Published online Mar 9, 2025. doi: 10.5492/wjccm.v14.i1.98419
Revised: October 23, 2024
Accepted: November 15, 2024
Published online: March 9, 2025
Processing time: 168 Days and 13.6 Hours
Diabetic foot attack (DFA) is the most severe presentation of diabetic foot disease, with the patient commonly displaying severe sepsis, which can be limb or life threatening. DFA can be classified into two main categories: Typical and atypical. A typical DFA is secondary to a severe infection in the foot, often initiated by minor breaches in skin integrity that allow pathogens to enter and proliferate. This form often progresses rapidly due to the underlying diabetic pathophy
Core Tip: Diabetic foot attack (DFA) represents the most severe presentation of diabetic foot disease, with typical and atypical forms that require distinct management strategies. A typical DFA results from a severe infection of the foot, propagated by the associated diabetic pathophysiology, leading to rapid spread of infection, tissue necrosis and potential systemic sepsis. An atypical DFA arises from either ischemia or Charcot arthropathy. Effective management of DFA necessitates early diagnosis, aggressive treatment of infections, and a multidisciplinary approach involving critical care, surgical intervention, and diabetes management teams. Current treatment practices are informed by guidelines for diabetic foot infections, but there is a critical need for dedicated research to develop standardized protocols for DFA management. This review highlights the urgent need for comprehensive care and research to optimize outcomes for patients experiencing DFA.
- Citation: Balakrishnan KR, Selva Raj DR, Ghosh S, Robertson GA. Diabetic foot attack: Managing severe sepsis in the diabetic patient. World J Crit Care Med 2025; 14(1): 98419
- URL: https://www.wjgnet.com/2220-3141/full/v14/i1/98419.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i1.98419
A diabetic foot attack (DFA) is the most severe presentation of diabetic foot disease, which can result in limb- or life-threatening sepsis[1,2]. DFA presents in two different forms: Typical and atypical DFAs. The typical DFA presents secondary to infection in the foot, while the atypical DFAs present either secondary to vascular insufficiency, or Charcot arthropathy.
The typical DFA is a severe infection in the foot, usually preceded by a minor break in skin integrity, which harbors a pathway for pathogen entry and local infection[3,4]. The preceding diabetic foot disease, with a combination of neuropathy, micro-vascular disease and elevated blood sugar levels, leaves the tissue susceptible to colonization, and this allows the infection to spread rapidly through the foot. The severe tissue damage that ensues can lead to tissue necrosis, both through micro-vascular and macro-vascular compromise, allowing further proliferation of the infection. The infection will target the soft tissues initially, though with prolonged colonization of the foot, osteomyelitis will subsequently ensue. Abscess formation can develop in the early stages of the process, especially when the initial skin breaks are of limited size.
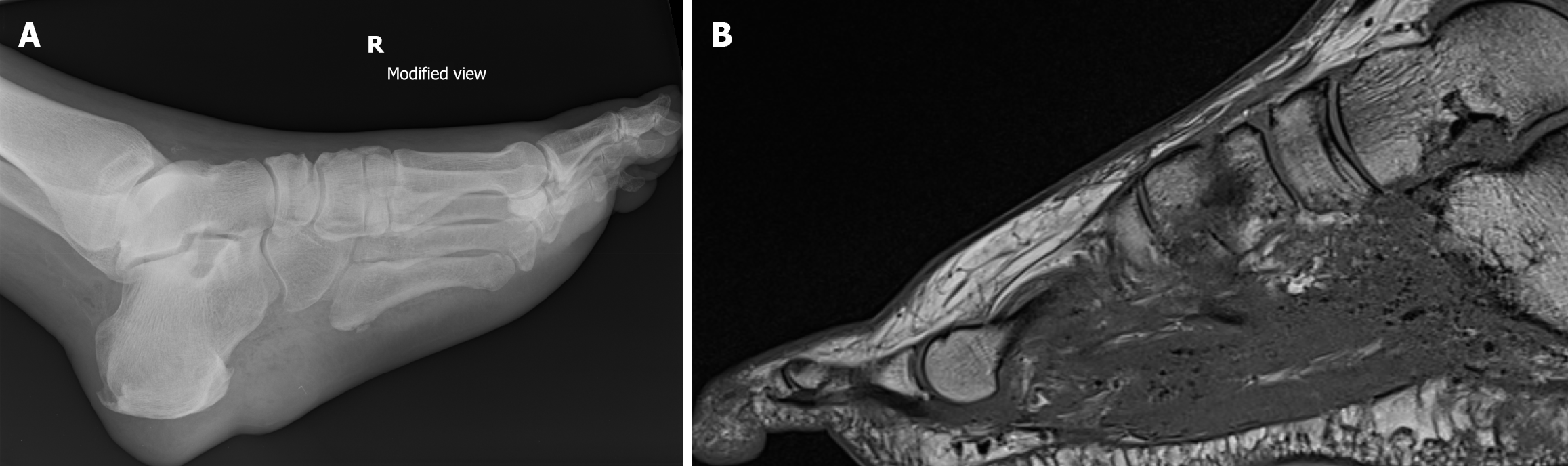
As the soft tissue infection increases in severity during the typical DFA, one of three major forms of infective pathology are often observed: Necrotizing fasciitis, pyomyositis, and myonecrosis (Figures 1 and 2). With pyomyositis, the bacteria penetrates the muscle tissue, resulting in intra-muscular abscess formation[5]. In necrotizing fasciitis, the bacteria release toxins and enzymes, which results in micro-vascular thrombosis of the fascial layers and sub-cutaneous tissues of the foot, secondary necrosis, further spread of serious infection, with a high risk of mortality[6]. Similar to necrotizing fasciitis, myonecrosis results in micro-vascular occlusion of the muscular tissue in the foot, resulting in necrosis, with further rapid spread of bacterial infection[1]. With any of these three infective pathologies, systemic sepsis can ensue. In the diabetic patient, often with pre-existing co-morbidities in the cardio-vascular and renal systems, this can rapidly precipitate renal failure and cardio-vascular collapse. Thus, the early recognition and management of such conditions is key, to prevent such effects. However, if the infection presents at an established stage, careful interplay is required between sepsis management, surgical management and critical care intervention, in order to optimize and recover the patient.
Atypical DFA is characterized by a non-infectious etiology and can be divided into two main subtypes: Ischemic DFA and Charcot neuropathic arthropathy. Unlike typical DFA, which arises from a breach in skin integrity and bacterial proliferation, atypical DFA develops due to microvascular and macrovascular insufficiencies or neuroarthropathic changes in the diabetic foot. The impaired vascularity and structural foot abnormalities create a permissive environment for chronic wounds and joint instability, respectively[1].
Ischemic DFA results from critical limb ischemia due to peripheral artery disease, which is commonly seen in patients with long-standing diabetes and multiple cardiovascular comorbidities. Severe ischemia leads to tissue necrosis and ulceration without an active infection. Studies have shown that diabetic patients with ischemic DFA have a significantly higher risk of limb loss due to delayed revascularization[2,6]. The pathophysiology of ischemic DFA is complicated by calcified and narrowed arteries, which limit perfusion even in the absence of acute infection[7].
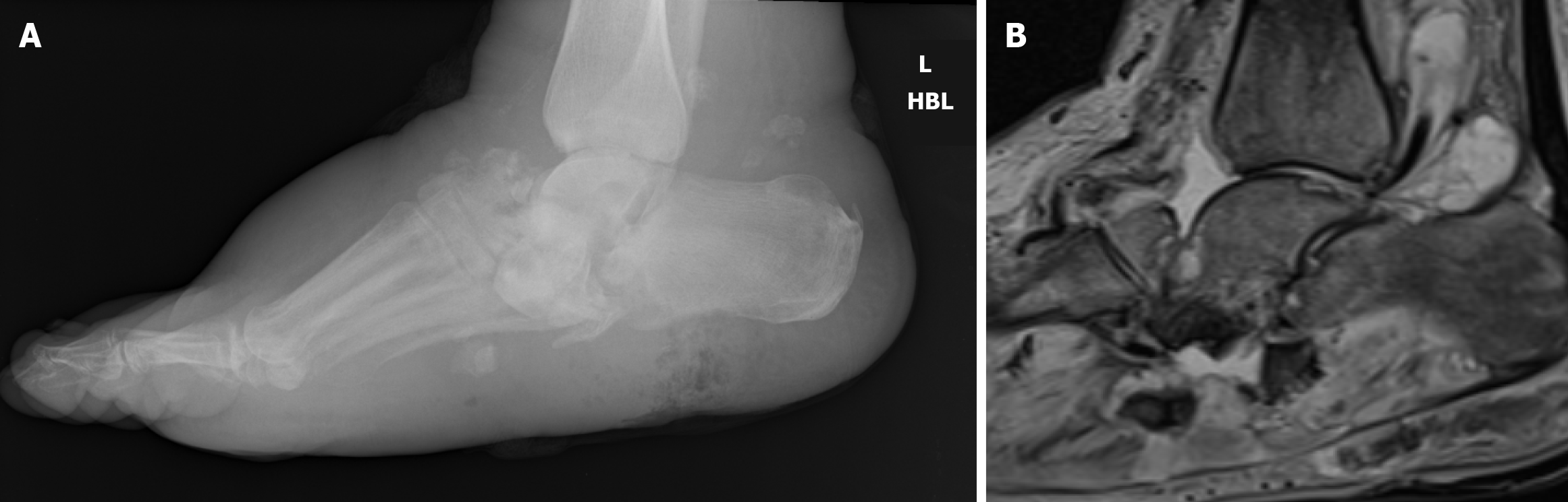
Charcot arthropathy is a neurodegenerative condition that results in joint disintegration, instability, and deformity. It typically manifests as a red, hot, swollen foot, mimicking acute infection but without an infective etiology[3]. The lack of protective sensation in diabetic neuropathy leads to repetitive trauma and unperceived injuries, which, in combination with a hyperemic response, result in bony destruction and collapse. Recent studies have highlighted that Charcot neuropathy can often be misdiagnosed as cellulitis or deep tissue infection, resulting in inappropriate management and increased risk of severe deformity[6].
While a formal guideline on the management of DFA does not yet exist, useful guidance can be obtained from the International Working Group on the Diabetic Foot/Infectious Diseases Society of America (IWGDF/IDSA) guidelines on the diagnosis and treatment of diabetes-related foot infections and the Charcot’s neuro-osteo-arthropathy (2023), and the combined British Orthopaedic Foot and Ankle Society (BOFAS)-Vascular Society guidelines. Given the lack of defined guidelines, controversies still surround the diagnosis and management of this condition, and further research is required to ensure the optimal management and outcomes for patients with DFA[3,4].
Diabetes mellitus is the umbrella term for a cohort of metabolic diseases encompassing persistently elevated blood sugar level as a result of faults in insulin secretion, its action or a conjoined effect due to the interplay of genetic and environmental factors. Diabetes leads to complications with myocardial infarction (MI) and cerebrovascular accident (CVA) being the leading macrovascular complications, whereas diabetic neuropathy, retinopathy and nephropathy are the predominant microvascular complications[7,8]. Diabetes is an epidemic throughout the world, with the reported prevalence of diabetes as high as 37.5% in the Western Pacific[9]. In the United Kingdom, 7% of the population were recorded to have diabetes in 2019[10]. This has almost doubled from the 4.3% recorded in 2005[11]. An annual public spending of £23.7 billion is accounted for diabetes and its complications: This accounts for 1.8% of the global gross domestic product[12,13]. This highlights the importance of vigilance in monitoring and treating diabetes in view of the economic impact.
Diabetes proves to be a major burden in the field of orthopaedics[14,15]. A wide spectrum of orthopaedic complications, secondary to diabetes, is noted, with foot pathologies, such as diabetic foot ulcers (DFUs), infections and attacks being the most prevalent in terms of hospital admission[16]. An interplay of neuropathy and ischaemia in the diabetic patient precipitates ulcerations in the foot, which usually results in patients attending the clinical setting. These ulcerations can be complicated by secondary infection, which without time-sensitive treatment, can result in limb or life threatening sepsis. The treatment of patients with diabetic foot pathologies is highly complex and necessitates a multi-disciplinary team approach to achieve a good clinical outcome[17].
Diabetes is a chronic metabolic disorder, which occurs secondary to abnormal function of the insulin pathways. Symptoms of diabetes include tiredness, polyuria, polydipsia, unintentional weight loss, opportunistic infections, slow healing of wounds and glucose in urine[18-21]. The pathophysiology in the development of diabetes can divided into: (1) Type 1 diabetes: This result following exhaustion of insulin production from the pancreas, driven mainly by an autoimmune reaction. The cells in the islet of the Langerhans are obliterated by T-cell mediated autoimmune response driven by an interplay of environmental and genetic factors[22-24]; and (2) Type 2 diabetes: This encompasses 90%-95% of diagnosis of diabetes[25], and is multifactorial in nature, and driven by an interplay of environmental and genetic factors resulting in hyperinsulinemia and resistance to insulin[26]. Insulin requirements are raised to achieve the same desired effect for the utilization of glucose by the cells. The pancreas becomes less efficient in its production of insulin, overtime resulting in development of the condition.
Diabetes is diagnosed as per the National Institute for Health and Care Excellence guidelines based on the following parameters: (1) Hemoglobin A1c levels of 48 mmol/mol (6.5%) or higher (hemoglobin A1c levels of 42-47 mmol/mol or 6.0%-6.4% signifies a raised risk of diabetes); (2) A fasting plasma glucose levels of 7.0 mmol/L (126 mg/dL) or higher; (3) A 2-hour plasma glucose level of 11.1 mmol/L (200 mg/dL) or higher after a 75 g oral glucose tolerance test, can as well be utilized as a parameter to diagnose diabetes; and (4) Patients presenting with symptoms suggestive of diabetes (i.e., polyuria or polydipsia) can be diagnosed with a random plasma glucose level of 11.1 mmol/L (200 mg/dL) or higher[27].
Sepsis is a reaction where a significant immune response is produced in response to an infection by the body, leading to systemic inflammation and organ dysfunction[28,29]. This can result in the systemic inflammatory response syndrome (SIRS). Pathogens can be identified by immune defense cells, such as macrophages, lymphocytes and mast cells. Once pathogens are recognized, immune cells release a large amounts of cytokines, such as interleukins and tumor necrosis factor, that leads to the activation of the immune system[28,30]. This immune system activation leads to cascade reaction, which in turn leads to systemic inflammation, resulting in the secretion of secondary chemicals, such as nitrous oxide which promotes vasodilation. The significant quantity of cytokines released causes increased permeability of the endothelial layer lining the blood vessels. These in turn allows fluid to migrate from the blood vessels into the extracellular space[28,31]. This results in oedema and intravascular volume depletion. Oedema creates a barrier between the blood and the tissues, reducing the efficiency of gas exchange, by reducing oxygen delivery to the tissue.
Sepsis also leads to the activation of the coagulation system which leads to fibrin deposition. This causes thrombosis formation along the entire circulation, leading to a compromise of organ and tissue perfusion. This process also results in consumption of platelets and clotting factors in the body, which results in thrombocytopenia and hemorrhage, in the form of disseminated intravascular coagulopathy[32]. This pathological cascade results in an inadequate supply of oxygen to the surrounding tissues, necessitating anaerobic respiration. The end product of anaerobic respiration, lactic acid, accumulates, causing a rise in serum lactate, and secondary metabolic acidosis[33]. In order to diagnose SIRS, the patient must have two of the following physiological parameters: (1) Temperature < 36 °C, or > 38 °C; (2) Heart rates > 90 beats/minute; (3) Respiratory rates > 20 breaths/minute, and/or partial pressure of CO2 < 32 mmHg; and (4) White cell count > 12000/μL, or < 4000 /μL.
The mechanism of sepsis in a diabetic foot infection (DFI) develops through a complex interplay of factors related to the impaired immune response (secondary to hyperglycemia) and pre-existing tissue damage (secondary to diabetic neuropathy, diabetic angiopathy and hyperglycemia). This process most commonly begins with a DFU formation. Such ulcer formation develops through the effects of diabetic-related peripheral neuropathy, peripheral vascular disease and diabetic related foot structure abnormality[34,35]. Not only do these conditions increase the risk of foot injuries, they also result in compromised healing. Once a foot ulcer develops, it provides a portal of entry for bacteria, commonly staphylococcus aureus (though this can vary based on patient characteristics and geographical location), into the deeper tissues. The compromised blood flow and impaired immune response in diabetic individuals make it difficult for the body to contain and clear the infection effectively[36].
As the infection progresses, local inflammation intensifies. The body’s immune response, characterized by the release of inflammatory mediators, aims to control the infection but can also cause collateral tissue damage. Without timely and appropriate treatment, the infection can spread beyond the local site, invading deeper tissues, muscles, bones, and potentially entering the bloodstream. The presence of severe infection triggers the SIRS response, which can progress to life-threatening sepsis. In sepsis, the body’s immune response becomes dysregulated, leading to widespread inflammation and potentially life-threatening organ dysfunction[37]. In diabetic foot sepsis, the combination of impaired wound healing, reduced tissue perfusion, and underlying comorbidities increases the risk of severe complications and mortality. Of particular concern, are the pre-existing diabetic co-morbidities seen in these patients: Systemic sepsis can often precipitate renal failure and cardio-vascular collapse, if not diagnosed early and managed effectively.
Most DFIs arise through superficial colonization of a DFU. However, the pathogens can then spread rapidly to the subcutaneous tissue and underlying tissues. These include the tendons, fascia, muscles bones and joints[38]. The anatomy of the foot, with several distinct but interconnected compartments, can promote rapid spread of infection throughout the foot structure. The tendon sheaths in particular facilitate proximal spread of the infection, and this spread normally occurs from an area of higher to lower pressure. The virulence of bacteria also plays a vital role in the progression of such infections. The pathological effects of the diabetic physiology also render the affected tissues more susceptible to infection. The accompanying microvascular disease limits the perfusion of antibiotics to the affected tissue. The concomitant neuropathy devoid the tissue of protective sensation, thus rendering such areas more prone to injury: The limited protective nociception pathways also further impair healing. Lastly, the elevated glucose levels, with the secondary tissue glycosylation, facilitates the colonization of tissue by bacteria, and the spread of infection.
The infection can induce a local inflammatory response, which can cause the pressure in the compartment to surpass the capillary pressure. This leads to tissue necrosis in the initial infected component and eventually progressive infection. The infection, when severe, can also precipitate micro-vascular thrombosis, and further tissue necrosis, with subsequent polymicrobial colonization. As the infection spreads and increases in severity, this can then lead to systemic symptoms such as pyrexia and malaise. Although uncommon in patients with a DFI, systemic symptoms such as fever, chills, or significant metabolic changes indicate serious infection that may pose a threat to limb or life. DFIs often worsen quickly if they are not identified and treated appropriately[38].
With a DFA, the patient is at risk of developing systemic sepsis and septic shock. Septic shock is a process whereby an initial localized infection spreads systemically, creates a systemic septic pathophysiology, through cytokine and cellular induced pathways, resulting in dysregulation of the cardio-vascular (e.g., vasodilation), coagulation, metabolic, and cellular functioning. In addition to systemic antibiotic therapy, this then necessitates the requirement of vasopressor therapy and organ support. Septic shock can be confirmed when: (1) A patient requires vasopressor therapy to sustain a mean arterial blood pressure > 65 mmHg; (2) A patient has an associated serum lactate level > 2 mmol/L; and (3) A patient has been adequately fluid resuscitated (i.e., not hypovolemic)[39].
The common systemic co-morbidities seen within diabetic patients include renal disease, cardiovascular disease and cerebrovascular disease[37]. The main concern during such episodes of severe sepsis is the effect that this can have on these already compromised systems. During episodes of septic shock, the patient is at risk of acute kidney injury (e.g., through renal shunting), cardio-vascular events (e.g., MI) and cerebrovascular events (e.g., CVAs). Patient suffering a DFA, with systemic sepsis, should require liaison with the critical care team, to ensure optimal monitoring and management of secondary renal failure and cardio-vascular strain. Close monitoring of renal function should be performed, with dialysis therapy commenced as required. Inotrope therapy will be required if septic shock develops, and this should be commenced and monitored within a critical care setting.
On a longer-term perspective, research demonstrates that, diabetic patients who suffer a DFU have a greater chance of dying from all causes as compared to those who do not suffer a DFU. In patients with DFU, trend points towards an increased risk of CVAs and an increased risk of fatal MI[40]. Although there are more fatalities from cerebro-vascular disease in the DFU group, it was shown that the overall mortality rates from cardiovascular causes are similar in DFU and non-DFU patient. Studies on all-cause and cardiovascular mortality found that in both the DFU and the non DFU groups, fatal MI and CVA accounted for 44% of all deaths. These results suggest that the increased mortality rate seen in DFU patients is not entirely explained by the additional cerebro-vascular risk. Sepsis and other non-cardiovascular consequences of foot ulceration, along with a more advanced stage of diabetes with a higher disease burden, may also contribute to excess mortality in DFU patients[37].
In the typical DFA, the main pathology is systemic sepsis, and rapid infection control is necessitated[38]. Once diagnosed, patients must be administered empirical broad-spectrum antibiotics, targeted towards the severe infective processes that underpin this condition (e.g., necrotizing fasciitis). The actual anti-biotic agents prescribed will be directed by each clinician’s local hospital microbiological policies. This should be combined with initial deep tissue sampling, and then further antibiotic therapy should be targeted the cultured organism. A culture-determined antibiotic treatment is of pivotal importance in all DFI, and the guidelines set by IWGDF strongly suggest proper sampling techniques to accurately culture the causative pathogen and target the antibiotic treatment. Careful renal dosing of antibiotics should be observed as such diabetic patients often suffer from a varying degree of renal impairment.
Fluid resuscitation is paramount in the management of sepsis to support blood pressure and tissue perfusion[41]. However, care must be used to prevent volume overload and prevent cardiac failure in the diabetic patients with associated heart failure. If hypotension persists, despite adequate fluid resuscitation, septic shock should be suspected. Urgent critical care review should be requested, and the use of vasopressors and inotropes may be required to maintain tissue perfusion and blood pressure[42].
Optimal glycemic control is another key factor in the management of the DFA. Sepsis and SIRS are stress response and hyperglycemia is a form of stress response in reaction to the systemic inflammatory response. Targeted glycemic management is important to prevent the detrimental effects of hyperglycemia and hypoglycemia to the patient. The ideal range is usually between 6-10 mmol/L. To maintain this, continuous glucose monitoring and appropriate insulin therapy, with input from the medical/diabetes team, is crucial[43]. Diagnostic imaging can help confirm the causative pathology, in the DFA. However, acquisition of specialist imaging should not delay time to theatre. An initial foot radiograph can show gas in the soft tissue, often suggestion of necrotizing fasciitis and myonecrosis. A magnetic resonance imaging (MRI) scan is the gold standard modality to quantify soft tissue and bone pathology in the foot.
However, access to MRI scanning out of hours is often not possible, and delays to surgical debridement should not be postponed to allow access to this. A standard computed tomography (CT), with soft tissue windowing techniques, can often provide satisfactory pre-operative imaging, to locate and quality the involved pathology. A CT angiogram can provide further useful information, regarding the associated peripheral vascular disease. However, this will be contra-indicated in a patient presenting with associated acute kidney injury.
After appropriate resuscitation and antibiotic administration, this should be followed by prompt surgical intervention. This should involve complete debridement of all necrotic and infected tissues, and investigation of any potential tracking pathways in conjunction. Local administration of antibiotics to the sites of infection, through antibiotic loaded bone void fillers (e.g., Cerament® and Stimulan®) should be considered[44,45]. Surgery and other infection control measures should not be postponed to perform vascular status evaluations, not even in cases of severe ischemia. If vascular status has not been assessed prior to initial surgical debridement, it is imperative to do so as soon as the initial infection is brought under control through administration of antibiotics and surgical intervention. Revascularization must happen quickly when there is vascular compromise. If the necrosis persists, further exploratory procedures will often be required.
Following initial surgical debridement and subsequent sepsis control, the emphasis switches to wound stabilization. Patients will often require further surgical debridement procedures, and these should be appropriately timed with the other supporting specialists. Plastic surgery consult should request for patients with significant post-surgical soft tissue defects. Non-weightbearing on the affected limb is crucial, to facilitate wound healing. Negative pressure wound therapy (NPWT) can also be considered, whilst continuing targeted antibiotic therapy based on culture results. Anti-biotic therapy with targeted, culture specific antibiotic should only be stopped when complete eradication of infection is achieved, as confirmed by clinical and serological evidence[1,38].
While traditionally considered as a primary treatment for the DFA, major limb amputation (i.e., below knee amputation) can often be avoided, at least in the initial phases of management. As long as all necrotic and infected tissues tissue is debrided, the limb can be provisionally salvaged. If, however, on primary debridement, there is noted to be proximal tracking of severe, rapidly spreading infection (i.e., necrotizing fasciitis), such that debridement of this would render the limb un-salvageable, then primary limb amputation may be considered. This decision should be made by two consultant surgeons, who can be from different specialties within the diabetic foot multidisciplinary team (i.e., orthopaedics and vascular). More often, limb amputation may be considered following secondary debridement procedures, when it becomes apparent that the limb is un-salvageable, or the infection cannot be controlled through debridement alone. In such cases, all reconstruction options should be considered, in conjunction with the plastic surgery team. However, if severe infection persists, or if pre-existing vascular disease and diabetic pathophysiology preclude complex reconstruction procedures, then limb amputation may be required. All such decisions should be made in conjunction with the multi-disciplinary team, along with in-depth discussions with either the patient or with family/next of kin, if the patient is intubated. A multi-disciplinary approach should be performed throughout, ensuring relevant medical/diabetic review of the patient to ensure optimal pre-operative and peri-operative control of glucose levels and metabolism, as well as addressing any other medical issues that patient is presenting with. For patients demonstrating evidence of septic shock or developing organ dysfunction, critical care review must be performed.
Guidelines were outlined in the management of DFA which were described in three different phases[4,46]: (1) Phase 1: Admit patient to the hospital and provide prompt surgical consultation. Observe local guidelines for sepsis and schedule the necessary imaging. Arterial duplex; this should not delay continuation to next phase; (2) Phase 2: Identify the proximal part of the infection and implement a thorough debridement of all diseased tissue. Proactive preparation should be done, a surgical follow-up for relook in 48 hours; and (3) Phase 3: Refrain from bearing weight on the injured limb and perform regular bedside examinations. Assessing and treating revascularization should be given first priority. Medical optimization and targeted antibiotics. Skin grafting in suitable cases. It has been demonstrated that completing these three processes results in improved patient outcomes and efficient care. A multidisciplinary approach is necessary in the treatment of DFA.
The management of atypical DFA differs significantly from typical DFA and requires a tailored approach depending on the subtype. The cornerstone of treatment for ischemic DFA is to restore perfusion as rapidly as possible. Endovascular interventions, such as angioplasty and stenting, are preferred due to their minimally invasive nature and reduced perioperative risks[2]. In cases where immediate revascularization is not feasible, hyperbaric oxygen therapy (HBOT) can be used to enhance oxygen delivery to ischemic tissues and promote healing. Recent evidence supports the use of adjunctive HBOT in reducing the extent of necrosis and improving limb salvage rates[5]. Antiplatelet agents and vasodilators are recommended to improve microcirculation, although their efficacy is limited in advanced cases[6].
When it comes to management of Charcot neuropathic arthropathy, early offloading using total contact casting or removable boots is essential to prevent further joint damage. Evidence shows that early offloading can reduce the progression to severe deformities by 40%[1]. Surgery is reserved for patients with severe joint instability or recurrent ulceration. Studies indicate that surgical correction using internal fixation can improve long-term outcomes, though the risk of postoperative complications remains high[2]. A multidisciplinary approach is crucial in the management of atypical DFA due to its complex etiology and high risk of complications. The involvement of diabetologists, vascular surgeons, orthopaedic surgeons, and radiologists is recommended to optimize care[1]. Patients with atypical DFA require long-term follow-up to monitor for recurrent ischemia or progressive Charcot changes. Telemedicine and structured foot care programs can reduce the risk of recurrence and improve patient outcomes[7]. Atypical DFA presents unique diagnostic and therapeutic challenges compared to typical DFA. While typical DFA primarily requires aggressive infection control, atypical DFA management must focus on vascular optimization and joint stabilization.
Protocols to salvage the limb includes timely, aggressive surgical debridement. Sufficient debridement is important in lowering the infective burden: This will cause a disturbance to the bio-film, which is anti-microbial resistant. It will also allow deep culture of the microbiome (the cause of the infection), which can in turn aid in providing effective antimicrobial therapy[47]. This was further supported in a subsequent paper where it is stated that for severe foot infections, clinicians need to define the extent of the infection through accurate radiological investigation, and ensure all infected and necrotic tissue is resected, to achieve eradication of the infection and attain healing of the ulcer.
A red-amber-green model described by Ahluwalia and Reichert[1] introduced a system for the debridement of DFI. The ‘red zone’ is all clinically infected and necrotic tissue, which must form part of surgical debridement. The surrounding fibrous tissue with no vascular supply which usually houses the infective pathogen is classified as the amber zone. The normal healthy tissue beyond this is known as the green zone. It is pivotal that all the tissue in the red and amber zone is completely excised. This classification system should be applied when performing surgical debridement of the typical DFA. Samples for culture from the deep tissue usually obtained from the border of the necrotic area, within the red area. This sample can then undergo culture to guide antimicrobial treatment. After debridement and debulking of the necrotic tissue is completed, elliptical incisions are designed. These are utilized to house the negative pressure therapy for the wound. Once the tissues are aggressively debrided, the tendon sheaths involved must be explored up to the muscle belly and lavaged, with copious warmed normal saline. All the wounds are then re-examined with further debridement of any remaining necrotic or infected tissue, before the surgery is concluded. This is then followed by bedside management of the wound and additional soft tissue debridement if needed.
Typical DFA is associated with a higher risk of systemic complications, such as sepsis and multi-organ failure, if not promptly treated. The mortality rate for patients with typical DFA ranges from 15% to 30%, with a significant risk of major amputation[2]. Amputation rates in typical DFA are notably high, particularly when infection is severe or not promptly managed. Studies indicate that the rate of major amputations in typical DFA, primarily driven by infection, also ranges from 15% to 30%, with outcomes heavily dependent on the timeliness of intervention and the use of multidisciplinary care[2,6]. Aggressive surgical debridement and intravenous antibiotics are the mainstays of treatment, with limb salvage rates improving when combined with early multidisciplinary intervention[1].
In contrast, atypical DFA, particularly ischemic DFA, has a poorer prognosis due to the difficulty in achieving adequate perfusion. Recent studies show that up to 40% of patients with ischemic DFA undergo major amputations within the first year of diagnosis, even with aggressive revascularization[6]. Mortality rates in ischemic DFA are significant, with studies reporting rates as high as 40% within one year of diagnosis, particularly in patients with severe ischemia and delayed revascularization[2,6]. Charcot-related DFA also has a prolonged treatment course, with an average time to stabilization exceeding 6 months[3]. Delays in diagnosis and inappropriate management often lead to irreversible deformities and recurrent ulceration, further complicating the clinical course.
Atypical DFA is generally associated with higher rates of limb loss and poorer functional outcomes compared to typical DFA. The lack of standardized protocols for atypical DFA and the complexity of its management contribute to these adverse outcomes. A study by Tanabe et al[5] found that limb salvage rates were 25% lower in atypical DFA compared to typical DFA due to the delayed recognition of ischemic and Charcot-related changes.
DFA is a complex condition requiring advanced therapeutic approaches to prevent limb loss and optimize patient outcomes. Traditional management strategies, such as debridement and systemic antibiotic therapy, are effective but often inadequate in severe cases or where comorbidities complicate the healing process. Recent advancements in DFA management have introduced novel therapies, including bioengineered tissue substitutes, NPWT, and HBOT. These emerging therapies aim to accelerate wound healing, enhance tissue regeneration, and reduce the need for major amputations in high-risk patients. This section provides an in-depth analysis of these innovative interventions, supported by recent clinical evidence.
Bioengineered tissue substitutes, such as dermal replacements and acellular matrices, have revolutionized the treatment of chronic and non-healing wounds in diabetic patients. These substitutes act as scaffolds that promote cellular regeneration and provide a protective barrier against further trauma and infection. Dermal replacements, such as Integra® and Apligraf®, have been widely studied in patients with DFUs, showing significant improvements in wound closure rates and reduced healing times[5]. In the study by Kirsner et al[48], Apligraf®, a bioengineered living cellular construct, demonstrated significantly higher wound closure rates in DFUs compared to standard care. Specifically, Apligraf®-treated wounds achieved a 63% wound closure rate at 12 weeks, compared to 49% in the standard treatment group. Additionally, patients treated with Apligraf® experienced faster healing times, reducing the risk of complications like infections and amputations. These findings highlight the potential of bioengineered tissues in promoting rapid wound healing and reducing the overall burden of chronic wounds.
Additionally, acellular dermal matrices have been explored for their role in complex wounds, including deep tissue infections and exposed bone. In a recent study by Ulusoy and Oruc[2], acellular dermal matrices were successfully used in conjunction with surgical debridement to cover exposed tendons and bone, resulting in a 40% improvement in healing rates compared to traditional dressings. These substitutes not only enhance the structural integrity of the wound bed but also reduce the risk of secondary infections by providing a sterile environment for tissue regeneration.
NPWT, also known as vacuum-assisted closure therapy, is a non-invasive modality that uses controlled negative pressure to promote wound healing. By applying sub-atmospheric pressure, NPWT removes excess exudate, reduces edema, and increases local blood flow, thereby creating a conducive environment for wound healing[49]. The therapy is particularly beneficial in managing complex diabetic foot wounds, including those with deep cavities and extensive tissue loss. A systematic review and meta-analysis by Liu et al[50] assessed the efficacy of NPWT in 450 patients with DFUs and found that NPWT was associated with a 50% reduction in wound size and a 35% increase in granulation tissue formation compared to standard care. The authors concluded that NPWT is effective in promoting wound closure and should be considered a first-line adjunctive therapy in high-risk diabetic foot wounds.
NPWT can also be combined with other advanced therapies, such as bioengineered tissue or antibiotic beads, to enhance outcomes in infected wounds. A study by Iacopi et al[6] reported that combining NPWT with a dermal substitute resulted in faster wound closure and a lower incidence of wound complications in patients with deep tissue infections and exposed bone. These findings suggest that NPWT may serve as a valuable adjunct in the multimodal management of complex DFA cases, particularly when standard therapies have failed.
HBOT involves the inhalation of 100% oxygen at elevated atmospheric pressure, which enhances oxygen delivery to ischemic tissues and promotes angiogenesis and fibroblast proliferation. HBOT has been explored as an adjunctive therapy in ischemic DFA, particularly in patients with peripheral artery disease who are not candidates for immediate revascularization. The therapy aims to increase local tissue oxygenation, reduce hypoxia, and stimulate the release of growth factors that accelerate wound healing[51]. A prospective cohort study by Ulusoy and Oruc[2] evaluated the impact of HBOT in 80 patients with severe ischemic DFA. The study found that 65% of patients receiving HBOT achieved limb salvage within six months compared to 45% in the control group receiving standard wound care. The authors concluded that HBOT is effective in improving limb salvage rates and should be considered in select cases where revascularization is not feasible or as a bridge to definitive surgical intervention.
Moreover, HBOT has been shown to reduce the severity of infections in patients with deep tissue involvement by enhancing the bactericidal effects of leukocytes and reducing anaerobic bacterial growth[51]. This makes HBOT a valuable adjunctive therapy in patients with necrotizing fasciitis or deep abscesses, where traditional antibiotics may be less effective due to poor tissue perfusion. However, despite its benefits, HBOT has limitations, including high cost, limited availability, and the need for specialized facilities. Therefore, its use should be carefully considered based on individual patient factors and the availability of resources[49]. In low-resource settings, the benefits of HBOT may not outweigh the logistical challenges, making it less applicable in such environments.
Emerging therapies, such as gene therapy, stem cell therapy, and platelet-rich plasma (PRP), are currently being explored for their potential to enhance wound healing and tissue regeneration in diabetic foot patients. Although these therapies are still in the experimental stage, preliminary studies have shown promising results in promoting angiogenesis and reducing inflammation in chronic wounds[43].
Gene therapy: A recent study on vascular endothelial growth factor gene therapy showed significant improvements in wound healing in diabetic models when combined with fibroblast growth factor-1. The study found that the combination of vascular endothelial growth factor-A and fibroblast growth factor-1 mRNA promoted faster wound closure and enhanced neovascularization in diabetic mice[52]. This combination approach underscores the potential of gene therapy for improving chronic wound healing in DFUs by targeting multiple pathways involved in revascularization and tissue repair.
Stem cell therapy: Stem cells have shown significant potential to differentiate into various cell types and secrete growth factors that enhance tissue regeneration. A recent systematic review demonstrated that stem cell therapy can significantly improve wound healing in patients with critical limb ischemia, leading to a reduction in major amputation rates. For example, bone marrow-derived stem cells and adipose tissue-derived stem cells were effective in promoting angiogenesis and tissue regeneration, resulting in improved blood flow and reduced ischemia-related complications[53].
PRP: PRP is derived from the patient’s own blood and contains a high concentration of growth factors that stimulate wound healing. A randomized controlled trial by Driver et al[54] showed that PRP accelerated wound healing in 40% of patients with non-healing DFUs compared to 15% in the placebo group. These emerging therapies hold great potential but require further validation through large-scale clinical trials before they can be integrated into standard DFA management protocols.
The management of the diabetic patient presenting with systemic sepsis necessitates a multi-disciplinary approach, that takes into account the distinctive challenges that posed by the diabetic patient, including: (1) The need to optimise glycaemic control; (2) The awareness to detect potential associated causative peripheral vascular diseases; and (3) The pre-existing comorbidities, with increased cardiovascular and cerebro-vascular risks, and the real risk of sepsis-related renal failure[41]. Early identification and diagnosis of developing sepsis is crucial[55]. Diabetic patients often present with occult signs of sepsis, hence careful observation is warranted. Healthcare providers should be cautious and monitor vital signs regularly and escalate care quickly when sepsis is identified. Early diagnostic testing, which include blood markers, blood lactate and relevant radiological imaging, should be performed in suspected patients, to confirm sepsis and identify the source.
Administration of antibiotics is crucial when treating sepsis[39]. National Institute for Health and Care Excellence states that broad-spectrum antibiotics should be started within the first hour of recognizing sepsis. Targeted antibiotics can then be commenced after culture results and sensitivities, to ensure optimal treatment of the infection. Careful renal dosing of antibiotics should be observed as diabetic patients often suffer from associated renal impairment.
Fluid resuscitation is similarly paramount in the management of sepsis to support blood pressure and tissue perfusion[41]. Care must be taken to prevent volume overload and prevent cardiac failure. If hypotension persists, the use of vasopressors and inotropes can be used to maintain tissue perfusion and blood pressure[42]. In parallel to this, optimal glycemic control is of key importance. The ideal range is usually between 6-10 mmol/L. To maintain this, continuous glucose monitoring and appropriate insulin therapy is crucial[43].
The monitoring of all organ systems is crucial during the treatment of severe sepsis[56]. Cardiovascular, respiratory, hepatic and liver function monitoring is crucial, and supportive treatments such as renal replacement therapy for renal failure and mechanical ventilation for respiratory failure can be provided in the high dependency unit and intensive therapy unit setting as needed[41]. Early enteral nutrition is also preferred to maintain gut integrity and support overall metabolic needs in sepsis. A multi-disciplinary team approach to include the specialties such as diabetes/endocrinology renal, critical care, orthopaedics, vascular, plastics and cardiology is necessary to ensure holistic care for the patient.
Long-term management is essential in reducing the morbidity and mortality associated with DFA. While acute management in the typical DFA focuses on debridement and infection control, sustained care is necessary to prevent complications and improve outcomes. A patient-centred approach focusing on structured rehabilitation, infection monitoring, and education is key to success[2,6].
Rehabilitation is critical to preventing re-ulceration and promoting functional recovery. A structured program involving physical therapy, podiatry, and diabetes education is recommended[7,8]. Physical therapy helps improve mobility and balance, with gait training essential for patients who have undergone partial amputations to prevent new ulcers[2]. Additionally, custom orthotics and offloading devices help reduce mechanical stress on the foot, a critical factor in preventing recurrence[6]. Recent studies have shown that structured rehabilitation reduces readmission rates and improves functional outcomes. For example, Iacopi et al[6] demonstrated a 30% reduction in recurrent ulcers with a structured rehabilitation protocol compared to standard care.
Continuous monitoring of infection is crucial for early detection and prevention of severe complications. Effective monitoring requires a combination of clinical assessments, imaging, and biomarkers. Advanced imaging, such as MRI, is recommended for detecting early osteomyelitis, a common complication in DFA[3]. Biomarkers like C-reactive protein and procalcitonin help guide therapy by tracking inflammation[7]. Telemedicine has become a valuable tool for remote monitoring, particularly for high-risk patients with limited access to healthcare. Digital platforms facilitate early detection and allow clinicians to intervene before complications worsen. Elafros et al[7] found that telemedicine reduced complication detection times by 50% and improved patient satisfaction.
Patient education is central to long-term DFA management, as it empowers individuals to take control of their care. Educational programs should include daily foot inspections, proper footwear, and blood sugar management[8]. Structured education, often provided by diabetes educators, has been shown to reduce the incidence of recurrent ulcers and lower amputation rates[5]. Digital tools, such as mobile apps, have made education more accessible. A study by Vas et al[3] found that patients using diabetes management apps had better glycaemic control and fewer foot complications than those receiving traditional pamphlets.
Effective long-term management of DFA requires a multidisciplinary team, including endocrinologist, vascular surgeons, orthopaedic surgeons, podiatrists, and infectious disease specialists. This comprehensive approach ensures that all aspects of DFA care - vascular health, glycaemic control, and wound care are addressed[6]. Studies show a 20% improvement in limb salvage rates when a standardized multidisciplinary protocol is employed[8].
Emerging technologies, such as bioengineered skin substitutes, NPWT, and 3-dimensional printed orthotics, offer promise in DFA care. Bioengineered tissues promote cellular regeneration, while NPWT accelerates wound closure and reduces hospital stays[3]. Additionally, 3-dimensional printed orthotics provide a personalized approach to offloading, addressing the specific biomechanical challenges faced by DFA patients[2,5].
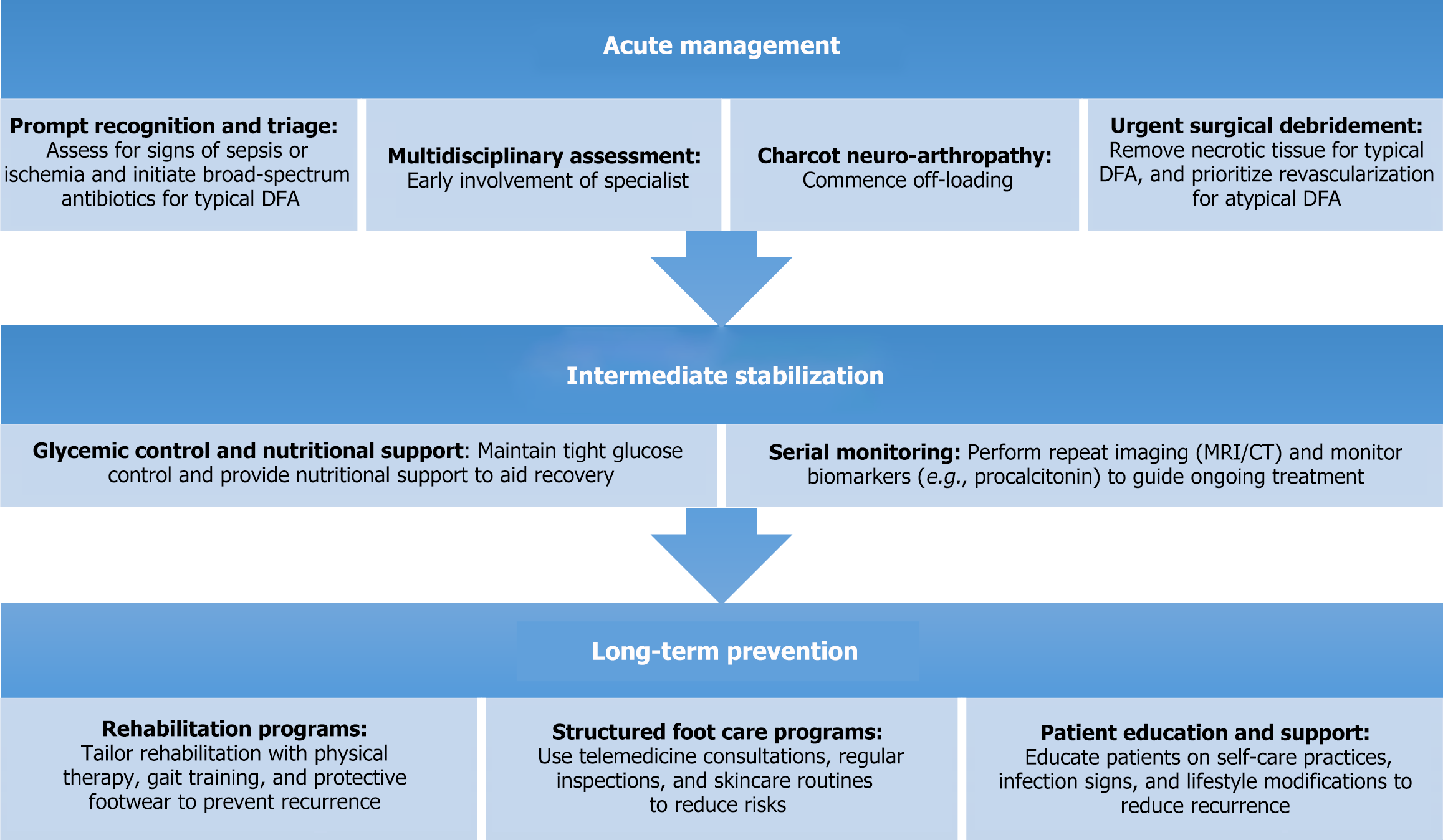
A comprehensive treatment framework is necessary to optimize outcomes in patients with DFA. The management of DFA can be divided into three key phases: Acute management, intermediate stabilization, and long-term prevention and rehabilitation. This proposed framework addresses both typical and atypical DFA with tailored approaches, integrating newer therapies and evidence-based interventions (Figure 3).
Clinical guidelines for managing DFA are essential for ensuring standardized, evidence-based care. Among the most referenced are the IWGDF/IDSA guidelines and the BOFAS-Vascular Society guidelines. Both offer valuable frameworks, yet they have distinct strengths and limitations, particularly when applied in different healthcare contexts. The IWGDF/IDSA guidelines primarily address DFIs, providing detailed recommendations on wound care, infection control, and antibiotic usage. These guidelines emphasize a multidisciplinary approach and early intervention to reduce limb loss. However, their scope can be limited when dealing with atypical DFA[1,38]. While the IWGDF/IDSA guidelines are well-structured for managing infected DFUs and promote infection control strategies, they can be limited in guiding the integration of surgical, vascular, and orthopaedic interventions for non-infective DFAs[6]. However, recent improvements have been made, with a specific Charcot neuro-osteo-arthropathy guidelines introduced as part of the new IWGDF/IDSA 2023 guidelines.
In comparison, the BOFAS-Vascular Society guidelines offer a holistic approach, encompassing not only infection management but also vascular and orthopaedic complications. These guidelines advocate for early vascular assessments and surgical debridement, which are crucial in preventing limb loss in ischemic DFA[2,3]. Additionally, BOFAS highlights the use of advanced imaging techniques, such as MRI and CT angiography, to assess disease severity and plan surgical interventions. However, the BOFAS guidelines can be resource-intensive, making them less feasible in low-resource settings where access to advanced imaging and specialist care may be limited. Furthermore, the BOFAS guidelines lack specific algorithms for managing conditions like Charcot neuroarthropathy, a common presentation in atypical DFA[3,6].
Both sets of guidelines face challenges in their applicability across low-income and middle-income countries, where healthcare resources are often limited. The reliance on advanced diagnostic tools, frequent specialist consultations, and complex surgical techniques renders both guidelines difficult to implement in these regions[43]. To address these challenges, there is a pressing need for context-specific adaptations that account for regional differences in healthcare delivery. Current guidelines also require optimization of management advice for atypical DFA, where the lack of standardized protocols for early detection and intervention contributes to higher rates of limb loss and poorer outcomes[6]. Furthermore, guidance on emerging therapies, like HBOT, bioengineered tissues, and NPWT remains limited in both guidelines, despite evidence supporting their efficacy in limb salvage[2].
To improve the management of DFA, guidelines need to be enhanced by incorporating better algorithms for atypical DFA, emphasizing early recognition and intervention for ischemic and Charcot-related cases. In resource-limited settings, guidelines should identify essential diagnostic and treatment strategies that do not rely heavily on advanced imaging, and promote the use of telemedicine and remote consultations to mitigate specialist shortages[7]. Emerging therapies like HBOT, NPWT, and bioengineered tissues should be further integrated into guidelines to offer more advanced treatment options for complex DFA cases[5]. Moreover, a stronger focus on long-term management, including rehabilitation, infection monitoring, and structured foot care programs, is necessary to reduce recurrence and improve quality of life[6]. By addressing these gaps, future guidelines can better align with diverse clinical needs and ensure more comprehensive DFA care, particularly in low-resource settings. This critical evaluation highlights the need for more flexible, resource-conscious, and comprehensive guidelines that address the full spectrum of DFA presentations, ensuring better patient outcomes globally.
| 1. | Ahluwalia RS, Reichert ILH. Surgical management of the acute severely infected diabetic foot - The 'infected diabetic foot attack'. An instructional review. J Clin Orthop Trauma. 2021;18:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Ulusoy S, Oruc M. Characteristics and management of patients undergoing emergency surgery for diabetic foot attack. Ulus Travma Acil Cerrahi Derg. 2023;29:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Vas PRJ, Edmonds M, Kavarthapu V, Rashid H, Ahluwalia R, Pankhurst C, Papanas N. The Diabetic Foot Attack: "'Tis Too Late to Retreat!". Int J Low Extrem Wounds. 2018;17:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complications. 2007;21:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Tanabe A, Kaneto H, Kamei S, Hirata Y, Hisano Y, Sanada J, Irie S, Kinoshita T, Tatsumi F, Shimoda M, Kohara K, Mune T, Kaku K. Case of disseminated pyomyositis in poorly controlled type 2 diabetes mellitus with diabetic ketoacidosis. J Diabetes Investig. 2016;7:637-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Iacopi E, Sbarbaro C, Pieruzzi L, Lorenzi I, Baroni L, Goretti C, Malacarne P, Piaggesi A. Necrotizing Fasciitis and Diabetic Foot: Results of a Prompt Identification, Surgery and Antibiotic Therapy (P.I.S.A.) Protocol. Int J Low Extrem Wounds. 2023;22:733-741. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Elafros MA, Callaghan BC, Skolarus LE, Vileikyte L, Lawrenson JG, Feldman EL. Patient and health care provider knowledge of diabetes and diabetic microvascular complications: a comprehensive literature review. Rev Endocr Metab Disord. 2023;24:221-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Sattar N. Prevention of Diabetes Macrovascular Complications and Heart Failure. Endocrinol Metab Clin North Am. 2021;50:415-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 9. | Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6:850-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 584] [Cited by in RCA: 575] [Article Influence: 57.5] [Reference Citation Analysis (42)] |
| 10. | Whicher CA, O'Neill S, Holt RIG. Diabetes in the UK: 2019. Diabet Med. 2020;37:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | González EL, Johansson S, Wallander MA, Rodríguez LA. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health. 2009;63:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 568] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 13. | Zhang P, Gregg E. Global economic burden of diabetes and its implications. Lancet Diabetes Endocrinol. 2017;5:404-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Al-Mayahi M, Cian A, Kressmann B, de Kalbermatten B, Rohner P, Egloff M, Jafaar J, Malacarne S, Miozzari HH, Uçkay I. Associations of diabetes mellitus with orthopaedic infections. Infect Dis (Lond). 2016;48:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Morriss N, Brophy RH. Diabetes in Orthopaedic Sports Medicine Surgeries Standard Review. J Am Acad Orthop Surg. 2024;32:51-58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Boulton AJ, Armstrong DG, Kirsner RS, Attinger CE, Lavery LA, Lipsky BA, Mills Sr. JL, Steinberg JS. Diagnosis and Management of Diabetic Foot Complications. Arlington (VA): American Diabetes Association, 2018. [PubMed] |
| 17. | British Orthopaedic Association; British Orthopaedic Foot and Ankle Society, Vascular Society, Diabetes UK; Association of British Clinical Diabetologists; Foot in Diabetes UK; British Association of Prosthetists and Orthotists. Operational Delivery of the Multi-Disciplinary Care Pathway for Diabetic Foot Problems. April 2016. [cited 12 April 2024]. Available from: https://vascularsociety.org.uk/_userfiles/pages/files/guidelines/030416-diabeticfoot-final-pdf.pdf. |
| 18. | Gavin JR, Rodbard HW, Battelino T, Brosius F, Ceriello A, Cosentino F, Giorgino F, Green J, Ji L, Kellerer M, Koob S, Kosiborod M, Lalic N, Marx N, Prashant Nedungadi T, Parkin CG, Topsever P, Rydén L, Huey-Herng Sheu W, Standl E, Olav Vandvik P, Schnell O. Disparities in prevalence and treatment of diabetes, cardiovascular and chronic kidney diseases - Recommendations from the taskforce of the guideline workshop. Diabetes Res Clin Pract. 2024;211:111666. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Atrese T, Fekadu L, Kune G, Shita A, Woldemikael K. Prevalence of undiagnosed diabetes mellitus and associated factors among adult residents of Mizan Aman town, Southwest Ethiopia: Community-based cross-sectional study. PLoS One. 2024;19:e0302167. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Pleus S, Tytko A, Landgraf R, Heinemann L, Werner C, Müller-Wieland D, Ziegler AG, Müller UA, Freckmann G, Kleinwechter H, Schleicher E, Nauck M, Petersmann A. Correction: Definition, Classification, Diagnosis and Differential Diagnosis of Diabetes Mellitus: Update 2023. Exp Clin Endocrinol Diabetes. 2024;132:e1. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Gonzalez-Samano M, Villarreal HJ. Diabetes, life course and childhood socioeconomic conditions: an empirical assessment for Mexico. BMC Public Health. 2024;24:1274. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Kim M. Diabetic ketoacidosis in type 1 diabetes mellitus in children and adolescents. J Korean Med Assoc. 2024;67:335-341. [DOI] [Full Text] |
| 23. | Cheng Z, Cao W, Sun B. Global epidemiology of diabetes from 1990 to 2019: A pan-database synthesis of risk factors and disease associations. Clin Chim Acta. 2024;558:118076. [DOI] [Full Text] |
| 24. | Wang Q, Chen Y, Xie Y, Xia Y, Xie Z, Huang G, Fan L, Zhou Z, Li X. Type 2 Diabetes Family History as a Significant Index on the Clinical Heterogeneity Differentiation in Type 1 Diabetes. J Clin Endocrinol Metab. 2023;108:e1633-e1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2128] [Article Influence: 425.6] [Reference Citation Analysis (0)] |
| 26. | Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 225] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 27. | National Institute for Health and Care Excellence. Diagnosis in adults, Diagnosis, Diabetes-type 2, CKS, NICE. [cited 12 April 2024]. Available from: https://cks.nice.org.uk/topics/diabetes-type-2/diagnosis/diagnosis-in-adults/. |
| 28. | Kanth SM, Torabi-Parizi P. Personalized Sepsis Treatment: Are We There Yet? Crit Care Med. 2021;49:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Sepsis best practices: Definitions, guidelines, and updates. Nursing. 2024;54:39-40. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Gauer R, Forbes D, Boyer N. Sepsis: Diagnosis and Management. Am Fam Physician. 2020;101:409-418. [PubMed] |
| 31. | Mierzchała-Pasierb M, Lipińska-Gediga M. Sepsis diagnosis and monitoring - procalcitonin as standard, but what next? Anaesthesiol Intensive Ther. 2019;51:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Rousan TA, Aldoss IT, Cowley BD Jr, Curtis BR, Bougie DW, Aster RH, George JN. Recurrent acute thrombocytopenia in the hospitalized patient: sepsis, DIC, HIT, or antibiotic-induced thrombocytopenia. Am J Hematol. 2010;85:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Farhan A, Sufyan A, Tahir MA, Ahmad A, Manzoor S. Serum Lactate as a predictor of in-hospital mortality in patients with sepsis. Ann Clin Anal Med. 2022;13:645-648. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, Tan JS; Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg. 2006;117:212S-238S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 35. | Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4:560-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1534] [Cited by in RCA: 1428] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 36. | Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1711] [Cited by in RCA: 1813] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 37. | Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, Hinchliffe RJ. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012;55:2906-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Senneville É, Albalawi Z, van Asten SA, Abbas ZG, Allison G, Aragón-Sánchez J, Embil JM, Lavery LA, Alhasan M, Oz O, Uçkay I, Urbančič-Rovan V, Xu ZR, Peters EJG. IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-related Foot Infections (IWGDF/IDSA 2023). Clin Infect Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 39. | Dugar S, Choudhary C, Duggal A. Sepsis and septic shock: Guideline-based management. Cleve Clin J Med. 2020;87:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 40. | Russo MP, Grande-Ratti MF, Burgos MA, Molaro AA, Bonella MB. Prevalence of diabetes, epidemiological characteristics and vascular complications. Arch Cardiol Mex. 2023;93:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 41. | Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 507] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 42. | Pool R, Gomez H, Kellum JA. Mechanisms of Organ Dysfunction in Sepsis. Crit Care Clin. 2018;34:63-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 43. | Carro GV, Saurral R, Witman EL, Braver JD, David R, Alterini PA, Illuminati G, Carrió LM, Torres JC. [Diabetic foot attack. Pathophysiological description, clinical presentation, treatment and outcomes]. Medicina (B Aires). 2020;80:523-530. [PubMed] |
| 44. | Drampalos E, Mohammad HR, Pillai A. Augmented debridement for implant related chronic osteomyelitis with an absorbable, gentamycin loaded calcium sulfate/hydroxyapatite biocomposite. J Orthop. 2020;17:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Niazi NS, Drampalos E, Morrissey N, Jahangir N, Wee A, Pillai A. Adjuvant antibiotic loaded bio composite in the management of diabetic foot osteomyelitis - A multicentre study. Foot (Edinb). 2019;39:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Roberts RHR, Davies-Jones GR, Brock J, Satheesh V, Robertson GA. Surgical management of the diabetic foot: The current evidence. World J Orthop. 2024;15:404-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Ahluwalia R, Vainieri E, Tam J, Sait S, Sinha A, Manu CA, Reichert I, Kavarthapu V, Edmonds M, Vas P. Surgical Diabetic Foot Debridement: Improving Training and Practice Utilizing the Traffic Light Principle. Int J Low Extrem Wounds. 2019;18:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Kirsner RS, Sabolinski ML, Parsons NB, Skornicki M, Marston WA. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. 2015;23:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1152] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 50. | Liu S, He CZ, Cai YT, Xing QP, Guo YZ, Chen ZL, Su JL, Yang LP. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:533-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015:CD004123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 52. | Tejedor S, Wågberg M, Correia C, Åvall K, Hölttä M, Hultin L, Lerche M, Davies N, Bergenhem N, Snijder A, Marlow T, Dönnes P, Fritsche-Danielson R, Synnergren J, Jennbacken K, Hansson K. The Combination of Vascular Endothelial Growth Factor A (VEGF-A) and Fibroblast Growth Factor 1 (FGF1) Modified mRNA Improves Wound Healing in Diabetic Mice: An Ex Vivo and In Vivo Investigation. Cells. 2024;13:414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 53. | Jeyaraman M, Nagarajan S, Maffulli N, R P P, Jeyaraman N, N A, Khanna M, Yadav S, Gupta A. Stem Cell Therapy in Critical Limb Ischemia. Cureus. 2023;15:e41772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 54. | Driver VR, Hanft J, Fylling CP, Beriou JM; Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68-70, 72, 74 passim. [PubMed] |
| 55. | Huang M, Cai S, Su J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 481] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 56. | Caraballo C, Jaimes F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J Biol Med. 2019;92:629-640. [PubMed] |