Published online May 4, 2014. doi: 10.5492/wjccm.v3.i2.55
Revised: October 19, 2013
Accepted: March 3, 2014
Published online: May 4, 2014
Processing time: 445 Days and 2 Hours
AIM: To characterize differences of arterial (ABG) and venous (VBG) blood gas analysis in a rabbit model of hemorrhagic shock.
METHODS: Following baseline arterial and venous blood gas analysis, fifty anesthetized, ventilated New Zealand white rabbits were hemorrhaged to and maintained at a mean arterial pressure of 40 mmHg until a state of shock was obtained, as defined by arterial pH ≤ 7.2 and base deficit ≤ -15 mmol/L. Simultaneous ABG and VBG were obtained at 3 minute intervals. Comparisons of pH, base deficit, pCO2, and arteriovenous (a-v) differences were then made between ABG and VBG at baseline and shock states. Statistical analysis was applied where appropriate with a significance of P < 0.05.
RESULTS: All 50 animals were hemorrhaged to shock status and euthanized; no unexpected loss occurred. Significant differences were noted between baseline and shock states in blood gases for the following parameters: pH was significantly decreased in both arterial (7.39 ± 0.12 to 7.14 ± 0.18) and venous blood gases (7.35 ± 0.15 to 6.98 ± 0.26, P < 0.05), base deficit was significantly increased for arterial (-0.9 ± 3.9 mEq/L vs -17.8 ± 2.2 mEq/L) and venous blood gasses (-0.8 ± 3.8 mEq/L vs -15.3 ± 4.1 mEq/L, P < 0.05). pCO2 trends (baseline to shock) demonstrated a decrease in arterial blood (40.0 ± 9.1 mmHg vs 28.9 ± 7.1 mmHg) but an increase in venous blood (46.0 ± 10.1 mmHg vs 62.8 ± 15.3 mmHg), although these trends were non-significant. For calculated arteriovenous differences between baseline and shock states, only the pCO2 difference was shown to be significant during shock.
CONCLUSION: In this rabbit model, significant differences exist in blood gas measurements for arterial and venous blood after hemorrhagic shock. A widened pCO2 a-v difference during hemorrhage, reflective of poor tissue oxygenation, may be a better indicator of impending shock.
Core tip: Recent studies regarding early goal directed therapy and damage control resuscitation have indicated a potential role for calculated arteriovenous pCO2 differences in monitoring resuscitative efforts. In a rabbit model of hemorrhagic shock, we demonstrate significant derangements between arterial and venous blood and, while not a novel concept, explore the potential of central venous pCO2 as an indicator of hemorrhagic shock. Our results demonstrate a widened arteriovenous pCO2 difference is significantly associated with hemorrhagic shock and may be a more reliable indicator of inadequate tissue perfusion and therefore impending circulatory collapse.
-
Citation: Williams KB, Christmas AB, Heniford BT, Sing RF, Messick J. Arterial
vs venous blood gas differences during hemorrhagic shock. World J Crit Care Med 2014; 3(2): 55-60 - URL: https://www.wjgnet.com/2220-3141/full/v3/i2/55.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v3.i2.55
Circulatory collapse is a definitive indicator of the shock state, but may manifest late during hemorrhage leading to delayed diagnosis, resuscitation, and treatment when clinical metrics of circulatory collapse (hypotension, tachycardia, decreased organ perfusion, altered mental status, etc.) are the sole measures of a patient’s physiologic status. Robust compensatory responses to injury in young, healthy patients can delay treatment of hemorrhage even further as clinical parameters defining shock may not be evident until later stages in the clinical course. Any delay in diagnosis and treatment during massive hemorrhage will likely result in increased morbidity and mortality, fueling the search for adequate trauma resuscitation protocols, such as damage control resuscitation, as well as reliable early markers of impending or ongoing shock[1].
