Published online Mar 9, 2025. doi: 10.5492/wjccm.v14.i1.97443
Revised: October 6, 2024
Accepted: November 20, 2024
Published online: March 9, 2025
Processing time: 195 Days and 2.1 Hours
Cardiac arrest caused by acute pulmonary embolism (PE) is the most serious clinical circumstance, necessitating rapid identification, immediate cardiopulmonary resuscitation (CPR), and systemic thrombolytic therapy. Extracorporeal CPR (ECPR) is typically employed as a rescue therapy for selected patients when conventional CPR is failing in settings where it can be implemented.
We present a case of a 69-year-old male who experienced a prolonged cardiac arrest in an ambulance with pulseless electrical activity. Upon arrival at the emergency department with ongoing manual chest compressions, bedside point-of-care ultrasound revealed an enlarged right ventricle without contractility. Acute PE was suspected as the cause of cardiac arrest, and intravenous throm
This case illustrates the potential of combining systemic thrombolysis with ECPR for refractory cardiac arrest caused by acute PE, but it also highlights the in
Core Tip: This case study evaluates the feasibility of combining systemic thrombolysis with extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest due to acute pulmonary embolism. Despite hemodynamic and contractility improvements post-treatment with tissue plasminogen activator, the patient succumbed to intracerebral hemorrhagic complications. It underscores the potential of this combined approach but highlights the increased risk of severe bleeding, including fatal intracranial hemorrhage.
- Citation: Yuan GX, Zhang ZP, Zhou J. Thrombolysis and extracorporeal cardiopulmonary resuscitation for cardiac arrest due to pulmonary embolism: A case report. World J Crit Care Med 2025; 14(1): 97443
- URL: https://www.wjgnet.com/2220-3141/full/v14/i1/97443.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i1.97443
Acute pulmonary embolism (PE) is a life-threatening disease, and cardiac arrest or circulatory collapse caused by acute PE is among the most serious clinical situations, challenging physicians with the need for rapid decision-making in an emergency[1]. Clinical practice guidelines recommend systemic thrombolysis with anticoagulation for patients with high-risk or massive PE and hemodynamic compromise[2,3], In cases where PE leads to refractory cardiac arrest, extracorporeal cardiopulmonary resuscitation (ECPR) using veno-arterial extracorporeal membrane oxygenation (ECMO) is typically employed as a rescue therapy when conventional CPR measures are unsuccessful in achieving a sustained return of spontaneous circulation[3,4]. However, systemic thrombolysis during CPR, especially during ECPR, is associated with an increased risk of bleeding, including intracranial hemorrhage. Here, we report a case of acute PE complicated by cardiac arrest who had intracerebral hemorrhagic complications after administration of tPA during mechanical chest compression and ECPR.
Dyspnea and syncope accompanied by sudden cardiac arrest due to pulseless electrical activity.
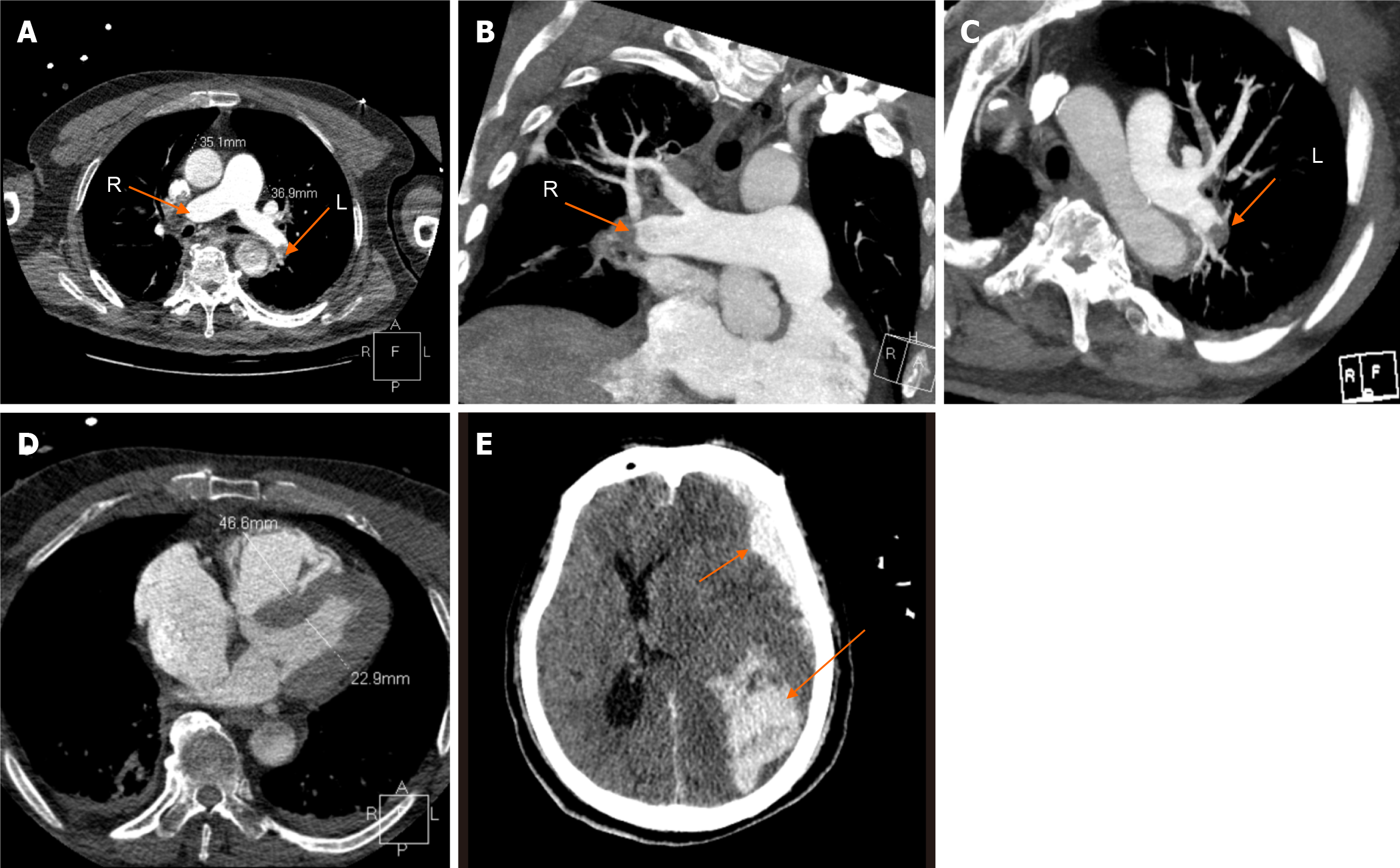
A 69-year-old man experienced a sudden cardiac arrest due to pulseless electrical activity while being transported by ambulance. The emergency medical staff performed continuous manual CPR. Upon arrival at our emergency department five minutes later, the patient received emergency tracheal intubation, repeated intravenous injections of epinephrine, and mechanical chest compressions using the LUCAS 2 device (Physio-Control Inc., Lund, Sweden). A bedside point-of-care ultrasound (POCUS) revealed an enlarged right ventricle (RV) without contractility. Given the severe dilation of the RV, acute PE was suspected as the cause of cardiac arrest. Systemic thrombolysis with 50 mg of tissue plasminogen activator (tPA), consisting of a 10 mg bolus followed by a 40 mg infusion over 2 hours, was administered as rescue therapy during mechanical chest compressions. Concurrently, the extracorporeal membrane team was called to initiate ECPR. Before the ECMO team established cannulation, manual or mechanical chest compressions had been ongoing for approximately 31 minutes; however, a sustained return of spontaneous circulation (ROSC) was not achieved until 8 minutes after the initiation of Veno-arterial ECMO. It took about 39 minutes to attain ROSC following the initial collapse. The patient experienced a sustained ROSC and stabilized on ECMO support along with vasoactive drugs infusions (epinephrine at 0.2 µg/kg/min, norepinephrine at 0.5 µg/kg/min, and dobutamine at 5 µg/kg/min). The patient's clinical events (Table 1). An urgent computed tomography (CT) with contrast media demonstrated thrombus in both pulmonary arteries, extending to the distal right interlobar artery and to the left interlobar pulmonary artery (Figure 1A-C). CT angiography revealed RV dilation with a diameter of 46.6 mm (Figure 1D).
| Time | Event description |
| 0 minute | Cardiac arrest: Patient experienced sudden cardiac arrest due to pulseless electrical activity while being transported by ambulance |
| 5 minutes | Point-of-care ultrasound findings: Point-of-care ultrasound at the emergency department revealed an enlarged right ventricle with no contractility |
| 15 minutes | Thrombolysis initiated: Pulmonary embolism was suspected, and systemic thrombolysis with tissue plasminogen activator was administered as rescue therapy during mechanical chest compressions |
| 31 minutes | Extracorporeal membrane oxygenation implementation: Extracorporeal cardiopulmonary resuscitation with veno-arterial extracorporeal membrane oxygenation was initiated |
| 39 minutes | Return of spontaneous circulation achieved: Return of spontaneous circulation was achieved after 8 minutes of extracorporeal membrane oxygenation support |
| Day 1 | Computed tomography scan results: Computed tomography scan demonstrated thrombus in both pulmonary arteries |
| Day 2 | Hemodynamic improvement: The hemodynamic situation improved, and the dilated right ventricle and myocardial contractility were significantly improved |
| Day 3 | Decannulation and complications: The patient was decannulated. Unfortunately, he experienced intracerebral hemorrhagic complications |
| Day 7 | Patient outcome: The patient had a severe hypoxic brain injury and died in the hospital |
Hypertension.
On admission, the patient was in a coma with normal pupil diameter on both sides and dull light reflexes. He was transferred to the intensive care unit, where he was cooled to a target temperature of 34 °C using a surface cooling pad device for cerebral protection.
POCUS findings: POCUS at the emergency department revealed an enlarged RV with no contractility.
CT scan results: CT scan demonstrated thrombus in both pulmonary arteries. Following admission, the patient's hemodynamic situation significantly improved, allowing for the weaning of vasoactive drugs such as epinephrine and norepinephrine. Bedside POCUS indicated significant improvement in the dilated RV and myocardial contractility. The ECMO flow was reduced from an initial 3.5 L/min to 2 L/min, and the ECMO was removed on the third day after admission. Despite successful decannulation, the patient remained in a deep coma with unequal pupil sizes and a complete absence of light reflex. Another CT revealed left frontal lobe, parietal lobe, and subarachnoid hemorrhage (Figure 1E).
Acute PE.
Thrombolysis initiated: PE was suspected, and systemic thrombolysis with tPA was administered as rescue therapy during mechanical chest compressions.
ECMO implementation: ECPR with veno-arterial ECMO was initiated.
The patient ultimately did not survive. He experienced intracerebral hemorrhagic complications and severe hypoxic brain injury, leading to his death a few days later in the hospital.
The present case report illustrates the critical role of simultaneous thrombolytic therapy alongside mechanical chest compression and ECPR in a patient with acute PE leading to refractory cardiac arrest. While this integrated approach has demonstrated efficacy in managing such critical conditions, it also poses a significant risk of bleeding complications, including intracranial hemorrhage, especially when systemic thrombolysis is combined with veno-arterial ECMO[4].
In the context of acute, massive PE, obstruction of pulmonary blood flow leads to increased right ventricular afterload, potentially resulting in right ventricular strain, tricuspid regurgitation, right ventricular failure, and hemodynamic instability, which can be fatal. Acute PE is associated with high mortality and the majority of deaths usually occur within one hour of presentation[5,6]. The potential for sudden and fatal deterioration in patients with acute PE highlights the need for a prompt diagnosis and appropriate intervention[7]. Pulseless electrical activity as the initial cardiac arrest rhythm is the most common electrocardiogram caused by obstructive PE. The most useful initial test in this situation is bedside echocardiography, which will yield evidence of acute right ventricular dilation and dysfunction if acute PE resulting in hemodynamic decompensation[6]. In a highly unstable patient, echocardiographic evidence of right ventricular dysfunction is sufficient to prompt immediate reperfusion without further testing. This aggressive mana
The decision-making process for treatment in massive PE is complex and must consider various options, including systemic or catheter-directed thrombolysis, catheter clot-retrieval techniques, surgical embolectomy, and hemodynamic support[10]. Current guidelines recommend thrombolytic therapy with anticoagulation as the first-line treatment[2,3]. In cases of circulatory collapse or cardiac arrest, the choice of treatment strategy must be made promptly to optimize the chance of a favorable outcome. Once PE is the suspected cause of cardiac arrest, thrombolytic therapy should be considered, and when thrombolytic drugs have been administered, consider continuing CPR attempts for at least 60-90 minutes before termination of resuscitation attempts[3].
Veno-arterial ECMO provides cardiopulmonary support, alleviating right ventricular strain and serving as a salvage therapy for PE, either allowing clot resolution with anticoagulation alone or as a bridge to surgical or catheter-directed therapy[11]. Early and aggressive use of veno-arterial ECMO has shown promise in managing massive PE, with several institutions reporting positive outcomes[12,13]. The timing of ECPR initiation is crucial, with shorter intervals between cardiac arrest and ECPR initiation associated with improved survival[14,15]. Guidelines recommend ECPR should be considered after 10–20 minutes of failed conventional resuscitation efforts[4].
While ECMO is a vital life-support measure, it does not remove the out flow obstruction caused by embolized thrombi[16]. Therefore, further treatment, either pharmacological or invasive, is necessary to eliminate the thromboembolic burden[17,18]. Normally, ECMO usually may be considered in combination with surgical embolectomy or catheter-directed treatment in patients with PE and refractory circulatory collapse or cardiac arrest[19]. The effect and safety of simultaneous systemic thrombolysis during ECMO support remained controversial. Some studies have reported success with this approach in patients with cardiac arrest due to acute PE[19,20]. Lin et al[20] retrospectively reported their experience of simultaneous thrombolysis-based therapeutic strategy while under ECMO support in 13 patients with acute massive PE, 8 of whom underwent conventional CPR before or during ECMO establishment. All 13 patients were successfully weaned off from ECMO and 11 survived to discharged. Therefore, they concluded that for patients with cardiac arrest due to acute PE, who underwent conventional CPR and stabilized with ECMO, thrombolytic therapy could still be applied in selected cases.
As a potentially lifesaving maneuver, systemic thrombolysis during chest compressions or ECPR seems to be effective. However, systemic thrombolysis in the context of cardiac arrest caused by massive PE may increase the risk of lethal bleeding complications[21], especially when administered simultaneously with veno-arterial ECMO. Bleeding is the most common complication associated with veno-arterial ECMO, and hemorrhage is also prevalent in thrombolytic therapy. Consequently, both thrombolysis and veno-arterial ECMO procedures may exacerbate hemorrhagic complications. Indeed, combining ECMO with thrombolysis nearly triples the risk of hemorrhage compared to thrombolysis alone[22].
Recent studies have suggested that catheter-directed thrombolysis and surgical embolectomy, when combined with ECMO, can offer effective treatment strategies with potentially fewer bleeding complications compared to systemic thrombolysis[23,24]. In our case, the decision to employ systemic thrombolysis and ECPR was driven by the urgency of the patient's critical state and the need for immediate reperfusion. However, it is also acknowledged that alternative strategies such as catheter-directed thrombolysis or surgical embolectomy may be preferable in situations where the risk of bleeding complications is high. These methods could potentially offer similar efficacy in treating the embolism while minimizing the risk of bleeding, especially intracranial hemorrhage.
A significant limitation of our approach is the inherent risk of bleeding complications when combining ECMO with systemic thrombolysis. The case report briefly mentions this risk but does not provide an in-depth analysis of risk mitigation strategies. Insights on managing anticoagulation and monitoring during ECMO could help prevent serious complications such as intracerebral hemorrhage. Furthermore, while the potential benefits of alternative strategies like catheter-directed thrombolysis and surgical embolectomy are discussed, but lack comparative data to our method. Larger studies comparing different treatments are needed to clarify their risks and benefits.
The integration of systemic thrombolysis with ECPR for managing cardiac arrest due to acute PE presents a double-edged sword. While it may improve the chances of survival, it significantly increases the risk of major bleeding complications, including intracranial hemorrhage. A careful assessment of each patient's condition, the potential benefits and risks, and the available resources is essential in making the best treatment decision. Future research should focus on refining these treatment strategies to maximize patient outcomes while minimizing complications.
| 1. | Camen S, Söffker G, Kluge S, Zengin E. Massive pulmonary embolism with intra-hospital cardiac arrest and full recovery of right ventricular function after veno-arterial extracorporeal membrane oxygenation therapy: a case report. Eur Heart J Case Rep. 2020;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Áinle FN, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 813] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 3. | Lott C, Truhlář A, Alfonzo A, Barelli A, González-Salvado V, Hinkelbein J, Nolan JP, Paal P, Perkins GD, Thies KC, Yeung J, Zideman DA, Soar J; ERC Special Circumstances Writing Group Collaborators. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation. 2021;161:152-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 417] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 4. | Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, Bernard S, Finney SJ, Grunau B, Youngquist ST, McKellar SH, Shinar Z, Bartos JA, Becker LB, Yannopoulos D, Bˇelohlávek J, Lamhaut L, Pellegrino V. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO J. 2021;67:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 5. | Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 516] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Amirghofran AA, Emami Nia A, Javan R. Surgical embolectomy in acute massive pulmonary embolism. Asian Cardiovasc Thorac Ann. 2007;15:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Giraud R, Banfi C, Siegenthaler N, Bendjelid K. Massive pulmonary embolism leading to cardiac arrest: one pathology, two different ECMO modes to assist patients. J Clin Monit Comput. 2016;30:933-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Koć M, Kostrubiec M, Elikowski W, Meneveau N, Lankeit M, Grifoni S, Kuch-Wocial A, Petris A, Zaborska B, Stefanović BS, Hugues T, Torbicki A, Konstantinides S, Pruszczyk P; RiHTER Investigators. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J. 2016;47:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Ferrari E, Benhamou M, Berthier F, Baudouy M. Mobile thrombi of the right heart in pulmonary embolism: delayed disappearance after thrombolytic treatment. Chest. 2005;127:1051-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Rouleau SG, Casey SD, Kabrhel C, Vinson DR, Long B. Management of high-risk pulmonary embolism in the emergency department: A narrative review. Am J Emerg Med. 2024;79:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Kabrhel C, Rosovsky R, Channick R, Jaff MR, Weinberg I, Sundt T, Dudzinski DM, Rodriguez-Lopez J, Parry BA, Harshbarger S, Chang Y, Rosenfield K. A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience With a Novel Approach to Delivery of Care to Patients With Submassive and Massive Pulmonary Embolism. Chest. 2016;150:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Corsi F, Lebreton G, Bréchot N, Hekimian G, Nieszkowska A, Trouillet JL, Luyt CE, Leprince P, Chastre J, Combes A, Schmidt M. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 13. | Pasrija C, Kon Z. A protocolized approach to veno-arterial extracorporeal membrane oxygenation for massive pulmonary embolism. Resuscitation. 2018;126:e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Debaty G, Babaz V, Durand M, Gaide-Chevronnay L, Fournel E, Blancher M, Bouvaist H, Chavanon O, Maignan M, Bouzat P, Albaladejo P, Labarère J. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 15. | Wengenmayer T, Rombach S, Ramshorn F, Biever P, Bode C, Duerschmied D, Staudacher DL. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 16. | Frenk NE, Tabori NE, Molina EJ, Sabri SS. Large-bore Suction Thrombectomy Therapy for Massive Pulmonary Embolism on Extracorporeal Membrane Oxygenation. J Vasc Interv Radiol. 2020;31:1932-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Yamamoto T. Management of patients with high-risk pulmonary embolism: a narrative review. J Intensive Care. 2018;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Elbadawi A, Mentias A, Elgendy IY, Mohamed AH, Syed MH, Ogunbayo GO, Olorunfemi O, Gosev I, Prasad S, Cameron SJ. National trends and outcomes for extra-corporeal membrane oxygenation use in high-risk pulmonary embolism. Vasc Med. 2019;24:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide-Chevronnay L, Aissaoui N, Neuschwander A, Zogheib E, Dupont H, Pili-Floury S, Ecarnot F, Schiele F, Deye N, de Prost N, Favory R, Girard P, Cristinar M, Ferré A, Meyer G, Capellier G, Sanchez O. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J. 2018;39:4196-4204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Lin TW, Tsai MT, Hu YN, Wang YC, Wen JS, Wu HY, Luo CY, Roan JN. Simultaneous Thrombolysis and Extracorporeal Membrane Oxygenation for Acute Massive Pulmonary Emboli. Ann Thorac Surg. 2021;111:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Janata K, Holzer M, Kürkciyan I, Losert H, Riedmüller E, Pikula B, Laggner AN, Laczika K. Major bleeding complications in cardiopulmonary resuscitation: the place of thrombolytic therapy in cardiac arrest due to massive pulmonary embolism. Resuscitation. 2003;57:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 551] [Article Influence: 50.1] [Reference Citation Analysis (1)] |
| 23. | Ismayl M, Ismayl A, Hamadi D, Aboeata A, Goldsweig AM. Catheter-directed thrombolysis versus thrombectomy for submassive and massive pulmonary embolism: A systematic review and meta-analysis. Cardiovasc Revasc Med. 2024;60:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Harvey JJ, Huang S, Uberoi R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst Rev. 2022;8:CD013083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |