Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.101864
Revised: March 2, 2025
Accepted: March 14, 2025
Published online: September 9, 2025
Processing time: 292 Days and 20.8 Hours
Sepsis is a critical medical condition, and poses a substantial global health burden, with significant morbidity, mortality, and economic costs, particularly pron
To assess the distribution of sepsis categories and the use of empirical antibiotics classified by the World Health Organization (WHO) Access, Watch, and Reserve (AWaRe) system in a tertiary care hospital in Northern India and to correlate antibiotic usage with sepsis classifications.
This longitudinal observational study in the Department of General Medicine, in a tertiary care hospital in Northern India, from 2023 to 2024, aimed to assess the use of empirical antibiotics classified by the WHO AWaRe system. The study also aimed to correlate antibiotic usage. Patients were categorized into sepsis classes (Asepsis, Possible Sepsis, Probable Sepsis, Confirmed Sepsis) and followed until discharge or Day-28. Descriptive and inferential statistical analyses were employed to assess sepsis categories and empirical antibiotic usage classified by the WHO AWaRe system.
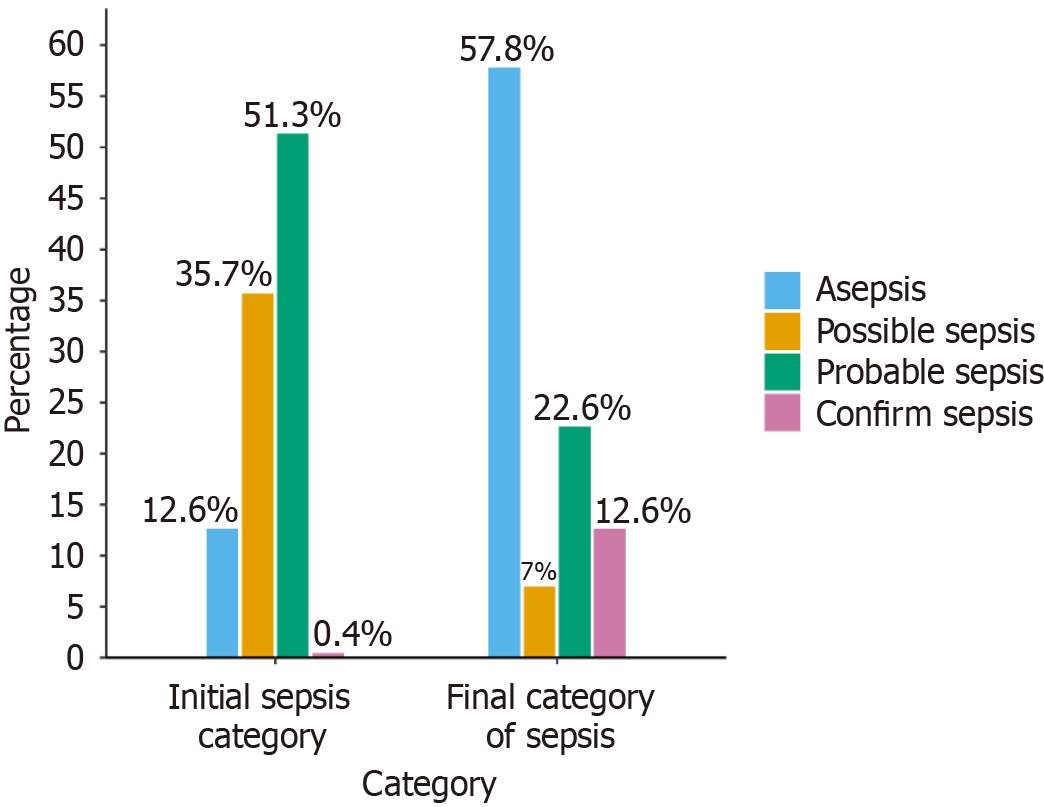
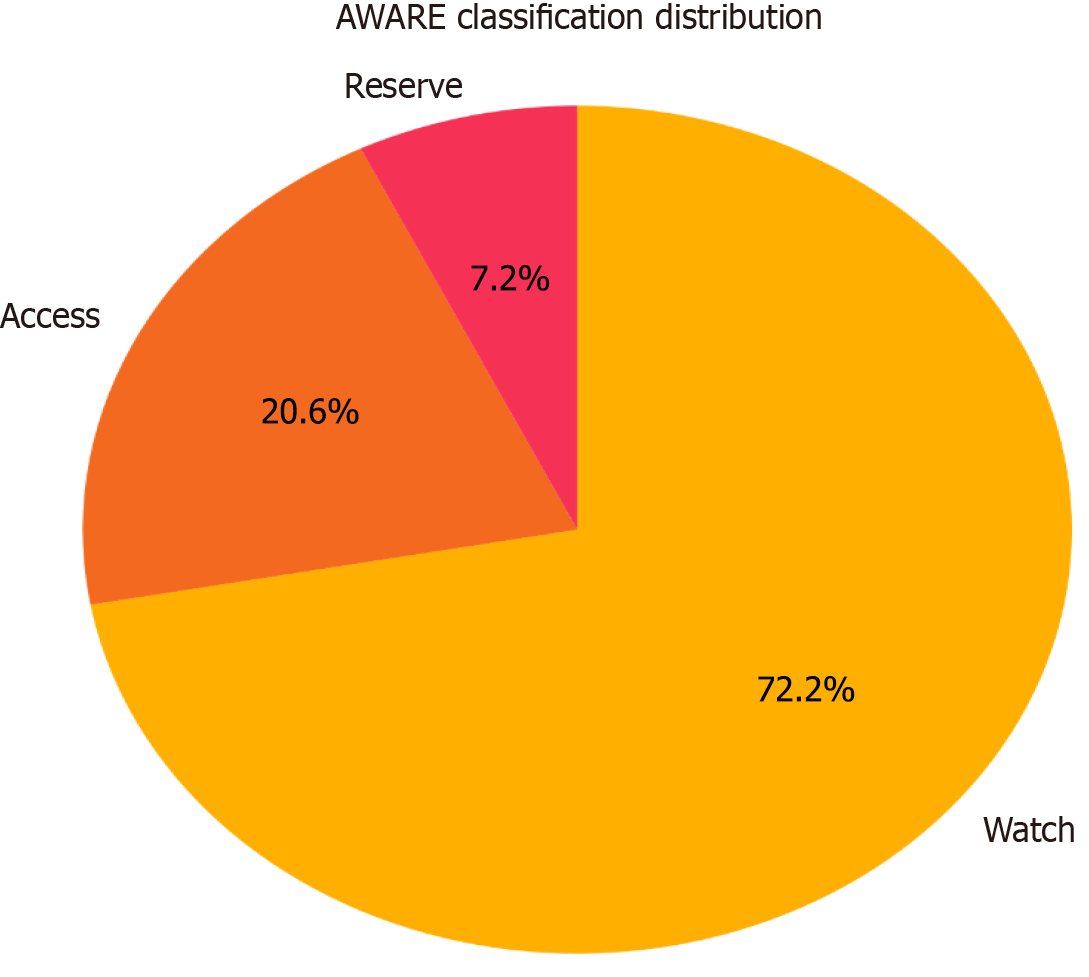
A total of 1867 patients admitted with suspected sepsis were screened, with 230 meeting the inclusion criteria. Among the study cohort (mean age 40.70 ± 14.49 years, 50.9% female), initial sepsis classification predominantly included probable sepsis (51.3%) and possible sepsis (35.7%), evolving to asepsis (57.8%) upon final classification, but all received antibiotics. Empirical antibiotic use showed a predominance of Watch group antibiotics (72.2%), with ceftriaxone and piperacillin-tazobactam being the most commonly prescribed; however, no statistical association could be established among the different classes of sepsis with the AWaRe groups.
Accurate sepsis classification is pivotal for clinical decision-making, optimizing antibiotic use, and combating antimicrobial resistance. The majority of the asepsis category was labelled as probable or possible sepsis and given antibiotics at initial hospitalization. The high reliance on Watch group antibiotics in empirical therapy signals a need for enhanced diagnostic strategies to refine treatment initiation, potentially reducing unnecessary antibiotic exposure. Future efforts should focus on establishing sepsis classification checklists as in this study and promoting adherence to antimicrobial stewardship principles to mitigate the global threat of antimicrobial resistance.
Core Tip: Accurate sepsis classification is pivotal for clinical decision-making, optimizing antibiotic use, and combating antimicrobial resistance. The majority of the asepsis category was labelled as probable or possible sepsis and given antibiotics at initial hospitalization. The high reliance on Watch group antibiotics in empirical therapy signals a need for enhanced diagnostic strategies to refine treatment initiation, potentially reducing unnecessary antibiotic exposure.
- Citation: Pilania J, Panda PK, Chauhan U, Kant R. Correct sepsis classification–A must for antimicrobial stewardship: A longitudinal observational study. World J Crit Care Med 2025; 14(3): 101864
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/101864.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.101864
Sepsis, a life-threatening condition caused by a dysregulated host response to infection[1], remains a leading cause of global morbidity and mortality, affecting over 49 million people annually and causing approximately 11 million deaths[1,2]. It is an extremely heterogeneous entity, both at the clinical and molecular level, can have various subtypes and there are even conditions which can mimic sepsis, i.e., sepsis mimics[3]. The clinical variability of sepsis, ranging from mild organ dysfunction to multi-organ failure, complicates treatment and underscores the need for rapid diagnosis and intervention. However, the growing prevalence of antimicrobial resistance (AMR) significantly hinders effective treat
As per The Global Burden of Disease study, it was estimated that there were around 48.9 million sepsis cases worldwide in the year 2017, with around 11 million deaths related to sepsis in the same year, which accounts for around 19.7% of all global deaths[5]. The majority of the sepsis-related burden is found in low- and middle-income countries in the world. In many developing countries, such as India, with a population of more than 1.4 billion people, the data regarding prevalence, apart from other epidemiological data, are missing and poorly understood, despite high rates of mortality related to sepsis. In 2017, the estimated number of cases of sepsis in India was around 11.3 million, and among them, 2.9 million deaths were noted[6]. To determine the latest epidemiological data regarding sepsis, a study named “Sepsis in India Prevalence Study” is ongoing.
There is also a growing incidence of AMR around the globe, which is emerging as a public health concern, and has led to the emergence of many multidrug resistance microbes, also called “superbugs”. In 2017, the World Health Organization (WHO) Expert Committee on Selection and Use of Essential Medicine developed the Access, Watch, and Reserve (AWaRe) classification of antibiotics as a tool to support antibiotic stewardship at various levels[7]. The antibiotics were categorized into 3 groups- Access, Watch and Reserve on the basis of their impact on AMR. This action is a step forward in reducing the burden of AMR by following the targets set by the WHO. To understand the importance of this, it should be noted that in 2019, it was estimated that AMR was responsible for around 1.3 million deaths worldwide[8]. Inappropriate use of antibiotics in humans is a major well-known and proven factor for AMR, in addition to other causes[9]. The misuse of antibiotics, including using them unnecessarily or selecting the incorrect antibiotic at the wrong dose, for the wrong duration, and by the wrong method, is a prevalent issue affecting 30% to 50% of all antibiotic prescriptions[10,11]. The decision to initiate empiric antibiotics must be based on some parameters and a ‘wait and watch’ strategy can be followed in some cases[12]. Hence, to quantify antibiotic use using the AWaRe classification, we also need an appropriate sepsis classification/definition. Both classifications are complementary to each other in righteousness.
This study was carried out at a tertiary care teaching hospital in northern India with the aim of assessing the distribution of various sepsis categories (asepsis, possible sepsis, probable sepsis, and confirmed sepsis) in admitted patients and the use of empirical antibiotics as per the WHO AWaRe classification. The study also aimed to highlight implications for antimicrobial stewardship by examining the use of AWaRe class antibiotics and their correlation with sepsis classifications.
This longitudinal study was designed to assess the distribution of various sepsis categories in a real-world clinical setting from admission to discharge and to observe the AWaRe classification.
The study was conducted at a tertiary care teaching hospital in northern India. Data were collected from the Department of General Medicine from January 1, 2023 to December 31, 2024. The study was approved by the Institute Ethics Committee, ensuring patient confidentiality, data protection, and adherence to ethical standards in research involving human participants.
Participants included adult patients aged 18 years or older admitted to the Department of General Medicine with suspected sepsis (need for antibiotics, evidence of infection, organ dysfunction not explained by non-infective causes, or improvement following antibiotic treatment) during the study period. Patients diagnosed with an alternative condition within 5 days of admission were excluded, including patients with incomplete data.
The study employed universal sampling due to the absence of prior reference studies for precise sample size calculation. All eligible patients during the study period were included.
Patients without evidence of dysregulated host response (step-1) were subjected to step-2 for evaluation of risk factors for infection (step-2) and patients without dysregulated host response and no evident risk factors for infection were categorized into the asepsis group. Patients with evidence of dysregulated host response (step-1) were directly subjected to step-3 for sepsis classification; however, risk factors for infection were also evaluated (Table 1).
| Step | Description |
| Step 1: Evidence of dysregulated host response | Assessed using the National Early Warning Score-2 ≥ 6 |
| Step 2: Risk factors for infection | Evaluated based on the presence of risk factors such as chronic illnesses, malnutrition, unhygienic living conditions, immunosuppressive states, age, trauma, structural diseases, recent surgery, travel history, animal bites, and previous hospitalizations |
| Step 3: Evidence of infection | Determined through: |
| 3 (A) Clinical evidence | Syndromic diagnosis including pyelonephritis, infective endocarditis, intra-abdominal infections, skin and soft tissue infections, meningitis, cerebrospinal fluid shunt infections, catheter-related infections, osteomyelitis, abscesses, and pneumonia |
| 3 (B) Supportive/suggestive evidence | Imaging (X-ray, ultrasonography, computed tomography, magnetic resonance imaging, positron emission tomography) and biomarkers (blood, urine, other fluids) |
| 3 (C) Confirmatory evidence | Direct visualization, endoscopic evidence, microscopy and culture growth, PCR/gene detection, and immunological methods |
| Interpretation | Sepsis categories/classification |
| (1) Step-1 = negative; (2) Step-1 = positive with step-2 and 3 = negative | Asepsis |
| Step-1, 2 and 3 (A) = positive | Possible sepsis |
| Step-1, 2 and 3 (B) = positive | Probable sepsis |
| Step-1, 2 and 3 (C) = positive | Confirmed sepsis |
Step 1: Evidence of dysregulated host response.
Step 2: Risk factors for infection.
Evaluated based on the presence of risk factors such as chronic illnesses, malnutrition, unhygienic living conditions, immunosuppressive states, age, trauma, structural diseases, recent surgery, travel history, animal bites, and previous hospitalizations.
Step 3: Evidence of infection. Determined through: 3 (A) Clinical evidence: Syndromic diagnosis including pyelo
Sepsis categories/classification: Asepsis: (1) Step-1 = negative; Or (2) Step-1 = positive with step-2 and 3 = negative.
Possible sepsis: Step-1, 2 and 3 (A) = positive.
Probable sepsis: Step-1, 2 and 3 (B) = positive.
Confirmed sepsis: Step-1, 2 and 3 (C) = positive.
Data were collected from patient records and medical charts using RedCap software (AIIMS Rishikesh version) and Microsoft Excel.
In this longitudinal study, statistical analysis was performed and the data were entered into an MS Excel sheet and RedCap software (AIIMS version). The data were summarized using descriptive and inferential statistics. Continuous variables were presented as mean ± SD. Categorical variables were presented as frequencies and percentages. A two-sided significance level of 0.05 was used for all statistical tests. Inferential statistics were performed using the χ² test. Results with a P value < 0.05 were considered statistically significant. All statistical analyses were conducted using the statistical software SPSS-25.
A total of 1867 patients were screened, and a cohort of 230 patients was included in the analysis. The results revealed that the mean age (in years) was 40.70 ± 14.49 years, and 113 (49.1%) were male and the remainder were females. Age was further subcategorized into 3 major groups, with 49.13% of patients aged 18-40 years, 40.87% aged 41-60 years, and the remainder aged > 60 years. Patients were classified into different sepsis categories based on a novel sepsis classification system, as shown in Table 1. There was a significant shift in sepsis classification from the day of hospital admission to discharge (Figure 1).
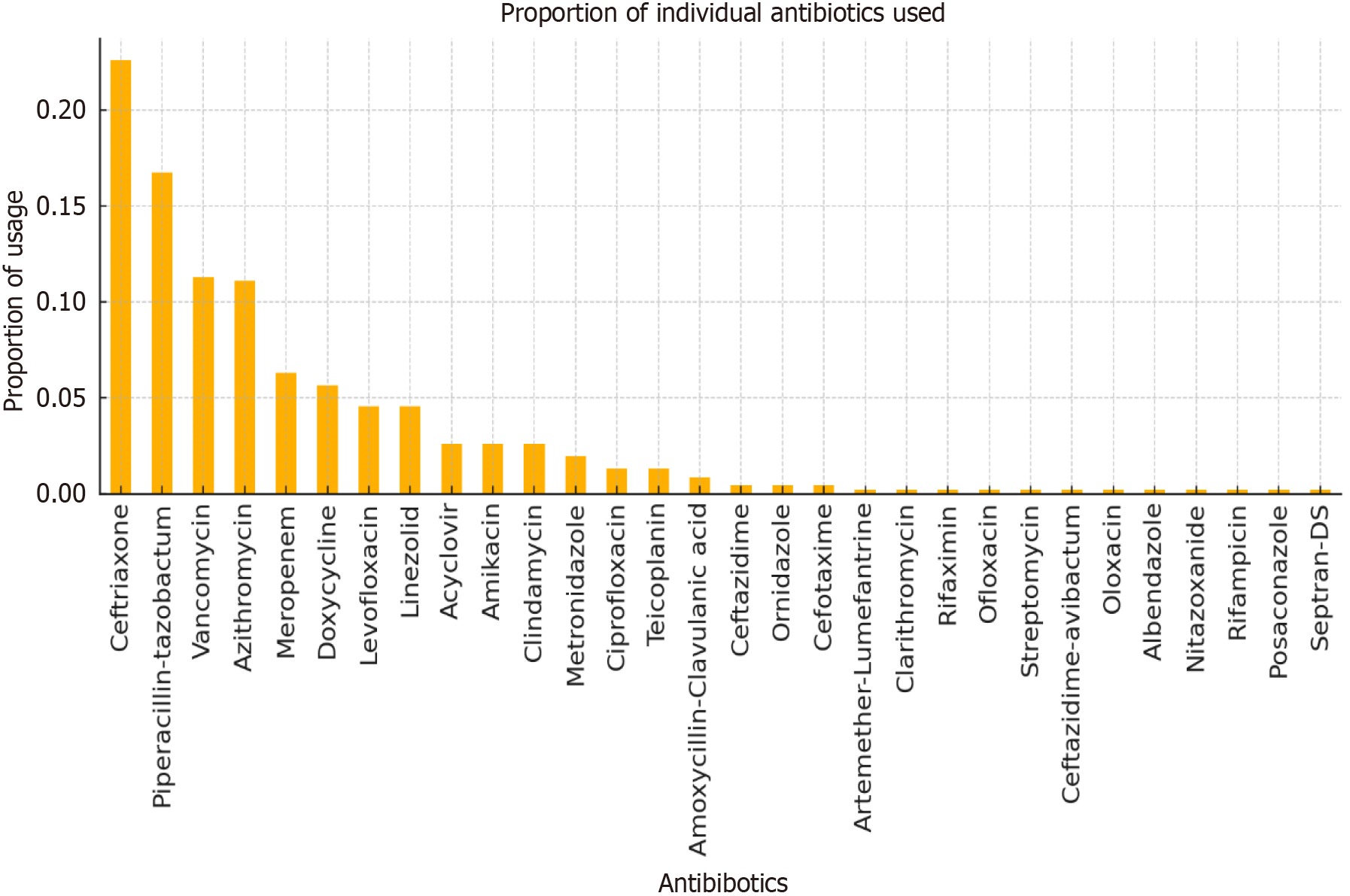
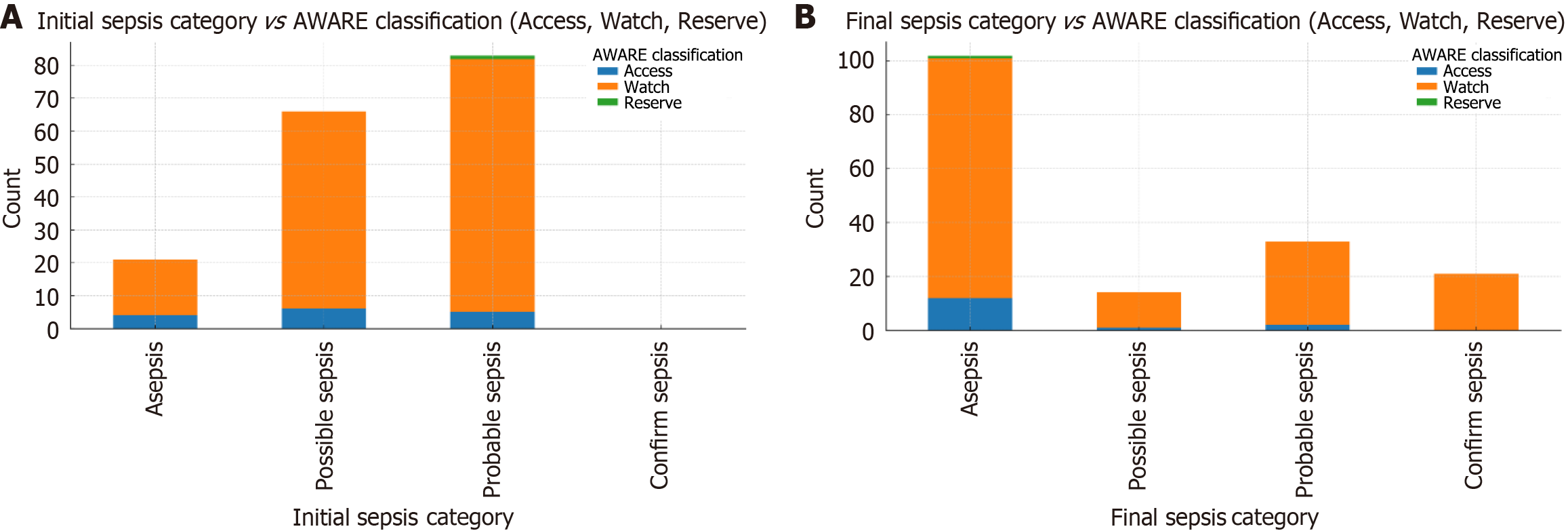
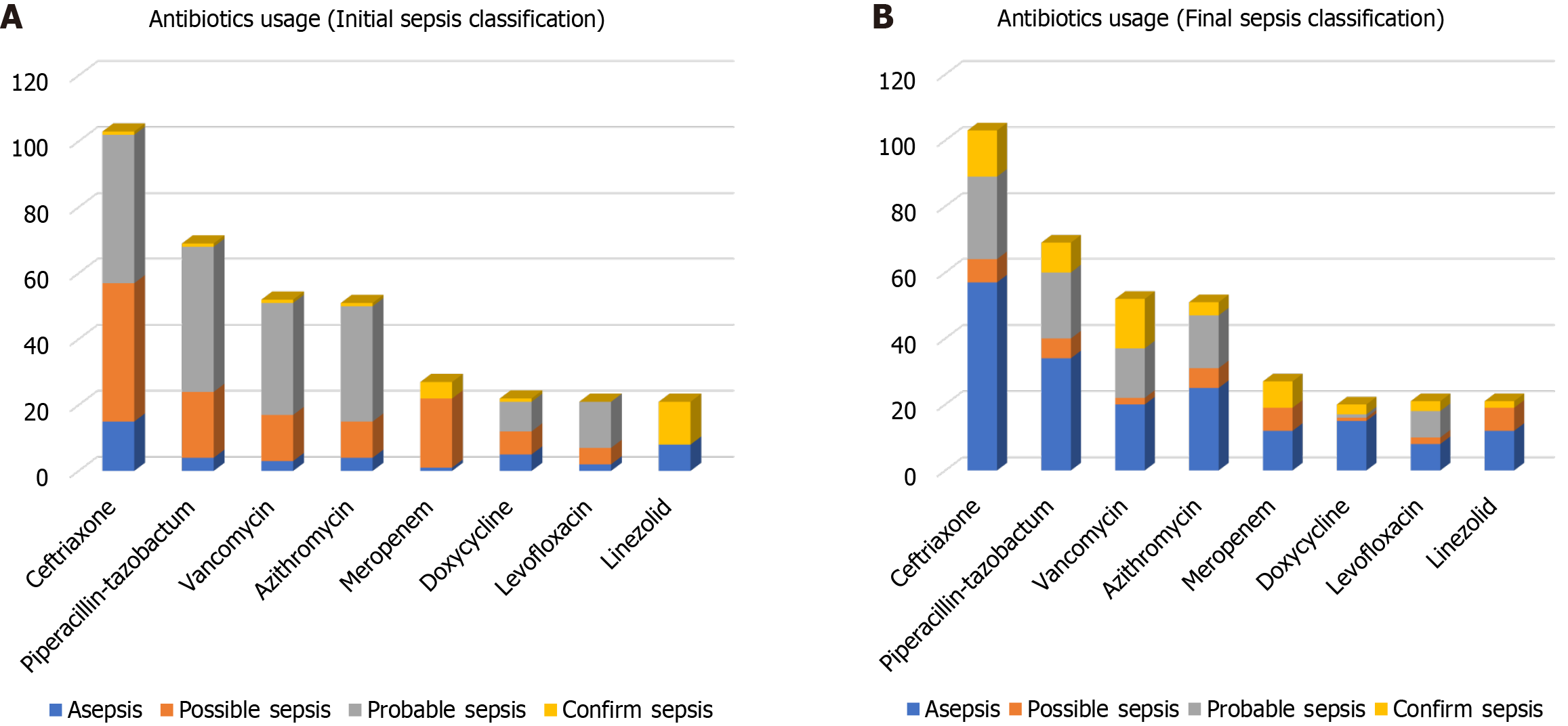
Empirical antibiotic usage was studied and classified as per the WHO AWaRe classification, as shown in Figure 2, which shows that the major antibiotic usage was from the Watch group. Individual antibiotic usage was also estimated, as shown in Figure 3, revealing the most frequently used antibiotics were ceftriaxone, piperacillin-tazobactam, vancomycin, azithromycin, meropenem, doxycycline, levofloxacin, and linezolid, followed by others. Figure 4 shows the relationship between the antibiotics used per AWaRe classification and the different sepsis categories at different time points. These commonly used antibiotics were then assessed for their usage as per the sepsis classification as shown in Figure 5. No statistical association (P > 0.05) was found among the different sepsis classes with the AWaRe groups or even different types of antibiotics when χ² tests were performed.
This single-center observational study at a tertiary care teaching hospital aimed to classify patients into different sepsis categories with the help of a novel approach and to study their antibiotics usage[13]. Initial sepsis classification was dominated by probable and possible sepsis groups, which were finally changed to the majority being in the asepsis group over the duration of the hospital stay. The patients with suspected sepsis were prescribed antibiotics based on the treating clinician's decision and Indian Council of Medical Research Treatment guidelines for antimicrobial use in common syndromes[14]. Antibiotics used were classified as per the WHO AWaRe classification, showing major usage from the Watch group (72.2%), followed by the Access (20.6%) and Reserve (7.2%) groups. Commonly prescribed empirical antibiotics were ceftriaxone, piperacillin-tazobactam, vancomycin, azithromycin, meropenem, doxycycline, levofloxacin and linezolid, whose utilization was approximately ≥ 5%. These commonly used antibiotics were then studied in terms of their usage and corresponding sepsis category, revealing shocking results. It was noted that antibiotics were commonly prescribed to asepsis patients on initial encounters at healthcare facilities. However, initiation of antibiotics in suspected sepsis patients is a risky situation, and delay can be associated with adverse outcomes and should be taken into account. The treating clinician should decide following local or standard antibiotic guidelines, and any structured approach as in this study is always productive.
The antibiotic usage per the AWaRe classification was studied; however, due to lack of available sepsis classification criteria/model, direct comparison could not be conducted. However, antibiotic prescribing patterns were studied by Negi et al[15] at the same institute, revealing 57.61% of antimicrobials in the Access category, 38.27% in the Watch and 4.11% in the Reserve category, which is significantly different to our study results. The study by Negi et al included the whole institute, hence, eccentric inference can be found in a specific department. The overzealous use of antibiotics from the Watch category poses a risk of disruption of the local microbiome or microecology, which maintains homeostasis and promotes the co-existence of multiple flora, which in turn leads to the emergence of drug resistant organisms and repeated infection, making it difficult to treat and increases patient morbidity and mortality.
Sepsis has evolved in its definition and understanding over time to its latest definition in Sepsis-3 guidelines reflecting a deficit in its proper understanding and diagnosis, which was addressed in this study using a novel approach to classify sepsis (1). Using this approach (Table 1), patients were classified as shown in Figure 1, demonstrating the dynamic nature of sepsis[13,16]. Various novel tools such as antibiotic bundle care (ABC-bundle) can improve the delivery of appropriate antibiotic therapy and antimicrobial stewardship[17]. AMR is exacerbated by the misuse and overuse of antibiotics, accelerating the development of resistant bacterial strains. A study by Ventola[18] highlighted the role of broad-spectrum antibiotic misuse in promoting multidrug-resistant infections, which are associated with poorer outcomes in septic patients[18]. The WHO has recognized AMR as a top global health threat, predicting that drug-resistant infections could cause 10 million deaths annually by 2050 without immediate intervention[19]. To address this and for optimization of antibiotic use, the WHO introduced a new classification system AWaRe in 2017, categorizing antimicrobials into three groups—AWaRe—based on their spectrum, the anticipated risk of resistance development, toxicity risk, and clinical utility, which was revised twice, in 2019 and 2021, respectively[20-22]. In this study, we also utilized the WHO AWaRe classification for the classification of used antibiotics, which revealed that the majority of patients received antibiotics from the Watch group (Figure 2), which is not in line with the WHO’s proposed target of using at least 60% of all consumed antibiotics to be from the “Access” group for a country[23]. Antibiotics utilized from different groups were also evaluated, revealing that a large portion of patients received antibiotics from the Watch group based on initial sepsis categorization, which was later classified as asepsis (Figure 4), highlighting the improper utilization of antibiotics contributing to AMR, considered a global pandemic. The AWaRe system of classification, introduced by WHO in 2017 as a part of its antimicrobial stewardship, is considered a milestone step in the fight against increasing AMR, as it was more objective in nature and a user-friendly tool for better organization of antibiotics[24,25]. Some intensive care unit (ICU) settings have better antibiotic consumption than others, as evaluated by various studies with many parameters[26,27]. There is evidence of irrational antibiotic prescriptions in hospitals, which may be due to inadequate available methods for the diagnosis of sepsis[28]. For antimicrobial stewardship, the most important thing is to make the right diagnosis and the right use of antibiotics for its management. The discontinuation of prophylactic antibiotics, giving antibiotics courses for the appropriate duration and their timely de-escalation are essential to improve antibiotic practices in ICU settings to meet international recommendations[29]. This study highlights the importance of the correct sepsis diagnosis, which will be followed by the appropriate treatment in the form of antibiotics, as many patients have received antibiotics when they were wrongly classified as sepsis, and later classified as asepsis, showing misuse rather than overuse, of antibiotics leading to ongoing AMR. The lack of availability of a gold standard test for sepsis, associated with very high mortality and morbidity, highlights the need for an appropriate diagnostic model/algorithm, guiding clinicians and treating physicians for better utilization of resources[30]. The misuse of antibiotics is a widespread issue, affecting 30% to 50% of all prescriptions. This includes instances where antibiotics are used unnecessarily, the incorrect antibiotic is chosen, or the dosage, duration, and method of administration are incorrect[10,11]. Molecular testing methods such as Biofire (a polymerase chain reaction-based test), when incorporated into conventional testing methods, are associated with improved clinical outcomes and effective antimicrobial stewardship programs and procalcitonin for reduction of antibiotic use[31,32].
Many studies across the globe have shown that 20%–50% of all prescribed antimicrobials are either unnecessary or inappropriate[33,34]. From the studies conducted in China and Bangladesh, the antimicrobials selected to treat proven bacterial infections were deemed inappropriate in 63% and 50%, respectively[35,36]. Antibiotics utilized as per the WHO AWaRe classification in this study had nearly similar utilization patterns as observed previously from the same institute[28,29], necessitating the urgent need for the correct sepsis diagnosis, and making the right selection and usage of antibiotics in different groups. Setting up a multidisciplinary team such as an endocarditis team and incorporating its suggestions and recommendations in treating and diagnosing patients is associated with better clinical outcomes, halts misuse of antibiotics and helps in antimicrobial stewardship[37,38]. In this study, we noted the undue usage of antibiotics in patients not rightly classified as sepsis, such as ceftriaxone, piperacillin-tazobactam, and vancomycin, who were later classified as asepsis, and may not have required antibiotics in the first place (Figure 5). This needs to be addressed with utmost importance to curb the upcoming global pandemic of AMR, and this novel approach could be helpful; with the initial correct sepsis diagnosis, the appropriate antibiotic becomes an easy task, except in some circumstances.
Sepsis still does not have a proper definition, is evolving, and diagnostic tests pose a challenge for doctors globally in terms of the correct initial sepsis diagnosis amounting to misdiagnosis, irrational usage of antibiotics, increasing morbidity and mortality of patients and increasing financial burden on the healthcare system with improper utilization of available resources, especially in developing countries, all contributing to increasing AMR. The modern world requires the correct sepsis diagnosis on initial contact with healthcare personnel or treating doctors, which, in turn, allows for better implementation of the 4Ds of antimicrobial stewardship practices in this study[39].
As with most research studies, this study has some limitations. These included the following: this was a single-center and single department observational study, limiting its generalizability to a broader population with different characteristics, for example, head and neck surgery patients, using a novel method to classify sepsis, not using routinely available methodology; unavailability of prior reference studies, making it much more difficult and challenging, and lastly external validity of the study, all of which needs to be considered when interpreting the study results. Other potential con
The correct diagnosis of sepsis and its classification are crucial steps in antimicrobial stewardship. High reliance on antibiotics can be overcome by using a structured approach to diagnose sepsis, giving more confidence to treating doctors in the right decision-making, ultimately improving patient outcomes and morbidity and mortality associated with sepsis. Global targets of antimicrobial utilization can be achieved with the correct sepsis diagnosis. Overutilization of antimicrobials from the Watch group of the AWaRe classification is concerning, especially in asepsis, and control of this situation can limit the emergence of resistant microorganisms. However, its validation involving diverse patient groups, including surgical fields and healthcare settings, may be needed before generalizing to the global population.
We acknowledge the contributions of our mentors and colleagues whose insights and guidance significantly enriched this research. Our heartfelt thanks go to the study participants for their cooperation and contribution. Finally, we recognize the role of anonymous reviewers and previous researchers whose work laid the foundation for this study. Their collective efforts were instrumental in the successful completion of this work.
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17127] [Article Influence: 1903.0] [Reference Citation Analysis (2)] |
| 2. | Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 2307] [Article Influence: 256.3] [Reference Citation Analysis (0)] |
| 3. | Kellum JA, Formeck CL, Kernan KF, Gómez H, Carcillo JA. Subtypes and Mimics of Sepsis. Crit Care Clin. 2022;38:195-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 4. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4003] [Article Influence: 500.4] [Reference Citation Analysis (0)] |
| 5. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4086] [Article Influence: 817.2] [Reference Citation Analysis (4)] |
| 6. | Jeganathan N. Burden of Sepsis in India. Chest. 2022;161:1438-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Moja L, Zanichelli V, Mertz D, Gandra S, Cappello B, Cooke GS, Chuki P, Harbarth S, Pulcini C, Mendelson M, Tacconelli E, Ombajo LA, Chitatanga R, Zeng M, Imi M, Elias C, Ashorn P, Marata A, Paulin S, Muller A, Aidara-Kane A, Wi TE, Were WM, Tayler E, Figueras A, Da Silva CP, Van Weezenbeek C, Magrini N, Sharland M, Huttner B, Loeb M. WHO's essential medicines and AWaRe: recommendations on first- and second-choice antibiotics for empiric treatment of clinical infections. Clin Microbiol Infect. 2024;30 Suppl 2:S1-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 8. | GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:2221-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 845] [Article Influence: 281.7] [Reference Citation Analysis (0)] |
| 9. | Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3674] [Cited by in RCA: 3317] [Article Influence: 221.1] [Reference Citation Analysis (0)] |
| 10. | Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, Lynfield R, Margolis DJ, May LS, Merenstein D, Metlay JP, Newland JG, Piccirillo JF, Roberts RM, Sanchez GV, Suda KJ, Thomas A, Woo TM, Zetts RM, Hicks LA. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA. 2016;315:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1241] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 11. | Zhao H, Wei L, Li H, Zhang M, Cao B, Bian J, Zhan S. Appropriateness of antibiotic prescriptions in ambulatory care in China: a nationwide descriptive database study. Lancet Infect Dis. 2021;21:847-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 12. | Önal U, Akalın H. Coronavirus Disease 2019 and Antibiotic Stewardship-Antibiotic Usage in Adult Patients: Is It Necessary? Balkan Med J. 2021;38:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Pilania J, Das A, Panda PK, Chauhan U. 3-step Model- An Explorative Novel Approach to Classify Sepsis: A Longitudinal Study. J Antimicrob Steward Pract Infect Dis. 2024;2:15-21. [DOI] [Full Text] |
| 14. | Walia K, Ohri VC, Madhumathi J, Ramasubramanian V. Policy document on antimicrobial stewardship practices in India. Indian J Med Res. 2019;149:180-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Negi G, Kb A, Panda PK. Ground level utility of Access, Watch, Reserve classification: Insights from a tertiary care center in North India. World J Exp Med. 2023;13:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Mutters NT, De Angelis G, Restuccia G, Di Muzio F, Schouten J, Hulscher M, Antonelli M, Tacconelli E. Use of evidence-based recommendations in an antibiotic care bundle for the intensive care unit. Int J Antimicrob Agents. 2018;51:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Kallen MC, Roos-Blom MJ, Dongelmans DA, Schouten JA, Gude WT, de Jonge E, Prins JM, de Keizer NF. Development of actionable quality indicators and an action implementation toolbox for appropriate antibiotic use at intensive care units: A modified-RAND Delphi study. PLoS One. 2018;13:e0207991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277-283. [PubMed] |
| 19. | Munkholm L, Rubin O. The global governance of antimicrobial resistance: a cross-country study of alignment between the global action plan and national action plans. Global Health. 2020;16:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Wirtz VJ, Hogerzeil HV, Gray AL, Bigdeli M, de Joncheere CP, Ewen MA, Gyansa-Lutterodt M, Jing S, Luiza VL, Mbindyo RM, Möller H, Moucheraud C, Pécoul B, Rägo L, Rashidian A, Ross-Degnan D, Stephens PN, Teerawattananon Y, 't Hoen EF, Wagner AK, Yadav P, Reich MR. Essential medicines for universal health coverage. Lancet. 2017;389:403-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 21. | Sharland M, Gandra S, Huttner B, Moja L, Pulcini C, Zeng M, Mendelson M, Cappello B, Cooke G, Magrini N; EML Expert Committee and Antibiotic Working Group. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. 2019;19:1278-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 22. | Kaur S, Sethi P, Panda PK. Knowledge-Practice Gaps of Practicing Doctors on Antimicrobial Stewardship-A Single Center Experience. Am J Infect Dis. 2022;18:9-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Zanichelli V, Sharland M, Cappello B, Moja L, Getahun H, Pessoa-silva C, Sati H, van Weezenbeek C, Balkhy H, Simão M, Gandra S, Huttner B. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Org. 2023;101:290-296. [RCA] [DOI] [Full Text] [Cited by in RCA: 106] [Reference Citation Analysis (0)] |
| 24. | Sharland M, Zanichelli V, Ombajo LA, Bazira J, Cappello B, Chitatanga R, Chuki P, Gandra S, Getahun H, Harbarth S, Loeb M, Mendelson M, Moja L, Pulcini C, Sati H, Tacconelli E, Zeng M, Huttner B. The WHO essential medicines list AWaRe book: from a list to a quality improvement system. Clin Microbiol Infect. 2022;28:1533-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 25. | Mudenda S, Daka V, Matafwali SK. World Health Organization AWaRe framework for antibiotic stewardship: Where are we now and where do we need to go? An expert viewpoint. Antimicrob Steward Healthc Epidemiol. 2023;3:e84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 26. | Young D, McKenzie CA, Gupta S, Sparkes D, Beecham R, Browning D, Dushianthan A, Saeed K. Exploring Antibacterial Usage and Pathogen Surveillance over Five Years in a Tertiary Referral Teaching Hospital Adult General Intensive Care Unit (ICU). Pathogens. 2024;13:961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Hanssens Y, Ismaeil BB, Kamha AA, Elshafie SS, Adheir FS, Saleh TM, Deleu D. Antibiotic prescribing pattern in a medical intensive care unit in Qatar. Saudi Med J. 2005;26:1269-1276. [PubMed] |
| 28. | Mugada V, Mahato V, Andhavaram D, Vajhala SM. Evaluation of Prescribing Patterns of Antibiotics Using Selected Indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) Classification by the World Health Organization. Turk J Pharm Sci. 2021;18:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Chin V, Harding HE, Tennant I, Soogrim D, Gordon-Strachan GM, Frankson MA. Dynamics of antibiotic usage in the intensive care unit at the University Hospital of the West Indies. West Indian Med J. 2010;59:159-164. [PubMed] |
| 30. | Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. 2020;24:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 449] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 31. | Senok A, Dabal LA, Alfaresi M, Habous M, Celiloglu H, Bashiri S, Almaazmi N, Ahmed H, Mohmed AA, Bahaaldin O, Elimam MAE, Rizvi IH, Olowoyeye V, Powell M, Salama B. Clinical Impact of the BIOFIRE Blood Culture Identification 2 Panel in Adult Patients with Bloodstream Infection: A Multicentre Observational Study in the United Arab Emirates. Diagnostics (Basel). 2023;13:2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Saeed K, Dryden M, Bourne S, Paget C, Proud A. Reduction in antibiotic use through procalcitonin testing in patients in the medical admission unit or intensive care unit with suspicion of infection. J Hosp Infect. 2011;78:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3171] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 34. | Camins BC, King MD, Wells JB, Googe HL, Patel M, Kourbatova EV, Blumberg HM. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30:931-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Hui L, Li XS, Zeng XJ, Dai YH, Foy HM. Patterns and determinants of use of antibiotics for acute respiratory tract infection in children in China. Pediatr Infect Dis J. 1997;16:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Hossain MM, Glass RI, Khan MR. Antibiotic use in a rural community in Bangladesh. Int J Epidemiol. 1982;11:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | McDonald EG, Aggrey G, Aslan AT, Casias M, Cortes-Penfield N, Dong MQD, Egbert S, Footer B, Isler B, King M, Maximos M, Wuerz TC, Azim AA, Alza-Arcila J, Bai AD, Blyth M, Boyles T, Caceres J, Clark D, Davar K, Denholm JT, Forrest G, Ghanem B, Hagel S, Hanretty A, Hamilton F, Jent P, Kang M, Kludjian G, Lahey T, Lapin J, Lee R, Li T, Mehta D, Moore J, Mowrer C, Ouellet G, Reece R, Ryder JH, Sanctuaire A, Sanders JM, Stoner BJ, So JM, Tessier JF, Tirupathi R, Tong SYC, Wald-Dickler N, Yassin A, Yen C, Spellberg B, Lee TC. Guidelines for Diagnosis and Management of Infective Endocarditis in Adults: A WikiGuidelines Group Consensus Statement. JAMA Netw Open. 2023;6:e2326366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 38. | El-Dalati S, Cronin D, Riddell J 4th, Shea M, Weinberg RL, Washer L, Stoneman E, Perry DA, Bradley S, Burke J, Murali S, Fagan C, Chanderraj R, Christine P, Patel T, Ressler K, Fukuhara S, Romano M, Yang B, Deeb GM. The Clinical Impact of Implementation of a Multidisciplinary Endocarditis Team. Ann Thorac Surg. 2022;113:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Dixit D, Ranka R, Panda PK. Compliance with the 4Ds of antimicrobial stewardship practice in a tertiary care centre. JAC Antimicrob Resist. 2021;3:dlab135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |