INTRODUCTION
Since the 1970’s, the most appropriate way for safe and prolonged administration of drugs and fluids in hospitalized patients are the central venous access devices (CVADs)-typically in the perioperative period and/or in the intensive care unit (ICU). In-hospital subjects requiring intravenous (iv) therapies for an extended time should be routinely assessed for an adequate and stable CVAD, considering the emerging evidence that its early use led to higher patient preference and adherence, reduced discontinuations in infusion associated to failure of CVAD, less catheter-related adverse events (i.e., dislocation and infiltration), conservation of “venous bed” of arms and forearms, reduced consuming-time for nurses to repeatedly insert a venous device, and decreased infusion therapy costs, compared with the use of peripheral short-term cannulas[1]. In particular, a CVAD is mandatory for a number of infusions, such as vesicant/irritant drugs, solutions with pH lower than 5 and higher than 9 (e.g., vancomycin, levofloxacin, dopamine) or hyperosmolar parenteral nutrition (i.e., whose osmolarity exceeds 800-900 mOsm/L).
The insertion and care of a CVAD are integral elements of management for adult and pediatric hospitalized patients. Based on data coming from United States, almost five million of CVADs are annually placed[2]. Therefore, no data are available about the exact number of CVAD placement in critically ill patients, but it is realistic to presume that the proportion is relevant, as it is widely accepted that critically ill patients require a central VAD for the optimal management of their disease.
However, healthcare professionals (HPs) are commonly worried about potential risks related to the presence of a CVAD, in particular catheter-related bloodstream infections (CRBSIs), because of morbidity, mortality, and costs[3]. The most expensive and dangerous healthcare-associated infection is CRBSI. In the United States it was reported an incidence of approximately 80000 CRBSI every year in hospitalized patients and almost 50% of cases occurred in ICU. The number of subjects dying each year for CVAD-related sepsis is between 14000-28000. Each central line sepsis costs $29000 and increases hospital length of stay by seven days[4].
The incidence of CRBSI is often related to an inadequate implementation of proper aseptic policies and insufficient training of the physicians and nurses who insert and maintain the CVAD. Moreover, emerging data describe other important but often overlooked risk factors for catheter-related complications (CRCs): the choice of a CVAD that is inappropriate for that clinical situation; the cannulation of a vein too small for the CVAD needed; the insertion of CVAD without ultrasound guidance, for instance by “blind” infraclavicular venipuncture of the subclavian vein (SV); inappropriate exit site of the CVAD; routine utilize of sutures to secure the CVAD; failure to recognize the importance of a right placement of the CVAD tip[5,6], etc.
In recent years, there have been a widespread dissemination of several technological innovations which improve the security of CVADs [ultrasound (US)-guided vein puncture, innovative materials, peripherally placement of CVAD, sutureless materials to secure CVADs], as well as novel actions apt to reduce the incidence of CRCs [well-defined and accepted procedures (the so-called “bundles”), meticulous adherence to protocols for hand-washing, training programs for HPs, utilize of accurate antisepsis of the skin, and so on][7,8].
The present review aims to discuss indications and management of CVADs in adult and pediatric critically ill patients; in particular, this review will focus on peripherally inserted central catheters (PICCs). We hope that this review may be clinically useful for guiding all HPs to proper choice, technique of insertion, and care of CVADs so to decrease the incidence of catheter-related complications and draw out the duration of the CVAD in critically ill patients.
CHOICE OF THE VAD
The term central venous catheter has been avoided, since it may be ambiguous (PICCs are central venous catheters, too) and replaced by centrally inserted central catheters (CICCs), as opposed to PICCs, or by “CVAD” (term which includes both PICCs and CICCs).
The list of VADs potentially usable in acutely ill patients includes midline catheters, CICCs, and PICCs.
Midline catheters and PICCs are commonly regarded as “medium term VADs”, while non-tunneled CICCs are regarded as “short term VADs”[8]. This review will not discuss “long-term CVADs” (tunneled and cuffed central devices or totally implantable catheters, i.e., ports), since they have no indication in the critical care setting. Pre-existing long term VADs in critically ill patients should not be used in ICU and even removed if suspected to be source of infection.
Midline are 20-25 cm long non-tunneled venous devices (polyurethane- or silicone-made), whose diameter is generally 3-5 Fr. Midline catheters are placed into antecubital or cephalic veins located in the region of the arm in front of the elbow (the so-called antecubital area)[9], employing the “blind” percutaneous procedure, or preferably by US-guided venipuncture of arm deep veins[10]. Therefore, the tip position of these VADs is not “central”, i.e., is not in superior vena cava (SVC) or in right atrium (RA), but in axillary vein (AV) or in SV. A grade B recommendation of the guidelines states that the Midline has to be considered an alternative choice whenever a parenteral therapy trough a peripheral vein is planned for a period longer than six days[7]. Thus, a use of midline catheters should be preferred because short vein cannulas usually lead to an important rate of infiltration and dislocation and ask for a strict surveillance. However, midline catheters have their major limitation in the risk of peripheral venous thrombosis (the so-called “thrombophlebitis”)[11]. Moreover, critically ill patients usually require a CVAD for infusions of drugs associated with endothelial damage or hyperosmolar parenteral nutrition or monitoring.
Centrally inserted central catheters are generally 20 to 30 cm long non-tunneled venous devices (polyurethane-made) placed in a deep central vein [internal jugular vein (IJV), SV, AV, or innominate vein] as well as in the femoral veins (FVs). These VADs are intended for continuous use; they are commonly inserted in ICU and non-ICU patients and used for a short period of time (days or weeks)[12]. CICCs may have either a single lumen or from 2 to 5 lumens.
Peripherally inserted central catheters are 50 to 60 cm long non-tunneled central catheters (silicone- or II-III generation polyurethane-made). PICCs are placed via a peripheral vein (i.e., basilic vein, brachial vein, or -less frequently- cephalic vein) of the arm. They can be used for continuous or intermittent iv fluid and drug administrations, either in inpatients or outpatients (in ambulatory or day-hospital), at home for home parenteral nutrition, or in hospice for palliative care for prolonged periods of time[12,13]. Generally, PICCs are inserted by doctors or nowadays more frequently by registered nurses at the bedside. Currently, they are preferably inserted in a deep vein of the upper midarm by US-guidance. One, two or three lumens PICCs are presently available.
Indications for PICCs
Nowadays, PICCs are being increasingly used in critical care settings[14] because of their benefits over CICCs. Firstly, their insertion is easy and safe, as it implies puncture and cannulation of a peripheral vein of the arm. Using the US-guidance for the PICC insertion, the risk of hemothorax and pneumothorax can be avoided, as well as the possibility of primary malposition is very low[7,15]. Also, PICC placement is appropriate to avoid post-procedural hemorrhage in patients with coagulative disorders who need a CVAD[7,8].
At present, PICCs are highly recommended in the following clinical conditions: major anatomic abnormalities of the chest and neck that may lead to difficulties in the placement and dressing of CICCs, tracheostomy and decreased platelet count or coagulation abnormalities[7]. Some authors suggests that PICCs are also highly recommended in critically ill patients with severe cardiopulmonary problems or severe malnutrition and obesity[15].
The commonly accepted contraindications to PICC insertion are: (1) small diameter of arm veins (basilic or brachial) (i.e., < 3-4 mm); (2) femoral access necessary because of a mediastinal syndrome; and (3) particular conditions of the arms (e.g., paresis, local infection of the skin, presence of devices due to orthopedic procedures with a block of the arm, local severe burns, earlier removal of lymphatic nodes of the axilla). PICCs are also contra-indicated in case of severe renal impairment associated with a potential dialysis indication due to critical need to preserve the deep veins of the arms for the placement of an arteriovenous fistula. Further, is mandatory a CICC in case of need of multiple lumens (i.e., > 3 lumens). Finally, all CVADs placed as emergency procedure should preferably be CICCs[15].
INSERTION OF THE VAD
Site of insertion
Generally speaking, the choice of site of insertion of the most appropriate CVAD should be “patient-oriented”; thus, related to his/her previous VAD placements, status of “vascular bed”, anatomy of deep veins, history of coagulation disorders, underlying disease, and, mainly, duration and characteristics of all planned therapies. The choice of the vein for the CVAD is influenced by aspects such as the venipuncture method, the likelihood of CRCs (i.e., infectious, thrombotic, and mechanical) and the practicability of proper management of the exit site of the CVAD[7].
Commonly, there are two methods for percutaneous insertion of the CICCs into central veins: (1) by using the anatomic landmarks (i.e., the “blind” technique); and (2) by the US-guided venipuncture. Using the anatomic landmarks, the SV (through an infraclavicular or supraclavicular via), the IJV (with different approaches: e.g., low lateral, high anterior or posterior, and axial among the 2 heads of sternoclavicular muscle), or the FV are the veins generally chosen. Comparison between technical success and outcome of CVADs via“blind” cannulation of IJV and SV demonstrated a decrease in adverse events associated with the insertion for an jugular approach[16]. A retrospective survey of more than 5400 CVAD insertions showed that the low lateral approach to the IJV (the technique of Jernigan) is the easiest and safest method for “blind” cannulation because of the lowest rate of insertion-related adverse events[17].
Adopting US-guided venipuncture, CICCs are inserted by supraclavicular approach to the innominate vein, the IJV, or the SV; by infraclavicular approach to the AV/SV; and into the FV. This latter approach is relatively contraindicated unless no other venous access can be obtained because the insertion of a non-tunneled VAD in the FV is related to a higher rate of complications (e.g., thrombosis and contamination at the exit site at the groin)[18]. In the case of VAD placed in the FV, its exit site has to be positioned properly distant from the groin to reduce the danger of contamination.
A review published in 2003[19] has suggested that choice of the SV seems to reduce the incidence of infections. Nevertheless, although this assertion was attested by a randomized controlled trial (RCT) bringing into comparison the incidence of infectious complications linked to the choice of SV or FV[20], it is not available a RCT that compares the infectious occurrence related to the jugular or subclavian approach. In 2007, a comprehensive analysis by Cochrane Collaboration did not find satisfactory RCTs regarding SV vs IJV and concluded that, to define the best method between the subclavian or the jugular approach, it would be necessary to collect more data[21]. A prospective analysis in about a thousand oncologic patients[22] showed that all CRCs (i.e., immediate complications, catheter malposition, venous thrombosis, and long-term morbidity) were significantly more recurring in the SV than in the IJV. A recent review investigating the risk of CRBSIs associated with VADs placed in the FV evaluated this approach with SV and IJV insertion and showed equivalence in the incidence of VAD-related infections between the three sites[23].
Although several studies compared complication rate associated to the SV, IJV, and FV placements in ICU, data are not conclusive. Lorente et al[24] reported in a case series of a thousand critically ill patients, the IJV and the FV were related to an increased rate of infectious complications at the catheter exit site; however, there was equivalence in reference to CVAD-related bloodstream infections[24]. A non-RCT evaluating the SV, IJV, and FV exit sites in ICU demonstrated that overall incidence of both colonization and infection of the VAD is not relevant and similar between the three sites, provided that optimal VAD management is adopted[25].
All these previous findings are biased by the fact that often these studies were carried out in the “pre-ultrasound” era, when the only choice was between IJV (mostly associated with an exit site at mid-neck, particularly unstable and at high risk for contamination) and SV (mostly associated with an exit site in the infraclavicular area): also, the actual diameter of the vein was not known, so that the real risk of thrombosis could not be assessed.
Since most guidelines currently recommend the use of ultrasound guided venipuncture[26], the choice is today between the catheter site in supraclavicular area (puncture of SV, IJV, or innominate vein by US guidance) vs the catheter site in infraclavicular area (insertion into the AV by US guidance). At mid-neck the catheter exit site should be avoided: if it is in the neck upper part, either a higher possibility of colonization or CRBSI, related to the mobilization of the neck, or a greater difficulty with the dressing of the exit site are to be expected[27]. Thus, it is preferable to choose an exit site that facilitates the changes of exit site-dressing, like the zone immediately above the clavicle (the supraclavicular technique to innominate vein or SV; the Jernigan’s method to IJV) or the infraclavicular area (AV).
On the left side, the insertion of CVADs can frequently lead to an increased possibility of primary malposition and thrombosis; for this reason, the insertion on the right side is to be preferred. Though, some specific clinical (e.g., chest or pulmonary diseases, skin abnormalities) or anatomic conditions (poor US vein view) may recommend the use of one or the contralateral side.
Current guidelines on prevention of catheter-related thrombosis[28,29] recommend the use of ultrasound, the appropriate match of vein diameter and catheter diameter, the appropriate “central” position of the tip, and the diameter of CVADs as little as possible[8].
US guidance
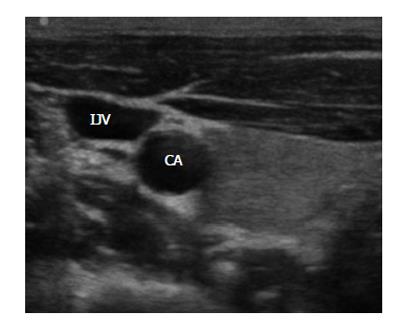
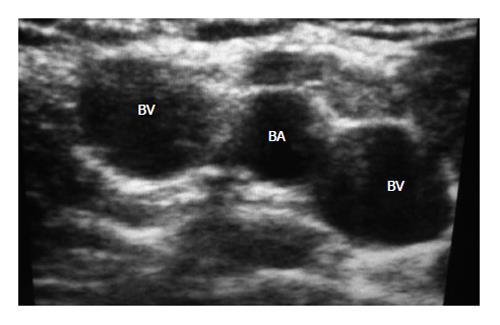
In a recent paper, an international panel of experts defined the US-guided insertion of a vascular access[26]. Summarizing, this definition states that US guidance is useful for verifying the location of an appropriate vascular access before the needle introduction, as well as for real-time US scanning to direct the tip of the needle all the way through the venipuncture (Figures 1 and 2).
Figure 1 Ultrasound visualization of the internal jugular vein and the carotid artery on the right side of the neck.
IJV: Internal jugular vein; CA: Carotid artery.
Figure 2 Ultrasound visualization of vessels of right upper arm: the brachial artery is the middle of two brachial veins.
BA: Brachial artery; BV: Brachial veins.
There are different evident clinical advantages with US vascular imaging; first, it shows a healthy vein before VAD insertion[30,31]. Second, the US-guided skin puncture increases the chances of the insertion of the needle into the vein during the initial attempt with the benefit of minimizing the possibility of adverse events[32,33]. Finally, US imaging can confirm correct position of the VAD tip[34]. The advantages of US-guidance are demonstrated for both CICCs or PICCs and long-term CVADs (tunneled or totally implanted)[35].
Since the late 1990’s, many RCTs and meta-analyses have demonstrated the benefits of the US-guided insertion of CVADs[36]. Several meta-analyses on this subject confirmed that the use of US guidance is characterized by many advantages in comparison to the “blind” technique: a reduced incidence of insertion failure, insertion-related adverse events (e.g., pneumothorax and accidental arterial puncture), and thus an increased incidence of successful insertion at the primary venipuncture, along with fewer CRCs (thrombotic and infectious complications) and of costs[37-39]. Therefore, since the beginning of new millennium, evidence-based guidelines produced by several scientific societies and healthcare organizations[7,29,40-49] have strongly recommended the use of US guidance for increasing efficacy and safety of CVAD insertion in both adults and children[7]. Prospective, nonrandomized investigations have demonstrated that the rate of catheter-related thrombosis is reduced avoiding the injury of the wall of the vein during the placement, as obtained via US-guided insertion[7].
In summary, evidence and broad consensus suggest that US guidance before skin puncture and through the vein cannulation is related to reductions of insertion-related adverse events and costs, as well as a more rapid placement of the CVAD[7,30,32,36-38,45,50-53]. Thus, nowadays it may be judged unethical to deny the employ of the US guidance[7,54].
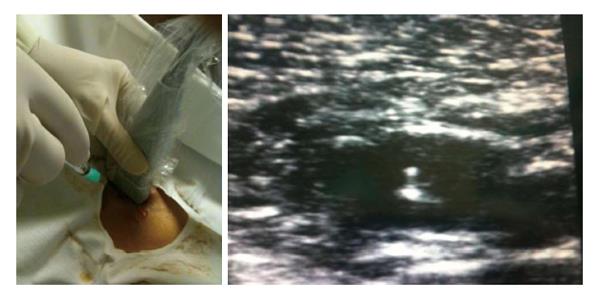
It is possible that the ideal venous approach for CVAD in the near future will be the US-guided puncture of the axillary vein, which combines the advantages to have an “ideal” catheter exit site (i.e., infraclavicular area) and an “ideal” complication-free insertion (Figure 3).
Figure 3 Ultrasound-guided venipuncture of the axillary vein.
Single vs multiple-lumen
The Center for Disease Control and Prevention (CDC) guidelines[44] and a RCT[55] stated that comparing multiple lumens to single lumen CVAD, the first one showed increased incidence of infections. The issue has been also evaluated by a meta-analysis and a review that investigated both the rate of colonization and CRBSI in multi-lumen and single-lumen CVADs. The meta-analysis demonstrated that multi-lumens CVADs are not an independent variable for higher colonization and CRBSI[56]. The review showed that findings from five randomized studies documented that, for each twenty single lumen CVADs placed in, one CRBSI that would have taken place if had multiple lumen CVADs been inserted, will instead be prevented[57].
When a multi-lumen CVAD is inserted, it is recommended to use one lumen just for parenteral nutrition. In fact, if nutrient emulsions, pharmacological agents, or any parenteral infusion of dissimilar pH get in contact, the possibility of precipitates and, therefore, the risk of infectious complications, increases. Moreover, lipid containing parenteral nutrition bags should be infused through the largest lumen of the CVAD, so to reduce the risk of lumen obstruction[40]. Obviously, each lumen has to be managed with an equal careful awareness to the asepsis. Although further studies are necessary in this regard, currently single lumen CVADs are to be preferred, except if a multi-lumen CVAD is necessary for patient care[7].
Stabilization
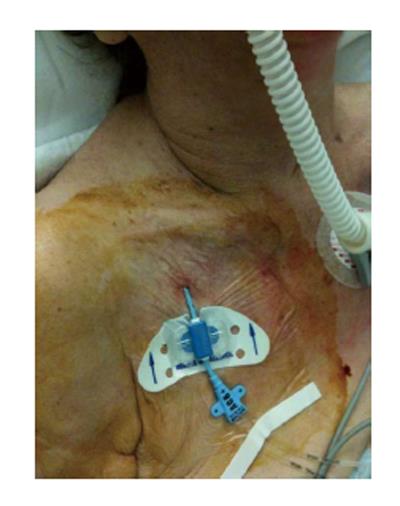
Different devices are commonly used to secure CVADs to the skin: strips, tapes, sutures, and manufactured catheter-stabilization devices (i.e., sutureless devices) (Figures 4 and 5); indeed, a fully consideration to sutureless devices should be nowadays given[7]. In particular, sutures would be better to be avoided because of the increasing of the incidence of vein phlebitis and thrombosis (in case of PICCs), CRBSIs (in case of CICCs), and exit site infection and catheter dislocation (in every CVADs)[58].
Figure 4 Stabilization with a sutureless device of a peripherally inserted central catheter.
Figure 5 Stabilization with a sutureless device of a centrally inserted central catheter.
Tip position
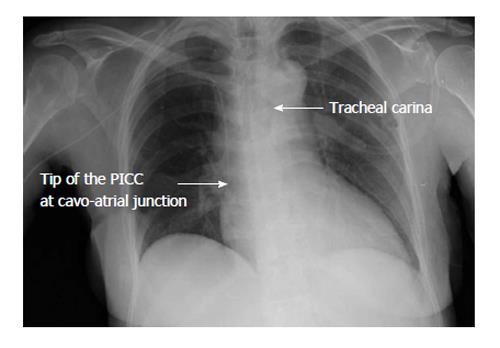
A crucial relevance has the position of the tip that must be always checked after every placement of a CVAD. However, there is no widespread agreement between the experts regarding the correct position for the tip of a CVAD[59]. Most American recommendations (Association for Vascular Access, Food and Drug Administration)[60-62] suggest that the tip has to be in the inferior 1/3 of superior vena cava, while European guidelines[7,46] often consider the position of the tip in the RA (specifically, in the upper area) as appropriate. A wide accepted opinion is that the optimal site is proximal to the area among SVC and RA[63] [i.e., the cavo-atrial junction-(CAJ)] (Figure 6).
Figure 6 Post-procedural chest X-ray.
The left arrow shows the tip position of the peripherally inserted central catheter (PICC) that is at cavo-atrial junction (between the superior vena cava and the right atrium). The right arrow shows the tracheal carina.
Evidence from several prospective studies suggests that an inappropriate tip position is one of the primary causes of CVAD thrombotic complications, as well as the principal cause of catheter failure (mainly, due to malfunction) and decreased catheter dwell time. In fact, if catheter tip is in a higher position (i.e., middle third or upper third of the SVC, or in the brachiocephalic, or in the IJV, or in the SV), there is an increased risk of malfunction[64] and an increased risk of venous thrombosis if compared to a lower position into the superior cava vein or close to the CAJ[65]. If the tip is positioned “too low” (right atrium, right ventricle or inferior vena cava), there is a risk of arrhythmias, tricuspid valve dysfunction or lesions, and thrombosis[19,66,67]. Malpositions are classified as “primary” when malposition occurs during the insertion (e.g., catheter tip into the ipsilateral IJV, as well as into the opposite brachiocephalic vein/SV/IJV) while as “secondary” when the catheter tip migrates spontaneously in the weeks or months following the insertion. The overall incidence of “primary” malpositions varies from 2% to 30%. Clinical experience suggests that malposition occurs more frequently when the left veins are chosen for CVAD placement.
Indeed, during insertion, some methods may significantly reduce the risk of a catheter being placed too “high” or too “low”. The most commonly used “post-procedural” method is the standard chest X-ray. This method is appropriate to verify the exact position of the tip, with few false negatives (mainly due to technical problems or X-ray artifacts) and very few false positive results (e.g., tip position into internal mammary veins and hemiazygos veins). However, “post-procedural” diagnosis of malposition requires a further intervention on the CVAD which implies logistical problems and additional costs. Thus, it is preferable to adopt a method for checking the position of the tip of the CVAD during the placement itself. During catheter insertion, the correct position of the tip can be checked by three different methods: (1) fluoroscopy; (2) trans-thoracic or trans-esophageal echocardiography; and (3) the electrocardiographic method [intracavitary electrocardiography (EKG)]. Many authors have shown that chest X-ray and fluoroscopy are not completely accurate in identifying the post-procedural tip location because “traditional” radiological landmarks of the CAJ are not reliable[34,68,69].
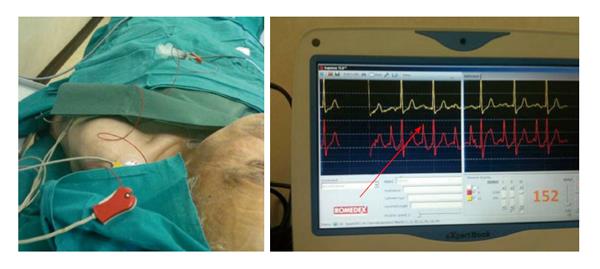
At present, the EKG method is widely employed in Europe (mainly in Germany and Italy) for positioning the tip of VADs[70-73]. The EKG method uses the catheter itself as an intracavitary (endovascular) electrode. If adopting the “column of saline” technique, the method can be applied to any type of CVADs (CICCs, PICCs, dialysis catheters, tunneled-cuffed catheters, ports, etc.), both with open-ended or closed ended tip. The electrocardiographic methodology presents several important features: has the same accuracy of the fluoroscopy, however, it is easier to use, more promptly accessible, more secure and, therefore, has a major cost-effectiveness[63,71-76] (Figure 7).
Figure 7 The electrocardiographic method (intracavitary electrocardiography).
The red arrow shows the maximal height of P-wave detectable when the catheter tip is at cavo-atrial junction (intracavitary EKG = red line, lead II). The yellow line is the surface EKG (lead III). EKG: Electrocardiography.
CARE OF THE VAD
Care of the CVAD is a crucial issue in critically ill patients and its importance is equal to catheter choice or insertion. CVAD care might be judged a simple procedure, but if strict adherence to guidelines recommendations is not adopted it can lead to even life-threatening harms[44]. In the early years of CVAD, it was shown that the most significant actions to reduce CRBSIs were accurate aseptic care during replace of the dressing and access to the catheter reserved only to specially trained nurses[77]. Nowadays, worldwide registered nurses play a fundamental role in evaluating the need of a VAD in each patient and in preserving the functionality of the device[78]. Moreover, in several countries nursing involvement in CVAD management is highly developed, as specially trained nurses choose and insert both peripheral and central catheters.
It should be highlighted that the risk of CVAD infection is also dependent (in addition to all other well-known factors) on the length of duration (i.e., cumulative risk with each day use). Indeed, the CDC guidelines recommend (Category IA recommendation) the removal of vascular devices that are not necessary as soon as possible[44].
Specific aspects regarding the care of the CVADs lie outside the aim of the present publication; however, their importance is essential. Detailed recommendations are published in literature[43,46], therefore, brief comments about dressing regimens of vascular access exit site, replacement of administration sets, and catheter flushing and locking are reported below.
Dressing of vascular access exit site
Accepted guidelines recommend semi-permeable, sterile, and transparent dressings (e.g., in polyurethane) as a cover of the exit site (see CDC and EPIC guidelines)[40,44]. Transparent dressings permit continuously the vision of the exit site and therefore the frequency of dressing change is reduced if compared to gauzes or other tapes. On the other hand, it is preferable to use gauze dressing in case of diaphoretic patients and of bleeding and oozing from the exit site of catheter[44].
Nevertheless, the choice of dressing is still controversial. Data from a large study about dressing procedures of VADs show that the incidences of colonization or phlebitis of exit sites covered with transparent ones and of those covered with gauze are almost equal[79]. Two meta-analyses have also investigated the CRBSI rate with transparent vs gauzes and they found no differences in colonization of skin or catheter tip and bloodstream infections comparing different types of dressing[80,81].
The CDC guidelines recommend replacing dressings of central lines after 48 h for gauzes (Category II) and after seven days for transparent ones (Category IB), respectively. Indeed, dressing of exit site has to be changed when it is moist, slackened off, or evidently dirty (Category IB)[44].
Less debate exists about the utilize of sponge dressing impregnated with chlorhexidine (i.e., a polyurethane scrap with a hole that permits it to fit in the area of the VAD, whose antibacterial activity persists for 7 d). The CDC guidelines recommend the use of these dressing for short term CICCs when the CRBSI rate is still high in spite of adequate “bundles” of CVAD care (Category IB). Chlorhexidine-impregnated dressings have been successfully employed for reducing CRBSI rate. In a large RCT in critically ill patients, normal were compared with chlorhexidine dressings showing that CRBSI rates were decreased[82]. However, a meta-analysis of 8 RCTs stated that the use of dressings with chlorhexidine determines a decrease of colonization but without a CRBSI decrease[83]. Although there are few studies investigating the utilize of chlorhexidine-impregnated dressings in children, one RCT showed a reduction of colonization in patients with CVADs covered with chlorhexidine dressings vs those with normal ones, but no difference in the CRBSI rate. For this reason, CDC guidelines indicate to do not use the dressings with chlorhexidine in children aged less than two months to avoid contact dermatitis in neonates very small at birth.
The international guidelines[84] stress the need to check regularly the exit site during dressing changes as well as by viewing and palpating the unchanged dressing. In case of local signs (e.g., tenderness) suggesting the infection of the exit site or clinical signs (e.g., fever suggesting a CRBSI) it is mandatory to remove the dressing to allow an accurate exit site inspection (Recommendation: category IB).
Administration set replacement
Meta-analyses and several controlled studies investigated the safe and cost-effective interval for routine replacement of all iv sets (i.e., those for continuous administration as well as add-on devices and secondary sets). Findings from these studies[85] inspired the CDC guidelines to recommend the substitution of iv sets at least 96 h after the beginning of their utilize (Recommendation: category IA). However, new trials suggested that iv sets might be utilized in safety for a period up a week whether infusions increasing the growth of pathogens (e.g., blood, plasma, platelets or fat emulsions) have not been administered[86,87]. Conversely, the CDC guidelines recommend replacing every tubing utilized to supply blood, plasma, platelets or lipids (either included in “all-in-one” bags or administered alone) not more than 24 h after the start of the administration (Recommendation: category IB) because they have been recognized as factors significantly associated with an increased risk of CRBSIs[88-91].
Catheter flushing and locking
Flushing the catheter is a basic practice necessary to maintain the patency of any CVAD by reducing drug precipitates and clot formation inside the lumen[92]. Flushing should be done before and after administration of drugs, parenteral nutrition or blood products, after obtaining blood samples, and before locking the device. 10-mL or larger syringes should be preferred, because excessive tension or pressure might harm the CVAD. Flushing protocols in adult patients usually recommend flushing with 10 mL saline in standard conditions (before and after each infusion) and 20 mL saline after infusion of blood products, lipids or contrast medium, or after blood sampling. Flushing should be performed with the so-called push/pause method, i.e., by infusing 2-3 mL at a time.
While a few old meta-analyses investigating the efficacy of heparin on duration of catheter patency had demonstrated some beneficial effect in the 90s[93-95], a more recent review covering a period from 1982 to 2008 did not found strength of evidence to affirm that 0.9% sodium chloride [i.e., normal saline (NS)] is less effective than heparin for flushing CVADs[96]. A recent randomized trial compared heparin and normal saline flush solutions for CICC maintenance in critically ill patients[97]. This study has confirmed that heparin and normal saline flushing solutions have similar rates of lumen occlusion. According to many guidelines[43,46] if CVAD is not in use for less than 8 h the heparin lock is not necessary. The heparin lock might be still be used when specifically recommended by manufacturers, in VADs utilized for hemapheresis and hemodialysis[98].
In conclusion, an adequate flushing using heparin is less essential than locking with NS. Particularly, we stress the key role of flushing both before and after utilize with NS for all CVADs, using the so-called pulsating “push/pause” method associated with a positive pressure technique.
NEONATES, INFANTS, CHILDREN
A CVAD is often required in the pediatric patient in several hospital settings (emergency room, ICU, oncology, hematology, surgery, etc.) and for different purposes (parenteral nutrition, chemotherapy, antibiotic or other drug infusions, hemodynamic monitoring, blood withdrawal, hemodialysis/hemapheresis procedures, etc.). As in adults, CVADs are needed not only because they are a stable, reliable route of fluid and drug administration, but also because some solutions-vesicant or frequently cause of injury of endothelium-must be administrated in high-flow veins[7,43].
Until few years ago, most CVADs in pediatric patients were placed with the surgical cut-down procedure or with the traditional landmark technique (i.e., “blind technique”). It is now well documented that both techniques have serious limitations and are potentially associated with severe complications[99]. In the past ten years, the availability of US guidance for vein cannulation has completely changed the decision-making in venous access in pediatrics[100-104]. Nevertheless, early it was manifest that the use of US lead to an increased rate of successful insertion (both on the whole and at the initial try), quicker cannulation and a reduced rate of insertion-related adverse events[102,105,106]. In recent years, several studies[100-102,107-110] and meta-analyses[38,105,111] compared the “blind” approach to US-guided approach in pediatric patients and stated that the latter is much more effective and safe. According to the guidelines recommendations, US-guided catheter insertion is recommend as the primary method and not as a subsequent choice or a “salvage” technique in case of failure[99].
Indeed, this skill in pediatrics requires a longer training curve in comparison with that in adult patients[26,99]; however, US-guided insertion is recommended to be utilized when the operator has gained even a minimal experience[106]. However, the CVAD insertion in neonates, infants, and children is a risky operation, even when carried out with US. Thus, there is a strong recommendation for the use of US also after the procedure to immediately identify potentially very dangerous adverse events[106].
A recent paper has described in detail US guidance for CVAD insertion in pediatrics[99]. Hereafter, we briefly described the advantage of US guidance in the different venous access. US-guided venipuncture may be utilized for all VAD insertions in the venous system of: neck (i.e., IJV and external JV); thorax (supraclavicular or infraclavicular area; i.e., brachio-cephalic and subclavian vein); mid-arm (i.e., axillary and cephalic vein; basilic vein and brachial veins); and groin (i.e., femoral and saphenous vein).
Puncture of the IJV in neonates is still challenging and nowadays there are no evidence-based recommendations; however, experts suggest that could be useful to employ US for venipuncture consistently, in everyday practice as well in difficult patients, since US allow location of the vein before the skin puncture and therefore constantly increases the probability of successful CVAD insertion at the first attempt[26,99].
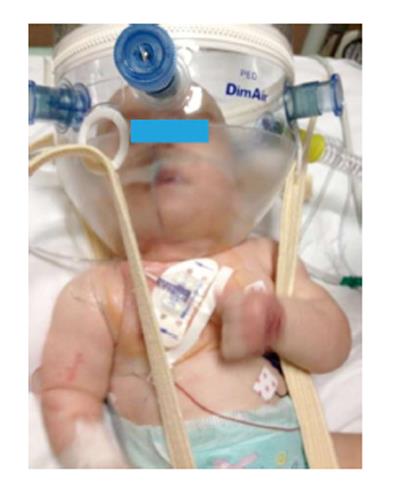
Usually, US allows the visualization of the subclavian vein all along its course (i.e., from clavicle to brachio-cephalic vein)[112]. Two methods are accepted for needle passing: infraclavicular[113] (i.e., passing below the clavicle) and supraclavicular[114] (i.e., above the bone). The infra-clavicular approach (Figure 8) offers a wider vision of the SV and, obviously, a catheter exit in the infraclavicular area. The second approach offers perfect needle visualization that is not hampered by the clavicle. In small children, particular attention is needed to visualize the nervous plexus (i.e., brachial)[44].
Figure 8 A neonate in intensive care unit with a centrally inserted central catheter.
In infants, US visualization of veins at the groin (i.e., femoral) is usually arduous because in this area the anatomic elements are noticeably less US-detectable in comparison with the neck area[115,116]. The skin puncture for catheter insertion into the FV has to be performed near to inguinal ligament; specifically, the FV lies medial to the common femoral artery). A compression of the low area of the abdomen can be employed to make easy the venipuncture; a thrombosis of the iliac vein may be supposed every time there is no increasing in size[115].
Peripheral venous cannulas are commonly inserted in neonates and children[106]; however, easily may happen that the superficial venous system becomes no more utilizable or an unfeasible venous access is expected. In this case, an US-guided catheter insertion in deeper non visible veins has to be the chosen method[117]; even if, the US visualization of the veins might be complicated due to vein squeeze by the probe[26].
Instead, in the deep veins of the mid-arm (i.e., brachial veins and basilic vein) PICCs may be inserted using US (Figure 9). However, PICCs can be inserted at mid-arm only if the veins of the child are of adequate diameter (at least ≥ 3 mm). PICCs (≥ 3 Fr) are different from the epicutaneo-caval VADs (1-2.7 Fr) which are usually inserted in the superficial veins of neonates. These devices are characterized by very small flow, impossibility of blood withdrawal, and high risk of mechanical complications; their insertion may be facilitated by the use of devices which adopt the Near InfraRed technology, ideal for the visualization of superficial veins.
Figure 9 A children in intensive care unit with a peripherally inserted central catheter.
HEMODIALYSIS CATHETERS
The central venous catheter is a very common access in patients who require the hemodialysis treatment. If it necessary to perform an acute hemodialysis, a non-tunneled and non-cuffed VAD is usually inserted. These catheters are mainly inserted in hospitalized patients with acute renal failure, as well as for a brief period in patients already in chronic hemodialysis that present a failure of their long-term catheters.
VAD complications are the main cause of hospitalization in chronic hemodialysis patients. In particular, central vein stenosis is not an uncommon problem in patients on hemodialysis. Earlier studies reported that subclavian vein stenosis occurs in 15% to 50% of chronic hemodialysis patients[118]. Therefore, the CDC guidelines recommend (Category IA)[44] that acute hemodialysis catheters have to be inserted in the IJV or FV due to the increased possibility of thrombosis and stenosis for catheters inserted into the SV[119].
In the authors’ opinion, the risk of venous stenosis after subclavian inserted lines is mostly related to the size of the catheter and to the possible occurrence of a catheter-related thrombosis of the SV (since the stenosis is often the final fibrotic consequence of a severe occlusive thrombosis with only partial recanalization). In our institution, subclavian stenosis is quite rare, since we avoid the placement of large bore dialysis catheter via the subclavian route [i.e., we follow the recommendations of the kidney dialysis outcomes quality initiative guidelines for dialysis access][120] and we do our best efforts to insert catheters in veins with inner diameter at least three times the outer diameter of the catheter (of course, this is possible only with a consistent use of US for a pre-puncture evaluation of the vein).
Additionally, some chronic hemodialysis patients may present acutely ill with an indwelling central access devices, such us pre-existing tunneled-cuffed catheters, whereas these devices are the actual source of illness. In these cases, if infection suspect, it is enormously important to remove these devices as soon as possible. In fact, some of these tunneled-cuffed accesses can be removed at bedside[121].
Finally, the need for dialysis or hemapheresis in the adult critically ill patient is still an absolute indication to a CICC, as no double lumen PICC appears to be useful in this regard. Specific aspects of hemodialysis catheters are beyond the scope of this review, since PICCs have little or no role in this regard. We refer the reader to a comprehensive review on the subject of hemodialysis catheters[122].
STRENGTHS AND LIMITATIONS OF PICCS
In 1996, the first case series of PICCs inserted in critically ill patients was described[123]. However, some technical limitations have limited the introduction of PICCs into daily practice in the ICU because of critically ill patients have special needs (e.g., high flow rates of iv infusions; simultaneous administration of potentially incompatible drugs; hemodynamic monitoring).
In the past, valved PICCs have been widely used. They were provided with pressure-sensitive valves to avoid the blood backflow, either distal (close-ended silicon catheters with Groshong valve) or proximal (open-ended polyurethane catheters with pressure safety valve or SOLO valve). More recently, non-valved polyurethane PICCs are becoming increasingly popular also the in critical care setting. In particular, power-injectable open-ended non-valved polyurethane PICCs are preferred in critically ill patients, since they are ideal for delivering high flows (up to 300 mL/min). Moreover, because are open-ended non-valved catheters, these PICCs may be used to monitor central venous pressure (CVP) as well as ScvO2 (central venous oxygen saturation)[124]. Currently, are available both single-lumen (3-5 Fr) and multi-lumen [double lumen (4-5 Fr) and triple lumen (6 Fr)] power-injectable PICCs. These PICCs have the additional advantage of tolerating high-pressure injection (up to 300-350 PSI) of contrast media during radiological procedures, while silicone-made VADs are resistant just about to 50-60 PSI, as standard polyurethane-made VADs up to 100 PSI.
The choice of the material of (silicone vs polyurethane PICCs) has been a matter of debate. For long time, the silicone has been regarded has more favorable than polyurethane in terms of biocompatibility and risk of venous thrombosis[125]. Though, more recent investigations have failed to detect any significant difference between silicone and new polyurethanes in terms of risk of infection, risk of thrombosis, or expected dwell time. On the other hand, those polyurethane-made are more preferred than those silicone-made thanks to some characteristics that greatly enhance infusion flow and decrease the rate of dislodgement or fracture of the catheter, such as high resistance to rupture, ample inner diameter, and thin wall. This is particularly true for last generation of ultra-resistant polyurethane power-injectable PICCs.
Pump-driven iv infusions may be administered without particular problems through both silicone- and polyurethane-made PICCs, specially using catheters whose diameter is 4 Fr or larger. However, polyurethane-made PICCs are related to a lower incidence of rupture rather than silicone-made PICCs[126]. Moreover, ultra-resistant polyurethane-made power-injectable catheters seems to reduce the rate of occlusion and dislocation more than silicon and standard polyurethane PICCs[127].
Conflicting results about the incidence of CRCs were reported for PICCs in hospitalized patients (principally, in critically ill patients). Two studies in ICU patients with PICCs, one regarding standard ones[128] and another power-injectable ones (three-lumen 6 Fr )[129] have described a high incidence of symptomatic PICC-related thrombosis. Conversely, two other studies in patients with power-injectable PICCs have found a lesser rate of thrombosis (≤ 5%)[15,130]. Chopra et al[131] in a recent meta-analysis reported a high incidence of PICC-related thrombosis. However, the results of this meta-analysis are difficult to accept because are based on a mixed pool of studies on PICCs of different materials, as well as PICCs inserted both with blind technique and US guidance.
Comparison between CICCs and PICCs
Since the 1970’s, in critically ill patients CICCs are inserted in central veins (IJV, SV, axillary, and innominate) for short duration therapy. In the last decade, some authors have proposed PICCs in place of CICCs also in critically ill patients. In fact, PICCs present many theoretical advantages-previously reported in this review-for any hospitalized patient but more relevant in the ICU setting.
Even though this matter is still questionable[3,132], PICCs are generally judged to be less risky for CRBSI, compared with CICCs, which may be an important advantage in ICU patients. This advantage is probably due to the exit site of PICCs, less prone to contamination if compared to the exit sites of CICCs; specifically, the number of bacteria is lower in the skin of upper arms than in the infraclavicular and neck skin[2,40]. Moreover, placement at the upper mid-arm presents the important benefit of removing secretions (nasal, oral, endotracheal) from catheter exit site[2,40]; though, such hypothesis has not yet been proven by RCTs[40]. Finally, a more favorable care of the catheter exit site is obtained by placing PICCs at the upper mid-arm[27]. Indeed, accepted guidelines suggest that PICC may be taken into account as the first option in tracheostomy patients[7].
In a large trial on CVADs in critically ill patients, CICCs were related to a relevant higher incidence of CRBSIs compared to PICCs[133]. Three recent papers in ICU patients reported a CRBSI rate equal to zero[15,129,134]. The hypothesis regarding a lower risk of CRBSI with PICCs than CICCs should be demonstrated by appropriately designed RCTs[135]. With regards to critically ill patients, at present there are not available evidence-based data showing clear advantages of PICCs vs CICCs, or viceversa.
Summarizing, the rate of VAD-related complications, as well the expected dwell time of a CVAD (CICC or PICC) depend on several factors: right ratio between vein and catheter diameter, technique of insertion (US guidance vs blind technique), proper tip position, appropriate exit site location, catheter securement technique (sutureless devices vs sutures), patient’s compliance, and, last but not least, the competence of nurses in the maintenance policies.
CONCLUSION
Two important take-home messages may be drawn from this review. First, the incidence of CRCs is different between different CVADs and physicians should be aware of the different clinical performance as well as of the different risks associated with each type of CVAD (CICCs or PICCs). Second, an inappropriate CVAD choice and, particularly, an inadequate insertion technique are relevant -and often not recognized- potential risk factors for complications in critically ill patients.
We strongly believe that all health-care professionals involved in the choice, insertion or management of CVADs in critically ill patients should know all potential risk factors of CRCs. This knowledge may yield to minimize complications and guarantee longevity to the CVAD optimizing the risk/benefit ratio of CVAD insertion and use. Summarizing, the proper care of CVADs in critical care save lines and save lives.
In conclusion, much evidence from the medical literature and from the clinical practice supports the belief that polyurethane-made power-injectable PICCs may be considered a convincing choice in critical care. Certainly, RCTs comparing CICCs with PICCs are mandatory for recommendations on CVAD choice in critically ill patients.