Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.102733
Revised: March 10, 2025
Accepted: March 21, 2025
Published online: September 9, 2025
Processing time: 264 Days and 9.7 Hours
Acute colonic pseudo-obstruction (ACPO) is defined as colonic obstruction without a mechanical or extrinsic inflammatory factor. Colonic decompression is advised for patients with ACPO after the failure of conservative and medical management.
To systematically review and analyze the efficacy and safety of colonoscopic decompression in ACPO.
A search was conducted in MEDLINE, EMBASE, and Scopus from inception to August 2024. Studies reporting the clinical success, perforation, recurrence, and need for surgery after colonoscopic decompression in ACPO were included. A random-effects inverse-variance model was used to calculate the pooled proportion.
Sixteen studies were included in the final analysis. The pooled rates of success after the first session of colonoscopic decompression and overall success were 78.8% (95%CI: 72.0-85.6) and 91.5% (95%CI: 87.0-96.0), respectively. The first session of colonoscopic decompression had a significantly higher success than the first dose of neostigmine with OR 3.85 (95%CI: 2.00-7.42). The pooled incidence of perforation was 0.9% (95%CI: 0.0-2.0), while recurrence was observed in 17.1% (95%CI: 12.9-21.3) of the patients after clinical success. The pooled rates of surgery in all cases undergoing colonoscopic decompression and those who had a successful procedure were 10.5% (95%CI: 5.0-15.9) and 3.7% (95%CI: 0.3-7.1), respectively. Subgroup analysis, excluding the low-quality studies, did not signi
Colonoscopic decompression for ACPO is associated with a clinical success rate of > 90% with a perforation rate of < 1%, demonstrating high efficacy and safety.
Core Tip: Colonoscopic decompression for acute colonic pseudo-obstruction (ACPO) was associated with a pooled success rate of 78.8% after the first session and an overall success rate of 91.5%. The first session of colonoscopic decompression had a significantly higher success than the first dose of neostigmine with OR 3.85. The pooled incidence of perforation remained less than 1%, while one-sixth of the patients had a recurrence after clinical success. Surgery was required in 3.7% of those who had a successful procedure and 10.5% of all cases receiving colonoscopic decompression. In patients undergoing colonoscopic decompression, tube placement should be attempted with the position of the tube at least until the transverse colon to improve clinical success and reduce recurrence. However, the predictors of failure of colonoscopic decompression in ACPO need to be studied further.
- Citation: Giri S, Krishnamurthy V, Shah D, Joseph A, Korrapati SK, Maharshi S, Sundaram S. Outcome of colonoscopic decompression in acute colonic pseudo-obstruction: A systematic review and meta-analysis. World J Crit Care Med 2025; 14(3): 102733
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/102733.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.102733
Colonic obstruction accounts for approximately 25% of all cases of intestinal obstructions[1]. Intestinal obstruction can be either functional or mechanical. Acute colonic pseudo-obstruction (ACPO) or Ogilvie's syndrome is defined as colonic obstruction without a mechanical or any extrinsic inflammatory factor[2-4]. Trauma, presence of sepsis, history of recent surgery, old age, neurologic causes, and use of opiates and chemotherapeutic agents are implicated in the development of ACPO[5]. The management options for ACPO include conservative treatment that consists of bowel rest, nasogastric decompression, electrolytes corrections, and halting of implicating factor. In patients not responding to this treatment, the available treatment options are the use of neostigmine and colonoscopic decompression[2].
American Society for Gastrointestinal Endoscopy (ASGE) recommends colonoscopic decompression with decom
The current meta-analysis was conducted as per the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[6].
The electronic databases of MEDLINE, Embase, and Scopus were searched from inception until August 31, 2024 using the following keywords: (Pseudo-obstruction AND (Colonic OR Intestinal) OR Ogilvie) AND Colonoscop). Before including the studies, two independent reviewers evaluated the titles and abstracts of the retrieved papers to determine their eligibility. Furthermore, the listed papers' bibliographies were examined for relevant studies. Any dispute was settled by a third reviewer.
Both prospective and retrospective studies fulfilling the following criteria were included: (1) Study population: Patients with ACPO; (2) Intervention: Colonoscopic decompression; and (3) Outcomes: Efficacy and safety of colonoscopic decompression in ACPO. Editorials, correspondences, case reports, case series (< 10 patients), conference abstracts, and review articles were excluded. Studies with insufficient or irrelevant clinical data were also excluded.
Two reviewers independently extracted the data, while a third reviewer arbitrated any conflicts. Each study's title, first author, year of publication, country, number of patients, age and sex distribution, etiology, cecal diameter, prior intervention, and outcomes. Two independent reviewers used a modified Newcastle-Ottawa scale for cohort studies for quality assessment[7]. In the event of a disagreement, a third reviewer was contacted.
The primary outcomes of the study were initial success (after the first session), overall success (after multiple sessions), perforation, recurrence after successful decompression, and the need for surgery. Due to heterogeneity in the definition of clinical success (clinical ± radiological), we defined clinical success based on clinical criteria only (reduction in abdominal distension with the passage of flatus and stool).
The pooled proportions were calculated using a random-effects inverse-variance model. I2 and the P value for heterogeneity were used to evaluate the studies' degree of heterogeneity. I2 values of 25%, 50%, and 75% were considered the cut-offs for low, moderate, and considerable heterogeneity, respectively[8]. A P value of less than 0.10 was considered statistically significant. Funnel plots were visually inspected to assess publication bias. Egger’s test was used to assess the small-study effect. The sensitivity analysis utilized a leave-one-out meta-analysis and sub-group analysis after the exclusion of low-quality studies. Meta-regression was utilized to evaluate the source of heterogeneity by the analysis of linear association between study-level variables and effect magnitude. STATA software (version 17, StataCorp., College Station, TX) was used for statistical analysis.
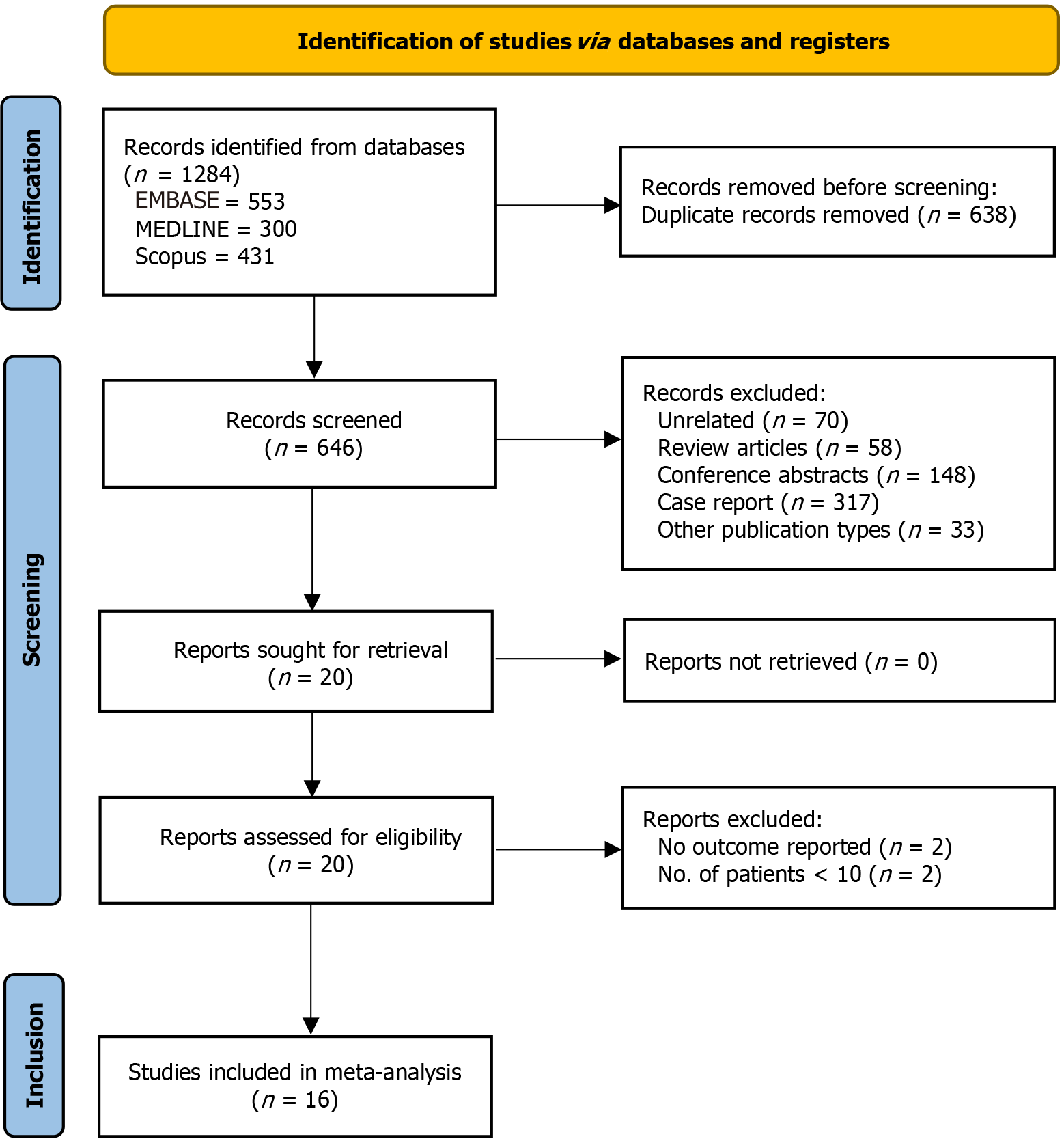
A total of 1284 records were identified with the search strategy, of which 16 studies were included in the final analysis[9-24]. Figure 1 shows the PRISMA flowchart for the study selection and inclusion process. Tables 1 and 2 show the baseline characteristics and outcomes of the individual studies included in the present analysis. All studies were retrospective, except for the study by Zhao et al[20], which was prospective. The majority of the studies were from the United States. The sample size of the studies varied from 16 to 83, with the median age varying from 56 to 72 years. Males constituted the majority of the patients, with the proportion of post-surgical cases varying from 27% to 81.8%. The average cecal diameter varied from 9 to 13 cm. Supplementary Table 1 shows the quality analysis for individual studies. The majority of the studies were of medium quality, while six studies were of low quality[17-20,23,24].
| Ref. | Study design, country | No. of patients | Age | Male sex | Etiology | Cecal diameter, in cm | Prior neostigmine | Tube placement |
| Nivatvongs et al[9] | Retrospective, United States | 22 | 56 (30-82) | 15 (68.2) | Post-surgical: 18 (81.8); Critically ill: 4 (18.2) | - | - | 0 |
| Strodel et al[10] | Retrospective, United States | 44 | 59 (25-89) | 31 (70.4) | Post-surgical: 12 (27); Critically ill: 29 (66) | 12.8 (9.5-17) | - | 0 |
| Bode et al[11] | Retrospective, United States | 22 | 64.3 (21-92) | 15 (68.2) | Post-surgical: 13 (59.1); Critically ill: 9 (40.9) | 12.9 (10-17) | - | 0 |
| Fausel and Goff[12] | Retrospective, United States | 19 | 46-80 | 17 (89.4) | Post-surgical: 8 (42.1); Critically ill: 11 (57.9) | 13 (12-15) | - | - |
| Lavignolle et al[13] | Retrospective, France | 29 | - | - | - | - | - | 15 (51.7) |
| Harig et al[14] | Retrospective, United States | 20 | 60-78 | 12 (60) | Post-surgical: 8 (40); Critically ill: 12 (60) | - | - | 10 (52.6) |
| Jetmore et al[15] | Retrospective, United States | 48 | 67 (36-90) | 33 (68.7) | Post-surgical: 27 (56); Critically ill: 21 (44) | 12.4 | - | 42 (87) |
| Geller et al[16] | Retrospective, United States | 50 | 68 ± 13 | 33 (66) | Post-surgical: 33 (66); Critically ill: 17 (34) | 13 (9-20) | - | 42 (84) |
| Pham et al[17] | Retrospective, United States | 24 | 65 (34-85) | 20 (83) | Post-surgical: 9 (38); Critically ill: 15 (62) | 12.6 ± 3.1 | - | 8 (75) |
| Tsirline et al[18] | Retrospective, United States | 52 | - | - | - | - | - | - |
| Peker et al[19] | Retrospective, Turkey | 54 | 59.4 ± 15.3 | - | - | 10.8 ± 1.2 | 16 (30.2) | - |
| Zhao et al[20] | Prospective, China | 59 | - | - | - | - | 59 (100) | - |
| Mankaney et al[21] | Retrospective, United States | 83 | 66.8 ± 16.2 | 60 (72.3) | Post-surgical: 27 (32.5); Critically ill: 13 (54.2) | - | 25 (30) | - |
| Liu et al[22] | Retrospective, United States | 24 | 72.2 (51-93) | 19 (79.2) | Post-surgical: 11 (45.8); Critically ill: 13 (54.2) | 12.5 | - | 14 (58.3) |
| Joechle et al[23] | Retrospective, Germany | 25 | 64 (34-86) | 19 (76) | Critically ill: 25 (100) | 9 (6-13) | - | 19 (76) |
| Williamson et al[24] | Retrospective, United States | 16 | 68 (62-84) | 12 (73) | - | - | - | - |
| Ref. | No. of patients | Initial success | Overall success | Perforation | Recurrence after decompression | Need for surgery overall after successful decompression |
| Nivatvongs et al[9] | 22 | 19 (86.4) | 19 (86.4) | 0 | 4/19 (21.0) | 3/22 (13.6) | 2/19 (10.5) |
| Strodel et al[10] | 44 | 32 (72.7) | 34 (77.3) | 1 (2.3) | 5/34 (14.7) | 7/44 (15.9) | 1/34 (2.9) |
| Bode et al[11] | 22 | 20 (91) | 20 (91) | 1 (4.5) | 4/20 (20) | 3/22 (13.6) | 2/20 (10) |
| Fausel and Goff [12] | 19 | - | 17 (89.5) | 0 | 3/17 (17.6) | 3/19 (15.8) | 2/17 (11.8) |
| Lavignolle et al[13] | 29 | - | 29 (100) | 0 | 7/29 (24.1) | - |
| Harig et al[14] | 20 | 18 (90) | 20 (100) | 0 | 4/20 (20) | - |
| Jetmore et al[15] | 45 | 29 (64.4) | 38 (84.4) | 1 (2.2) | 6/38 (15.8) | 5/45 (11.1) |
| Geller et al[16] | 50 | 39 (78) | 44 (88) | 1 (2) | 8/44 (18.2) | 1/50 (2) |
| Pham et al[17] | 24 | 22 (91) | 23 (95.8) | 0 | - | - |
| Tsirline et al[18] | 52 | 39 (75) | 44 (85) | 1 (1.9) | - | - |
| Peker et al[19] | 54 | 44 (81.5) | - | - | - | - |
| Zhao et al[20] | 59 | 27 (45) | - | - | - | - |
| Mankaney et al[21] | 83 | 73 (87.9) | - | 0 | 11 (15.1) | - |
| Liu 2021[22] | 24 | 22 (91.6) | 23 (95.8) | 1 (4.2) | - | 1/24 (4.2) | 1/23 (4.3) |
| Joechle et al[23] | 25 | 17 (68) | - | 6 (24) | - | 8/25 (32) | 0/17 |
| Williamson et al[24] | 16 | 11 (68.7) | - | 0 | 1/11 (9.1) | 1/16 (6.2) | 0/11 |
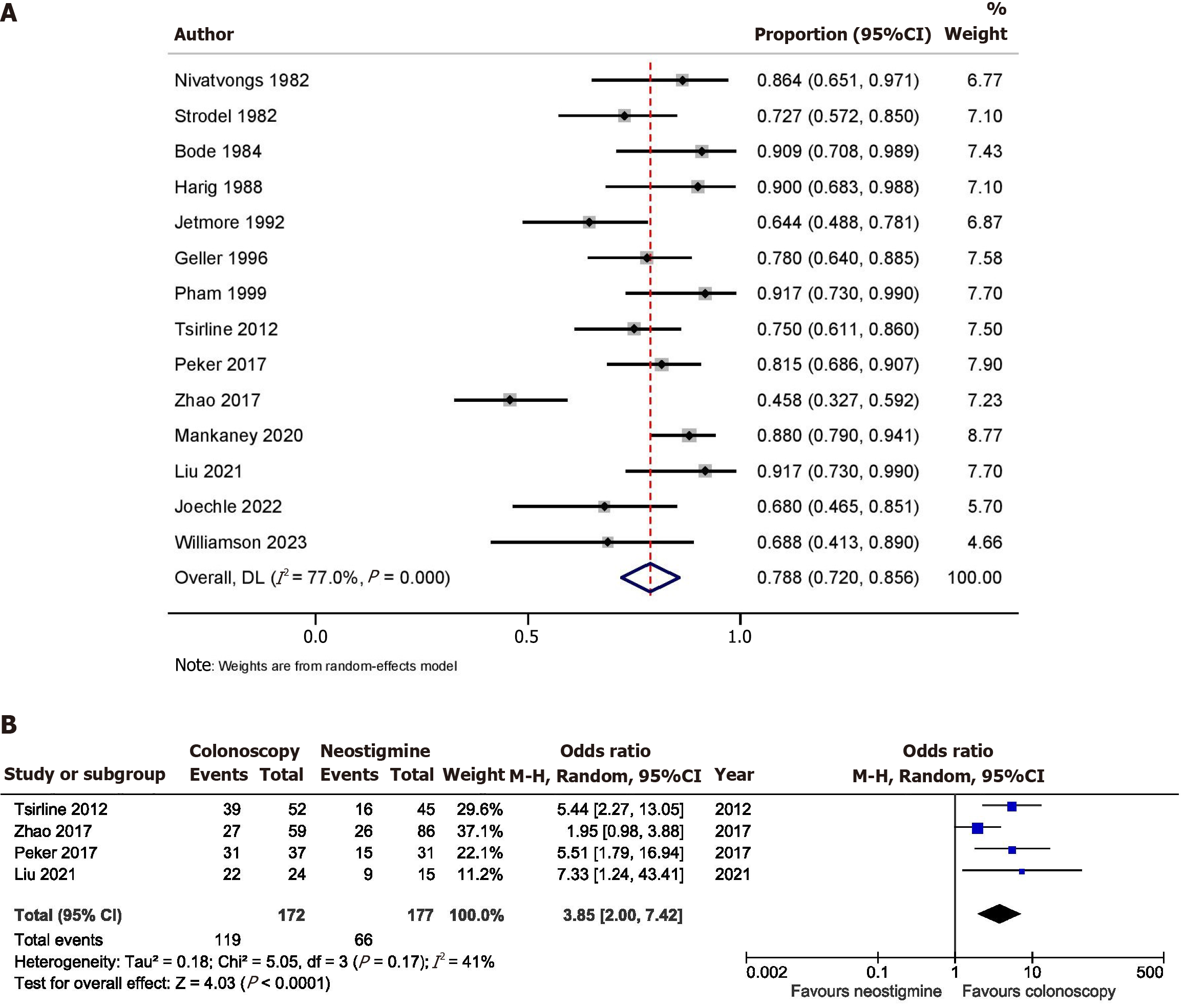
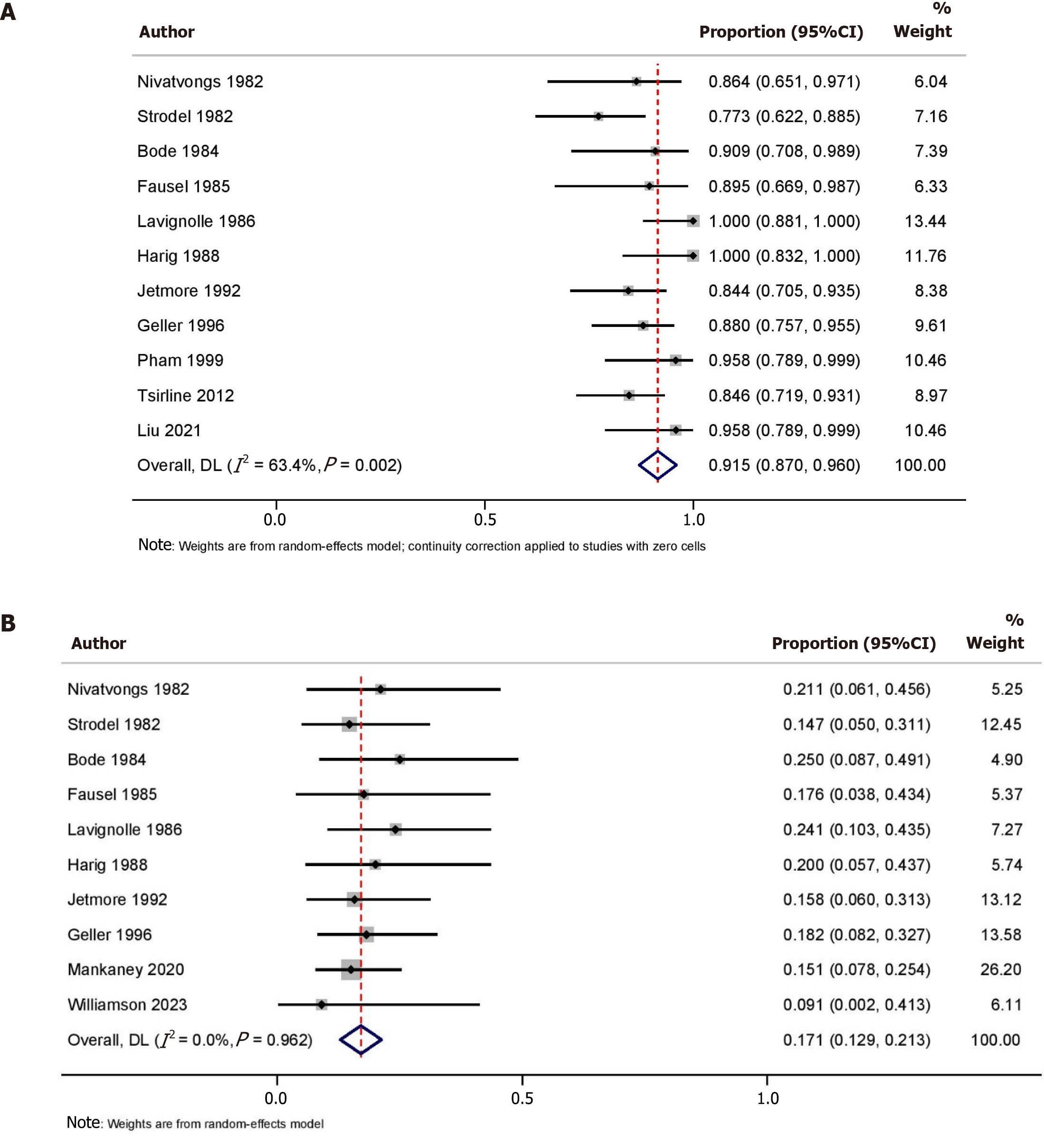
Data on success after the first session of colonoscopic decompression for ACPO was available from 14 studies (n = 540). The pooled initial success rate was 78.8% (95%CI: 72.0-85.6; I2 = 77.0%) with significant heterogeneity (Figure 2A). Leave-one-out analysis showed that the removal of the study by Zhao et al[20] increased the pooled initial success rate to 82.0% (95%CI: 77.0-87.0; I2 = 52.7%) with moderate heterogeneity. Four studies (n = 349) compared the success after the first session of colonoscopic decompression and the first dose of neostigmine. Colonoscopic decompression had a significantly higher success with OR 3.85 (95%CI: 2.00-7.42; I2 = 41.0%) (Figure 2B). Data on the overall success after one or more sessions of colonoscopic decompression for ACPO was available from 11 studies (n = 351). The pooled overall success rate was 91.5% (95%CI: 87.0-96.0; I2 = 63.4%) with moderate heterogeneity (Figure 3A).
Data on the incidence of perforation after colonoscopic decompression for ACPO was available from 14 studies (n = 475). The pooled incidence of perforation was 0.9% (95%CI: 0.0-2.0; I2 = 0.0%) (Supplementary Figure 1). Leave-one-out analysis showed that the removal of the study by Mankaney et al[21] increased the perforation rate to 1.6% (95%CI: 0.1-3.1; I2 = 0.0%) without heterogeneity.
Data on the recurrence of pseudo-obstruction after successful colonoscopic decompression for ACPO was available from 10 studies (n = 305). The pooled incidence of recurrence was 17.1% (95%CI: 12.9-21.3; I2 = 0.0%) (Figure 3B).
The need for surgery after colonoscopic decompression for ACPO was reported in 9 studies (n = 267). The pooled rate for surgery was 10.5% (95%CI: 5.0-15.9; I2 = 59.9%) (Supplementary Figure 2A). Leave-one-out analysis showed that the removal of the study by Joechle et al[23] reduced the rate of surgery to 8.3% (95%CI: 3.8-12.9; I2 = 0.0%) without heterogeneity. The need for surgery after successful colonoscopic decompression for ACPO was reported in 7 studies (n = 141). The pooled rate for surgery after successful colonoscopic decompression was 3.7% (95%CI: 0.3-7.1; I2 = 0.0%) (Supplementary Figure 2B).
There was evidence of publication bias for the outcome of initial success but not for other outcomes (Supplementary Figure 3). Egger’s test did not show any evidence of a small-study effect for any of the outcomes (Supplementary Table 2). A sub-group analysis after exclusion of the low-quality studies did not show any significant difference in the pooled event rates but reduced the heterogeneity in the pooled rates of initial success (Table 3). Meta-regression analysis using sample size, year of publication, proportion of non-surgical patients, and proportion of patients having tube placement did not have any significant contribution from any of the covariates towards heterogeneity.
| Outcomes | Overall | Exclusion of low-quality studies |
| Initial success | 78.8% (95%CI: 72.0-85.6), I2 = 77.0% | 83.4% (95%CI: 77.0-89.7), I2 = 57.8% |
| Overall success | 91.5% (95%CI: 87.0-96.0), I2 = 63.4% | 91.6% (95%CI: 86.4-96.8), I2 = 65.7% |
| Perforation | 0.9% (95%CI: 0.0-2.0), I2 = 0.0% | 0.7% (95%CI: 0.0-1.9), I2 = 0.0% |
| Recurrence | 17.1% (95%CI: 12.9-21.3), I2 = 0.0% | 17.6% (95%CI: 13.2-21.9), I2 = 0.0% |
| Need for surgery | 10.5% (95%CI: 5.0-15.9), I2 = 59.9% | 8.9% (95%CI: 3.7-14.0), I2 = 50.8% |
| Need for surgery after success | 3.7% (95%CI: 0.3-7.1), I2 = 0.0% | 5.2% (95%CI: 1.2-9.3), I2 = 0.0% |
Colonoscopic decompression is primarily used for patients with ACPO who fail conservative and pharmacological management. The present meta-analysis showed that the success of colonoscopic decompression after the first session was 78.8%, while the pooled overall success rate was 91.5%. The first session of colonoscopic decompression had a significantly higher success than the first dose of neostigmine with OR 3.85 (2.00-7.42). The pooled incidence of perforation was 0.9% (0.0-2.0), while recurrence was observed in 17.1% (12.9-21.3) of the patients. The pooled rate of surgery after colonoscopic decompression and those who had a successful procedure was 10.5% (5.0-15.9) and 3.7% (0.3-7.1), respectively.
The ASGE guidelines recommend neostigmine as the 1st line therapy for the management of patients with ACPO after the failure of conservative treatment, and colonoscopy is recommended as an alternative therapy due to a lack of comparative studies regarding the same[2]. However, Tsirline et al[18] compared the outcome of neostigmine vs colonoscopic decompression as first-line therapy. They reported a higher success rate (defined as no further therapy requ
Loftus et al[25] analyzed the factors associated with a sustained response to neostigmine for ACPO and reported that younger age was associated with a significantly lower response rate. Zhao et al[20] compared the outcome of patients with ACPO with no obvious thickening (edema) of the colonic wall (ACPO-NT) group and ACPO with acute thickening of the colonic gut wall (ACPO-T) group. The authors reported a higher response rate with non-surgical treatment in the ACPO-NT group, compared to the ACPO-T group (97.91% vs 64.4%, P < 0.01), although the response rate of colonoscopic decompression was comparable between both groups (75% vs 42.86%, P = 0.318). Thus, the younger age group and those with thickening of the colonic wall may represent the subset of patients who are at risk of failure to respond with neostigmine and may benefit from upfront colonoscopic decompression. However, further studies are required to validate these findings.
Randomized studies regarding the benefit of decompression tube placement after colonoscopic decompression are not available. Harig et al[14] compared the outcome of colonoscopic decompression with and without tube placement and reported a higher clinical success rate (100% vs 77.8%) with a lower incidence of reintervention (0% vs 44%) with tube placement. Geller et al[16] reported a higher clinical success rate of 80% (43 of 54) with colonoscopic decompression and tube placement, compared to 25% (2 of 8) in those without tube placement. The success rate of colonoscopic decom
Despite the benefit of colonoscopy, it is associated with the risk of adverse events as it is invasive. The reported incidence of perforation with colonoscopic decompression varied from 0 to 24%, with a pooled event rate of 0.9% (0.0-2.0). The incidence of perforation with colonoscopy and neostigmine were 1.9% and 4.4%, respectively, in the study by Tsirline et al[18] and Mankaney et al[21] reported no perforation in either of the groups. Thus, the overall incidence rate of perforation with colonoscopic decompression remains comparable to those treated with pharmacotherapy.
Joechle et al[23] reported that a colonic diameter ≥ 11 cm was the only independent factor for predicting non-response with colonoscopic decompression in ACPO. However, this inference was based on univariate analysis and not multivariate analysis. Peker et al[19] showed that old age, male sex, and associated cardiac disease were associated with poor response to colonoscopic treatment on univariate analysis. Mankaney et al[21] performed a multivariate analysis, and colonoscopic decompression (OR 2.6), reducing dose or stopping opioids (OR 2.7), and initial colonic diameter (OR 0.8) were found to be independent predictors of resolution of ACPO. Tsirline et al[18] reported that the number of doses of neostigmine before colonoscopy did not affect the success rate. Similarly, Peker et al[19] reported no difference in the response rate in patients undergoing upfront colonoscopy vs those who underwent colonoscopy after failed neostigmine. Thus, these factors should be kept in mind while proceeding with colonoscopic decompression for ACPO.
The present meta-analysis is the first to summarize the current evidence on the efficacy and safety of colonoscopic decompression in ACPO. The findings of our study may assist healthcare providers and patients while deciding colonoscopic decompression for ACPO and aid international organizations in formulating guidelines for safety and establishing acceptable thresholds for adverse events during the procedure. However, despite the encouraging outcomes, these results should be interpreted cautiously as the meta-analysis has several limitations. First, most of the studies were retrospective, increasing the risk of selection bias. Second, the definition of success varied between studies, contributing to heterogeneity. Third, all the included studies were of medium to low quality, reducing the level of evidence. Lastly, there was evidence of publication bias for the outcome of initial success. Publication bias in meta-analysis can lead to misleading conclusions by overestimating the true effect size of a phenomenon due to the tendency for studies with positive results to be published more readily than those with negative results, potentially distorting the overall interpretation of the research literature and impacting decision-making based on the meta-analysis findings. However, the exclusion of the low-quality studies removed the publication bias. Future prospective studies on the role of colonoscopic decompression in ACPO and randomized studies comparing colonoscopic decompression with neostigmine in ACPO are required to improve reliability and reduce the risk of selection bias.
To conclude, around three-fourths of the patients with ACPO respond after the first session of colonoscopic decom
| 1. | Markogiannakis H, Messaris E, Dardamanis D, Pararas N, Tzertzemelis D, Giannopoulos P, Larentzakis A, Lagoudianakis E, Manouras A, Bramis I. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Naveed M, Jamil LH, Fujii-Lau LL, Al-Haddad M, Buxbaum JL, Fishman DS, Jue TL, Law JK, Lee JK, Qumseya BJ, Sawhney MS, Thosani N, Storm AC, Calderwood AH, Khashab MA, Wani SB. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of acute colonic pseudo-obstruction and colonic volvulus. Gastrointest Endosc. 2020;91:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Pereira P, Djeudji F, Leduc P, Fanget F, Barth X. Ogilvie's syndrome-acute colonic pseudo-obstruction. J Visc Surg. 2015;152:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | De Giorgio R, Knowles CH. Acute colonic pseudo-obstruction. Br J Surg. 2009;96:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Chudzinski AP, Thompson EV, Ayscue JM. Acute colonic pseudoobstruction. Clin Colon Rectal Surg. 2015;28:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40022] [Article Influence: 10005.5] [Reference Citation Analysis (2)] |
| 7. | Giri S, Kale A, Shukla A. Efficacy and Safety of Transjugular Intrahepatic Portosystemic Shunt Creation for Budd-Chiari Syndrome: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol. 2022;33:1301-1312.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 395] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 9. | Nivatvongs S, Vermeulen FD, Fang DT. Colonoscopic decompression of acute pseudo-obstruction of the colon. Ann Surg. 1982;196:598-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Strodel WE, Nostrant TT, Eckhauser FE, Dent TL. Therapeutic and diagnostic colonoscopy in nonobstructive colonic dilatation. Ann Surg. 1983;197:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Bode WE, Beart RW Jr, Spencer RJ, Culp CE, Wolff BG, Taylor BM. Colonoscopic decompression for acute pseudoobstruction of the colon (Ogilvie's syndrome). Report of 22 cases and review of the literature. Am J Surg. 1984;147:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Fausel CS, Goff JS. Nonoperative management of acute idiopathic colonic pseudo-obstruction (Ogilvie's syndrome). West J Med. 1985;143:50-54. [PubMed] |
| 13. | Lavignolle A, Jutel P, Bonhomme J, Cloarec D, Cerbelaud P, Lehur PA, Galmiche JP, Le Bodic L. [Ogilvie's syndrome: results of endoscopic exsufflation in a series of 29 cases]. Gastroenterol Clin Biol. 1986;10:147-151. [PubMed] |
| 14. | Harig JM, Fumo DE, Loo FD, Parker HJ, Soergel KH, Helm JF, Hogan WJ. Treatment of acute nontoxic megacolon during colonoscopy: tube placement versus simple decompression. Gastrointest Endosc. 1988;34:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Jetmore AB, Timmcke AE, Gathright JB Jr, Hicks TC, Ray JE, Baker JW. Ogilvie's syndrome: colonoscopic decompression and analysis of predisposing factors. Dis Colon Rectum. 1992;35:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Geller A, Petersen BT, Gostout CJ. Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc. 1996;44:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Pham TN, Cosman BC, Chu P, Savides TJ. Radiographic changes after colonoscopic decompression for acute pseudo-obstruction. Dis Colon Rectum. 1999;42:1586-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Tsirline VB, Zemlyak AY, Avery MJ, Colavita PD, Christmas AB, Heniford BT, Sing RF. Colonoscopy is superior to neostigmine in the treatment of Ogilvie's syndrome. Am J Surg. 2012;204:849-55; discussion 855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Peker KD, Cikot M, Bozkurt MA, Ilhan B, Kankaya B, Binboga S, Seyit H, Alis H. Colonoscopic decompression should be used before neostigmine in the treatment of Ogilvie's syndrome. Eur J Trauma Emerg Surg. 2017;43:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhao C, Xie T, Li J, Cheng M, Shi J, Gao T, Xi F, Shen J, Cao C, Yu W. Acute Colonic Pseudo-Obstruction with Feeding Intolerance in Critically Ill Patients: A Study according to Gut Wall Analysis. Gastroenterol Res Pract. 2017;2017:9574592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Mankaney GN, Sarvepalli S, Arora Z, Kamal A, Lopez R, Vargo JJ, Burke CA. Colonic Decompression Reduces Proximal Acute Colonic Pseudo-obstruction and Related Symptoms. Dis Colon Rectum. 2020;63:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Liu JJ, Venkatesh V, Gao J, Adler E, Brenner DM. Efficacy and Safety of Neostigmine and Decompressive Colonoscopy for Acute Colonic Pseudo-Obstruction: A Single-Center Analysis. Gastroenterology Res. 2021;14:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Joechle K, Guenzle J, Utzolino S, Fichtner-Feigl S, Kousoulas L. Ogilvie's syndrome-is there a cutoff diameter to proceed with upfront surgery? Langenbecks Arch Surg. 2022;407:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Williamson S, Muller A, Butts CA, Geng TA, Ong AW. Acute Colonic Pseudo-obstruction: Colonoscopy Versus Neostigmine First? J Surg Res. 2023;288:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Loftus CG, Harewood GC, Baron TH. Assessment of predictors of response to neostigmine for acute colonic pseudo-obstruction. Am J Gastroenterol. 2002;97:3118-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |