Published online Feb 4, 2016. doi: 10.5492/wjccm.v5.i1.96
Peer-review started: September 6, 2015
First decision: October 27, 2015
Revised: November 18, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: February 4, 2016
Processing time: 147 Days and 11 Hours
This review summarizes the epidemiology, pathophysiological consequences and impact on outcome of mild to moderate (Grade I to II) intra-abdominal hypertension (IAH), points out possible pitfalls in available treatment recommendations and focuses on tasks for future research in the field. IAH occurs in about 40% of ICU patients. Whereas the prevalence of abdominal compartment syndrome seems to be decreasing, the prevalence of IAH does not. More than half of IAH patients present with IAH grade I and approximately a quarter with IAH grade II. However, most of the studies have addressed IAH as a yes-or-no variable, with little or no attention to different severity grades. Even mild IAH can have a negative impact on tissue perfusion and microcirculation and be associated with an increased length of stay and duration of mechanical ventilation. However, the impact of IAH and its different grades on mortality is controversial. The influence of intra-abdominal pressure (IAP) on outcome most likely depends on patient and disease characteristics and the concomitant macro- and microcirculation. Therefore, management might differ significantly. Today, clear triggers for interventions in different patient groups with mild to moderate IAH are not defined. Further studies are needed to clarify the clinical importance of mild to moderate IAH identifying clear triggers for interventions to lower the IAP.
Core tip: This review summarizes the epidemiology, pathophysiological consequences and impact on outcome of mild to moderate intra-abdominal hypertension (IAH) and focuses on tasks for future research in the field. More than half of IAH patients present with IAH Grade I and approximately a quarter with IAH grade II. Even mild IAH can have a negative impact on tissue perfusion and be associated with impaired clinical outcomes. However, the impact of IAH and its different grades on mortality is controversial. Clear triggers for interventions in different patient groups with mild to moderate IAH are not defined.
- Citation: Maddison L, Starkopf J, Reintam Blaser A. Mild to moderate intra-abdominal hypertension: Does it matter? World J Crit Care Med 2016; 5(1): 96-102
- URL: https://www.wjgnet.com/2220-3141/full/v5/i1/96.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v5.i1.96
Intra-abdominal hypertension (IAH) occurs in 20%-40% of intensive care patients and has a significant impact on outcome[1-3]. IAH is defined as intra-abdominal pressure (IAP) of 12 mmHg or above, whereas normal IAP is in the range of 0 to 11 mmHg[4]. The cut-off point of 12 mmHg for IAH was initially selected empirically[5,6], but is now supported by several epidemiological studies[1,5,7-11]. Based on severity, IAH is graded into four levels. Grade I refers to IAP from 12 to 15 mmHg, grade II 16 to 20 mmHg, grade III 21 to 25 mmHg, and grade IV above 25 mmHg, respectively. The most severe form is abdominal compartment syndrome (ACS), defined as sustained IAP ≥ 20 mmHg (with or without an abdominal perfusion pressure (APP) of ≤ 60 mmHg) in association with the new onset or worsening of existing organ failure[4]. This is a life-threatening syndrome[6], which, however, seldom occurs in clinical practice[2,3,12]. In these cases, the deterioration of cardiac, respiratory and renal performance is usually clearly evident and determines the immediate need for life-saving treatment. The management of IAP below 20 mmHg is much more controversial[2,13] as there is no clear trigger for when and to what extent to initiate the treatment of IAH.
This review was undertaken to differentiate IAH grade I and II from higher grades of IAH regarding the pathophysiological changes and impact on outcome, and to discuss possible differences in management based on the severity of IAH.
There are several recent studies describing the epidemiology of IAH and investigating its impact on outcome[10,14,15].
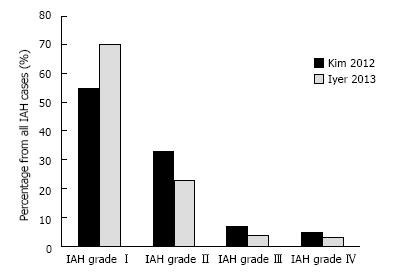
Kim et al[15] included 100 consecutive patients in a prospective observational single-center study on a mixed intensive care unit (ICU) population. The overall incidence of IAH was 42%, while IAH grade I occurred in 23%, grade II in 14%, grade III in 3% and grade IV in 2% of IAH patients (Figure 1). Patients with IAH had higher APACHE II and III scores, body mass index (BMI) and more sepsis on admission. However, there was no difference in the length of ICU stay or hospital mortality in patients with IAH (irrespective of the grade) vs patients without IAH.
Iyer et al[14] included 403 consecutive patients to investigate the incidence of IAH/ACS and to develop a screening tool for the early identification of patients requiring IAP monitoring. The incidence of IAH was 39% and of ACS, 2%. IAH grade I occurred in 27%, grade II in 9%, grade III in 2% and grade IV in 1% (Figure 1). Regarding outcomes, patients with IAH had significantly longer duration of mechanical ventilation and, length of stay in the ICU and in the hospital. No difference in mortality was detected between the patients with or without IAH; however, patients with IAH of higher grades (II-IV) had higher ICU mortality (13% vs 3.4%, P = 0.003)[14].
A recent systematic review and individual patient data meta-analysis reported distribution of IAP values among 1669 critically ill patients upon admission to ICU[10]. The mean IAP was 9.9 mmHg; whereas 27.7% of patients had IAH and 2.7% ACS on admission. Although the exact data on different IAH grades were not given, the IAP distribution histogram supports the studies discussed above (Kim et al[15] and Iyer et al[14]). Concerning the outcomes, the ICU length of stay, the ICU and the hospital mortality were significantly increased in the IAH group.
It is somewhat surprising that outcomes differ significantly when the data from Reintam et al[2], Malbrain et al[10] and Blaser et al[16] and our own earlier studies are compared to the results of Kim et al[15] or Iyer et al[14]. One possible explanation might be that the meta-analysis is based on studies published predominantly between 1995 and 2008. Almost two decades of research has very likely improved our understanding and management of IAH/ACS[12,17], and this may explain the decrease in morbidity and mortality. Moreover, most of the earlier studies (including the ones in the meta-analysis by Malbrain et al[10]) enrolled only selected patients (e.g., patients with pancreatitis, trauma, mechanically ventilated or presenting other risk factors for IAH). Therefore, the patient groups are not entirely comparable. Furthermore, assigning patients with IAP of 12 to 15 mmHg to the same group as ACS most likely confuses the results in all the above-mentioned studies. As the incidence of ACS is decreasing and the outcome simultaneously improving[12,16,17], its impact to the overall group of IAH patients is diminishing.
Considering these factors, the clinical importance of IAH is much dependent on the severity (grade) of IAH, but these associations have been poorly studied.
Next to the severity of IAH, the nature and course of underlying pathology needs to be taken into account. Higher grades of IAH may be less deleterious if the disease is cured (e.g., after abdominal surgery), whereas moderate IAH may have additional aggravating effect on the patients with uncontrolled primary pathology (e.g., shock with continuing need for fluid resuscitation).
In patients with pancreatitis or intra-abdominal infections already the mild IAH deserves close attention as a sign of increased oedema formation. In these patients development of IAH/ACS is primarily caused by the inflammatory process inside the abdominal cavity and may be further exacerbated by aggressive fluid therapy[18,19].
Intraperitoneal bleeding should be recognized promptly and therefore in trauma patients any increase of IAP, even at the low grades, deserves particular attention.
During pregnancy IAP increases physiologically, but the effect of the IAH has been poorly studied in this specific patient group. Recently, it has been postulated that the inability to adapt to the increasing intra-abdominal volume, reflected as sustained increase of IAP above 12 mmHg may be involved in the etiopathogenesis of pre-eclampsia[20].
Children, not specifically addressed in this review, require different approach. Organ dysfunction in paediatric patients has been reported to occur at the IAP 10 to 15 mmHg, and the ACS may develop already at the IAP of 16 mmHg[19,21,22].
In contrary, after elective abdominal hernia repair transient increase of IAP up to 18 mmHg may be well tolerated[23].
An experimental study on pigs demonstrated that a pneumoperitoneum-caused IAP of 12 mmHg combined with positive end-expiratory pressure of 10 cmH2O decreases blood flow in the hepatic and mesenteric arteries and portal vein, impairs the hepatic and intestinal mucosal microcirculation, attenuates the hepatic pO2 and intestinal mucosal pH, this all indicating to seriously disturbed splanchnic blood flow[24]. Olofsson et al[25] showed in pigs that increased IAP correlates with decreased gastrointestinal microcirculation measured by laser Doppler flowmetry, whereas microcirculation in mucosal layer was significantly less affected than in serosa. Cheng et al[26] demonstrated that, in rabbits, microvascular blood flow is decreased by 40% during IAP 15 mmHg for 2 h, and by 81% when the IAP was 25 mmHg for 6 h, while markers of intestinal injury increased significantly, in proportion to IAP and exposure time. After prolonged exposure to increased IAP, erosions and necrosis of the jejunal villi, mitochondrial swelling and discontinuity of intracellular tight junctions were microscopically observed[26]. These findings are clearly contrasted by recent study on mechanically ventilated sheep without sepsis, which demonstrated decreased renal blood flow and diuresis but preserved blood flow in superior mesenteric artery at IAP of 20 mmHg and no changes in microcirculatory variables. However, sheep with IAH received large amounts of fluids compared to sham and still developed relevant lactic acidosis. As shown by Dubin et al[27] in septic sheep, resuscitation was able to normalize mean arterial blood pressure, cardiac output, superior mesenteric artery blood flow and sublingual and serosal intestinal microvascular flow indexes, but not to restore percentage of perfused ileal villi, which could explain elevation of lactate. These different findings may be explained by several aspects: the level, mechanism and duration of the elevated IAP, presence or absence of sepsis, resuscitation strategy as well as the methodology of assessment of microcirculation all may play an important role.
In humans, the effect of IAP on gut perfusion is not well studied, but significant reduction of splanchnic blood flow has been demonstrated after the IAP increases from 7 to 14 mmHg[28]. As the splanchnic area is difficult to access in clinical setting, the monitoring of sublingual microcirculation might be considered for indirect evaluation of splanchnic microcirculation[29], although it is not clear whether this is well applicable in case of IAH where splanchnic bed is directly affected through the extravascular pressure.
So far, the associations between IAP and sublingual microcirculation have been evaluated in two studies[30,31]. No significant alterations in sublingual microcirculation were demonstrated at IAH grades I and II, neither in elective surgery nor in critically ill patients. In elective laparoscopic surgery the microcirculatory perfusion indices were relatively low at the baseline and did not improve during the study period[30].
In heterogeneous group of critically ill patients, the moderate but prolonged increase of IAP (median 14.5 mmHg) for up to 24 h exhibits a negligible influence on the sublingual microcirculation[31]. Ten out of 15 of these patients were in circulatory shock, and it is somewhat surprising that no significant alterations in the sublingual microcirculation were detected[31]. Observed positive correlations between microvascular flow index, MAP and APP, however, may support the importance of APP in clinical management of this situation[31].
Relevant alterations in microcirculation should result in the increased anaerobic metabolism and this hypothesis has led to studies investigating influence of IAP on tissue metabolism[30-35]. The findings suggest that the deterioration of tissue metabolism in the abdominal area may occur well before IAH-related organ dysfunction become evident[30-35].
In animal experiments, tissue microdialysis has been increasingly used to evaluate metabolic status in different vascular beds and in different conditions. By this method, Meier et al[35] were able to demonstrate the accumulation of metabolites of anaerobic glycolysis in the rectus abdominis muscle (RAM) of rats subjected to IAH. The RAM is surrounded by a tight sheet of fascia, which makes the muscle-fascia compartment relatively non-compliant. The pressure in the intra-abdominal cavity directly influences the muscle tissue and its perfusion. This muscle is easily accessible and makes the RAM microdialysis minimally invasive; therefore the RAM serves as a good model of intra-abdominal organ[32].
We performed RAM microdialysis in elective laparoscopic surgery (median IAP 12.5 mmHg) and in critically ill patients (median IAP 14.5 mmHg)[32,33]. In both groups the RAM tissue metabolism was seriously disturbed when compared to the baseline before surgery or to the references from other studies[36]. Elevated lactate, lactate-to-pyruvate ratio, and glutamate levels indicated anaerobic metabolism during moderately raised IAP. The correlation analysis revealed a negative correlation of APP, pyruvate and glycerol, supporting the relevance of APP as a resuscitation end-point when setting the targets for MAP and vasopressor therapy[33].
Considering these factors, some experimental studies indicate that increased IAP results in impaired splanchnic microcirculation and metabolism. Limited data suggests that similar changes may occur also in humans although direct assessment of the splanchnic area would be desirable to confirm these findings. Setting the APP or any other macrocirculatory variable as a resuscitation endpoint in patients with IAH remains controversial.
The Abdominal Compartment Society management algorithm suggests initiating medical treatment at IAP of 12 mmHg or higher and tailoring treatment to keep IAP below 15 mmHg[4]. Several mechanisms may lead to IAH: increased baseline IAP (e.g., obesity), increased intra-abdominal volume (e.g., ascites or oedema), and decreased abdominal wall compliance (e.g., tight abdominal closure after hernia repair)[6,37]. It is clear that these different mechanisms require different therapeutic approaches. However, the severity of IAH (grade of IAH) should also guide the selection of the best management option in these different situations. It is not clear whether in some cases lower grade IAH could merely be observed, whereas in others it should be aggressively treated with, e.g., sedatives and muscle relaxants. Current IAH/ACS guidelines suggest stepwise IAH treatment: From medical and minimally invasive techniques to aggressive surgical decompression[4]. A stepwise approach in general is definitely wise. However, different grades of IAH and different patient groups are not considered. As most of the options for IAP reduction are not without risks (e.g., drainages) and side effects (e.g., sedation, muscle relaxation), it is important not to apply these strategies without clear indication. It is possible that not treating moderate IAH could impair the outcome in one patient, whereas aggressive treatment (e.g., muscle relaxation) could be harmful in the other.
Future studies should form the basis for more detailed recommendations, whereas currently these decisions should be made during careful bedside evaluation, and require deep knowledge and experience.
Based on existing evidence, it is likely that lower grades of IAH are relevant in terms of both pathophysiology and clinical consequences. However, the relationships are most likely complex, and the influence of IAP depends on patient characteristics (e.g., obese vs non-obese; ventilated vs spontaneously breathing, critically ill vs ward patients), concomitant macro- (dependent on MAP) and microcirculation and disease characteristics (e.g., pancreatitis vs pneumonia). As IAH has been often assessed as a yes-or-no variable, these issues are not yet clarified. Accordingly, there are several issues that should be studied more closely in future studies.
Normal IAP in critically ill patients is thought to be between 5-7 mmHg[4], but there is not enough data to identify normal values in spontaneously breathing patients. Moreover, “normal” or expected IAP levels in patients with abdominal pathology, e.g., after elective major abdominal surgery, are unknown.
Signs of organ dysfunction, duration of mechanical ventilation, ICU length of stay and ICU mortality seem to increase in patients with elevated IAH, whereas in most cases, IAP ranges between 12-16 mmHg. Dalfino et al[11] showed that elevated IAP and low APP are associated with the development of acute renal failure (ARF) in critically ill patients after shock and identified a cut-off IAP of 12 mmHg for increasing the risk of ARF. A cut-off value of 12 mmHg for IAH is also supported by several other studies[1,5,7-10]. However, it is not clear whether the current IAH grading system can be translated to gradually increasing mortality or worsening organ function.
None of the studies has specifically addressed IAH in spontaneously breathing patients. Therefore, it is not known, whether 12 mmHg is also applicable to this subset of patients. Moreover, the likelihood of more unstable measurement conditions in spontaneously breathing patients may lead to wide variations, and muscle tonus may play an important role.
Obesity and pregnancy are chronic states of low-graded IAH to which the patient has adapted[6,38]. Both conditions develop relatively slowly, and the human organism adapts to these (patho) physiological changes, but there is still an issue if such patients need to be admitted to an ICU. IAP levels that trigger specific treatment should be most likely higher, but how much so, remains unclear.
Post-operative complications after abdominal surgery are frequent and are associated with increased morbidity and mortality[39-42]. Intra-abdominal hypertension and its pathophysiological consequences may contribute to the development of postoperative complications[43,44], but no specific data are available thus far. Therefore, a cut-off that triggers interventions directed to lower IAP and/or to increase APP in such patients is warranted.
Ideally, the management of hemodynamics should ensure normal microcirculation and organ function in different vascular beds. We currently lack reliable tool(s) to assess microcirculation at the bedside, and the clinical signs (diuresis, mottled skin) and macrohemodynamic variables (MAP, cardiac output) rather than microcirculatory changes direct the patient management in clinical practise. However, in the case of increased abdominal pressure, “normal” MAP and cardiac output might be insufficient to assure the adequate perfusion of abdominal and retroperitoneal organs. Moreover, due to heterogeneous aspects of microcirculatory perfusion, effect of classical hemodynamic interventions on microcirculation will most likely be limited[45]. Whether microcirculatory measurements may be supportive for hemodynamic management during IAH remains to be elucidated.
The identification of patient groups where even mild IAH leads to microcirculatory dysfunction with anaerobic metabolism would be desirable for the more precise adjustment of management suggestions.
Higher grades of IAH are infrequent; most patients with IAH qualify as grade I of IAH. Therefore, further studies should focus on mild to moderate IAH to clarify, which patients should be treated aggressively and which patients can simply be observed. Studies are needed to allow revision of the algorithm based on evidence. More specific recommendations including treatment triggers in spontaneously breathing patients, obese patients, patients after abdominal surgery and other specific groups, are warranted.
IAH occurs in about 40% of the ICU population. More than half of these patients present with IAH grade I and approximately a quarter with IAH grade II. Patients with IAH have a significantly longer duration of mechanical ventilation and, longer length of stay in the ICU and in the hospital. The impact of IAH and its different grades on mortality is controversial.
Preliminary data suggest that grades I and II IAH has a negative impact on tissue perfusion and microcirculation. Further studies are needed to clarify whether and in which particular sub-group of patients the occurrence of mild to moderate IAH should trigger immediate interventions directed at lowering the IAP.
P- Reviewer: Qiu HB S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 442] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Primary and secondary intra-abdominal hypertension--different impact on ICU outcome. Intensive Care Med. 2008;34:1624-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Reintam Blaser A, Parm P, Kitus R, Starkopf J. Risk factors for intra-abdominal hypertension in mechanically ventilated patients. Acta Anaesthesiol Scand. 2011;55:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 856] [Article Influence: 71.3] [Reference Citation Analysis (2)] |
| 5. | Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 6. | Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 863] [Article Influence: 45.4] [Reference Citation Analysis (2)] |
| 7. | Regueira T, Bruhn A, Hasbun P, Aguirre M, Romero C, Llanos O, Castro R, Bugedo G, Hernandez G. Intra-abdominal hypertension: incidence and association with organ dysfunction during early septic shock. J Crit Care. 2008;23:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Vidal MG, Ruiz Weisser J, Gonzalez F, Toro MA, Loudet C, Balasini C, Canales H, Reina R, Estenssoro E. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med. 2008;36:1823-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Santa-Teresa P, Muñoz J, Montero I, Zurita M, Tomey M, Alvarez-Sala L, García P. Incidence and prognosis of intra-abdominal hypertension in critically ill medical patients: a prospective epidemiological study. Ann Intensive Care. 2012;2 Suppl 1:S3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Malbrain ML, Chiumello D, Cesana BM, Reintam Blaser A, Starkopf J, Sugrue M, Pelosi P, Severgnini P, Hernandez G, Brienza N. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol. 2014;80:293-306. [PubMed] |
| 11. | Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Carr JA. Abdominal compartment syndrome: a decade of progress. J Am Coll Surg. 2013;216:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Reintam A, Parm P, Kitus R, Starkopf J, Kern H. Gastrointestinal failure score in critically ill patients: a prospective observational study. Crit Care. 2008;12:R90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Iyer D, Rastogi P, Åneman A, D’Amours S. Early screening to identify patients at risk of developing intra-abdominal hypertension and abdominal compartment syndrome. Acta Anaesthesiol Scand. 2014;58:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kim IB, Prowle J, Baldwin I, Bellomo R. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care. 2012;40:79-89. [PubMed] |
| 16. | Blaser AR, Sarapuu S, Tamme K, Starkopf J. Expanded measurements of intra-abdominal pressure do not increase the detection rate of intra-abdominal hypertension: a single-center observational study. Crit Care Med. 2014;42:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Balogh ZJ, Lumsdaine W, Moore EE, Moore FA. Postinjury abdominal compartment syndrome: from recognition to prevention. Lancet. 2014;384:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | De Waele JJ, Leppäniemi AK. Intra-abdominal hypertension in acute pancreatitis. World J Surg. 2009;33:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | De Waele JJ, Ejike JC, Leppäniemi A, De Keulenaer BL, De Laet I, Kirkpatrick AW, Roberts DJ, Kimball E, Ivatury R, Malbrain ML. Intra-abdominal hypertension and abdominal compartment syndrome in pancreatitis, paediatrics, and trauma. Anaesthesiol Intensive Ther. 2015;47:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Sawchuck DJ, Wittmann BK. Pre-eclampsia renamed and reframed: Intra-abdominal hypertension in pregnancy. Med Hypotheses. 2014;83:619-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Beck R, Halberthal M, Zonis Z, Shoshani G, Hayari L, Bar-Joseph G. Abdominal compartment syndrome in children. Pediatr Crit Care Med. 2001;2:51-56. [PubMed] |
| 22. | Ertel W, Oberholzer A, Platz A, Stocker R, Trentz O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747-1753. [PubMed] |
| 23. | Petro CC, Raigani S, Fayezizadeh M, Rowbottom JR, Klick JC, Prabhu AS, Novitsky YW, Rosen MJ. Permissible Intraabdominal Hypertension following Complex Abdominal Wall Reconstruction. Plast Reconstr Surg. 2015;136:868-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Kotzampassi K, Paramythiotis D, Eleftheriadis E. Deterioration of visceral perfusion caused by intra-abdominal hypertension in pigs ventilated with positive end-expiratory pressure. Surg Today. 2000;30:987-992. [PubMed] |
| 25. | Olofsson PH, Berg S, Ahn HC, Brudin LH, Vikström T, Johansson KJ. Gastrointestinal microcirculation and cardiopulmonary function during experimentally increased intra-abdominal pressure. Crit Care Med. 2009;37:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Cheng J, Wei Z, Liu X, Li X, Yuan Z, Zheng J, Chen X, Xiao G, Li X. The role of intestinal mucosa injury induced by intra-abdominal hypertension in the development of abdominal compartment syndrome and multiple organ dysfunction syndrome. Crit Care. 2013;17:R283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Dubin A, Edul VS, Pozo MO, Murias G, Canullán CM, Martins EF, Ferrara G, Canales HS, Laporte M, Estenssoro E. Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med. 2008;36:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Windberger UB, Auer R, Keplinger F, Längle F, Heinze G, Schindl M, Losert UM. The role of intra-abdominal pressure on splanchnic and pulmonary hemodynamic and metabolic changes during carbon dioxide pneumoperitoneum. Gastrointest Endosc. 1999;49:84-91. [PubMed] |
| 29. | Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, Rodriguez H, Pries AR, Vincent JL. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med. 2009;37:2875-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Maddison L, Riigor KM, Karjagin J, Starkopf J. Sublingual microcirculatory changes during transient intra-abdominal hypertension--a prospective observational study in laparoscopic surgery patients. Clin Hemorheol Microcirc. 2014;57:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Maddison L, Karjagin J, Buldakov M, Mäll M, Kruusat R, Lillemäe K, Kirsimägi U, Starkopf J. Sublingual microcirculation in patients with intra-abdominal hypertension: a pilot study in 15 critically ill patients. J Crit Care. 2014;29:183.e1-183.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Maddison L, Karjagin J, Tenhunen J, Starkopf J; Moderate intra-abdominal hypertension is associated with an increased lactate-pyruvate ratio in the rectus abdominis muscle tissue: a pilot study during laparoscopic surgery. Ann Intensive Care. 2012;2 Suppl 1:S14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Maddison L, Karjagin J, Tenhunen J, Kirsimägi U, Starkopf J. Moderate intra-abdominal hypertension leads to anaerobic metabolism in the rectus abdominis muscle tissue of critically ill patients: a prospective observational study. Biomed Res Int. 2014;2014:857492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Benninger E, Laschke MW, Cardell M, Holstein JH, Lustenberger T, Keel M, Trentz O, Menger MD, Meier C. Early detection of subclinical organ dysfunction by microdialysis of the rectus abdominis muscle in a porcine model of critical intra-abdominal hypertension. Shock. 2012;38:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Meier C, Contaldo C, Schramm R, Holstein JH, Hamacher J, Amon M, Wanner GA, Trentz O, Menger MD. Microdialysis of the rectus abdominis muscle for early detection of impending abdominal compartment syndrome. Intensive Care Med. 2007;33:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Hörer TM, Skoog P, Norgren L, Magnuson A, Berggren L, Jansson K, Larzon T. Intra-peritoneal microdialysis and intra-abdominal pressure after endovascular repair of ruptured aortic aneurysms. Eur J Vasc Endovasc Surg. 2013;45:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Blaser AR, Björck M, De Keulenaer B, Regli A. Abdominal compliance: A bench-to-bedside review. J Trauma Acute Care Surg. 2015;78:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Chun R, Baghirzada L, Tiruta C, Kirkpatrick AW. Measurement of intra-abdominal pressure in term pregnancy: a pilot study. Int J Obstet Anesth. 2012;21:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Straatman J, Cuesta MA, de Lange-de Klerk ES, van der Peet DL. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg. 2015;32:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | McCoy CC, Englum BR, Keenan JE, Vaslef SN, Shapiro ML, Scarborough JE. Impact of specific postoperative complications on the outcomes of emergency general surgery patients. J Trauma Acute Care Surg. 2015;78:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res. 2015;198:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 42. | Albisinni S, Oderda M, Fossion L, Varca V, Rassweiler J, Cathelineau X, Chlosta P, De la Taille A, Gaboardi F, Piechaud T. The morbidity of laparoscopic radical cystectomy: analysis of postoperative complications in a multicenter cohort by the European Association of Urology (EAU)-Section of Uro-Technology. World J Urol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Jhanji S, Lee C, Watson D, Hinds C, Pearse RM. Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med. 2009;35:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care. 2010;14:R151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent JL. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |