INTRODUCTION
Overview of gastrointestinal bleeding
Gastrointestinal (GI) bleeding is a common and potentially life-threatening medical condition that occurs when there is bleeding from any part of the digestive tract, which includes the esophagus, stomach, small intestine, and large intestine. The severity of the bleeding can range from mild to severe, with the latter often requiring immediate medical attention and intervention[1].
Patients with GI bleeding (GIB) may present with a variety of symptoms, including hematemesis (vomiting of blood), melena (black, tarry stools), and hematochezia, depending on the location and severity of the bleeding[2]. When a patient presents with GIB, prompt assessment and resuscitation are vital. The initial management includes stabilizing the patient, assessing the severity of the bleeding, and determining the source of the bleeding[3-6].
Overall, the management of GIB requires a multidisciplinary approach, involving prompt assessment, resuscitation, risk stratification, and the timely application of appropriate therapeutic interventions.
Epidemiology and incidence in intensive care unit
GIB is a serious clinical event that poses a significant risk to life, leading to over 400000 hospital admissions annually in the United States. This condition also places a considerable financial strain on healthcare systems[7].
The incidence of upper GI bleed (UGIB) is estimated to be around 67 cases per 100000 individuals, whereas lower GIB (LGIB) occurs at a rate of approximately 36 cases per 100000 individuals. Notably, LGIB is more prevalent in men than in women, which can be attributed to the higher prevalence of vascular diseases and diverticulosis in the male population[8]. Peptic ulcer disease (PUD) remains the leading cause of acute UGIB, accounting for up to 67% of all cases[9].
The overall incidence of acute UGIB, especially cases related to PUD, showed a marked decline toward the end of the twentieth century. In recent decades, however, this trend has plateaued, with the incidence rates stabilizing in the twenty-first century within the United States[10].
Pathophysiology of GIB
GIB is a complex and multifaceted medical condition that arises from various underlying pathologies affecting the digestive tract. The normal stomach mucosa maintains a delicate balance between protective and aggressive factors, and disruption of this equilibrium can lead to the development of GI ulcers and subsequent bleeding. Aggressive factors, such as gastric acid, abnormal motility, pepsin, bile salts, use of alcohol and non-steroidal anti-inflammatory drugs, as well as infection with microorganisms, can overwhelm the protective mechanisms, including mucus secretion, bicarbonate production, gastroprotective prostaglandin synthesis, and normal tissue microcirculation[11]. The severity of macroscopic lesions developed in GIB can be assessed using a semiquantitative scale[12].
The incidence of GIB varies with age, gender, and geographical location, and it is associated with severe complications, including hemorrhages, perforations, GI obstruction, and malignancy. Consequently, this clinical condition represents a significant health problem worldwide due to its high morbidity, mortality, and economic burden. Pharmacists practicing in critical care settings require a comprehensive understanding of the causes and consequences of bleeding, as well as the mechanisms of hemostasis, in order to provide appropriate management and treatment for patients with GIB[11,13].
The hemostatic mechanism plays a crucial role in the pathophysiology of GIB. Platelets, coagulation, and the vessel wall work in intimate cooperation to maintain hemostasis. The unique acidic environment of the stomach can have a significant impact on the various physiological stages of hemostasis, potentially contributing to the development and progression of GIB. Additionally, patients with inherited and acquired bleeding disorders, as well as those undergoing therapeutic anticoagulation, are at an increased risk for severe bleeding events[13].
Stress ulcers are gastric lesions that occur in critically ill patients due to factors like mucosal ischemia, increased gastric acid production, and impaired protective mechanisms, often exacerbated by conditions such as hypotension, mechanical ventilation, or corticosteroid use. Steroids can worsen the risk of stress ulcers by increasing gastric acid secretion, altering electrolyte balance, and suppressing the immune system. Hypotension, resulting from shock, bleeding, or sepsis, disrupts tissue perfusion and oxygen delivery, contributing to organ dysfunction. Shock further compounds this by leading to inadequate tissue oxygenation and cellular injury. Mechanical ventilation, while essential for respiratory support, can alter hemodynamics, increase the risk of lung injury, and contribute to acid-base imbalances, thereby exacerbating shock and increasing the likelihood of developing stress ulcers in critically ill patients[14].
CLASSIFICATION OF GIB
UGIB
UGIB are defined as bleeding along the GI tract from the mouth to the duodenum proximal to the ligament of Treitz[15]. Common causes of UGIB include duodenal ulcer (20%-30%), gastric or duodenal erosions (20%-30%), esophageal varices (15%-20%), gastric ulcer (10%-20%), Mallory-Weiss tears (MWT, 5%-10%), erosive esophagitis (5%-10%), angioma (5%-10%), arteriovenous malformations (< 5%), GI stromal tumors (GIST), and hemobilia[16].
As mentioned above, the most common causes of UGIB are ulcers of the stomach or duodenum, manifestations of PUD, which presents with a lifetime incidence rate between 5% and 10% in Western countries[17]. The two most common causative agents of GI bleeds from PUD are Helicobacter pylori (H. pylori) infection and chronic nonsteroidal anti-inflammatory drug (NSAID) use, with an estimated 90% of patients with duodenal ulcers and over 70% of patients with gastric ulcers testing positive for H. pylori infection. Alcohol and smoking of tobacco products have also both been implicated in increased risk of developing PUD[18,19]. Erosion of the stomach lining falling short of ulceration is another common cause of UGIB, with H. pylori infection and NSAID use [particularly aspirin (ASA) and other nonselective COX-inhibitors] also demonstrated to be the main causative agents[20].
Gastroesophageal varices are another common cause of UGIB, with 80% of variceal bleeding occurring along the esophagus itself, although one study found that 27.3% of fundal varices present with active bleeding compared to 19.6% of esophageal varices[21]. Esophageal varices are commonly a consequence of portal hypertension, the most common cause of which is cirrhosis, with ruptured esophageal varices the most common cause of mortality in patients with cirrhosis[22].
MWT and erosive esophagitis, also known as esophageal erosion, are two other common causes of UGIB originating in the esophagus itself. MWT are longitudinal lacerations in the mucosa of the distal esophagus, are most commonly involved in episodes of vomiting following acute alcohol abuse, and present with hematemesis in 85% of cases. The bleeding is generally petechial and mild with a self-limiting course[23,24]. Erosive esophagitis is caused by breaks in the esophageal mucosa, most commonly as a consequence of gastroesophageal reflux disease[25]. Dieulafoy lesions are rare submucosal vascular malformations that can progress to bleeding along the GI tract, occurring without any ulceration or erosion and most commonly seen along the lesser curvature of the stomach[26].
Gastric hemangiomas are rare benign tumors that can progress to UGIB, although they more commonly cause occult bleeding. Due to the scarcity of these tumors, a definitive diagnostic approach does not exist, and they are often not biopsied in order to avoid progression of further bleeding[27-29].
GIST are rare tumors arising in the mesenchyme of the GI tract that can progress to bleeding of the stomach and present as UGIB. They occur sporadically without any apparent heritability pattern or known environmental or lifestyle risk factors, but KIT oncogene expression has been connected to increased incidence of GIST occurrence[30].
Hemobilia is defined as bleeding from the liver and the biliary tree. Iatrogenic injury after hepatobiliary intervention or surgery is the most common cause of hemobilia, but rare cases have been documented of hemobilia secondary to accidental trauma or a neoplastic, infectious, or inflammatory process[31].
Lower GI bleeds
Lower GI bleeds are classified as bleeding along the GI tract distal to the ligament of Treitz. Common causes of lower GI bleeds include anal fissures, angiodysplasia (vascular ectasia), colonic carcinoma and colonic polyps, diverticular disease, inflammatory bowel disease (IBD) (ulcerative proctitis/colitis & Crohn's disease), and internal hemorrhoids[16].
Diverticular bleeding of the large intestine is a common cause of lower GI hemorrhage, with patients generally presenting with massive but painless rectal hemorrhage. It is the most common cause of acute severe LGIB. They develop when arteries within the diverticulum of the colon erode or tear and bleed directly into the colon, and like many other lower GI bleeds, their exact pathophysiological pathways of development aren’t fully understood. Several causes have been implicated in increasing risk of diverticular bleeding common to other lower GI bleeds, such as advanced age, increased intraluminal pressure, medications such as NSAIDs, and dehydration or diets low in fiber[32,33].
Angiodysplasias, or vascular ectasia, are an abnormally dilated small blood vessel in the mucosal and submucosal layers of the GI tract and are the most common vascular abnormality in the GI tract[34]. The pathophysiological mechanisms causing vascular ectasia progressing to GI bleed are not known, but there are causal relationships shown between development of angiodysplasias leading to lower GI bleeds and incidence of end-stage renal disease, systemic sclerosis, and aortic stenosis[34,35].
Internal hemorrhoids, swollen veins in the rectum or anus, are a common cause of LGIB, often presenting as blood in stool or on toilet paper. Risk factors for hemorrhoids include straining during bowel movements, prolonged toilet sitting, chronic constipation or diarrhea, low fiber diets, aging (especially over 50), pregnancy, and heavy lifting[36,37]. IBD, including Crohn’s disease and ulcerative colitis, also contributes to LGIB, with unclear causes but a suspected combination of genetic and environmental factors. Infectious agents, ischemia, and radiation can also lead to colitis[38-43]. Malignancies like colorectal cancer and polyps, particularly in those over 50 or with certain risk factors (e.g., family history, smoking, obesity), are common causes of LGIB[44,45]. Additionally, anal fissures, which are tears in the anal mucosa often associated with constipation, can cause severe anorectal pain, though other underlying vascular factors may also contribute to their development[46].
INITIAL ASSESSMENT AND DIAGNOSIS
Triaging
Thorough physical exam and history are the most useful tools for evaluation of patients presenting with GIB, and many of the signs of GI bleeds will present to the clinician as massive or frankly apparent. The most common clinical presentations of UGIB are melena (black, tarry stool) and hematemesis, and severe bleed of the upper GI tract can also present as hematochezia (significant amounts of bright red or maroon blood in the stool), although hematochezia is more commonly a sign of lower GI bleed due to the relative proximity of lower GI bleeds to the rectal canal[47-49]. Lower GI bleeds are the more common cause of hematochezia, and can also often lead to occult bleeding, defined as bleeding that does not present with visible signs of blood loss[50,51]. Occult blood loss is especially difficult to diagnose, underscoring the importance of careful history-taking and purposeful testing modalities for evidence of abnormal bleeding.
The most relevant piece of history taking for GI bleed is previous history of GI bleed. Even in the absence of previous incidents of GI bleed, a full and comprehensive medical history is of utmost importance, with focus on common causes of GI bleed as described above (varices, portal hypertension or cirrhosis, alcohol and tobacco use, chronic NSAID use or abuse, PUD, diverticulitis, hemorrhoids, IBD, H. pylori infection, and known colorectal cancers being the most common and relevant factors to consider). Symptoms associated with bleeding, such as level of pain upon bleeding or bowel movements, difficulty swallowing, eating, or defecating, unintentional weight loss within the same timeframe as the suspected blood loss, emesis or retching, or any change in bowel habits, including frequency, urgency, and consistency, should also be carefully evaluated[49,52]. Patients should also be carefully evaluated for contributory medications with known associations with bleeding or bleeding disorders such as NSAIDs, anticoagulants or antiplatelets, bismuth, iron or vitamin K, or any blood-thinning supplements[53].
In the event of reported blood loss without confirmation of signs of blood in the stool or vomit, or in the case of suspected occult blood loss, the physical exam should include thorough work-up of any signs of dynamic instability associated with hemorrhage. The most critical signs are resting tachycardia and orthostatic hypotension, associated with the loss of less than 15% total blood volume; positional/supine hypotension associated with the loss of approximately 40% total blood volume[54]. Abdominal pain, particularly upon palpation, may raise suspicion for perforation or ischemia and can be helpful in diagnosing the location and cause of a GI bleed[49].
In the case of blood present in the stool or suspected occult blood loss, a physical exam of the anus and rectum are important for the evaluation of any apparent causes of GI bleed. During the rectal exam, the clinician should evaluate for internal or external hemorrhoids, anal fissures, or any visible or palpable anorectal masses. Stool exams to evaluate for color, consistency, and for microscopic smears are also indicated for patients with GI complaints regardless of blood loss or suspected bleeding[50,55].
Diagnostic modalities
As is the case with any suspected cause of blood loss, GI bleeds are indications for blood work following patient presentation to the clinical or emergency setting. The following laboratory tests are indicated as first-line diagnostic tests to assess the potential source and severity of GI bleeds:
Complete blood count, hemoglobin, and hematocrit levels are indicated to evaluate the presence of any anemias or hematological disorders that could be contributing generally to a state of blood loss. International normalized ratio (INR), prothrombin time, and activated partial thromboplastin time are all indicated in order to assess the severity and potential chronicity of the patient’s known or suspected blood loss. Lactate and liver function tests, used respectively to indicate hypoxia and liver failure or blockage, can be used to both assess the severity of the GI bleed and may indicate location in the absence of diagnostic imaging[49,56].
The array of imaging for the GI tract is both varied and purposeful, as complete imaging of the entire GI tract is made difficult by its length, various tissue types, variable pH, and the need for full sedation for many imaging modalities.
The most commonly utilized imaging techniques are endoscopy, either of the upper GI tract or lower GI tract; endoscopy of the lower GI tract is more commonly referred to as colonoscopy due to the structures in the lower intestine that are best visualized with this modality. Both techniques generally require full sedation. Upper GI endoscopy, or esophagogastroduodenoscopy (EGD), allows for visualization of the upper GI tract from the mouth to the proximal duodenum. This imaging modality can also be diagnostic, paired with localized treatment of injection of medication, thermal or electrocautery to coagulate visible bleeding sites, or application of clips to establish hemostasis[57-60]. Lower GI colonoscopy allows for many of the same benefits of visualizations and therapeutic techniques (coagulation, injection, and application of hemostatic clips) to the lower structures of the GI tract, namely the large intestine and terminal ileum[59,61].
Push enteroscopy (PE) and deep small bowel enteroscopy are two additional modalities beyond traditional EGD and colonoscopy that are commonly used to visualize structures within the small intestine that are not typically seen on EGD or colonoscopy, due to the twisting nature of the GI tract. These techniques are able to use one or more balloons to inflate and stabilize the intestinal tract in order to better visualize the distal duodenum and jejunum, and can be indicated if traditional EGD or colonoscopy are unable to identify the suspected source of a GI bleed[62,63].
Endoscopic ultrasound (EUS) is a procedure often used to visualize structures along and adjacent to the GI tract, and is commonly used to visualize the liver, gallbladder, and pancreas. EUS can be performed with or without contrast for visualization purposes and requires a specialized technician or trained physician to assist or operate and is increasing in use as the modality of choice for non-variceal UGIB diagnostic imaging[64,65].
Standard angiography allows for identification of a bleeding vessel and potential treatment via embolization or intra-arterial vasopressin, both of which can be administered or performed during the angiography itself. This imaging modality requires the active bleeding to be present during imaging at a rate of 0.5 to 1.0 mL/minute to visualize the site, and for this reason is often less effective for pediatric patients[66]. Computed tomography (CT) angiography is a less invasive form of angiography that can allow for similar imaging and diagnostic information, but they are generally considered less precise than standard angiography, and should the bleeding source be identified, a separate procedure would need to be undertaken to perform an angioplasty, whereas angioplasty can be performed in real time during most standard angiographies. Tagged red blood cell (RBC) scans can detect bleeding occurring at a rate of 0.1 to 0.5 mL/minute using technetium-99m, a tag that can be seen on nuclear scintigraphy. These scans can only detect active bleeding and are often used for pediatric patients due to their lower volume of blood and bleeding. They are also used in conjunction with other traditional angiographic studies and can be paired with active surgical interventions to establish GI hemostasis[67,68].
A Meckel scan is an imaging modality used specifically to diagnose Meckel diverticulum, most often performed on pediatric patients. The Meckel scan is a nuclear scan to look for ectopic gastric mucosa suggestive of or definitively diagnostic for Meckel’s diverticulum, the most common congenital intestinal abnormality, and the use of technetium-99m pertechnetate is considered to be the most accurate modality[69].
Risk stratification
There are several risk stratification systems for patients presenting with GI bleed, the most commonly used among them the Glasgow-Blatchford, the AIMS65, and the Rockall scores for UGIB and the Oakland score for LGIB.
The Glasgow-Blatchford score is currently recognized as the best risk assessment modality for evaluation of patients presenting to the emergency department with UGIB[70]. Separate values are provided to different levels of instability or evidence of blood loss in the categories of blood urea, hemoglobin, systolic blood pressure, heart rate, melena, syncope, hepatic disease, and heart failure. A score of 0 or 1 is considered to present a patient at no risk of immediate morbidity or mortality who is indicated to be transferred to outpatient care, although there is debate as to whether that threshold can be further raised to a score of 2[71].
The Rockall score and the AIMS65 score are both used in the intensive care unit (ICU) setting to assess for the risk of mortality in UGIB, while the Oakland score is similarly used to assess for mortality risk for LGIB. The Rockall assessment has two separate scores, one of which is calculated before endoscopy and identifies pre-endoscopy mortality, and the second of which is calculated post-endoscopy and calculates overall mortality and risk of recurrence of bleeding. The AIMS65 score takes a similar approach to evaluating risk of mortality, assessing age, serum albumin level, systolic blood pressure, prothrombin time (INR), and mental status; the use of any of these scores over the other is a matter of debate among clinicians and researchers who argue for their efficacy and ease of use[72,73]. The Oakland score is a risk calculator that attempts to help calculate the probability of a safe discharge in lower GI bleeds. The categories for assessment are set as biological sex, age, history of previous LGIB admission, presence of findings on digital rectal exam, heart rate, systolic blood pressure, and hemoglobin levels. Those with an Oakland score below 8 are generally considered safe for discharge to the outpatient setting[74].
ACUTE MANAGEMENT OF GI BLEED
Many patients are hemodynamically stable at presentation, but for those with major bleeding, early resuscitation is essential. A minimum of 2 Large-bore (at least 18-gauge) peripheral access catheters should be placed, and intravenous fluids should be administered to maintain adequate blood pressure and hemodynamic stability. Hypotension is associated with increased mortality[75]. Early intensive resuscitation of patients with UGIB significantly decreases mortality[76]. The recommended threshold for initiating blood transfusion in a patient presenting with acute UGIB is hemoglobin < 7 g/dL. A large meta-analysis showed better outcomes with restricted blood transfusion protocols[77]. Patients with severe bleeding and hemodynamic compromise, who were generally excluded from the trials, should have a higher threshold for transfusion, as their hemoglobin will equilibrate to much lower levels as their intravascular volume is repleted with fluid. In patients with existing cardiovascular disease or experiencing acute coronary syndrome, initiating transfusion when hemoglobin falls below 8 g/dL is advised. If patients are unable to protect their airway or have ongoing severe hematemesis, elective endotracheal intubation is advised. The data about elective intubation is controversial. Elective endotracheal intubation in patients with ongoing hematemesis or altered respiratory or mental status may facilitate endoscopy and decrease the risk of aspiration, although it is associated with worse outcomes. Other data show significantly more episodes of pneumonia occurred with prophylactic intubation, and aspiration and mortality, although not statistically significant[78]. The routine use of nasogastric tube placement in patients with suspected acute UGIB is not recommended, as studies have failed to demonstrate a benefit with regard to clinical outcomes. Some studies show the use of prokinetics like erythromycin, although it did not show better clinical outcomes, did show it helps in better visualization of stomach contents prior to endoscopy[79-81]. Studies also show less requirement for a repeat procedure if prokinetics are used[82]. The use of proton pump inhibitor (PPI) is a very common practice and is considered superior to H2 blockers. Although no strict guidelines or data are available for pre-endoscopy, a high-dose PPI, preferably in intravenous form, is used every 12 hours until the endoscopy is performed[83]. There is data showing that intermittent use of PPI is non-inferior to continuous infusion and is a safer and economically advantageous alternative[84]. Data on new PPIs are limited, but a study on Potassium-competitive acid blockers (P-CABs) indicates that P-CAB is noninferior to other treatments in healing artificial ulcers and PUD, with comparable rates of treatment-emergent adverse events. However, it may offer an advantage in preventing delayed bleeding and drug-induced ulcers, suggesting the need for further standardized studies to draw more definitive conclusions[85]. Octreotide has been well studied and is found to be a better option for patients with acute variceal bleed in the control of esophageal variceal bleeding. Recent studies show that the efficacy of terlipressin was not inferior to octreotide as an adjuvant therapy for the control of esophageal variceal bleeding and in-hospital survival[86,87]. No strong data is available to support the use of octreotide in non-variceal bleeding[88]. Antibiotic prophylaxis with ceftriaxone 1 g can prevent infection, reduce bacterial infections, reduce all-cause mortality, decrease bacterial infection mortality, reduce rebleeding events, and shorten hospitalization length, as well as decrease the amount of blood transfused for patients with acute GEVB[89,90]. Massive transfusion protocol (MTP), i.e., rapid allogeneic blood-product-based transfusions at a fixed ratio approximating those found in whole blood, should be initiated. The main purpose of administering RBCs, fresh frozen plasma (FFP), and platelets during MTP is to maintain physiological levels and prevent a deficit of blood constituents. The main goals of hemostatic management are to treat hyperfibrinolysis early and to restore clot formation and strength, which depends on the interaction of several factors, including the fibrin network, activated platelets, and activated FXIII. The ASGE recommends a target platelet count of > 20000/μL for diagnostic EGD and > 50000/μL for biopsies in their guideline on adverse outcomes in upper endoscopy. This recommendation is also not specific to patients presenting with bleeding[91]. Generally, a platelet count of > 50000 is considered safe for endoscopy. Platelet transfusion should not be given if ITP is suspected. Fibrinogen, also known as coagulation factor I, is a blood plasma protein that plays a crucial role in the coagulation process and is also the most abundant of the coagulation factors. Fibrinogen concentration of < 1.5-2 g/L is associated with an increased bleeding risk and needs to be addressed first in the hemostatic treatment. Moreover, fibrinogen levels < 1 g/L are a strong independent risk factor for death after injury in patients requiring a massive transfusion[92]. Cryoprecipitate should be considered in patients with low fibrinogen levels (< 1.5-2 g/L).
Coagulation factors can be substituted by administering FFP, cryoprecipitate, or specific factor concentrates, such as FC, PCC, or FXIII concentrate. The infusion of large FFP volumes exposes patients to the risk of transfusion-associated circulatory overload. A study by Khan et al[93] reported that patients receiving mixed transfusion packages of FFP with RBCs with late or no fibrinogen supplementation did not improve in either lactate levels, a prognostic marker of tissue hypoperfusion and hemorrhagic shock, nor any ROTEM parameters characterizing clot strength and clotting time. More concentrated agents such as cryoprecipitate or CFCs (i.e., FC) appear to be superior to FFP as first-line treatment[93-95]. The use of anticoagulant reversal agents should be reserved for patients presenting with a life-threatening GI hemorrhage with hemodynamically unstable patients on vasopressors, a drop in Hgb of ≥ 5 g/dL, blood transfusion of ≥ 5 units of PRBCs, or patients at risk of death.
The use of anticoagulant reversal agents should be reserved for patients presenting with life-threatening GI hemorrhage, particularly those who are hemodynamically unstable, on vasopressors, have a drop in hemoglobin of ≥ 5 g/dL, or require blood transfusions of ≥ 5 units of packed RBCs (PRBCs). In such cases, where there is a risk of death, the decision to use reversal agents is critical. However, it is important to recognize that high-quality evidence from randomized trials supporting these strategies is lacking[96].
Warfarin reversal: For patients on warfarin, studies argue against the use of FFP or vitamin K. Vitamin K, despite being a conventional treatment, does not rapidly achieve hemostasis in patients with acute bleeding. Prothrombin complex concentrate (PCC) is suggested as a more effective option compared to FFP. In the setting of clinically significant GIB requiring therapeutic intervention, vitamin K (2-5 mg orally or intravenously) can reverse the anticoagulant effect (to INR ≤ 1.3) within 24-48 hours[97].
Direct oral anticoagulants: The CHEST 2018 guidelines state that most Direct oral anticoagulant (DOAC)-associated bleeds can be managed with temporary discontinuation of the DOAC and supportive measures to enhance renal excretion. Reversal agents should only be used in severe or life-threatening bleeding, with four-factor PCC listed as a second-line treatment[98].
Dabigatran: For reversal of dabigatran, the American College of Gastroenterology (ACG) recommends using idarucizumab in life-threatening GI bleeds if the medication was taken within the last 24 hours[99,100]. Routine use of idarucizumab for non-life-threatening GIB, or in patients who took dabigatran more than 24 hours prior, is not strongly recommended due to the low quality of evidence and high cost[101].
Eliquis and Rivaroxaban: Clinical trials have shown that andexanet alfa can reduce apixaban activity by 94% and rivaroxaban activity by 92%, restoring thrombin generation in 100% of patients within 2-5 minutes[102]. However, the ACG does not recommend routine use of andexanet alfa in patients with GIB. It should only be considered in life-threatening GIB in hospitalized patients who have taken apixaban or rivaroxaban within the past 24 hours, as the supporting trials lacked control groups.
Antiplatelet interruption: In patients with GIB who are on ASA for secondary cardiovascular prevention, temporary interruption of ASA is unlikely to have a significant impact on the initial clinical course due to the persistent antiplatelet effect of ASA for the first 1-2 days after presentation. The ACG suggests resuming ASA as soon as hemostasis is confirmed endoscopically. If the patient is taking dual antiplatelet therapy, a temporary interruption of the P2Y12 receptor inhibitor is recommended while continuing ASA. Meta-analyses suggest that monotherapy with a P2Y12 inhibitor may be sufficient if acute coronary syndrome or stent placement occurred more than 1-3 months prior[103].
MANAGEMENT OF UGIB
Variceal bleeding from liver cirrhosis
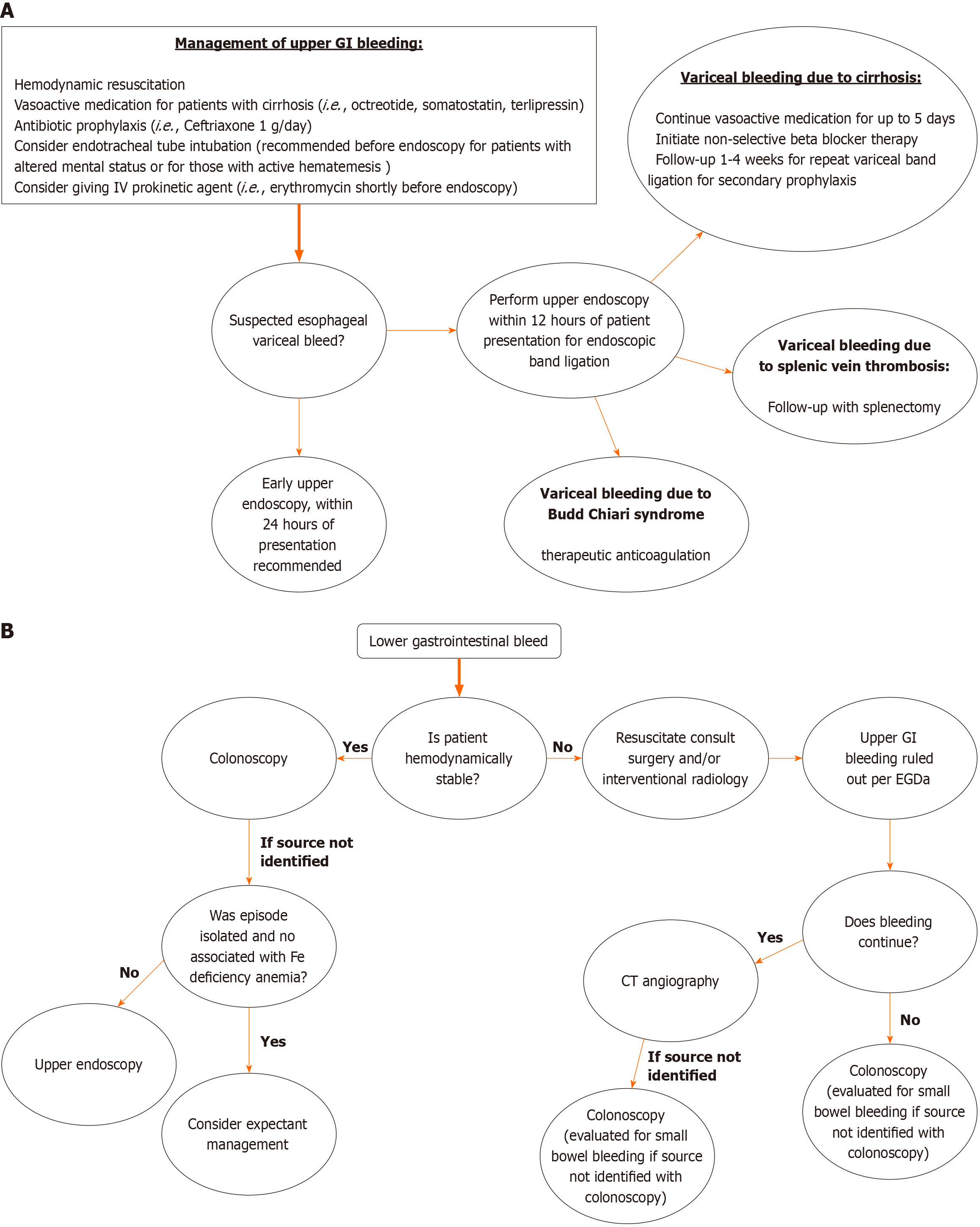
Liver cirrhosis is the most common cause of variceal bleeding. Esophageal and gastric varices develop due to increased portal pressure, and these varices can rupture, leading to life-threatening bleeding. For patients with acute variceal bleeding the ultimate goal is to perform an upper endoscopy which is the preferred method for diagnosis with current guidelines recommending within 12 hours of presentation. To tolerate the procedure, initial management should focus on optimizing the patient hemodynamically[104,105].
Fluid resuscitation should begin immediately, PRBC transfusion should be considered with target hemoglobin above 7 g/dL, intubation is also recommended before endoscopy for patients with altered mental status or for those with active hematemesis[105]. Antimicrobial prophylaxis and vasoactive therapy (octreotide, somatostatin, terlipressin) should be started immediately on presentation. Also, prokinetic agent erythromycin can be considered shortly before endoscopy to clear the stomach of blood[106,107]. The idea behind the combination of antibiotic prophylaxis and use of a prokinetic agent addresses the most common infection risk in these clinical scenarios. Patients with UGIB commonly vomit posing a serious risk for aspiration pneumonia; the antibiotic of choice is typically ceftriaxone 1 g/day for up to 7 days. The use of prokinetic agents aims at mitigating episodes of vomiting and also helps with clearing the upper GI tract making way for a smoother endoscopic procedure[108,109]. After attaining adequate organ perfusion and oxygenation, appropriate timing for endoscopy should be planned next; endoscopic therapy is the treatment of choice for bleeding esophageal varices[104,107].
There are two commonly used forms of endoscopic therapy for bleeding esophageal varices; endoscopic band ligation (EBL) and injection sclerotherapy. EBL alone is currently the standard therapy; injection sclerotherapy is an older technique associated with more complications than band ligation likely due to deeper tissue injury[106,110]. With EBL patients generally require several sessions to achieve variceal ablation. A recent retrospective study evaluated the efficacy of band ligation alone vs band ligation with combined injection sclerotherapy; 84 patients were evaluated, of which 40 received band ligation alone and 44 received combined treatment with band ligation and injection sclerotherapy. The study showed at 6 months, the rebleeding and recurrence rates were significantly lower in the combined band ligation and injection sclerotherapy group; notably this group also had significantly lower rates of chest pain[108].
Another endoscopic technique currently being applied is endoscopic argon plasma coagulation (APC), a type of monopolar electrosurgery. APC can be used as an adjunct to variceal band ligation or injection sclerotherapy. Some recent studies have demonstrated variceal band ligation combined with APC was superior to simple variceal band ligation alone. A number of randomized controlled trials have demonstrated that this combination can significantly reduce the recurrence and re-bleeding rates as well as death when compared to variceal band ligation alone[107].
In patients with persistent or recurrent esophageal variceal bleeding despite initial pharmacological and endoscopic interventions, urgent rescue transjugular intrahepatic portosystemic shunt (TIPS) should be considered. A recent study showed rescue TIPS placement performed within 8 hours of refractory variceal bleeds improves short term and long term survival[109,111].
Post-endoscopy, after achieving hemostasis for an acute variceal bleed, patients should be scheduled for follow-up for secondary prophylaxis in efforts to eradicate the esophageal varices. The European society of GI endoscopy (ESGE) strongly recommends follow-up esophageal band ligations at 1-4 weeks intervals until variceal eradication. Another strong recommendation by ESGE is the use of non-selective beta blockers for their beneficial effects on portal hypertension (decreased CO, b-1 blockade; decreased splanchnic vasoconstriction, b-2 blockade)[110,111].
Non-variceal bleeding
Non-variceal bleeding is predominantly caused by peptic ulcers but can also result from other rare causes.
Peptic ulcers: Peptic ulcers remain a leading cause of non-variceal UGIB, with H. pylori and chronic NSAID use being the primary etiologies[14]. Patients presenting with overt UGIB should be assessed using a risk stratification tool like the Glasgow-Blatchford score to identify patients who require hospitalization. The first step is to assess the hemodynamic status and initiate resuscitative efforts as needed, including fluids and blood transfusions[19]. Endoscopy should be performed within 24 hours of presentation, and endoscopic therapy is indicated for ulcers with active spurting, oozing, or non bleeding visible vessels[112-114]. Recommended therapies include bipolar electrocoagulation, heater probe, and ethanol injection, with alternatives such as clips, APC, or soft monopolar electrocoagulation. For ulcers with active bleeding, TC-325 hemostatic powder spray and over-the-scope clips are suggested for recurrent bleeding. Importantly, epinephrine injection should not be used alone but must be combined with another endoscopic modality for effective hemostasis. Post-endoscopic therapy involves high-dose PPI administration for three days, followed by a twice-daily PPI regimen for two weeks. If bleeding recurs, repeat endoscopy is suggested, and if endoscopic therapy fails, interventional radiology (IR) intervention with transcatheter arterial embolization is recommended[112,113,115].
If a bleeding ulcer is found to be the culprit lesion, efforts should be taken to prevent the recurrence of bleeding. If the patient is found to have H. pylori, eradication should be a target. If NSAIDs were the likely cause of the bleeding, they should be stopped, and if NSAIDs cannot be stopped, concurrent PPI should be used for GI prophylaxis. For patients on anticoagulation, resumption of therapy should occur once bleeding is controlled, ideally within seven days, considering the patient’s thromboembolic risk[113].
Other rare causes of GIB
MWT, characterized by a mucosal tear in the distal esophagus or proximal stomach, typically presents with hematemesis or coffee ground emesis after non-bloody vomiting. While most cases are self-limited, severe hemorrhage can occur, requiring urgent assessment. It accounts for about 5%-7% of UGIB cases, with many requiring only acid suppressive therapy. However, persistent or significant bleeding may necessitate endoscopic intervention[116,117]. Although epinephrine injection has a high hemostasis rate (93%), it carries a higher rebleeding risk (5.8%-6.6%) compared to other methods, making combined therapy more effective[116,117]. Mechanical hemostasis, such as hemoclips and band ligation are preferred for their near 100% success and low rebleeding rates. Contact thermal therapy should be used cautiously due to the risk of complications, including perforation. The 30-day mortality rate for MWT is around 5.3%, similar to that of peptic ulcer bleeding[117]
Dieulafoy lesions are rare (approximately 1.5%) but significant causes of UGIB, characterized by a large submucosal artery protruding through the mucosa without an ulcer, typically in the proximal stomach. Despite the intermittent nature of the bleeding and the normal appearance of the mucosa, these lesions can cause recurrent or massive hemorrhage[115]. Endoscopic therapy[116,117], which is now the standard approach, includes treatment options like injection therapy, thermal coagulation, and mechanical methods such as hemoclips or band ligation, with the latter two being preferred due to their high success rates (96% and 91%, respectively) and minimal tissue damage[117,118]. In cases of rebleeding, endoscopic tattooing is advised to facilitate easier localization[116]. If endoscopic treatment fails or if bleeding is intractable, selective arterial embolization or surgery may be necessary[117].
Gastric antral vascular ectasia (GAVE), or “watermelon stomach,” involves vascular lesions in the antrum causing occult or overt bleeding. It accounts for about 4% of non-variceal UGIB[119] and is more prevalent in cirrhotic patients, potentially leading to chronic anemia and transfusion dependency in up to 62% of cases[119]. Diagnosis is typically made via EGD, and endoscopic therapy is preferred due to the limited efficacy of pharmacological treatments and the high morbidity of antrectomy[119]. Endoscopic treatments for GAVE include APC, EBL, and radiofrequency ablation (RFA). EBL, which uses elastic bands to mechanically strangulate lesions, has higher success rates compared to APC in terms of eradication, recurrence, and transfusion requirements[120]. RFA, another thermal method, is effective, particularly for APC-refractory cases, offering better hemostasis, fewer sessions, and fewer complications[119](Figure 1A).
Figure 1 Flow chart.
A: Showing management of upper gastrointestinal bleed; B: Showing management of lower gastrointestinal bleed. GI: Gastrointestinal; EGD: Esophagogastroduodenoscopy; CT: Computed tomography.
MANAGEMENT OF LOWER GI BLEED
Diverticular bleed
Diverticular bleeding is the leading cause of LGIB, responsible for 20.8%-41.6% of cases in the Western world. Current management strategies involve resuscitation, diagnostic assessment through direct visualization, CT imaging, endoscopic procedures, angioembolization, and surgery when necessary[1]. Colonoscopy is the preferred method as it not only identifies the source of the bleed but also allows for potential therapeutic interventions. Other diagnostic options include flexible sigmoidoscopy, tagged red blood cell scans, or angiography. When the source of bleeding remains unclear, intraoperative enteroscopy (IOE) has become a useful technique for detecting obscure GI bleeds in select patients[121].
In a large multicentric study published in 2021 by Gobinet-Suguro et al[122], revealed that endoscopic treatment of confirmed chronic diverticular bleeding (CDB) has proven to be the most effective approach in reducing recurrent bleeding in both the short and long term, compared to leaving confirmed or presumed CDB untreated. Physicians should make efforts to identify and treat stigmata of recent hemorrhage in cases of suspected CDB[122].
Additionally, a dual-center retrospective study conducted by Hayasaka et al[123], revealed that clipping is a safe and effective treatment for preventing early recurrent bleeding in confirmed CDB, and it remains a viable therapeutic option[123].
Ischemic colitis
The management of ischemic colitis (IC) is guided by the severity of the condition at presentation and the presence or absence of poor prognostic indicators. Optimal care involves a multidisciplinary team comprising gastroenterologists and surgeons. Early recognition and appropriate diagnostic steps, beginning with CT and followed by lower GI endoscopy, are essential for diagnosis and patient risk stratification. While most cases resolve with conservative treatment, some will require surgery, with a high mortality rate in this group. Understanding the factors that influence surgical outcomes is critical for making key management decisions and providing appropriate patient counseling[124]. The cornerstone of medical management involves meticulous supportive care, addressing any underlying precipitating factors. Along with intravenous fluid resuscitation and blood glucose management (for diabetic patients), treatment typically includes bowel rest and intravenous antibiotics.
Lee and Park[125], published a case report on the management of congestive IC. Congestive IC is a rare form of IC that necessitates a thorough diagnostic evaluation. Their study concluded that the use of anti-inflammatory agents, such as glucocorticoids and 5-aminosalicylate, shows promise and may lead to novel effective therapies. Therefore, further research and clinical trials are necessary to validate and optimize the efficacy and safety of these treatment options for congestive IC[126].
Mesenteric vein thrombosis
Acute mesenteric venous thrombosis (MVT) accounts for up to 20% of acute mesenteric ischemia cases in high-income countries, with diagnosis increasingly made by portal-phase contrast-enhanced CT. Early-stage MVT without peritonitis is managed non-operatively with IV unfractionated heparin, or subcutaneous low-molecular-weight heparin in milder cases, followed by transition to oral anticoagulants. Exploratory laparotomy remains essential for diagnosis worldwide, and bowel resection with antibiotics is necessary for transmural bowel infarction[125].
Enteroscopy techniques for GIB
Small intestinal bleeding is relatively rare, representing only 5%-10% of all GI bleeds. These bleeds can either be occult or overt. Occult bleeding is characterized by a positive fecal occult blood test without any visible signs of blood loss, while overt bleeding involves visible symptoms such as melena, hematochezia, or iron deficiency anemia. Due to the nature and location of these lesions, they often cannot be detected through standard endoscopic evaluations or imaging, necessitating further investigation using capsule endoscopy (CE), PE, device-assisted enteroscopy (DAE), radiological assessments, or IOE[127].
IOE
IOE is an invasive procedure used to examine the small intestine during surgery, requiring collaboration between an endoscopist and a surgeon. Although IOE has a high diagnostic success rate, it has significant associated complications. These complications are often linked to the critical condition of the patients who undergo this procedure, rather than the procedure itself. Nevertheless, it is the most comprehensive method for inspecting the entire small intestine for both serosal and mucosal lesions, often revealing new or additional lesions that were not detected before surgery[128].
PE
For nearly three decades, PE was the go-to method for evaluating the small intestine. PE typically only examines the small bowel up to 150 cm distal to the ligament of Treitz. It involved using a long, standard-diameter endoscope to visualize the esophagus, stomach, duodenum, and proximal jejunum. With this approach, bleeding sources in the proximal small intestine, up to 50-70 cm from the pylorus, could be quickly ruled out, though it did not allow for a complete view of the entire small bowel. Compared to other forms of DAE, PE offers shorter sedation and procedure times. However, antegrade balloon enteroscopy achieves significantly deeper insertion (230 cm vs 80 cm, P < 0.001) and a higher diagnostic yield (63% vs 44%, P < 0.001). Moreover, deep enteroscopy often uncovers additional lesions in the deeper sections of the small intestine in patients who have already had positive findings with PE[129].
CE
In patients with acute severe GIB and negative upper endoscopy findings, emergency video CE can be valuable for promptly identifying the source of bleeding and helping to direct subsequent treatment[130].
Approach to management of acute LGIB
In any patients with acute LGIB initial focused history and physical examination along with the hemodynamic resuscitation is required. Focused history includes inquiry regarding co-morbidities, prior GI surgeries, recent endoscopy, associated GI symptoms including alteration of bowel habits, abdominal pain or unintentional weight loss. Recent guidelines from ESGE and ACG support use of risk stratification tools like Oakland score to supplement clinical decision making and identify patients with low risk with LGIB, who are appropriate for early discharge and outpatient evaluations[50,131].
Hemodynamically unstable patients should receive resuscitation with crystalloids to achieve normalization of blood pressure and heart rate before undergoing diagnostic and therapeutic intervention. CT angiography (CTA) is the preferred imaging modality in patients with hemodynamically significant hematochezia as it allows for rapid acquisition of images without the need for bowel preparation. CTA is an important tool to triage management of patients. In patients with negative CTA, a conservative management strategy may be preferable. In patients with positive CTA, with extravasation of contrast, and brisk bleeding, subsequent referral to IR with transcatheter arteriography and embolization is recommended. Colonoscopy can be considered in these patients if the bleeding has stopped.
In hemodynamically stable patients, non-emergent colonoscopy is reasonable. Although an ongoing matter of debate, in a meta-analysis including 4 randomized clinical trials (RCTs), early colonoscopy within 24 hours didn’t show to reduce mortality or further bleeding in patients hospitalized with acute LGIB[76]. ACG recommends a restrictive blood transfusion strategy in patients with hemodynamically stable LGIB with a hemoglobin cut-off threshold of < 7 g/dL compared to ESGE which supports a liberal transfusion with a cut-off of < 8 g/dL. The role of restrictive transfusion has been extrapolated from evidences including meta-analysis of RCTs for UGIB, which showed lower all-cause mortality and overall rebleeding in patients with restrictive transfusion strategy[132].
Other causes
Angioectasias: Colonic angioectasias are usually located in the right side and are common in elderly. Bleeding angioectasias are managed with endoscopic hemostasis. Both contact and non-contact thermal therapies can be performed and there is limited data comparing different types of therapies for treatment of colonic angiodysplasias. However, non-contact thermal anticoagulation e.g., APC is preferred for bleeding colonic angioectasias due to ease of use, and safety. It also has been shown to improve hemoglobin levels and reduce the need for blood transfusions[133]. Injection of 2-3 mL of submucosal adrenaline (1:200000) prior to APC is useful in right sided colonic angiodysplasia, where the risk of perforation is higher compared to rest of the colon[134].
Post-polypectomy bleeding: Post-polypectomy bleeding is the most common complication following polypectomy. While immediate bleeding can be recognized during the procedure with intervention, delayed post-polypectomy bleeding hours to days later can manifest as acute LGIB. When a patient is hospitalized following post-polypectomy bleeding, endoscopic intervention is usually indicated. Endoscopic intervention includes through the scope clips, which are shown to be highly effective in controlling the bleeding[135]. Other methods include direct thermal therapy, APC and over the scope clips. Studies comparing these modalities are lacking and decisions should be made depending on the experience, preference and availability of equipment.
Role of emergency colonoscopy: The role of emergency colonoscopy in LGIB is still debated, with some studies suggesting that urgent procedures within 12-24 hours of admission improve diagnostic outcomes and reduce rebleeding or surgery rates, while others do not. Urgent colonoscopy, performed after colon purge, is generally safe and can help identify and treat bleeding lesions, though its effectiveness varies, as seen in a study where cecal intubation was 41% without full preparation and 74% with proper preparation[136-138](Figure 1B).
MONITORING AND SUPPORTIVE CARE IN THE ICU
In the management of critically ill patients, particularly those suffering from GI hemorrhage, monitoring and supportive care in the ICU play a critical role in improving patient outcomes. Continuous hemodynamic monitoring, especially the use of central venous pressure (CVP) and echocardiographic (echo) measurements, has been associated with significantly better survival rates and reduced lengths of ICU stays. A retrospective analysis of the MIMIC-IV database demonstrated that these measurements help guide fluid resuscitation and hemodynamic management, leading to improved patient outcomes[139,140].
In terms of monitoring, the frequency of hemoglobin checks in patients with acute GIB has been questioned. Over-monitoring may lead to unnecessary blood transfusions, which carry their own risks. Evidence suggests that a more measured approach to hemoglobin monitoring - focusing on clinical signs of instability rather than frequent lab tests - may be more effective in managing these patients[141]. Advancements in monitoring technology have also been explored. For instance, artificial intelligence-based prediction models have been developed to assess the need for transfusion in ICU patients with GIB, potentially improving the precision of care[142]. Furthermore, continuous noninvasive arterial blood pressure monitoring using vascular unloading technology has been shown to be effective during complex GI endoscopic procedures, enhancing patient safety[143].
Another critical aspect of ICU care is the assessment of hemodynamic status using innovative techniques. For example, the use of a miniaturized transesophageal echocardiography probe has proven effective in providing real-time hemodynamic assessment in critically ill patients, offering a less invasive option compared to traditional methods[144]. Additionally, perioperative outcomes can be improved through minimally invasive and non-invasive hemodynamic monitoring techniques. These methods allow for better management of patients’ fluid status and cardiac output, reducing the risk of complications during and after surgery[145]. Moreover, CVP and echo measurements, as previously noted, are associated with better outcomes in GI hemorrhage, reaffirming the value of comprehensive monitoring strategies in the ICU[137,138].
There is also evidence supporting the use of point-of-care lactate testing to predict the occurrence of hypotension in stable patients with non-variceal UGIB. Elevated lactate levels can serve as an early warning sign, allowing for timely intervention and potentially preventing further deterioration[146,147].
Fluid and electrolyte management is a cornerstone of ICU care. The selection and administration rate of crystalloid fluids are particularly important in patients with acute GIB. Research indicates that using balanced crystalloids over saline can reduce the incidence of acute kidney injury (AKI) and lower mortality rates. This highlights the importance of carefully selecting fluids and controlling the infusion rates in critically ill patients[148].
Nutritional support is another vital component of care, with enteral feeding being the preferred method over parenteral nutrition due to its association with better patient outcomes. The role of nutrition in critically ill patients, with studies indicating that tailored nutritional support can significantly impact recovery and overall outcomes[149]. A study comparing stress ulcer prophylaxis combined with enteral nutrition vs enteral nutrition alone in patients at risk for GIB found that the combination significantly reduced the incidence of bleeding. This suggests that stress ulcer prophylaxis, when integrated with enteral feeding, can enhance patient recovery without increasing the risk of complications[150].
Beyond immediate resuscitative efforts, the management of stress ulcers through prophylaxis and nutrition support remains a debated topic. A study found that combining stress ulcer prophylaxis with enteral nutrition reduced the risk of GIB in critically ill patients, suggesting a synergistic benefit of these interventions[150]. However, the optimal approach to fluid resuscitation - whether the composition or rate of fluid administration - continues to be a subject of ongoing research, with some studies emphasizing the need for individualized care based on patient-specific factors[148].
In summary, the integration of continuous monitoring, precise fluid and electrolyte management, and tailored nutritional support forms the foundation of effective ICU care for patients with GI hemorrhage. The use of advanced monitoring technologies, alongside evidence-based practices, helps optimize outcomes and reduce complications in this vulnerable patient population.
Complications associated with procedural interventions
As described above, GIB, both upper and lower, can have a significant impact on patient outcomes and mortality. Around one-third of patients with GIB can develop hemodynamic instability, requiring aggressive resuscitation, with an estimated mortality of up to 10 percent mortality[151,152]. Older age groups, medical co-morbidities, anti-coagulant use, and bleeding disorders are all at higher risk for severe bleeding. Depending on the cause of the bleed, there can be a wide range of complications, including but not limited to peptic ulcer and intestinal perforation, prolonged hospitalization, and the need for endoscopic interventions.
Common diagnostic tools for GIB diagnosis include CTA and endoscopy. Although CTA can be a powerful tool to accurately detect the source of bleeding in an active bleed with a sensitivity of 85.7%, it has limited use in resolved or intermittent bleeds[153]. In addition, the procedure involves radiation exposure and uses intravenous contrast, which carries with it the risk of allergic reactions, anaphylaxis, and possible nephrotoxicity. Colonoscopy is the gold standard procedure for the diagnosis of the source of GIB, however, a colonoscopy may be difficult to perform in cases of acute LGIB for various reasons, including but not limited to the inability to tolerate prep and poor visualization during the procedure.
Therapeutic interventions in GIB initially involve pharmacological interventions, followed by invasive endoscopic interventions when there is continued bleeding. In the case of bleeding due to esophageal varices, a commonly used procedure is the use of endoscopic variceal ligation, which involves the placement of an elastic band around the base of the varix. Although an effective procedure for the treatment of bleeding varices, it can have postoperative complications such as severe pain, bleeding from the ligation site, and persistent bleeding from other non-variceal sources. An alternative technique used is the injection of sclerosing agents[154]. However, this may be associated with transient bacteremia and postoperative fevers[155]. Data regarding the risk of rebleeding after endoscopic intervention has been mixed, with some studies showing the 30-day rebleeding rate was increased in GIB where endoscopy was performed within 24 hours, whereas other trials have shown no difference[156,157].
Other therapeutic interventions used for GIB include mechanical interventions such as clips, the use of thermal cautery and particularly in the case of angiodysplasias and radiation proctopathy, the use of lasers such as APC and Nd-Yag lasers. Among laser-based techniques for treating haematochezia, APC is most commonly used and is relatively inexpensive; however, it can cause ulceration of the mucosa, severe postoperative pain, diarrhea, prolonged long-term tenesmus, and rectal pain[158].
Procedural complications after GI endoscopy include postoperative pain, nausea and vomiting, dental trauma, and complications related to the use of anaesthesia. Intestinal perforation can occur after upper or lower endoscopy, and can present as chest pain, dysphagia, odynophagia and/or subcutaneous emphysema. Treatment may be conservative or surgical, and is dependent on a variety of factors such as the size and site of the perforation, and patient-related factors including whether they will be suited for surgery[159].
One of the most severe complications after GI endoscopy for GIB is the incidence of sepsis and multi-organ dysfunction syndrome. Infections after an endoscopic procedure for a GIB can be either exogenous or endogenous, with exogenous caused by introduction of external organisms though the scope itself, whereas endogenous refers to translocation of the gut microbial flora. Most of these endoscope-associated infections are caused by translocation of organisms present in gut flora, including gram-negative rods, such as Pseudomonas and anaerobes[159]. The incidence of infections after a routine colonoscopy has been estimated to range from 0%-25%, and after a upper endoscopy 0%-8%[160]. Some of the solutions proposed for reducing the incidence of infections after endoscopic interventions include the use of disposable instruments, proper sterilization, and initiating quality improvement methods geared towards infection reduction goals. A prospective study found that the incidence of colonoscopy-related perforation was 0.66 per 1000 procedures, with a higher rate observed in therapeutic colonoscopy[161].
Overall, GIB is an essential cause of admissions and complicated hospitalizations across the United States, and current therapeutic interventions for GIB, although effective, are not without complications.
OUTCOMES AND PROGNOSIS
GIB remains a significant cause of morbidity and mortality in the United States, with outcomes largely influenced by the underlying cause, severity of the bleed, and comorbid conditions. Non-variceal GIB, which often results from peptic ulcers, erosive gastritis, or the use of NSAIDs, has seen improved mortality rates thanks to advances in therapeutic interventions, such as endoscopic hemostasis and the routine use of PPIs[162]. However, the rising prevalence of cardiovascular diseases has led to increased use of antiplatelet and anticoagulant medications, such as ASA, clopidogrel, warfarin, and DOACs like rivaroxaban and apixaban, which in turn has contributed to a greater incidence of GIB[163]. This trend presents new challenges in the management of these patients, requiring careful balance between treating cardiovascular conditions and minimizing the risk of GIB.
Non-variceal GIB remains a significant concern in clinical practice. Despite notable improvements in mortality rates over recent decades - primarily attributable to advances in medical therapies such as PPIs and refined endoscopic techniques - non-variceal GIB continues to pose considerable clinical challenges. Current estimates indicate an overall mortality rate for non-variceal GIB ranging from 2% to 10%. This variability depends on factors including patient age, underlying comorbidities, and the presence of complications[164]. In contrast, the mortality rate associated with variceal GIB, which is often related to esophageal or gastric variceal rupture due to portal hypertension, ranges from 10% to 20%. This rate is influenced by the severity of the liver disease, the effectiveness of initial therapeutic interventions, and the occurrence of complications such as liver failure or recurrent bleeding[165]. The management of GIB frequently necessitates substantial healthcare resources, including admission to ICUs for vigilant monitoring and treatment, blood transfusions to address significant hemorrhage, and prolonged hospital stays[166]. Rebleeding, which occurs in approximately 10%-20% of patients after initial hemostasis, is a significant predictor of poor outcomes[167]. It increases mortality rates and often necessitates additional interventions such as repeat endoscopy, IR, or surgery. Factors influencing rebleeding include initial endoscopic findings like active bleeding or visible vessels, which are associated with higher rebleeding rates. The need for these repeat interventions not only raises the risk of complications but also increases the length of hospital stay and healthcare costs.
In addition to the risk of rebleeding, several other complications can contribute to increased morbidity in patients with non-variceal GIB. These complications include infections, aspiration pneumonia, and the development of AKI, which are more common in elderly patients and those with pre-existing chronic illnesses[168]. The presence of comorbid conditions, such as chronic kidney disease (CKD), liver cirrhosis, heart failure, and cancer, further complicates management and is associated with poorer outcomes. Older patients are especially vulnerable, as age-related physiological changes and a higher prevalence of comorbidities reduce their ability to recover from acute bleeding episodes[169].
Several factors can predict poor outcomes in patients with non-variceal GIB, including advanced age, chronic illnesses such as CKD, heart failure, malignancy and the use of anticoagulant or antiplatelet medications.
Advanced age is a significant predictor of adverse outcomes in patients, with older adults demonstrating increased vulnerability to complications and exhibiting higher mortality rates, ranging from 12% to 25%, compared to younger populations with mortality rates around 10%[170]. This heightened risk is multifactorial, stemming from age-associated physiological changes, including diminished mucosal blood flow and reduced regenerative capacity of the GI epithelium[171]. Furthermore, age-related decline in cardiovascular reserve compromises the ability of elderly patients to effectively compensate for acute blood loss, rendering them more susceptible to hemodynamic instability and hypovolemic shock. The elevated prevalence of comorbidities such as hypertension, diabetes mellitus, and chronic obstructive pulmonary disease in this demographic exacerbates their clinical course[172]. These comorbid conditions can lead to complex clinical presentations, impair the body’s compensatory mechanisms, and decrease overall resilience, thereby increasing the likelihood of adverse outcomes following acute GI hemorrhage.
The severity of the initial bleed also plays a crucial role in predicting outcomes, as patients who require extensive blood transfusions or experience significant blood loss are at a higher risk of mortality. Patients with hemodynamic instability, characterized by low blood pressure, tachycardia, or signs of shock at presentation, are more likely to have severe bleeding and may need urgent and intensive care, including potential ICU admission.
Patients with CKD often experience platelet dysfunction due to uremia, which impairs platelet aggregation and adhesion, increasing the risk of ongoing or recurrent bleeding[173]. Moreover, CKD can lead to fluid overload and hypertension, exacerbating bleeding risks and complicating fluid management. Liver cirrhosis, another common comorbidity, is associated with coagulopathy due to impaired synthesis of clotting factors and thrombocytopenia from splenic sequestration. The presence of portal hypertension in cirrhotic patients increases the risk of variceal bleeding, although it also exacerbates non-variceal GIB by increasing the vascular pressure within the GI tract, making hemostasis more challenging. Patients with cirrhosis are also at higher risk for spontaneous bacterial peritonitis and sepsis, which can further complicate bleeding episodes[174].
Heart failure or reduced cardiac output in certain patients leads to compromised organ perfusion, which can impair the healing of GI lesions and increase the likelihood of ischemic complications. Heart failure patients often require anticoagulant therapy to manage thromboembolic risks, further increasing their susceptibility to bleeding[175]. Malignancy, particularly GI and hematologic cancers, is frequently associated with bleeding complications as tumors can directly invade blood vessels. Patients with cancer may also be on chemotherapy or other treatments that can cause thrombocytopenia or disrupt normal coagulation pathways, heightening the risk of bleeding and complicating hemostasis[176].
Early and timely intervention is crucial to prevent progression and improve prognosis. Patients who seek medical care late or face delays in diagnosis are more likely to be in a compromised state, with developed complications by the time they receive treatment. Additionally, the use of anticoagulants and antiplatelet agents heightens the risk of bleeding complications, making management more complex. Those on dual antiplatelet therapy or combined anticoagulant and antiplatelet therapy face particularly high risks[177].
Admission hemoglobin levels serve as a vital prognostic indicator; lower levels correlate with more severe bleeding and worse outcomes. Patients presenting with hemoglobin levels below 8.0 g/dL are at higher risk of requiring transfusions, experiencing rebleeding, and facing increased mortality[178]. The Rockall and Glasgow-Blatchford scoring systems, which assess factors like age, comorbidities, vital signs, and endoscopic findings, also predict outcomes effectively. High scores on these systems are associated with increased mortality and the need for intensive interventions.
Finally, endoscopic findings can provide critical insights into the prognosis. High-risk lesions, such as those with active arterial bleeding, non-bleeding visible vessels, or large ulcers, are linked to higher rebleeding rates and mortality. Prompt and effective endoscopic intervention is essential for managing these high-risk lesions and improving patient outcomes.
FUTURE DIRECTIONS AND RESEARCH GAPS
Endoscopic doppler probe
Endoscopic Doppler Probe continues to be an alternative under investigation proposed as a guide for the diagnosis and treatment of UGIB. Its use is not yet stipulated within the medical guidelines, but several isolated cases have reported good outcomes with its use[179]. More studies are needed to determine its efficacy and cost-benefit, but by looking for a positive US Doppler signal both before and after achieving hemostasis through endoscopic procedures, its use could reduce the rates of GI rebleeding, which remains as one of the most common complications of conventional endoscopic treatments of UGIB[179,180].
Hemostatic techniques
Endoscopic management of GIB has its limitations, and procedures can be challenging depending on the degree of bleeding or the ability to access the source. Based on these limitations, the development of homeostatic agents in the form of powders and gels has been playing an important role in the management of GIB. Some of these agents are TC-325 (Hemospray), EndoClot, Ankaferd Blood Stopper, and most recently: CEGP-003, UI-EWD and TDM-621 (PuraStat)[181].
Their mechanism of action varies from one agent to another, but in general they act by forming a homeostatic seal by activating platelets and the coagulation pathways. They can be used in combination with conventional therapies, as an adjuvant if first line treatment fails or as a single agent in cases of significant active bleeding that are poorly visualized or in cases with difficult access[182].
Areas requiring further research
The time to perform EGD and colonoscopy in a patient admitted with GIB remains controversial. Guidelines are still recommending the performance of endoscopy/colonoscopy within the first 24 hours of presentation followed by the hemodynamic stabilization, but it remains challenging to determine when during those 24 hours period an endoscopic intervention would change patients’ outcomes.
A retrospective study with 934 high risk patients with UGIB found lower mortality rates and decreased length of hospital stay in patients who underwent endoscopy during the first 13 hours when compared to later endoscopy, however another study with 6474 patients admitted with acute UGIB showed that endoscopy within 6 hours resulted in worse outcomes compared with those who underwent endoscopy between 6-24 hours and between 24-48 hours[183].
On the other hand, a multicenter retrospective cohort study with 10342 patients admitted with acute hematochezia in different hospitals in Japan showed that performance of colonoscopy in less than 24 hours of presentation shortened the hospital stay and improved the rate of identification of stigmata of recent bleeding but resulted in an increased risk of rebleeding and didn’t impact the mortality or the need of surgery or other interventions[184]. Based on these differences, more studies are needed to determine which factors or indicators might influence the outcomes in patients admitted with GI bleed.
Something that has been proposed to improve the management of patients with GI bleed presenting to the emergency department is the creation of a GI bleed code protocol such as those that exist for stroke, STEMI, and traumas. Brigham and Women's Hospital in Boston, MA, United States published the process that they followed to create one and commented on future directions for this topic. Creations of GI bleed code protocols may help to avoid delays in care of patients with signs and symptoms of severe GIB by making available resources more efficient and by shortening the period between patient’s arrival to the emergency room and transfer to endoscopic units or critical care[185].
Impact of AI- machine learning
Early identification of patients with GIB who require immediate care and admission to an ICU vs those who can be managed as an outpatient plays an important role in determining the prognosis and survival rate of patients. There are tools such as the Acute Physiology and Chronic Health Evaluation (APACHE) score that help to estimate the mortality of patients admitted to the ICU, however, their limitations have led to the development of other tools that seek better accuracy such as Machine Learning Prognostic Models.
Machine Learning Prognostic Models can determine patterns by studying repeated algorithms on electronic databases. Currently, these tools are only recommended to identify low-risk patients that can be managed as outpatient. Although some studies have shown that these tools are superior to the APACHE score in critically ill patients admitted to the ICU, its use has not yet been validated[179,186]. With its ability to analyze multiple electronic records and predict the different outcomes based on the analyzed patterns, this tool could provide useful information when determining how to manage patients, estimate their outcomes, thus improving physicians’ rationale to make important decisions[187].
development of other tools that seek better accuracy such as Machine Learning Prognostic Models.
Machine Learning Prognostic Models can determine patterns by studying repeated algorithms on electronic databases. Currently, these tools are only recommended to identify low-risk patients that can be managed as outpatient. Although some studies have shown that these tools are superior to the APACHE score in critically ill patients admitted to the ICU, its use has not yet been validated[179,186]. With its ability to analyze multiple electronic records and predict the different outcomes based on the analyzed patterns, this tool could provide useful information when determining how to manage patients, estimate their outcomes, thus improving physicians’ rationale to make important decisions[187].
CONCLUSION
GIB in the ICU setting remains a significant challenge despite advancements in critical care practices and prophylactic measures. The condition is associated with high morbidity and mortality, particularly among patients with risk factors such as coagulopathy, mechanical ventilation, and renal failure. While UGIB continues to be more prevalent, the management of both upper and LGIB necessitates a comprehensive, multidisciplinary approach involving resuscitation, endoscopic intervention, pharmacologic therapy, and sometimes surgical procedures. Although recent developments have reduced the overall incidence of GIB in critically ill patients, the complexity of the condition and its potential complications underscore the need for ongoing research and innovation. Future efforts should focus on refining prophylactic strategies, enhancing early detection, and developing targeted therapies to further improve patient outcomes in the ICU setting.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade B
P-Reviewer: Ray G; Sano W S-Editor: Li L L-Editor: A P-Editor: Zheng XM