Serologic markers including pH, base deficit, central venous oxygen saturation and lactate have been used to identify and quantitate shock[2-6]. Arterial blood gas analysis is considered the gold standard to determine oxygenation and acid-base status in the acutely injured as well as critically ill and repeat testing offers a means of monitoring resuscitation efforts. However, serious, albeit rare, complications of arterial cannulation (pseudoaneurysm, hematoma, hemorrhage, limb ischemia, infection, neurologic injury)[7] have led to a search for less invasive means of detecting impending shock, quantitating the degree of shock as well as measuring adequate resuscitation. As such, many studies have examined the reliability and accuracy of central venous blood gas in acid-base monitoring as an alternative to arterial blood gas analysis[8-11]. In previous animal models of severely reduced cardiac output, venous hypercarbia has been shown to correlate with inadequacy of tissue perfusion[12,13] and changes in venous blood were noted to occur with greater magnitude and earlier in the process of clinical deterioration than those of arterial blood[14,15]. Similar discrepancies in arterial and venous pCO2 have been reported in human studies of shock states, as well as the paradox of venous acidemia occurring simultaneously with arterial alkalemia, and have been suggestive of the role of serum pCO2 differences as an indicator of tissue perfusion[16-20]. In a recent clinical study highlighting the importance of serum pCO2 in surgical outcomes, Silva et al[21] showed a preoperative arteriovenous pCO2 gap greater than 5.0 mmHg in high risk patients to be predictive of increased in-hospital mortality, circulatory shock, renal failure, intensive care unit (ICU) infection, and length of stay. These previous studies suggest the usefulness of venous blood gas analysis in identifying hemorrhagic shock earlier than other serum markers obtained from arterial blood analysis as well as the potential to accurately monitor adequate resuscitative efforts.
The purpose of this study was to examine the effectiveness of venous blood gas analysis in comparison to the gold standard of arterial blood gas analysis in a rabbit model of hemorrhagic shock.
Following approval by the Institutional Animal Care and Use Committee of the Carolinas Medical Center, fifty New Zealand white rabbits weighing 3 to 6 kg were anesthetized with 1.0 to 1.5 mL/kg of sodium pentobarbital (25 mg/mL) through an ear vein. Anesthesia was maintained throughout the experiment with 0.5-1.0 mL/kg of intravenous sodium pentobarbital (12.5 mg/mL) as needed, determined by response to a pain stimulus. Adequately anesthetized animals then underwent a tracheotomy and endotracheal ventilation. Tidal volumes of 10 mL/kg were administered by a mechanical ventilator (Siemens 900C Servo ventilator, Berlin, Germany) and fraction of inspired oxygen (FiO2) was maintained at 0.5.
In all animals, bilateral groin dissection was performed to adequately expose femoral vasculature. Venous access was obtained via right femoral vein using a 5.0 French catheter advanced into the level of the right atrium and was utilized for drug infusion as well as withdrawal of venous blood samples. Arterial access was secured via left femoral artery utilizing a 3.5 French catheter advanced into the distal abdominal aorta for monitoring of blood pressure, heart rate and arterial blood sampling.
Following baseline arterial and venous blood gas measurements (Radiometer analyzer, ABL-520 #2, Copenhagen, Denmark), animals were hemorrhaged to a mean arterial pressure of 40 mmHg as determined by a multichannel recorder (MT95k2, Astro-Med, Inc., West Warwick, RI). Simultaneous arterial and venous blood gases were obtained every 3 min until hemorrhagic shock was observed, as defined by an arterial pH less than 7.2 and a base deficit greater than or equal to -15 mmol/L. Once the shock state was obtained, animals were then euthanized by intravenous administration of sodium pentobarbital. To minimize procedural variation, all animals were anesthetized, instrumented, hemorrhaged, and euthanized using identical technique by the same investigator.
Data was stored and analyzed using SAS software version 9.3 (SAS Inc., Cary, North Carolina). Obtained arterial and venous blood gases were compared to baseline measurements with regard to pH, base deficit and pCO2. Arteriovenous differences for each parameter (pH, base deficit, pCO2) were then calculated at baseline and shock. Statistical analysis was performed using the unpaired t-test or Wilcoxon rank sum test where appropriate. For all comparisons, statistical significance was set at a P value of less than 0.05.
RESULTSAll 50 animals underwent successful administration of anesthesia, groin dissection, instrumentation, hemorrhage to a state of shock and euthanization without any unexplained or premature losses. Data are expressed as mean ± SD.
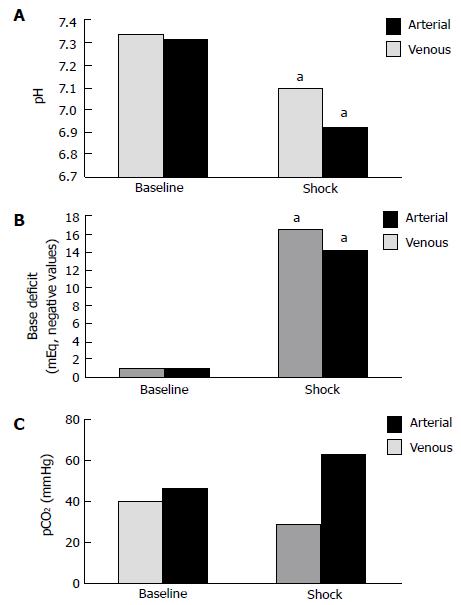
Mean values for pH were significantly decreased from baseline to shock (P < 0.05) in both arterial (7.39 ± 0.12 to 7.14 ± 0.18) and venous (7.35 ± 0.15 to 6.98 ± 0.26) blood gases (Figure 1A). Figure 1B compares mean values obtained for base deficit at the 2 physiologic states; a significant increase (P < 0.05) was seen in arterial (-0.9 ± 3.9 mEq/L vs -17.8 ± 2.2 mEq/L) and venous (-0.8 ± 3.8 mEq/L vs -15.3 ± 4.1 mEq/L) base deficit during shock. In comparing pCO2 at baseline and shock, a non-significant decrease was observed in arterial pCO2 (40.0 ± 9.1 mmHg vs 28.9 ± 7.1 mmHg, P > 0.05), while venous blood samples demonstrated a non-significant trend towards increased pCO2 (46.0 ± 10.1 mmHg vs 62.8 ± 15.3 mmHg, P > 0.05), as shown in Figure 1C.
Arteriovenous differences in pH, base deficit, and pCO2 at baseline and the hemorrhagic shock state are represented. No significant differences were seen in calculated differences at baseline for pH (0.04 ± 0.03), base deficit (0.01 ± 3.07 mEq/L), or pCO2 (5.8 ± 7.5 mmHg), although venous pH demonstrated a larger non-significant trend toward acidosis and a larger non-significant base deficit was seen for arterial samples. In the shock state, a significant difference was noted for arteriovenous pCO2 difference (34.0 ± 3.10 mmHg, P < 0.05), however, calculated differences for pH (0.16 ± 0.08) and base deficit (2.56 ± 3.10 mEq/L) were not significant.
Our results demonstrated significant parallel trends of acidosis and increased base deficit in both arterial and venous blood during hemorrhagic shock in a rabbit model (Figure 1A and B). The arteriovenous pCO2 difference during shock was also statistically significant as venous hypercarbia was observed with simultaneous arterial hypocarbia (Figure 1C).
During hemorrhagic shock, oxygen delivery to tissues is reduced due to lack of red blood cell mass and, subsequently, hemoglobin concentration is insufficient to meet tissue oxygen demands[22]. Contributing to the drop in oxygen carrying capacity, decreased cardiac output secondary to reduced venous return slows the delivery and elimination of venous CO2 in the lungs and augments ongoing venous hypercarbia[13,18,23]. Reduced oxygen delivery to tissues results in a shift from aerobic toward anaerobic cellular metabolism effecting subsequent production of organic acids, such as lactate, and ensuing acidosis and hypercarbia[17]. When the oxygen supply can be restored quickly, metabolic function can return to normal; however, when the oxygen insufficiency is prolonged, cells become irreversibly damaged and are unable to function in normal energy metabolism[24]. Serum and tissue acidosis develop in direct proportion to the amount and acuity of hemorrhagic shock[2,3,6,14,25].
Studies in both animal models and humans have demonstrated a pronounced dissociation between the arterial and venous pCO2 during periods of decreased oxygen delivery as a consequence of decreased cardiac output, such as cardiac tamponade[15], severe hemorrhagic shock[2,14], hemodynamic instability[5,17,18,20] or septic shock[16,19]. Carbon dioxide accumulates very rapidly during hemodynamic compromise, as with massive blood loss, before significant amounts of organic acids are detectable in blood since the normal liver is capable of upregulating lactate metabolism early in the hemorrhagic process[15,21]. Mixed venous CO2 (CvCO2) can be represented according to the Fick equation, CvCO2 = VCO2/Q + CaCO2, where VCO2 represents CO2 production in tissues, Q signifies cardiac output, and CaCO2 denotes the arterial CO2 content. Carbon dioxide is released into the circulation at the tissue-venous interface, represented by VCO2/Q, and is eliminated at the alveolar-arteriole interface in the lungs, represented by CaCO2. Under conditions of normal cardiac output and venous return, there is adequate ventilatory elimination of CO2 that is produced in the tissues and acid-base equilibrium is established. However, as cardiac output and venous return decrease, the increased CO2 produced from anaerobic tissues cannot be effectively eliminated by the lungs, resulting in a disconnect between arterial and venous vascular trees whereby arterial blood gases reflect CO2 exchange at the alveolar-arterial level while venous blood gases are indicative of acid-base status and oxygenation at the level of the tissues. Examining the Fick equation, it can be seen that as cardiac output (Q) declines with simultaneous increased tissue CO2 production (VCO2), mixed venous CO2 (CvCO2) will increase. This was demonstrated in our rabbit hemorrhage model in which the animals were adequately ventilated but hypoperfused, resulting in hypercarbia detected in venous but not arterial blood gas analysis, representing insufficient oxygen delivery to tissues. Therefore, venous blood gas values may better reflect insufficient oxygen delivery to the tissues and subsequent impending shock. Although our results did not show a statistically significant difference in the arteriovenous pH gradient, the venous samples were markedly more acidic than the arterial samples taken during the shock state (Figure 1A).
The clinical utility of arteriovenous pCO2 differences in goal directed therapy (GDT) has recently been addressed in the literature. In a series of septic ICU patients resuscitated to a mixed venous oxygen saturation goal of 70% or greater, Vallée et al[16] demonstrated those patients with arteriovenous pCO2 differences greater than 6 mmHg had higher lactate concentrations and lower lactate clearance rates than those with arteriovenous pCO2 differences less than 6 mmHg, subsequently reflecting the status of global tissue perfusion. They further demonstrated lower cardiac indexes in those patients with arteriovenous pCO2 values greater than 6 mmHg following “adequate” resuscitation to a mixed venous oxygen saturation of 70% as compared to the cohort of patients with arteriovenous pCO2 values less than 6 mmHg. Similarly, Futier et al[26] demonstrated larger arteriovenous pCO2 differences to be significantly associated with post-operative complications in “adequately resuscitated” patients (to a mixed venous oxygen saturation goal greater than 71%) undergoing major abdominal surgery. These clinical results indicate further optimization of GDT may be obtained through a combination of mixed venous oxygenation and arteriovenous pCO2 difference analysis, potentially playing vital roles in progressive damage control resuscitation models in trauma[1].
Some shortcomings of the current study deserve discussion. First, this study was not conducted in a spontaneously-breathing animal model, effectively eliminating the possibility of respiratory compensation which likely will occur in cases of acute injury and hemorrhage. To address this criticism, Mathias et al[15] performed acid-base comparisons between arterial and venous blood gases in a spontaneously-breathing, under-anesthetized canine model of acute cardiac tamponade and found a similar paradox of venous acidosis and hypercarbia with concomitant arterial alkalemia and hypocarbia, even in the early stages of decreased cardiac output (20%) prior to any reduction in arterial blood pressure. Although the criticisms of performing these studies in animal models with blunted or absent respiratory compensatory mechanisms are valid concerns, the results of Mathias et al[15] indicate the paradoxical acid-base trends are still evident in a minimally-anesthetized, spontaneously-breathing, non-ventilated animal model. Also, performing these studies in live animal models without sufficient sedation or supportive measures, such as ventilatory support, would certainly be considered distressful to the animals. A second shortcoming of this study is the lack of a temporal metric for the onset of acid-base changes in our animal model, as well as a lack of serum lactate analysis. It would be beneficial to define the chronological relationship of arterial hypocapnea, venous hypercapnea and acidosis in comparison to the accumulation of lactate during the course of hemorrhagic shock in the rabbit model to better define the usefulness of venous blood gas in the course of hemorrhagic shock.
Our study in a rabbit model indicates hemorrhage shock results in significant acidosis and base deficit in both arterial and venous blood with a significant arteriovenous pCO2 difference of venous hypercarbia and arterial hypocarbia, consistent with previously reported disparities between arterial and venous pCO2 in the setting of severely hypoperfused states. These results indicate that venous blood gas analysis may be a superior indicator of cellular hypoperfusion in hemorrhagic shock, as evidenced by pronounced hypercarbia, and may be more reflective of tissue oxygenation compared to arterial blood gas analysis. Further studies are needed to determine if venous blood gas analysis is a more rapid indicator of impending circulatory collapse or is a more accurate gauge of adequate resuscitative efforts.
Early and adequate tissue perfusion is a key tenet of goal-directed therapy and damage control resuscitation, employed in critical care and trauma practices, respectively. Arteriovenous differences in pCO2 have demonstrated potential in the early detection of insufficient tissue perfusion as well as the quantification of resuscitative efforts.
Establishment of an early, reliable, and easily obtainable marker for impending circulatory collapse in hemorrhagic shock would contribute significantly to treatment algorithms, possibly allowing supportive measures (fluid resuscitation, blood product administration, vasopressor circulatory support, etc.) to be initiated prior to classic physiologic indicators of circulatory collapse. However, no such definitive marker has been elucidated. In this study, the authors demonstrate significant similarities and differences in arterial and venous blood gas derangements, focusing on the arteriovenous differences noted in a rabbit model of hemorrhagic shock in an effort to further define arteriovenous pCO2 differences as a potential early indicator of inadequate tissue perfusion.
Previous studies have examined arterial and venous blood gas derangements (pH, base deficit, lactate levels, oxygen saturation) in states of hypoperfusion in animal models as well as humans. Paradoxical venous hypercarbia with arterial hypocarbia associated with decreased cardiac output has also been reported in the literature, suggesting a role for pCO2 monitoring in cases of hypoperfused states. In this study, authors conclusively demonstrated a widened pCO2 difference (venous hypercarbia with concomitant arterial hypocarbia) is associated with hemorrhagic shock in a novel rabbit model.
The results of this study, viewed in light of recent work regarding venous blood gas analysis in hypoperfused states, further supports the prospect that central venous blood gas pCO2 differences may indicate effectiveness of resuscitative efforts in the acutely injured hemorrhagic state. Certainly, further human studies in the setting of acute hemorrhage deserve attention so that a more rapid, accurate and easily obtainable mechanism of resuscitation may be elucidated.
The term arteriovenous pCO2 difference is used to describe the absolute value of the quantifiable variance between arterial blood gas pCO2 and venous blood gas pCO2. This is represented in units of mmHg, a standard unit of measurement for partial pressure.
This is a well-written manuscript which analyzes the effects of hemorrhagic shock on arterial and venous blood gases in a rabbit animal model. An added caveat is the significant widened arteriovenous pCO2 difference seen in the shock state. The manuscript also reviews pertinent publications on the subject, highlighting recent clinical studies which suggest a role for arteriovenous pCO2 differences in monitoring resuscitation. Although not novel, the results certainly provide further evidence that widened pCO2 differences are indicative of worsening shock and may therefore possibly be another tool in our armament to monitor resuscitation.
P- Reviewers: Chen XL, Mohta M, Nayci A S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 2. | Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441-453. [PubMed] |
| 3. | Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 480] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | van Beest P, Wietasch G, Scheeren T, Spronk P, Kuiper M. Clinical review: use of venous oxygen saturations as a goal - a yet unfinished puzzle. Crit Care. 2011;15:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Hobbs TR, O’Malley JP, Khouangsathiene S, Dubay CJ. Comparison of lactate, base excess, bicarbonate, and pH as predictors of mortality after severe trauma in rhesus macaques (Macaca mulatta). Comp Med. 2010;60:233-239. [PubMed] |
| 6. | Kaplan LJ, Frangos S. Clinical review: Acid-base abnormalities in the intensive care unit -- part II. Crit Care. 2005;9:198-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6:199-204. [PubMed] |
| 8. | Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Rudkin SE, Kahn CA, Oman JA, Dolich MO, Lotfipour S, Lush S, Gain M, Firme C, Anderson CL, Langdorf MI. Prospective correlation of arterial vs venous blood gas measurements in trauma patients. Am J Emerg Med. 2012;30:1371-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Middleton P, Kelly AM, Brown J, Robertson M. Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J. 2006;23:622-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Malinoski DJ, Todd SR, Slone S, Mullins RJ, Schreiber MA. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg. 2005;140:1122-1125. [PubMed] |
| 12. | Dubin A, Edul VS, Pozo MO, Murias G, Canullán CM, Martins EF, Ferrara G, Canales HS, Laporte M, Estenssoro E. Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med. 2008;36:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Vallet B, Teboul JL, Cain S, Curtis S. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J Appl Physiol (1985). 2000;89:1317-1321. [PubMed] |
| 14. | Oropello JM, Manasia A, Hannon E, Leibowitz A, Benjamin E. Continuous fiberoptic arterial and venous blood gas monitoring in hemorrhagic shock. Chest. 1996;109:1049-1055. [PubMed] |
| 15. | Mathias DW, Clifford PS, Klopfenstein HS. Mixed venous blood gases are superior to arterial blood gases in assessing acid-base status and oxygenation during acute cardiac tamponade in dogs. J Clin Invest. 1988;82:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Vallée F, Vallet B, Mathe O, Parraguette J, Mari A, Silva S, Samii K, Fourcade O, Genestal M. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med. 2008;34:2218-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, Teboul JL. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002;28:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Teboul JL, Mercat A, Lenique F, Berton C, Richard C. Value of the venous-arterial PCO2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Crit Care Med. 1998;26:1007-1010. [PubMed] |
| 19. | Mecher CE, Rackow EC, Astiz ME, Weil MH. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit Care Med. 1990;18:585-589. [PubMed] |
| 20. | Cuschieri J, Rivers EP, Donnino MW, Katilius M, Jacobsen G, Nguyen HB, Pamukov N, Horst HM. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med. 2005;31:818-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Silva JM, Oliveira AM, Segura JL, Ribeiro MH, Sposito CN, Toledo DO, Rezende E, Malbouisson LM. A large Venous-Arterial PCO(2) Is Associated with Poor Outcomes in Surgical Patients. Anesthesiol Res Pract. 2011;2011:759792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Hsia CC. Respiratory function of hemoglobin. N Engl J Med. 1998;338:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Funk DJ, Jacobsohn E, Kumar A. Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med. 2013;41:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Bruegger D, Kemming GI, Jacob M, Meisner FG, Wojtczyk CJ, Packert KB, Keipert PE, Faithfull NS, Habler OP, Becker BF. Causes of metabolic acidosis in canine hemorrhagic shock: role of unmeasured ions. Crit Care. 2007;11:R130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Theusinger OM, Thyes C, Frascarolo P, Schramm S, Seifert B, Spahn DR. Mismatch of arterial and central venous blood gas analysis during haemorrhage. Eur J Anaesthesiol. 2010;27:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Futier E, Robin E, Jabaudon M, Guerin R, Petit A, Bazin JE, Constantin JM, Vallet B. Central venous O₂ saturation and venous-to-arterial CO₂ difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care. 2010;14:R193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |