Published online Aug 4, 2015. doi: 10.5492/wjccm.v4.i3.258
Peer-review started: February 14, 2015
First decision: March 20, 2015
Revised: April 30, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: August 4, 2015
Processing time: 186 Days and 4.5 Hours
AIM: To describe the intensive care unit (ICU) outcomes of critically ill cancer patients with Acinetobacter baumannii (AB) infection.
METHODS: This was an observational study that included 23 consecutive cancer patients who acquired AB infections during their stay at ICU of the National Cancer Institute of Mexico (INCan), located in Mexico City. Data collection took place between January 2011, and December 2012. Patients who had AB infections before ICU admission, and infections that occurred during the first 2 d of ICU stay were excluded. Data were obtained by reviewing the electronic health record of each patient. This investigation was approved by the Scientific and Ethics Committees at INCan. Because of its observational nature, informed consent of the patients was not required.
RESULTS: Throughout the study period, a total of 494 critically ill patients with cancer were admitted to the ICU of the INCan, 23 (4.6%) of whom developed AB infections. Sixteen (60.9%) of these patients had hematologic malignancies. Most frequent reasons for ICU admission were severe sepsis or septic shock (56.2%) and postoperative care (21.7%). The respiratory tract was the most frequent site of AB infection (91.3%). The most common organ dysfunction observed in our group of patients were the respiratory (100%), cardiovascular (100%), hepatic (73.9%) and renal dysfunction (65.2%). The ICU mortality of patients with 3 or less organ system dysfunctions was 11.7% (2/17) compared with 66.6% (4/6) for the group of patients with 4 or more organ system dysfunctions (P = 0.021). Multivariate analysis identified blood lactate levels (BLL) as the only variable independently associated with in-ICU death (OR = 2.59, 95%CI: 1.04-6.43, P = 0.040). ICU and hospital mortality rates were 26.1% and 43.5%, respectively.
CONCLUSION: The mortality rate in critically ill patients with both HM, and AB infections who are admitted to the ICU is high. The variable most associated with increased mortality was a BLL ≥ 2.6 mmol/L in the first day of stay in the ICU.
Core tip: Several factors have been associated with poor prognosis among critically ill patients with infections caused by Acinetobacter baumannii (AB) in the intensive care unit (ICU) including renal failure, thrombocytopenia, neutropenia, history of prior immunosuppressive therapy use, the need for invasive mechanical ventilation, and development of severe sepsis. In this study the mortality rate in patients with both hematological malignancies, and AB infections who are admitted to the ICU is high. The variable most associated with increased mortality was a blood lactate levels ≥ 2.6 mmol/L in the first day of stay in the ICU.
-
Citation: Ñamendys-Silva SA, Correa-García P, García-Guillén FJ, González-Herrera MO, Pérez-Alonso A, Texcocano-Becerra J, Herrera-Gómez A, Cornejo-Juárez P, Meneses-García A. Outcomes of critically ill cancer patients with
Acinetobacter baumannii infection. World J Crit Care Med 2015; 4(3): 258-264 - URL: https://www.wjgnet.com/2220-3141/full/v4/i3/258.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i3.258
Acinetobacter baumannii (AB) is an aerobic, gram-negative coccobacillary rod that grows at 20 °C-30 °C on standard laboratory media[1]. AB infections may be fatal in patients with suboptimal immune defenses[2]. The mortality attributable to infections caused by AB in critically ill patients ranges from 40.7% to 73%[3-5]. The intensive care unit (ICU) and hospital mortality rate of patients with both hematologic malignancies and AB infection is 83%[6]; however, patients with solid tumors and bacteremia caused by AB have a relatively good prognosis with a mortality rate of 14.5%[7].
Risk factors associated with AB colonization or infection include prolonged hospitalization, admission to the ICU, recent surgical procedures, exposure to antibiotics, use of central venous catheter, hospitalization and nursing home residence before hospital admission[8]. Several factors have been associated with poor prognosis among critically ill patients with infections caused by AB in the ICU, including renal failure, thrombocytopenia[9], low Glasgow coma scale, neutropenia, history of prior immunosuppressive therapy use, the need for mechanical ventilatory support, and development of severe sepsis[6].
In Latin America Acinetobacter spp has been reported as one of the most commonly isolated species (9.6%) from patients with suspected hospital-acquired pneumonia[10]. In Mexico, information on the prevalence and incidence of AB infections is limited[11,12]. The aim of the present study was to describe the ICU outcomes of critically ill cancer patients with AB infection.
This was an observational study that included 23 consecutive cancer patients who acquired AB infections during their stay at ICU of the National Cancer Institute of Mexico (INCan), located in Mexico City. Data collection took place between January 2011, and December 2012. Data on the characteristics, organization, and recommendations for admission to our ICU have been previously reported[13,14]. Patients who had AB infections before ICU admission, and infections that occurred during the first 2 d of ICU stay were excluded. This investigation was approved by the Scientific and Ethics Committees at INCan (Rev/02/13). Because of its observational nature, informed consent of the patients was not required.
Data were obtained by reviewing the electronic health record of each patient. Data obtained included: the Eastern Cooperative Oncology Group scale for performance status[15] prior to hospitalization, malignancy types, reasons for ICU admission, the need for invasive mechanical ventilation (IMV), the need for vasopressor therapy, durations of vasopressors, length of IMV, the length of stay (LOS) in the hospital before ICU admission, the LOS in hospital wards before ICU, use of antibiotics 30 d before ICU admission, infection sites, and the ICU and hospital mortality rate. The LOS in the ICU was measured by the number of hours or days spent there by the patient. The LOS in the hospital before ICU admission was quantified as the number of days from date of hospital admission until ICU admission. The AB was categorized as follows: multidrug-resistant (MDR), pandrug-resistant (PDR), and pansensitive (PDS). AB MDR was defined as non-susceptible to ≥ 1 agent in ≥ 3 antimicrobial categories. AB PDR was defined as non-susceptible to ≥ 1 agent in all but ≤ 2 categories. AB PDS was defined as susceptible to all antimicrobial agents[16]. The Acute Physiology and Chronic Health Evaluation II score[17], and the Sequential Organ Failure Assessment (SOFA) score[18] were calculated within the first day ICU stay. In this study we have defined organ dysfunction as a SOFA score ≥ 1 point[14]. Malignancies were grouped into either hematological malignancies (HM) or solid tumors. Patients were divided into two groups based on their blood lactate levels (BLL): BLL ≥ 2.6 mmol/L or BLL < 2.6 mmol/L.
The Kolmogorov-Smirnov test was performed to verify the normality of the distributions of the data; all of continuous variables were normally distributed. Data are presented as the mean ± SD. The continuous variables were compared using student’s t test and the chi-square or the Fisher exact test was used to compare categorical data.
Discrimination was assessed using the area under the receiver operating characteristic (ROC) curve to evaluate the potential for using the lactate levels to discriminate between patients who die from those who survive. The sensitivity and specificity of the BLL cuoffs for predicting ICU mortality were examined. We constructed a multivariable model to identify factors associated with ICU mortality. We entered parameters into the model that were statistically significant on univariate analysis at a level of P < 0.20. Results were summarized as odds ratios (OR) with 95%CI. We assessed model discrimination using the area under the ROC curve[19]. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test and an adequate fit was assumed if P > 0.05[20]. Survival curves were estimated by the Kaplan-Meier method and differences between survival curves were checked with the log-rank test. Statistical analysis was done using the Statistical Package for the Social Sciences version 20.0. All tests were two-tailed, and a P < 0.05 was predetermined for statistical significance. All reported P values are 2 sided.
The statistical methods of this study were reviewed by Silvio A Ñamendys-Silva, Department of Critical Care Medicine, Instituto Nacional de Cancerología, Mexico City 14080, Mexico. Telephone: +52-55-47471020-13015, 13016.
Throughout the study period, a total of 494 patients with cancer were admitted to the ICU of the INCan, 23 (4.6%) of whom developed AB infections. Sixteen (60.9%) of these patients had HM. Most frequent reasons for ICU admission were severe sepsis or septic shock (56.2%) and postoperative care (21.7%). In Table 1 are presented demographic and clinical data of patients. The mean time between the admission to the ICU and the development of AB infection was 13 ± 9.9 d. The respiratory tract was the most frequent site of AB infection (91.3%). The most frequent co-morbidity associated with AB infection was diabetes mellitus 3/23(13%), followed by cardiovascular disease (8.7%).
| Characteristics | Values |
| No. of patients | 23 |
| Age (years), mean ± SD | 44.09 ± 17.10 |
| Gender (women), n (%) | 11 (47.8) |
| Length of ICU stay (d), mean ± SD | 21.9 ± 28.9 |
| Length of hospital stay (d), mean ± SD | 23.9 ± 12.3 |
| Need for vasopressors, n (%) | 23 (100) |
| Need for invasive mechanical ventilation, n (%) | 23 (100) |
| Length of mechanical ventilation (d), mean ± SD | 21.4 ± 11.8 |
| In hospital ward time before ICU admission, n (%) | 20 (86.9) |
| Length of stay in hospital wards before ICU admission (d), mean ± SD | 8.8 ± 10.6 |
| Use of antibiotics 30 days before ICU admission, n (%) | 17 (73.9) |
| Infection site, n (%) | |
| Respiratory | 21 (91.3) |
| Blood culture | 3 (13) |
| Surgical site | 1 (4.3) |
| Pansensitive, n (%) | 2 (8.7) |
| Pandrug-resistant, n (%) | 5 (21.7) |
| Multidrug-resistant, n (%) | 16 (69.6) |
| APACHE II score, mean ± SD | 13.3 ± 5.8 |
| SOFA score, mean ± SD | 8.7 ± 2.4 |
| Performance status 0-2, n (%) | 22 (95.7) |
| ICU mortality, n (%) | 6 (26.1) |
| Hospital mortality, n (%) | 10 (43.5) |
The most common organ dysfunction observed in our group of patients were the respiratory (100%), cardiovascular (100%), hepatic (73.9%) and renal dysfunction (65.2%). The ICU mortality of patients with 3 or less organ system dysfunctions was 11.7% (2/17) compared with 66.6% (4/6) for the group of patients with 4 or more organ system dysfunctions (P = 0.021) (Table 2).
| Characteristics | Survivors | Nonsurvivors | P |
| Age, years, mean ± SD | 42.6 ± 16.6 | 48.1 ± 19.3 | 0.510 |
| Women, n (%) | 8 (47) | 3 (50) | 0.901 |
| APCAHE II score, mean ± SD | 12.7 ± 5.0 | 15.3 ± 7.9 | 0.357 |
| SOFA score, mean ± SD | 8.4 ± 2.4 | 9.6 ± 2.6 | 0.318 |
| PEEP, cmH2O | 8.4 ± 2.8 | 7.3 ± 2.3 | 0.422 |
| Durations of vasopressors | 7.59 ± 4.2 | 11 ± 4.9 | 0.122 |
| Leukocytes, × 109/L | 8.6 ± 6.9 | 11.6 ± 13.1 | 0.487 |
| Absolute neutrophil count, cells/mm3 | 7.5 ± 6.2 | 10.2 ± 11.2 | 0.472 |
| Lymphocytes, cells/mm3 | 682 ± 542 | 666 ± 871 | 0.959 |
| Platelets, × 109/L | 184.4 ± 149.2 | 112.8 ± 105.0 | 0.291 |
| Sodium, mmol/L | 138 ± 5.85 | 135.3 ± 6.4 | 0.330 |
| Potassium, mmol/L | 3.9 ± 0.51 | 4.0 ± 0.71 | 0.800 |
| Chloride, mmol/L | 109.1 ± 8.74 | 109.3 ± 4.2 | 0.967 |
| Lactate, mmo/L | 2.01 ± 1.29 | 5.2 ± 3.2 | 0.002 |
| Magnesium, mmol/L | 0.93 ± 0.24 | 0.97 ± 0.13 | 0.722 |
| Phosphorus, mmol/L | 1.33 ± 0.46 | 1.28 ± 0.66 | 0.816 |
| Hemoglobin, g/L | 91.3 ± 19.2 | 94.5 ± 18.8 | 0.739 |
| Creatinine, μmol/L | 75.8 ± 39.01 | 133.2 ± 34.4 | 0.004 |
| Glucose, mmol/L | 8.34 ± 3.5 | 8.09 ± 2.2 | 0.877 |
| Bilirubin, total, μmol/L | 17.8 ± 12.5 | 22.5 ± 15.5 | 0.465 |
| Uric acid, μmol/L | 219.0 ± 94.0 | 189.3 ± 149.0 | 0.576 |
| ARDS, n (%) | 13 (76.4) | 4 (66.6) | 0.632 |
| Number of organ dysfunction (≥ 4) | 2 (11.7) | 4 (66.6) | 0.021 |
| Malignancies | |||
| Hematological malignancy, n (%) | 8 (47) | 6 (100) | 0.030 |
| Solid tumor, n (%) | 9 (52.9) | 0 (0) |
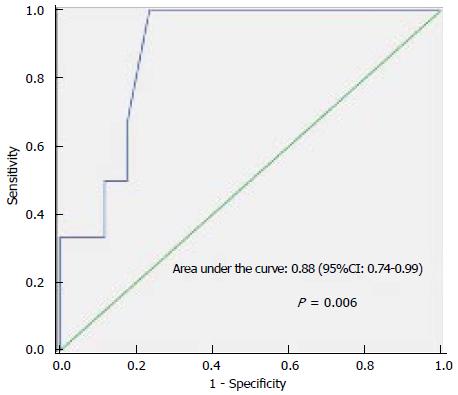
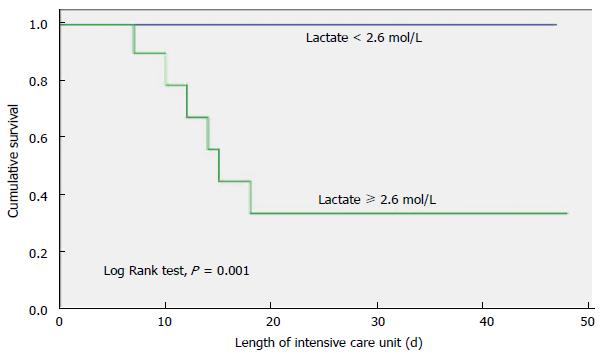
The primary outcome variable of interest was ICU mortality. Univariate analysis indicated that the following three factors were associated with ICU death: BLL, four or more organ dysfunctions, and creatinine level (Table 3). Multivariate analysis identified BLL as the only variable independently associated with in-ICU death. The area under the ROC curve was 0.88 (95%CI: 0.74-0.99), P = 0.006, demonstrating a good discriminatory power to predict ICU mortality. The cut-off point was a BLL ≥ 2.6 mmol/L, with 100% sensitivity and 77% specificity (Figure 1). ICU and hospital mortality rates were 26.1% and 43.5%, respectively. ICU survival by BLL is presented in Figure 2, indicating that the patients who had a BLL ≥ 2.6 mmol/L in the first day ICU stay were less likely to survive.
| Variables | Univariate | P | Multivariate | P | ||
| OR | 95%CI | OR | 95%CI | |||
| Age (yr) | 1.02 | 0.96-1.08 | 0.491 | |||
| Gender (male) | 1.12 | 0.17-7.24 | 0.901 | |||
| APACHE II score | 1.07 | 0.92-1.25 | 0.345 | |||
| SOFA score | 1.23 | 0.82-1.84 | 0.308 | |||
| Length of stay in hospital wards before ICU admission (d) | 0.76 | 0.51-1.12 | 0.171 | |||
| Duration of vasopressors (d) | 1.18 | 0.95-1.48 | 0.129 | |||
| Blood lactate level (mmol/L) | 2.59 | 1.04-6.43 | 0.04 | 2.59 | 1.04-6.43 | 0.04 |
| Number of organ dysfunction (≥ 4) | 15.00 | 1.58-142.1 | 0.018 | |||
| Creatinine (μmol/L) | 1.03 | 1.004-1.064 | 0.024 | |||
| Total bilirubin (μmol/L) | 1.02 | 0.95-1.10 | 0.449 | |||
| Albumin g/L | 1.06 | 0.89-1.27 | 0.494 | |||
| Platelets (× 109/L) | 0.99 | 0.98-1.00 | 0.295 | |||
| Absolute neutrophil count/μL | 1.04 | 0.92-1.17 | 0.459 | |||
| Absolute lymphocytes count/μL | 1.03 | 0.93-1.15 | 0.479 | |||
In this study, the incidence of AB infection in cancer patients who were admitted to the ICU was 4.6%, and ICU and hospital mortality rates were 26.1% and 43.5%, respectively, which is lower than the mortality rates reported by other authors[4,6]. All of the patients who died had HM. Patients who had four or more organ system failures at the time of admission to the ICU had a high mortality rate. In the multivariate analysis, the only variable independently associated ICU mortality was a BLL ≥ 2.6 mmol/L. Patients with a BLL ≥ 2.6 mmol/L in the first day ICU stay were less likely to survive.
The overall ICU mortality rate found in our study could be related to the implementation of medical management protocols. Patients with severe sepsis and septic shock had received standard guidelines-based treatment[21]. Patient care rounds were performed daily with an infectious diseases attending physician[22]. Levy and collaborators[23] reported that the implementation of guidelines for management of severe sepsis and septic shock is associated with sustained, continuous quality improvement in sepsis care, and with a significant reduction in hospital mortality among patients with severe sepsis and septic shock.
The patients with HM admitted to our ICU had higher ICU mortality rates than those with solid tumors (21.4% vs 46.1%)[13,14]. Sepsis remains a frequent complication in patients with cancer, and is associated with high mortality[24]. Immune dysfunction has been documented in patients with cancer. Predisposing factors for infection include the tumor site, intravenous devices, neutropenia because of an underlying disease, corticosteroids, monoclonal antibodies, and treatment with chemotherapy or radiation therapy[25].
Risk factors for developing AB infections in patients with HM include advancing age, prior exposure to aminoglycosides, central venous catheterization, and the presence of nasogastric tube[6]. Turkoglu et al[6] reported that a low Glasgow coma scale, neutropenia, history of prior immunosuppressive therapy use, the need for IMV, and development of severe sepsis were associated with mortality in patients with HM. Infection with AB an APACHE II score ≥ 21 points are variables associated with a poor clinical outcomes for patients with solid tumors and AB complex bacteremia[7]. In our study all of the patients who died in the ICU had HM, and required vasopressors. Univariate analysis primarily identified three factors that were related with ICU mortality; BLL, four or more organ dysfunctions, and creatinine levels. Multivariate analysis identified BLL as an independent prognostic factor for in-ICU death. The patients with BLL ≥ 2.6 mmol/L in the first day of stay in the ICU were less likely to survive. Increased BLL have been related to morbidity and mortality[26]. BLL are frequently elevated in critically ill patients and correlate well with disease severity. Hyperlactatemia (> 2 mmol/L) is observed in shock states when oxygen consumption becomes critically dependent on oxygen delivery[27]. The results of the current study suggest that in critically ill patients with cancer, and sepsis caused by AB, BLL may be used to identify patients at an increased risk of an adverse outcome. This may help to identify patients who may benefit from early admission to ICU. This report confirms that BLL is a valuable biomarker in the treatment of critically ill cancer patients with septic shock caused by AB infection. There have been no new cases reported since July 2014 in our ICU.
This study has the following limitations: (1) The clinical data were obtained from a single institution; and (2) A small number of patients was included.
The mortality rate in critically ill patients with both HM, and AB infections who are admitted to the ICU is high. The variable most associated with increased mortality was a BLL ≥ 2.6 mmol/L in the first day of stay in the ICU.
We thank the nurses and medical staff of the intensive care unit at INCan, Mexico City who were involved in the care of these patients for their assistance.
The mortality attributable to infections caused by Acinetobacter baumannii (AB) in critically ill patients ranges from 40.7% to 73%. The intensive care unit (ICU) and hospital mortality rate of patients with both hematologic malignancies and AB infection is high. Risk factors associated with AB colonization or infection include prolonged hospitalization, ICU admission, recent surgical procedures, antimicrobial agent exposure, central venous catheter use, prior hospitalization and nursing home residence. Several factors have been associated with poor prognosis among critically ill patients with infections caused by AB in the ICU, including renal failure, thrombocytopenia, the presence of neutropenia, prior immunosuppressive therapy, the need for invasive mechanical ventilation, and development of severe sepsis.
In Latin America Acinetobacter spp has been reported as one of the most frequent species isolated from patients hospitalized with suspected pneumonia. In Mexico, information on the prevalence and incidence of AB infections is limited.
Blood lactate level (BLL) is a valuable biomarker in the treatment of critically ill cancer patients with septic shock caused by AB infection and thereby the importance of providing ICU treatment.
The results of our study suggest that in critically ill cancer patients with sepsis caused by AB, BLL may be used to identify patients at an increased risk of an adverse outcome. This may help to identify patients who may benefit from early admission to ICU.
The manuscript is well conceived and indicates that lactate is a valuable biomarker in the treatment of critically ill cancer patients with septic shock caused by AB infection and thereby the importance of providing ICU treatment.
P- Reviewer: Boucek C, Krishnan T, Nagata T S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692-699. [PubMed] |
| 2. | Montefour K, Frieden J, Hurst S, Helmich C, Headley D, Martin M, Boyle DA. Acinetobacter baumannii: an emerging multidrug-resistant pathogen in critical care. Crit Care Nurse. 2008;28:15-25; quiz 26. [PubMed] |
| 3. | Lee NY, Lee JC, Li MC, Li CW, Ko WC. Empirical antimicrobial therapy for critically ill patients with Acinetobacter baumannii bacteremia: combination is better. J Microbiol Immunol Infect. 2013;46:397-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Prates CG, Martins AF, Superti SV, Lopes FS, Ramos F, Cantarelli VV, Zavascki AP. Risk factors for 30-day mortality in patients with carbapenem-resistant Acinetobacter baumannii during an outbreak in an intensive care unit. Epidemiol Infect. 2011;139:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Fagon JY, Chastre J, Domart Y, Trouillet JL, Gibert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brush. Clin Infect Dis. 1996;23:538-542. [PubMed] |
| 6. | Turkoglu M, Mirza E, Tunçcan OG, Erdem GU, Dizbay M, Yağcı M, Aygencel G, Türköz Sucak G. Acinetobacter baumannii infection in patients with hematologic malignancies in intensive care unit: risk factors and impact on mortality. J Crit Care. 2011;26:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Chiang MC, Kuo SC, Chen SJ, Yang SP, Lee YT, Chen TL, Fung CP. Clinical characteristics and outcomes of bacteremia due to different genomic species of Acinetobacter baumannii complex in patients with solid tumors. Infection. 2012;40:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Katsaragakis S, Markogiannakis H, Samara E, Pachylaki N, Theodoraki EM, Xanthaki A, Toutouza M, Toutouzas KG, Theodorou D. Predictors of mortality of Acinetobacter baumannii infections: A 2-year prospective study in a Greek surgical intensive care unit. Am J Infect Control. 2010;38:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Gales AC, Sader H HS, Jones RN. Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia in Latin America: frequency of occurrence and antimicrobial susceptibility profile: results from the SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn Microbiol Infect Dis. 2002;44:301-311. [PubMed] |
| 11. | Garza-González E, Llaca-Díaz JM, Bosques-Padilla FJ, González GM. Prevalence of multidrug-resistant bacteria at a tertiary-care teaching hospital in Mexico: special focus on Acinetobacter baumannii. Chemotherapy. 2010;56:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Llaca-Díaz JM, Mendoza-Olazarán S, Camacho-Ortiz A, Flores S, Garza-González E. One-year surveillance of ESKAPE pathogens in an intensive care unit of Monterrey, Mexico. Chemotherapy. 2012;58:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Namendys-Silva SA, Texcocano-Becerra J, Herrera-Gómez A. Prognostic factors in critically ill patients with solid tumours admitted to an oncological intensive care unit. Anaesth Intensive Care. 2010;38:317-324. [PubMed] |
| 14. | Namendys-Silva SA, González-Herrera MO, García-Guillén FJ, Texcocano-Becerra J, Herrera-Gómez A. Outcome of critically ill patients with hematological malignancies. Ann Hematol. 2013;92:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 16. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8746] [Article Influence: 624.7] [Reference Citation Analysis (0)] |
| 17. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] |
| 18. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [PubMed] |
| 19. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [PubMed] |
| 20. | Hosmer DW, Taber S, Lemeshow S. The importance of assessing the fit of logistic regression models: a case study. Am J Public Health. 1991;81:1630-1635. [PubMed] |
| 21. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 3979] [Article Influence: 331.6] [Reference Citation Analysis (0)] |
| 22. | Ñamendys-Silva SA, González-Herrera MO, Texcocano-Becerra J, Herrera-Gómez A. Clinical characteristics and outcomes of critically ill cancer patients with septic shock. QJM. 2011;104:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 24. | Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, Salluh JI, Soares M. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care. 2012;27:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Rapoport BL. Management of the cancer patient with infection and neutropenia. Semin Oncol. 2011;38:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 27. | Okorie ON, Dellinger P. Lactate: biomarker and potential therapeutic target. Crit Care Clin. 2011;27:299-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |