492/wjccm.v5.i3.171
Peer-review started: February 14, 2016
First decision: May 19, 2016
Revised: July 4, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: August 4, 2016
Processing time: 181 Days and 23.9 Hours
Ethical standards in the context of scientific publications are increasingly gaining attention. A narrative review of the literature concerning publication ethics was conducted as found in PubMed, Google Scholar, relevant news articles, position papers, websites and other sources. The Committee on Publication Ethics has produced guidelines and schedules for the handling of problem situations that have been adopted by professional journals and publishers worldwide as guidelines to authors. The defined requirements go beyond the disclosure of conflicts of interest or the prior registration of clinical trials. Recommendations to authors, editors and publishers of journals and research institutions were formulated with regard to issues of authorship, double publications, plagiarism, and conflicts of interest, with special attention being paid to unethical research behavior and data falsification. This narrative review focusses on ethical publishing in intensive care medicine. As scientific misconduct with data falsification damage patients and society, especially if fraudulent studies are considered important or favor certain therapies and downplay their side effects, it is important to ensure that only studies are published that have been carried out with highest integrity according to predefined criteria. For that also the peer review process has to be conducted in accordance with the highest possible scientific standards and making use of available modern information technology. The review provides the current state of recommendations that are considered to be most relevant particularly in the field of intensive care medicine.
Core tip: Ethical standards in the context of scientific publications are increasingly gaining attention. Recommendations to authors, editors and publishers of journals and research institutions were formulated by The Committee on Publication Ethics with regard to issues of authorship, double publications, plagiarism, and conflicts of interest, with special attention being paid to unethical research behavior and data falsification. As scientific misconduct with data falsification damage patients and society, it is important to ensure that only studies are published that have been carried out with highest integrity according to predefined criteria and that also the peer review process has to be conducted in accordance with the highest possible scientific standards.
- Citation: Wiedermann CJ. Ethical publishing in intensive care medicine: A narrative review. World J Crit Care Med 2016; 5(3): 171-179
- URL: https://www.wjgnet.com/2220-3141/full/v5/i3/171.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v5.i3.171
Clinicians and researchers must be able to rely on the integrity and fair presentation of biomedical publications. They have, after all, a vested interest in it[1]. In recent years, the traditional relationship of trust between authors of publications of clinical studies, editors of medical journals, and their readers has come to falter because of numerous examples of open scientific misconduct[2-7]. Numerous journals in intensive care medicine have been affected by the increased number of published articles that they have had to retract. Measures to preserve scientific integrity are therefore becoming increasingly important. These include recommendations how to perform and present clinical studies. What publishers of scientific journals undertake to ensure the integrity of the scientific literature has become a recognized performance criterion[8]. The integrity of a biomedical journal depends on the ethical conduct of those who carry the greatest responsibility for the research publications, namely the authors, on the one hand, and the publishers, on the other, who need to understand that honest mistakes are inevitable, and are able to distinguish them from deliberate wrongdoing.
The editors need to ensure that all articles published in their journals fulfill the highest standards of scientific integrity[9]. Previously, when confronted with integrity problems, editors behaved as though unethical behavior of authors was not in their area of responsibility. Today, most of them have recognized that time and energy need to be invested in the investigation of allegations of scientific misconduct in order to ensure the scientific integrity of the journal. According to a recent survey of 200 leading journals, only two-thirds have fixed rules on withdrawal of publications, and in 95% they would be allowed to opt for such a move even against the will of the authors[10].
Usually, accusations of wrongdoing are raised by referees or readers. Publishers may and can assume that the whistleblower is acting in good faith and that their anonymity must be protected. Accused authors again must be considered as innocent until the suspected misconduct has undergone careful examination and proven to be such. The principles underlying such an investigative procedure are the subject of this review paper. In this context, collaboration between journals and research institutions is of key importance[11]. Based on the experiences of the recent past, the relevant issues include questions about misrepresentation of study designs, faulty statistics, double publications, data falsification, withdrawal of unreliable publications, and assessment of submitted manuscripts, including peer review, authorship issues and conflicts of interest. This review describes the principles of ethical publishing. It gives an overview on the subject. Statements are based on the available literature and the recommendations of the Committee on Publication Ethics (COPE) (http://publicationethics.org). The problems addressed relate to allegation or evidence of various types of reporting bias, plagiarism, double publications, multiple submissions, fragmented multiple publications of research findings of individual studies, and selective reporting; authorship; falsification and fabrication of data; and withdrawal of published articles.
This narrative review has been made to address the problems of publication ethics in intensive care medicine. Author reviewed available literature, reports and surveys on the integrity of publications on critical care medicine as found in PubMed, Google Scholar, relevant news articles, position papers, websites and other sources.
Retractions of publications are a sign that a journal takes seriously its responsibility for the integrity of its publications. Erroneous, unethical or fraudulent studies must be indicated to be such by using the possible formats “Expression of Concern”, “Erratum”, “Corrigendum” and “Notice of Retraction” or “Retraction Note” in order to ensure the scientific community that the publications in question have been assessed correctly and can be quickly identified as such in the literature databases.
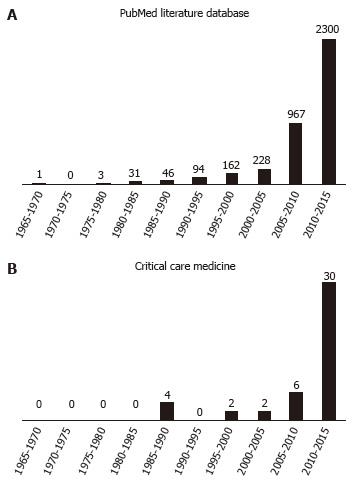
Until a few years ago, relatively few retracted publications in the field of intensive care medicine were made public (Table 1). Recently, there has been an exponential growth in publication retractions both in biomedical literature and in the field of intensive care (Figure 1). This has as much to do with the capabilities of modern information technology and their impact on academic medicine and medical research as with changes in career opportunities for researchers and the changing financial environment for research. And the number of publications retracted can be expected to rise in the future[12].
| Journal | Retractions (n) | Retracted Fujii papers (n) | Retracted Boldt papers (n) |
| American Journal of Critical Care | - | - | - |
| American Journal of Respiratoryand Critical Care Medicine | 7 | - | - |
| Anaesthesia and Intensive Care | 6 | 6 | - |
| Anästhesiologie IntensivmedizinNotfallmedizin Schmerztherapie | 6 | - | 6 |
| Annals of Intensive Care | - | - | - |
| Burns | - | - | - |
| Chest | 5 | - | - |
| Critical Care | - | - | - |
| Critical Care and Resuscitation | - | - | - |
| Critical Care Clinics | - | - | - |
| Critical Care Medicine | 5 | - | 2 |
| Critical Care Nurse | - | - | - |
| Current Opinion in Critical Care | 1 | - | - |
| Injury | 2 | - | - |
| Intensive Care Medicine | 7 | - | 6 |
| Journal of Critical Care | - | - | - |
| Journal of Intensive Care Medicine | - | - | - |
| Journal of Neurotrauma | 1 | - | - |
| Journal of Trauma and Acute CareSurgery | - | - | - |
| Journal of Trauma Nursing | - | - | - |
| Medicina Intensiva | - | - | - |
| Minerva Anestesiologica | 2 | 1 | 1 |
| Neurocritical Care | - | - | - |
| Pediatric Critical Care Medicine | - | - | - |
| Respiratory Care | 1 | - | - |
| Resuscitation | 3 | - | - |
| Seminars in Respiratory andCritical Care Medicine | - | - | - |
| Shock | 2 | - | - |
| Total | 48 | 7 | 15 |
In the field of intensive care medicine, the majority of article withdrawals were made by leading international scientific journals of the United States and Europe (Am J Resp Crit Care Med, Chest, Crit Care Med, Intensive Care Med). These are rather high-impact and not low-impact journals[13]. It is interesting to note that out of 28 involved journals, two national journals, namely “Anaesthesia and Intensive Care” and “Anaesthesiology Intensive Care Emergency Medicine Pain Therapy” from Australia and Germany, respectively are responsible for a quarter of all withdrawals in the field of intensive care (Table 1): All six articles retracted by “Anesthesia and Intensive Care” were articles of the Japanese author Fujii and all six withdrawals by the journal “Anaesthesiology Intensive Care Emergency Medicine Pain Therapy” were publications of Boldt in Germany. These two cases of scientific misconduct represent almost half (22/48) of all publication retractions in this area of medical research and therefore need further scrutiny. In seven of the 48 retracted articles in the area of intensive care, “Intensive Care Medicine” was involved and six of them were publications of Boldt. The exact scope of his fraud has neither been clearly determined, nor fully investigated. What is clear is that Joachim Boldt as an author of more than 215 publications on clinical trials had no authorization from the relevant ethics committees at both places where he worked (University Hospital of Giessen and Klinikum Ludwigshafen) for carrying out research on patients. Therefore, a total of 88 of his publications were retracted in March 2011 for the time being.
The Fujii fraud: In 2000, a letter to the editor was published in “Anesthesia and Analgesia” that questioned the credibility of information on adverse drug reactions, because they were almost always identical in the 47 articles of the Japanese author Dr. Yoshitaka Fujii[2]. Against this background of suspicion of falsifying data, many years later, when the author submitted a manuscript to another journal, the matter was thoroughly investigated in cooperation with the publisher and the author’s research institution with the result that it was found that no ethics committee approval had been obtained for the study, and furthermore, data manipulation was detected[3]. At the same time, the British anesthesiologist Dr. John Carlisle checked the integrity of the data of a total of 168 randomized controlled trials that Dr. Fujii had published over the years. He gave overwhelming statistical evidence that it was highly unlikely that the statistical distributions of continuous and categorical variables described in the publications are what could be expected to occur by chance[4]. After further examination of several Japanese universities where Dr. Fujii had worked continuously only for a few years each, the suspicion of falsification could not be discounted. Finally, a hitherto unprecedented number of 189 publications in anesthesia and intensive care medicine journals were recommended for retraction by the Japanese Society for Anesthesia.
In the case of the Japanese anesthesiologist Dr. Yoshitaka Fujii, who had worked in six Japanese universities and falsified a large number of publications, the involved academic institutions, in collaboration with the Japanese Society of Anesthesiology, quickly analyzed 300 articles after a group of editors and researchers suspected fundamental problems in many of his publications[2-4].
Although the fraudulent publications were discovered to be such only years later, recommendations to have these retracted were made to the responsible editors in a relatively short time, because the involved Japanese institutions and journals worked together constructively. Although research scandals are rated negatively by the public, in the end, particularly research institutions can benefit from this kind lively professionalism.
The Boldt fraud: In announcing the retraction of an article by Dr. Joachim Boldt, a group of editors declared that lack of ethics committee approval of a study does “not (...) mean that the research results per se are fraudulent”[5]. Data fraud was found in 10 of the publications[14]. The Klinikum Ludwigshafen could not find study documents on 92% of patients recruited for studies[14]. Suspicious homogeneity in the mortality data was seen in five publications[15]. Six publications on cardiac and major abdominal surgery showed suspiciously low interleukin-6 measurement variability[16-21]. For two of the six articles[17,19] data for comparative analysis were available in a thesis[22]. The dissertation showed that the articles misrepresented a single study as two separate studies, and that data had been manipulated to conceal the double publication.
Dissertations as a data source for fraudulent publications were found in two other retractions, one of which had already been withdrawn due to lack of ethics committee approval[23-25]. As of today, 89 publications have been retracted because they had failed to obtain ethics committee approval[5]; there are additional articles that have been retracted because of data falsification and double publications: two because of proven fabrication of data[26,27], and two because of proven data manipulation[28,29].
In 2012, the Klinikum Ludwigshafen pointed out that only those publications of Dr. Boldt had been examined that had appeared after 1999[14]. Because nearly 40% of clinical trials were carried out at the University Hospital Giessen, and articles based on these trials were published prior to 1999 and because thesis data were falsified in publications[17,19,22-25], it can be assumed that falsification occurred prior to 1999.
In the meantime, comparative analysis of theses and publications are being carried out at the University of Giessen. Initial results have led to a series of further retractions, all of which are explained by systematic data falsification and partly with simultaneous dual publication[30-33] and trial design change[34]. From a confidential communication from the University of Giessen to the editors of the journals involved, from which “Retraction Watch”[35] was permitted to quote, it can be assumed that there are still large numbers of other publications of clinical studies of Boldt that will be retracted because of scientific misconduct going beyond lack of approval from ethics committee[34]. Among the most important issues that arise from the fraudulent series of Boldt is: How was it possible for Boldt to publish over a period of 25 years, working at two research facilities only, at least 217 articles on clinical trials involving thousands of patients with more than 180 co-authors (Christian J Wiedermann, unpublished survey) without arousing any suspicion of misconduct in institutions where he worked and the co-authors?
Research-based institutions as well as scientific journals are obliged to fulfill their different responsibilities. Institutions are responsible for the conduct of research and the promotion of a healthy research environment. Journals are responsible for assuring that their editors uphold the high scientific quality of all their publications. On issues of integrity of the research, it is important for both sides to communicate and to cooperate with each other effectively. To achieve this, COPE has issued recommendations[36], according to which the obligations are defined (Table 2).
| Who should | Do what |
| Institutions | Have a representative or an office for research integrity with highly visible contact details |
| Inform magazines about cases of misconduct, in which the reliability of published data is doubtful | |
| Respond to journals when requested for information on issues such as disputed authorship, questionable data quality, existing conflict of interest or other issues that could affect the reliability of the published works, including honest errors | |
| Initiate investigations into allegations of scientific misconduct or unacceptable publication | |
| Have guidelines to support responsible research and provisions for implementation of investigative procedures in cases of suspected scientific misconduct | |
| Journals | Give the contact details of the publisher responsible for questions of research and publication integrity |
| Inform institutions if they suspect that wrongdoing by their researchers and submit evidence which support these concerns | |
| Cooperate with the institutions in question and in investigations suspected misconduct | |
| Be ready to announce retraction or correction of publications according to the guidelines on COPE if investigations confirm misconduct | |
| Have guidelines for responding to institutions and other organizations that investigate suspected cases of scientific misconduct |
Data falsification, plagiarism, double publications and irregularities in the authorship are the most common reasons for journal editors for having to deal with the question whether published articles should be retracted. Other problems are those of patients’ rights and whether they were taken into consideration and whether permission was obtained from ethics committees. The retraction of publications should not be confused with “errata” or “corrigenda” - these are necessary when journals make some mistakes during production or when authors seek to retrospectively correct honest mistakes.
With word processors, it has become easy to copy data and texts when writing scientific articles and exchange texts between documents and thus inadvertently or intentionally produce plagiarized texts. It is therefore important that citations and paraphrasing are properly done. It must be clear that the copying of existing documents is only permitted if the copied sections are clearly labeled as such, for example, by the text being enclosed in quotation marks and by correctly specifying the sources. Many institutions and scientific journal, particularly in English-speaking countries, now check submitted texts with commercial plagiarism software. One such text-comparative software is “iThenticate”, which, in conjunction with a large database of published scientific documents called “Crosscheck” provided by publishers, detects plagiarism and redundant publication. One disadvantage of these systems is that analysis is limited to determining the number of copied words, and the number of copied words that is acceptable is defined by the institutions themselves and the journals[37]. Another disadvantage is that figures cannot be compared. The publishers of journals must themselves specify their evaluation criteria for text and picture similarities.
In surveys made, on average 2% of scientists admitted to having falsified research data at least once, and up to 34% admitted to having used other questionable research practices[38]. The actual frequency is likely to be even higher.
The approach to statistically identify potentially fraudulent data in publications of randomized clinical trials (RCTs) was developed and refined by Carlisle et al[39] so that “improbability” in the distribution of data in RCTs can be determined with increasing accuracy. It is conceivable that, in the foreseeable future, such statistical methods will be introduced in the publication routine - analogous to the use of software for detection of plagiarism to check plausibility of data integrity[40], which should become possible at least for prolific authors.
Retractions of unreliable publications are important for scientific but also for economic reasons. After an investigation for misconduct, retracted publications of research projects that were funded by the “National Institutes of Health” in the United States lost about $58 million in direct financing in 1992-2012, representing on average US$ 392.582 per article). Researchers affected by withdrawal of one of their articles suffered a 90% decline in their publication output and large losses in the further financing of their projects[41]. Coauthors are not privy enough in publications also suffer from being under suspicion of participation in falsification and often without their knowledge. Their interest in correct publication practice can be used in strategies against unethical publishing[42].
Editorial efforts necessary for retracting a fraudulent publication are often enormous. Not least, the public loss of confidence arising from the misconduct and retraction of publications causes harm to scientific research itself. Although retracted publications represent only a small percentage of the total literature[43,44], it can be assumed that the number of unreported cases of falsified research reports is much higher than is currently known. Only a fraction of the cases of scientific misconduct is actually uncovered and made public[38,45]. Worse still, the results of the retracted article continue to be cited[46-48]. Only in a fifth of the cases of announced retraction of scientific publications is research or publication misconduct cited as a reason by the journals for the retraction; in two-fifths of the cases, merely loss of credibility of data or their interpretation is cited as a reason[48].
From the fact of a journal withdrawing an article, is it permitted to conclude that the reason for retraction was scientific misconduct on the part of the authors? There are demands that this should not be done since there are several reasons why journals retract an article. This, however, is not a justifiable demand because authors identified as having falsified their data in one publication appear as authors in numerous retracted publications and thus distort the interpretation of the situation. Thus, although numerous articles of the anesthesiologist Dr. Joachim Boldt were retracted only for lack of ethics committee approval, suspicions of falsification were not investigated[49,50]. This shows how important the involvement of universities and research institutions in the review of falsification suspicion is, mainly because they have the ability to prove fraudulent intent and scientific fraud. This is underlined by the recent observation that even if regulatory authorities such as the American “Food and Drug Administration” (FDA) detects significant deviations from good clinical practice in clinical trials, they are seldom reflected in the clinical literature, and this happens even when there was clear evidence of data manipulation and other forms of scientific fraud[51,52]. As an example of misconduct in publication ethics, the FDA study for approval of the infusion solution Voluven® in United Sates can also be cited in this context: The nephrotoxic potential of this drug was indeed reported to the authorities, but was not been included in the publication, and this situation continues to this day without any relevant note of caution related to selective outcome reporting being added[53]. Another example is the FIRST trial[54], where the trial design has been published beforehand, but the final publication was different from the stated parameters[55].
COPE guidelines explain when articles should be retracted, when corrections should be made and when only the “expression of concern” might be more appropriately used. Decisions that editors of journals must make still remain difficult. Thus, an analysis of the response of individual journals to a recent series of unethical publications of the German anesthesiologist Dr. Joachim Boldt that would need to be retracted according to the research institutions involved shows that only a small percentage of these have been dealt according to the COPE criteria[56].
For the researchers themselves and for the public, withdrawal of publications and the reasons for it[44,57-59], are of increasing interest. Both the absolute and relative number of retracted articles has increased dramatically. To what extent this represents an increase of scientific misconduct is unclear because journals also have better ways to detect especially plagiarism and multiple publications. Undoubtedly, researchers are under great pressure to publish and be “cited”[60].
Editors and publishers have the important duty to draw the attention of readers to scientific misconduct when publications have proven to be unreliable. In times of the conventional printing and traditional library catalogs, it was difficult to make any necessary corrections and any retractions of publications known in such a way that they could be brought into relation with the original articles. Today, the electronic publishing and cataloging system has simplified this task enormously. Readers are referred to corrections or retractions of texts at the very beginning of their electronic search. In this respect, supplementary information is already added to the table of contents and the article itself. Corrections and retractions are built directly into the affected article in this way. CrossMark (http://www.crossref.org/crossmark/) provides additional opportunities for cross-reference to refer the reader to comments and modifications of scientific publications. Thus, publishers can meet their responsibilities, so that retracted publications do not continue to be cited.
In case serious misconduct is suspected, the investigation of which takes more time to complete than expected, editors can warn readers of potential problems with an individual article even before completion of the investigations. In such cases, the publication of an “expression of concern” is advised by COPE.
Even when intentional fraud seems obvious, ethical problems in publication may not be intentional and may arise out of ignorance or carelessness. This must be considered while investigating scientific misconduct. In the transparent description of such investigations, scientific journals as well as research institutions must handle the issue appropriately in accordance with the severity of misconduct involved. When plagiarism is suspected, there are differences in responsibility between senior researchers and young scientists in manuscript preparation, which should be reflected in the response of the journal to the submitted article, as well as the disciplinary measures taken by the institutions. The COPE algorithms describe in as differentiated a way as possible, how the publisher can respond to different types of ethical publication problems. Of course, not all aspects could be anticipated and some had to be left open and left to the co-operation between publishers, publishing houses and research institutions. One such issue is how to react to an anonymous informant.
Steps that need to be carried out by journal editors when confronted with unethical publishing include notifying the affected authors and research institutions, and investigation of the incident and publishing a report on it. It is important to be vigilant in order to detect breaches of publication ethics whenever they take place.
All authors must adhere to the principles of ethical publishing and agree with and conform to the policy of the journal in this regard. The corresponding author has obtained the consent of all the listed co-authors for the submission and publication of all versions of the manuscript. This is confirmed by all authors. All of the authors make their email address available, over which they are kept informed about all the steps up to the final step of publication or rejection.
All individuals have been added to the group of authors that have made a significant independent contribution to the manuscript.
The submitted manuscript is original and not already published elsewhere, except as oral presentation or poster with an abstract of no more than one page. In addition, the integrity of submitted articles is assured by the obligatory peer review process using all possible information technology and statistical tools.
The data of the manuscript have been obtained according to modern ethical standards taking into consideration the guideline recommendations such as those of PRISMA and free of decidedly non-authorized texts or data copies from other sources. All contents derived from previously published sources, either their own or those of others, are properly cited. Should any of the above-mentioned conditions be unmet, the authors are obliged to notify the journal as soon as possible about it. Correct statistics are important.
Editors, authors and reviewers must follow the basic rules of ethical publishing when submitting articles for publication, do peer reviewing or when they identify potential integrity problems when reading the articles. Most published articles are free of unethical behavior. Articles that, despite careful review process, violate good publication ethics, must be identified, analyzed and corrected or, where appropriate, retracted. In the work-up of problem cases, the methods formulated in the recommendations of COPE (http://publicationethics.org/resources/guidelines) can be put into use. “Intensive Care Medicine” makes full use of these recommendations. Rapid and close cooperation between authors, research institutions, the publisher of the journal and the publishing house is of the highest importance. It is emphasized that the critical reader plays an important role in the identification of irregularities and possible violation of good publication ethics. While respecting the reader anonymity, all concerned are encouraged to report suspected misconduct to the publication editor of the magazine.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cattermole GN, Delgado MCM, den Uil CA, Llompart-Pou JA S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Caelleigh AS. Role of the journal editor in sustaining integrity in research. Acad Med. 1993;68:S23-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Kranke P, Apfel CC, Roewer N, Fujii Y. Reported data on granisetron and postoperative nausea and vomiting by Fujii et al. Are incredibly nice! Anesth Analg. 2000;90:1004-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Miller DR. Retraction of articles written by Dr. Yoshitaka Fujii. Can J Anaesth. 2012;59:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Carlisle JB. The analysis of 168 randomised controlled trials to test data integrity. Anaesthesia. 2012;67:521-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Editors-in-Chief statement regarding published clinical trials conducted without IRB approval by Joachim Boldt. Minerva Anestesiol. 2011;77:562-563. [PubMed] |
| 6. | Shafer SL. Shadow of doubt. Anesth Analg. 2011;112:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | China’s medical research integrity questioned. Lancet. 2015;385:1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Tobin MJ. Assessing the performance of a medical journal. Am J Respir Crit Care Med. 2004;169:1268-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Bailar JI, Angell M, Boots S, Myers E, N Palmer, Shipley M, Woolf P. Ethics and Policy in scientific publication. Council of Biology Editors, Bethesda (MD). 1990;. |
| 10. | Resnik DB, Wager E, Kissling GE. Retraction policies of top scientific journals ranked by impact factor. J Med Libr Assoc. 2015;103:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Wager E, Kleinert S. Cooperation between research institutions and journals in research integrity cases: guidance from the Committee on Publication Ethics. London: Committee on Publication Ethics. [accessed 2015; Oct 12] Available from: http//publicationethics.org. |
| 12. | Marcus A, Oransky I. What studies of retractions tell us. J Microbiol Biol Educ. 2014;15:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Fang FC, Casadevall A. Retracted science and the retraction index. Infect Immun. 2011;79:3855-3859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Hospital presents results of final report: committee completes investigation in the case of Dr Boldt [accessed 2015 Oct 13]. Available from: http//www.klilu.de/content/veranstaltungen___presse/pressearchiv/2012/hospital_presents_results_of_final_report_committee_completes_investigation_in_the_case_of_dr_boldt/index_ger.html. |
| 15. | Ioannidis JP, Trikalinos TA, Zintzaras E. Extreme between-study homogeneity in meta-analyses could offer useful insights. J Clin Epidemiol. 2006;59:1023-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Lang K, Suttner S, Boldt J, Kumle B, Nagel D. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anaesth. 2003;50:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Boldt J, Ducke M, Kumle B, Papsdorf M, Zurmeyer EL. Influence of different volume replacement strategies on inflammation and endothelial activation in the elderly undergoing major abdominal surgery. Intensive Care Med. 2004;30:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Boldt J, Schölhorn T, Mayer J, Piper S, Suttner S. The value of an albumin-based intravascular volume replacement strategy in elderly patients undergoing major abdominal surgery. Anesth Analg. 2006;103:191-199, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Boldt J, Brosch Ch, Röhm K, Papsdorf M, Mengistu A. Comparison of the effects of gelatin and a modern hydroxyethyl starch solution on renal function and inflammatory response in elderly cardiac surgery patients. Br J Anaesth. 2008;100:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Boldt J, Suttner S, Brosch C, Lehmann A, Röhm K, Mengistu A. The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. Intensive Care Med. 2009;35:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Boldt J, Mayer J, Brosch C, Lehmann A, Mengistu A. Volume replacement with a balanced hydroxyethyl starch (HES) preparation in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2010;24:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Papsdorf M. Auswirkungen einer Volumenersatztherapie auf Makro- und Mikrozirkulation und deren Regulatoren: Ein Vergleich von Hydroxyethylstärke und Humanalbumin beim kritisch kranken Patienten. Dissertation: Justus-Liebig-Universität Gießen 2004; . |
| 23. | Schellhaaß A. Untersuchungen über den Einfluss von Volumenersatzmitteln auf die Blutgerinnung in der Abdominalchirurgie - Vergleich zweier kristalloider und zweier kolloidaler Volumenersatzmittel. Dissertation: Justus-Liebig-Universität Gießen 2003; . |
| 24. | Boldt J, Haisch G, Suttner S, Kumle B, Schellhaass A. Effects of a new modified, balanced hydroxyethyl starch preparation (Hextend) on measures of coagulation. Br J Anaesth. 2002;89:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Are lactated Ringer’s solution and normal saline solution equal with regard to coagulation?: Retraction. Anesth Analg. 2011;112:1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Retraction note: Volume therapy in the critically ill: is there a difference?. Intensive Care Med. 2014;40:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Boldt J, Suttner S, Brosch C, Lehmann A, Röhm K, Mengistu A. Cardiopulmonary bypass priming using a high dose of a balanced hydroxyethyl starch versus an albumin-based priming strategy. Anesth Analg. 2009;109:1752-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Notice of formal retraction of an article by Dr Joachim Boldt. Br J Anaesth. 2014;112:397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Shafer SL. Editor’s note: notice of retraction. Anesth Analg. 2014;119:1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Blood Conservation Techniques and Platelet Function in Cardiac Surgery: Retraction. Anesthesiology. 2015;123:492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Boldt J, Zickmann B, Rapin J, Hammermann H, Dapper F, Hempelmann G. Influence of volume replacement with different HES-solutions on microcirculatory blood flow in cardiac surgery. Acta Anaesthesiol Scand. 1994;38:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Boldt J, von Bormann B, Kling D, Börner U, Mulch J, Hempelmann G. Volume replacement with a new hydroxyethyl starch preparation (3 percent HES 200/0.5) in heart surgery. Infusionsther Klin Ernahr. 1986;13:145-151. [PubMed] |
| 33. | Boldt J, von Bormann B, Kling D, Börner U, Mulch J, Hempelmann G. Retraction Statement. Transfus Med Hemother. 2015;42:266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Chaudry IH, Lang CH. Expression of concern. Shock. 2015;43:620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Palus S. Three more retractions for former record-holder Boldt, maybe more to come [accessed 2015 Sept 30]. Available from: http//retractionwatch.com/2015/08/13/three-more-retractions-for-former-record-holder-boldt-maybe-more-to-come. |
| 36. | Wager E, Kleinert S. Cooperation between research institutions and journals on research integrity cases: guidance from the Committee on Publication Ethics (COPE). Maturitas. 2012;72:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Wager E. Defining and responding to plagiarism. Learn Publ. 2014;27:33-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Fanelli D. How many scientists fabricate and falsify research? A systematic review and meta-analysis of survey data. PLoS One. 2009;4:e5738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 746] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 39. | Carlisle JB, Dexter F, Pandit JJ, Shafer SL, Yentis SM. Calculating the probability of random sampling for continuous variables in submitted or published randomised controlled trials. Anaesthesia. 2015;70:848-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Miller DR. Probability screening in manuscripts submitted to biomedical journals--an effective tool or a statistical quagmire? Anaesthesia. 2015;70:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Stern AM, Casadevall A, Steen RG, Fang FC. Financial costs and personal consequences of research misconduct resulting in retracted publications. Elife. 2014;3:e02956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Foo JY, Tan XJ. Analysis and implications of retraction period and coauthorship of fraudulent publications. Account Res. 2014;21:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Cokol M, Iossifov I, Rodriguez-Esteban R, Rzhetsky A. How many scientific papers should be retracted? EMBO Rep. 2007;8:422-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Steen RG. Retractions in the medical literature: Who is responsible for scientific integrity? Am Med Writers Ass J. 2011;26:2-7. |
| 45. | Titus SL, Wells JA, Rhoades LJ. Repairing research integrity. Nature. 2008;453:980-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Budd JM, Sievert M, Schultz TR, Scoville C. Effects of article retraction on citation and practice in medicine. Bull Med Libr Assoc. 1999;87:437-443. [PubMed] |
| 47. | Wiedermann CJ. Hydroxyethyl starch effects on tissue perfusion and oxygenation in patients undergoing liver surgery. Int J Clin Exp Med. 2014;7:1623. [PubMed] |
| 48. | Bornemann-Cimenti H, Szilagyi IS, Sandner-Kiesling A. Perpetuation of Retracted Publications Using the Example of the Scott S. Reuben Case: Incidences, Reasons and Possible Improvements. Sci Eng Ethics. 2015; Jul 7; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Wilkes MM, Navickis RJ. The Boldt affair: a quandary for meta-analysts. Anesthesiol News. 2013;39:8-9. |
| 50. | Grieneisen ML, Zhang M. A comprehensive survey of retracted articles from the scholarly literature. PLoS One. 2012;7:e44118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 51. | Hamrell MR. Raising suspicions with the Food and Drug Administration: detecting misconduct. Sci Eng Ethics. 2010;16:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Seife C. Research misconduct identified by the US Food and Drug Administration: out of sight, out of mind, out of the peer-reviewed literature. JAMA Intern Med. 2015;175:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Hartog CS, Reinhart K. CRYSTMAS study adds to concerns about renal safety and increased mortality in sepsis patients. Crit Care. 2012;16:454; author reply 454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | James MF, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth. 2011;107:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 55. | Finfer S. Hydroxyethyl starch in patients with trauma. Br J Anaesth. 2012;108:159-160; author reply 160-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Elia N, Wager E, Tramèr MR. Fate of articles that warranted retraction due to ethical concerns: a descriptive cross-sectional study. PLoS One. 2014;9:e85846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Wager E, Williams P. Why and how do journals retract articles? An analysis of Medline retractions 1988-2008. J Med Ethics. 2011;37:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Williams P, Wager E. Exploring why and how journal editors retract articles: findings from a qualitative study. Sci Eng Ethics. 2013;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Steen RG. Retractions in the scientific literature: is the incidence of research fraud increasing? J Med Ethics. 2011;37:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 60. | Kornhaber RA, McLean LM, Baber RJ. Ongoing ethical issues concerning authorship in biomedical journals: an integrative review. Int J Nanomedicine. 2015;10:4837-4846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |