Published online Dec 9, 2024. doi: 10.5492/wjccm.v13.i4.97631
Revised: August 26, 2024
Accepted: September 12, 2024
Published online: December 9, 2024
Processing time: 149 Days and 1.7 Hours
Hemoperfusion (HP) is an extracorporeal blood purification modality utilized to remove small- to medium-sized molecules, such as toxins and cytokines, that are difficult to remove by conventional hemodialysis. In clinical practice, HP has been successfully used as a salvage therapy for drug overdose and occasionally in pa
To summarize the clinical outcomes of a series of patients with severe coronavirus disease 2019 (COVID-19) who received HP.
Here, we summarize the clinical outcomes of a series of 18 patients with severe COVID-19 who received HP in our institution during the COVID-19 pandemic. A review of the literature was also performed.
HP was well-tolerated, and after an average of three sessions, respiratory and cardiovascular parameters as well as blood inflammatory markers improved in most patients. Ten patients were discharged alive. Our literature search identified a total of 20 studies (873 patients) in which HP was used for COVID-19. Nine studies reported improvements in respiratory parameters, and 13 studies (438 patients in total) reported better survival rates in patients undergoing HP.
HP was well-tolerated in patients with severe COVID-19, and most studies reported improved clinical parameters, including better survival rates, when HP was used in patients with severe COVID-19. Further research, especially prospective studies, is needed to evaluate the utility of HP as an early and supportive therapy for critically ill patients due to infectious diseases, such as those with COVID-19 or severe sepsis.
Core Tip: Hemoperfusion (HP) is an extracorporeal blood purification therapy that is increasingly being utilized in the intensive care unit. We show that HP improved respiratory and cardiovascular parameters and various inflammatory markers in a series of coronavirus disease 2019 patients in critical condition, in agreement with various reports from the literature. However, the absence of data from randomized controlled trials and a lack of consensus guidelines remain important issues for the utilization of HP in clinical practice.
- Citation: Vásquez-Torres J, Dávila-Collado R, Abdalah-Perez L, Jarquin-Duran O, Latino JS, Espinoza JL. Beyond conventional care: The therapeutic potential of hemoperfusion in severe COVID-19. World J Crit Care Med 2024; 13(4): 97631
- URL: https://www.wjgnet.com/2220-3141/full/v13/i4/97631.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i4.97631
Hemoperfusion (HP), also referred to as hemadsorption, is an extracorporeal blood purification modality that consists of the passage of anticoagulated whole blood through a column device that contains adsorbent particles for the removal of small- to medium-sized molecules (ranging from 100 to 40000 Daltons) such as toxins and cytokines that are difficult to remove by conventional hemodialysis[1]. Adsorbent particles can be made of activated charcoal or resins, such as polystyrene and polymyxin B. Charcoal has a high affinity for water-soluble molecules. In contrast, resins have a greater affinity for lipid-soluble molecules, and polymyxin B is typically used for the removal of bacterial endotoxins in the setting of sepsis.
HP has been clinically available since the 1970s, and despite its relatively low cost and potential usefulness in a broad spectrum of clinical conditions, its major clinical application has been largely the removal of drugs or poisons from the blood in cases of acute poisoning or drug overdose. Less frequently, it has been used as a supportive treatment for patients with severe liver dysfunction before and after liver transplantation, and occasionally, in conjunction with other extracorporeal techniques. HP is utilized for removing waste products from the blood in patients with kidney failure[1,2]. In addition, HP and other extracorporeal therapies, such as therapeutic plasma exchange, have been successfully utilized in patients with sepsis, where they have been associated with better clinical outcomes and an improved survival rate[3,4]. HP also showed beneficial effects in patients with severe influenza A, ameliorating the harmful effects of the cytokine storm[5]. Therefore, based on the crucial role of the dysregulated inflammatory response in the pathogenesis and clinical course of coronavirus disease 2019 (COVID-19)[6,7], along with the lack of specific therapeutic options, the use of extracorporeal therapies was utilized in patients with severe COVID-19, and several case reports or small case series[8,9] reported from around the world have been reported in the literature with variable results. Here, we re
This case series included a total of 18 adult patients in critical condition associated with COVID-19 who were consecutively admitted to the Baptist Hospital of Nicaragua (BHN) between August 2021 and November 2021. Epidemiological, clinical, and laboratory data were sourced and extracted from the hospital information system. Informed consent was obtained from all patients or their next of kin to publish their cases. Institutional Review Board approval was not required for this observational, retrospective study of routinely transmitted patient information, and written informed consent was waived owing to the rapid emergence of this infectious disease. The criteria for HP therapy are indicated in Supple
Blood cell count and differential were measured at the clinical laboratory of BHN with an ABX Pentra XL 80 automated cell counter system (Horiba Ltd., Kyoto, Japan). Additional blood examinations included liver function tests, ferritin, magnesium, C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and procalcitonin.
HP was carried out using the HA380 disposable HP cartridge (Jafron Biomedical Co., Ltd., Beijing, China), which is filled with neutral macroporous resin, mainly adsorbing molecules from 10 kDa to 60 kDa. In addition to supportive care and antiviral therapy, the standard treatment protocol for patients with acute respiratory failure included the use of no
A literature review was performed using standardized search terms, MeSH (medical subject headings) in MEDLINE and PubMed, and Emtree in Embase, organized in a hierarchal structure. We included in the analysis only peer-reviewed original articles that were published in the English language. The search terms were "hemoperfusion COVID-19", "he
Categorical variables were described as the total number and percentages, and continuous variables were described as either the means with SD or the median interquartile range. Data analysis was performed using Stata version 16.1 (Stata Corp., College Station, TX, United States), and statistical significance was set at a P value of ≤ 0.05.
A total of 18 patients with severe COVID-19 underwent HP, which was performed after the diagnosis of cytokine storm within the first 48 hours of hospital admission. Most patients (77.7%) in this series were men and relatively young (mean age 53 ± 15.80), and the most common comorbidity was hypertension (33.3%) followed by type 2 diabetes mellitus (27.7%). An incremental dosage of anticoagulation was used both to maintain circuit patency and to control thrombophilia. Unfractionated heparin was started at 10 IU/kg/h, but in some patients, a higher dosage of up to 20 IU/kg/h was required to ensure circuit patency. The demographic baseline characteristics of patients and clinical parameters before and after HP are shown in Table 1.
| Variable | Data |
| Sex | Male: 77 (n = 14) |
| Female: 23 (n = 4) | |
| Body mass index, mean ± SD | 30.6 ± 7.85 |
| Age, mean ± SD | 53 ± 15.80 |
| Comorbidities | |
| Hypertension | 33.33 (n = 6) |
| Heart disease | 0 |
| Diabetes mellitus | 27.77 (n = 5) |
| CKD | 22.22 (n = 4) |
| Asthma | 0 |
| Smoking | 5.55 (n = 1) |
| Days from onset to severe pneumonia diagnosis, mean ± SD | 8.94 ± 3.90 |
| APACHE II score, median (range) | 14.5 (29-6) |
| Initial ARDS respiratory support | |
| Low flow oxygen cannula | 5.55 (n = 1) |
| Oxygen mask with bag | 44..44 (n = 8) |
| Invasive mechanical ventilator | 50 (n = 9) |
The median (IRQ) length of the inpatient stay was 8 ± 4 days. Among the patients included in this study, eight were intubated. Nine patients received four or more sessions of HP, three patients received three sessions, and six patients received one or two sessions. Overall, improvements in respiratory cardiac parameters were evident following the application of HP, with oxygen saturation improving in 10 patients and reaching 95% in nine of them. Similarly, CRP levels and total leukocyte counts decreased in the majority of patients; however, ferritin levels slightly increased after receiving HP (Table 2 and Supplementary Table 2). Finally, 10 patients survived, and eight died. We believe that in pa
| Variable | Before HP | After HP | Difference | P value |
| SBP, mmHg | 102.77 ± 25.48 | 119.66 ± 30.93 | + 16.9 | 0.01 |
| DBP, mmHg | 57.11 ± 17.94 | 71.77 ± 16.16 | + 14.6 | 0.11 |
| RR, breath/min | 23 ± 3.89 | 21.94 ± 3.07 | - 1.06 | 0.19 |
| HR, pulse/min | 92.55 ± 17.15 | 87.05 ± 24.73 | - 5.5 | 0.14 |
| Temperature, °C | 36.26 ± 0.53 | 36.32 ± 0.82 | - 0.06 | 0.94 |
| PEEP | 12.94 ± 1.77 | 12.22 ± 1.20 | - 0.72 | 0.12 |
| SpO2, % | 89.11 ± 7.87 | 91.94 ± 5.16 | + 2.83 | 0.04 |
| CRP, mg/dL | 158.56 ± 73.98 | 85.68 ± 98.02 | - 72.88 | 0.01 |
| Ferritin, ng/mL | 2199.44 ± 2577.07 | 2294.46 ± 2548.26 | + 95 | 0.09 |
| Leukocytes/per mm3 | 14.85 ± 4.92 | 9.81 ± 3.66 | - 5.04 | 0.05 |
| Lymphocytes/per mm3 | 9.99 ± 5.61 | 8.01 ± 5.75 | - 1.98 | 0.07 |
| Neutrophils/per mm3 | 84.94 ± 5.71 | 86.85 ± 7.07 | + 1.91 | 0.13 |
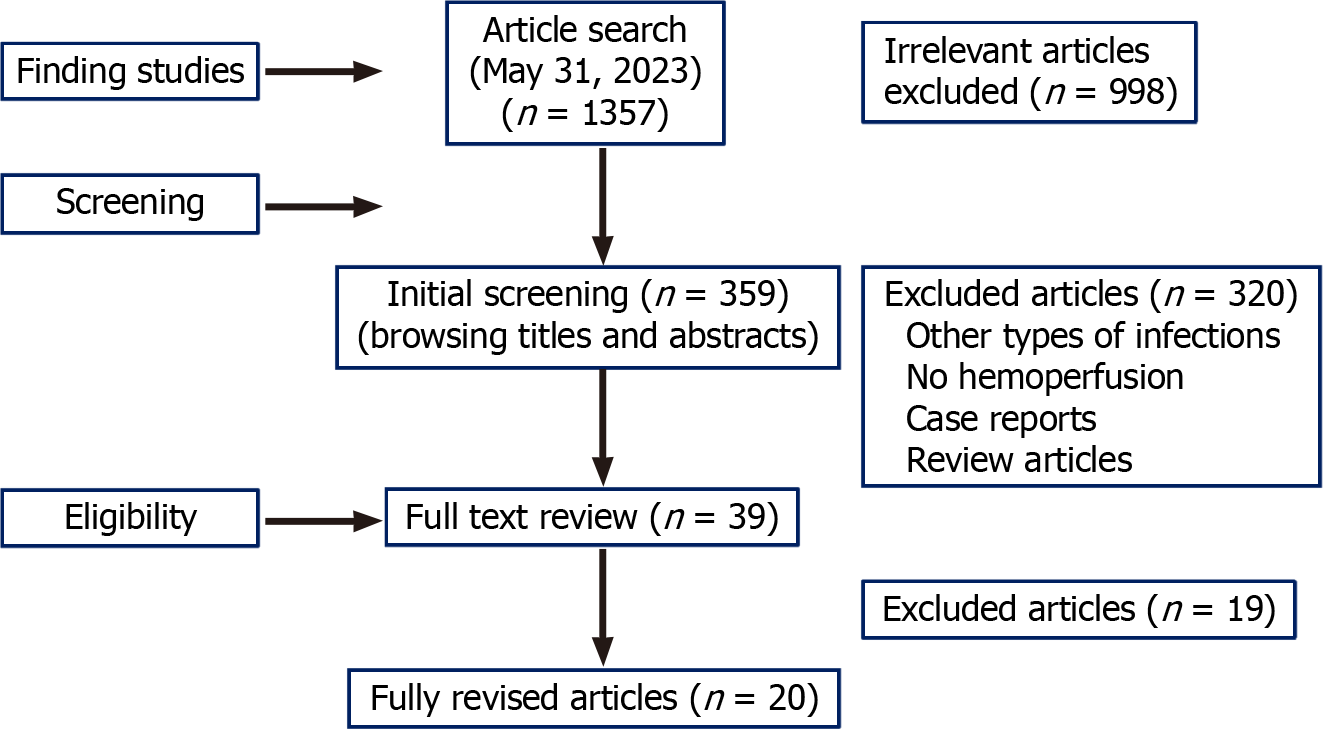
We identified 20 publications (873 total patients) that met the criteria for inclusion in this review (Figure 1). Among them, seven articles described retrospective cohorts (n = 320 patients), three were prospective studies, and 10 were case series. Most reported studies utilized non-selective HP with the help of the CytoSorb® cartridge, which has been described as an extracorporeal therapy with anti-inflammatory properties[1]. Regarding the HP strategy utilized, intermittent therapy was used in 11 studies (409 patients in total)[10-21], while in eight studies (total patients = 324)[22-28] HP was carried out using continuous therapy.
Among the selected studies, seven were conducted in Iran (= 342 patients in total)[10,12-14,22,28,29], three studies were from China[17,23,27], two from Italy[15,25] and Thailand[11,21], and the remaining seven were one each from Turkey[30], Saudi Arabia[24], Japan[16], North Macedonia[26], Germany[18], Colombia[19], and the United States of America[20]. The majority of included studies had small sample sizes, with an average of 37 patients participating. Table 3 sum
| Ref. | Number of subjects, age, M/F ratio | Study design/HP strategy | Effects on inflammatory markers | Effects on respiratory markers | Main clinical outcomes |
| Barriga-Moreno et al[19] | n = 116, Controls = 84; HP = 32; 57 year (range: 47-71 year); Males: 65% | Matched control retrospective Jafron© HA330 cartridge | Serum creatinine (1.4 mg/dL vs 0.5 mg/dL); Serum ferritin (2868 vs 1675) | NR | Mortality rate: 61% in controls (receiving standard care) vs 31% in HP group |
| Chiewroongroj et al[21] | n = 272 | Matched control retrospective | HP patients had less DIC (13% vs 33%; P = 0.046) and less sepsis (38% vs 64%; P = 0.02) | HP patients had reduced mechanical ventilation duration (15 days vs 35 days; P < 0.001) and less pulmonary complications (20% vs 42%; P = 0.04) | Mortality rates did not differ between the groups (33% vs 38%, P = 0.83) |
| Controls = 227; HP = 45; 57 year (range: 47-71 year); Males: 65% | Jafron© HA330 cartridge | HP group had a significantly shorter ICU stay (22 days vs 32 days; P = 0.017) | |||
| Hayanga et al[20] | n = 100 (63% male); 44 ± 11 year | Multicenter combination of VV-ECMO and CytoSorb hemoadsorption. Two post hoc groups: Early HP: ≤ 87 hours and late HP > 87 hours | Early HP had shorter median duration of mechanical ventilation (7 days)[2-26] than late HP (17 days)[7-37], P = 0.02 | Survival rates were 86% at 30 days and 74% at 90 days. Earlier HP was associated with shorter need for organ support and ICU stay | |
| Uysal et al[30] | 55 (34, 61.8%) Mean age 585 ± 12.5 year. Mean (SD), year = 59.6. Male sex, n (%) = 82 (64.1) | Case series. Resin-directed Jafron© (HA330) hemadsorption cartridges | Fibrinogen, LDH, CRP, and platelets decreased after HP | NR | In total, 9 patients (16.4%) survived. Ferritin levels correlated with survival status |
| Alavi Darazam et al[22] | 128 (55 HP vs 73 controls). Mean age, (SD), year = 59.6. Male sex, n (%) = 82 (64.1) | Matched control retrospective study. Jafron© (HA330) and CytoSorb® 300 cartridges | NR | Median SpO2 statistically higher in HP group than in the controls and median PaCO2 was lower | Mortality rate in the HP group significantly lower than controls (67.3% vs 89%; P = 0.002). The median length of ICU stay was 8 days in HP group vs 12 days in controls (P < 0.001) |
| Mikaeili et al[10] | 68 (35 HP vs 33 controls) | Prospective study | NR | Dyspnea decreased significantly in HP group | Significant mortality rate reduction in HP group compared with controls (37.1% vs 63.6%; P = 0.02) |
| Mean age: Control 5748 ± 15.63, 18/15; HP group 56.62 ± 15.60, 23/12; sex (M/F): HP group: 32/12; Control: 18/15 | HA330 D HP for 4 hours, in 3 consecutive days. Early started | Saturation of oxygen (SpO2) and P/F ratio significantly increased after HP unlike the control group | After 2 weeks, imagen lung opacities improved in 55% of the patients in HP vs 15.6% in the controls | ||
| Surasit et al[11] | 29 (15 HP vs 14 controls). HP group: 12/15, 54.5 ± 14.4; Control group: 7/7, 64.3 ± 10.2 | Prospective study. HA-330 3 HP sessions. Early started | Except for Hb levels (higher in HP group), blood and inflammatory parameters (CRP, ferritin, LDH, CBC) were not statistically different | NR | Significantly lower 28-day mortality rate in HP group compared to control group. Improvement of CXR RALE score and decreased SOFA score in HP group compared to control group |
| Darban et al[29] | 40 patients, 57.5 ± 15.9; year 24/16 | Retrospective cross-sectional study to determine the complications of HP patients with COVID-19 hospitalized in ICU | NR | NR | Arrhythmia, bleeding, thrombocytopenia, and coagulation disorders were the most common short-term complications mostly occurring on the second and third days after HP. Mortality occurred in 20 (50%) patients |
| Peng et al[23] | 10 patients, median age 677 year (range = 50-85). Sex (male/female): 8/2 | Case series Cytosorb HP. Median: 3 HPs (range = 1-6) | The level of IL-6 significantly decreased after HP. Lactate levels improved after HP | Significant improvement was found in PaO2/FiO2 [118 (81-220) mmHg vs 163 (41-340) mmHg, P = 0.04] | Albumin mildly decreased after HP. No significant changes were found in WBC |
| Abbasi et al[12] | 37 patients, 55 ± 14.1; year 12/25 | Retrospective cross-sectional study. Patients divided into three groups: (1) HP without (MV); (2) HP before MV; and (3) HP after MV | CRP and ferritin significantly improved after HP in the three groups | HR, RR, and PaO2/FIO2, significantly improved after HP in all groups | No statistically significant difference between the three groups in terms of length of hospital stay and ICU stay |
| Soleimani et al[13] | 48 patients in total. HP cases: 24; Controls: 24 | Retrospective observational study. HP: HA330 and HA280 filters for four hours | CRP levels decrease in HP patients compared to controls | RR and HR decreased after HP. SpO2 Levels significantly increased after HP. in the and a significant (P = 0.009) | HP improved respiratory distress in patients with severe COVID-19 but has no effect on mortality |
| Alharthy et al[24] | 50 patients, 50 ± 9; Sex: Male (n, %): 39 (78%) | Case series | Nonsurvivors had higher levels of inflammatory biomarkers (CRP, ferritin, and IL-6), and more unresolved shock, ARDS | Pulmonary emboli more common in non survivors | Posttherapy values of IL-6 predicted in-hospital mortality for critically ill COVID-19 patients. No side effects of therapy were recorded |
| All received CRRT and CytoSorb. Compared outcomes and biomarkers between survivors (39) and non survivors (11) | |||||
| Villa et al[25] | 37 patients, 31/6; 59 ± 9 year | Prospective non randomized. Blood purification with AN69ST (oXiris) hemofilter | Levels of IL-6 lowered in the first 24 hours of HP. IL-6 levels correlated with organ function | NR | Early HP treatment yielded the best outcomes. A slight decrease in observed vs predicted mortality rates observed. No complications reported |
| Ugurov et al[26] | 15 patients, 13/2; 60 ± 13 year | Case series; Blood purification using the AN69ST (oXiris) hemofilter | CRP (mg/L) pre 109 (73) Decreasing levels of IL-6, IL-8 and TNF-α | Blood purification was associated with decreasing levels of IL-6, IL-8, TNF-α, and CRP | Median intensive care unit length of stay was 9.3 days. 2 out of 15 patients died |
| Dai et al[27] | Treatment group: 40/10, 60 ± 13 year; control group: 35/16, 60 ± 15 year | Case-control multicenter, prospective; Plasma exchange + HP (50 treated + 51 controls) | IL-6 Level decreased in the treatment group and increased in the control group | No statistical differences in the duration of invasive assisted ventilation between the two groups | The 28-day mortality rates were 16% (8/50) in the treatment group and 50.98% (26/51) in the control group |
| Hashemian et al[14] | 14 patients, 9/6; 58 ± 12 year | Case-series Plasmapheresis Cytosorb | Inflammatory mediators (CRP, TNF-, IL-6, and Ferritin) were significantly reduced after plasmapheresis within a week | Plasmapheresis was associated with significant and rapid improvements in oxygenation status | Nine out of fifteen patients on NIPPV survived while the six patients undergoing IMV died |
| De Rosa et al[15] | 12 patients with COVID-19 + septic shock 9/3; 60 ± 10 year | Case series Polymyxin-B HP (PMX-HP) | SOFA score progressively improved over the next 120 hours following PMX-HP and it was | NR | COVID-19 patients with endotoxic shock, PMX-HP was associated with organ function recovery, hemodynamic improvement, and contemporary EAA level reduction. No PMX-HP-related complications were observed |
| Asgharpour et al[28] | 11 patients, 57 ± 18 year | Cases series; Tree sessions of extracorporeal resin-directed hemoadsorption | Serum IL-6 and CRP improved after intervention | Mean SpO2 increased after the three HP sessions | Six out of ten patients improved after the intervention |
| Katagiri et al[16] | 15 patients, 66 (47) year | Case series Toramixyn | Except for one non-survivor, IL-8, IL-10, and IL-17 Levels remained almost unchanged or trended downward | NR | 25% (NR) |
| Guo et al[17] | 17 patients, 62 ± 14 year | Case series | 32 cytokines (including IL-6 and TNF-α) out of 34 dosed were significantly decreased after each ALS course | NR | 100% |
In the largest study conducted so far, authors retrospectively analyzed data from 128 COVID-19 patients in Iran, with 55 patients receiving HP (using either Jafron© HA330 or CytoSorb® 300 HP cartridges) and 73 patients categorized as a matched control group. There was a significantly lower mortality rate in the HP group compared to the matched control group (67.3% vs 89%; P = 0.002), and patients receiving HP also showed a higher SpO2 than those in the matched group[22].
Four publications reporting prospective studies of HP in COVID-19 were found. The first one was an open-label study conducted in China between January and May 2020, and it reported that the mortality rate at day 28 was significantly lower in the treatment group (plasma exchange with HP) than in the control group (8/50 vs 26/51; P < 0.001). Also, whereas serum IL-6 levels increased in the control group, IL-6 levels significantly decreased in the treatment group[27]. Another prospective study was conducted in Italy and examined the effects of HP on 37 patients with severe COVID-19 hospitalized in intensive care units between February and April 2020. In addition to a reduction in serum IL-6 Levels, there were improvements in markers of systemic inflammation and multiorgan dysfunction, and a reduction in the expected ICU mortality rate, which were attributed to HP therapy, although no control group was included in the study[25]. Another prospective study was conducted between April and June 2020 in Iran and included 68 patients with severe critical symptoms of COVID-19 receiving either single standard therapy (33 controls) or a combination of standard treatment for COVID-19 combined with HP (35 patients) for 4 hours, in 3 consecutive days. Patients in the HP group had a significantly lower mortality rate compared with controls (37.1% vs 63.6%; P = 0.02)[10]. A small randomized controlled study investigated the effects of early initiation of HP via Cytosorb® therapy in patients with severe COVID-19 pneu
In this article, we have described the clinical outcomes of a series of patients with life-threatening COVID-19 who were treated with intermittent HP. Our results showed that HP was associated with improvements in various indicators of respiratory and cardiovascular function, including oxygen saturation, blood pressure, and respiratory rate. There was also a consistent decrease in CRP levels and total leukocyte counts. Importantly, even though all patients in this series were in critical condition and eight of them required invasive ventilation, a total of 10 patients survived, which is highly remarkable considering that they were treated in a low-income country, which is a factor that has been shown to directly conditionate provision of healthcare[31].
The beneficial effects of HP observed in this series are consistent with those reported in the literature, as revealed by our systematic search of publications testing the use of HP in COVID-19 patients. Using specific search criteria, we performed a literature search for articles reporting the use of HP in patients with severe COVID-19 and found 20 publications (839 total patients), which included mainly retrospective cohorts and case series (17 articles). Notably, about half of the patients (seven studies, 342 patients in total)[10,12-14,22,28,29], were treated in Iran, including the largest study reported so far (128 patients). Even though most studies published so far are small and there is considerable heterogeneity in terms of study design, populations, and study endpoints, it is possible to conclude that the vast majority of these studies reported positive results. HP was safe in COVID-19 patients, with no apparent complications associated with the procedure. Moreover, HP resulted in measurable clinical benefits, including an improvement in respiratory and cardiovascular parameters, amelioration of inflammatory responses, and better survival rates compared to controls. How
Data from studies in patients with sepsis indicate that the beneficial effects of HP are associated with the removal of circulating cytokines triggered by the infection, which results in significant mitigation of the host's inflammatory res
Direct HP using polymyxin B-immobilized fiber column (PMX-DHP) has been associated with better survival rates among patients with sepsis as shown by previous systematic review and meta-analyses of 17 trials[33,34]. However, the beneficial effects of PMX-DHP appear to be dependent on the patient’s baseline Sequential Organ Failure Assessment (SOFA) score as suggested by a more recent nationwide study conducted in Japan that included 44177 patients with sepsis treated between April 2018 to March 2020, where PMX-DHP significantly improved the survival of the patients in the SOFA score categories of 7-9 and 10-12 but did not improve survival rate in those with SOFA score categories of 0-6, 13-15, and 16-24[35]. Among the 18 studies included in our review, two case series[15,16] reported the use of PMX-DHP in patients with COVID-19, although it is unclear if a concomitant sepsis was confirmed in those cases, and hence, it is unknown whether this strategy may result in any additive beneficial effects compared to HP without polymyxin in pa
Thrombocytopenia and even the emergence of bleeding complications have been a recurrent concern for clinicians utilizing extracorporeal therapies[36]; however, in our study, no such complications were observed, which is consistent with what was reported in our review, where no significant changes in platelet counts or bleeding complications were reported[10,11,14,15,19-23,25-28].
Another important issue to consider is the potential influence of the filtration devices or cartridges and the courses of HP (number and length of sessions) on the efficacy of HP in severe COVID-19. Data from in vitro studies showed that both CytoSorb and oXiris were equally effective at removing cytokines and other inflammatory mediators, being drasti
It is important to notice that in our study, not all patients benefited from HD blood purification, and the reason for the failure of this therapy in some patients is currently unknown. It is plausible that genetic factors, variability in host im
HP is increasingly being utilized in the ICU, and with the advances in this technology and the development of new biocompatible devices, new indications for this extracorporeal technique are being developed[32]. While the use of polymyxin-B-bound HP is now a well-established therapy in patients with sepsis, where it can reduce 28-day mortality among patients with sepsis and septic shock[38]. New conditions where HP is currently utilized include bacterial endo
There are limitations associated with this study, including the small number of patients treated with HP, and also due to the lack of resources, we were unable to directly measure cytokines before and after the HP session, so we could not directly assess if the beneficial effects of HP, if any, were mediated via a reduction in the cytokine levels. Finally, although the number of patients enrolled in this study was relatively small, the results are encouraging, considering that HP is a technology that could be affordable even in low-income countries. Based on the broad experience of the use of HP in patients with sepsis and the encouraging data from a few studies reporting a beneficial effect of this extracorporeal therapy in patients with other viral infections, its safety and clinical utility could be tested in the setting of other severe viral infections or critically ill patients with acute inflammatory syndromes associated with other pathogen infections. However, currently, most experts recommend the application of HP on a personalized basis. Therefore, further studies are needed, particularly using randomized controlled trials and recruiting a larger number of patients to verify the results presented here. In addition, some aspects and parameters of HP in the context of severe viral infections, including COVID-19, need to be refined. For example, the optimal time for its implementation, the number of sessions required, and its combination with any additional supporting therapies must be properly determined. In summary, the available evidence suggests that HP is safe when utilized as an early and supportive therapy for critically ill patients with COVID-19 and may offer beneficial effects.
This case series and the available evidence suggest that HP is safe when utilized as an early and supportive therapy for critically ill patients with COVID-19 and may offer beneficial effects.
| 1. | Ronco C, Bellomo R. Hemoperfusion: technical aspects and state of the art. Crit Care. 2022;26:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Holubek WJ, Hoffman RS, Goldfarb DS, Nelson LS. Use of hemodialysis and hemoperfusion in poisoned patients. Kidney Int. 2008;74:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Li X, Liu C, Mao Z, Qi S, Song R, Zhou F. Effectiveness of polymyxin B-immobilized hemoperfusion against sepsis and septic shock: A systematic review and meta-analysis. J Crit Care. 2021;63:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Putzu A, Schorer R, Lopez-Delgado JC, Cassina T, Landoni G. Blood Purification and Mortality in Sepsis and Septic Shock: A Systematic Review and Meta-analysis of Randomized Trials. Anesthesiology. 2019;131:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Patel P, Nandwani V, Vanchiere J, Conrad SA, Scott LK. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A--an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011;12:e87-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Monserrat J, Gómez-Lahoz A, Ortega MA, Sanz J, Muñoz B, Arévalo-Serrano J, Rodríguez JM, Gasalla JM, Gasulla Ó, Arranz A, Fortuny-Profitós J, Mazaira-Font FA, Teixidó Román M, Martínez-A C, Balomenos D, Asunsolo A, Álvarez-Mon M; On Behalf Of The Covid-Hupa Group. Role of Innate and Adaptive Cytokines in the Survival of COVID-19 Patients. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 7. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1858] [Cited by in RCA: 1960] [Article Influence: 392.0] [Reference Citation Analysis (0)] |
| 8. | Rifkin BS, Stewart IJ. Seraph-100 Hemoperfusion in SARS-CoV-2-Infected Patients Early in Critical Illness: A Case Series. Blood Purif. 2022;51:317-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Hartomuljono A, Sugiarto A, Jennefer. Hemoperfusion techniques using Jafron HA330 cartridge combined with BBraun Dialog+ dialysis machine in patient with coronavirus disease 2019 pneumonia and septic shock: a case report. J Med Case Rep. 2023;17:156. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Mikaeili H, Taghizadieh A, Nazemiyeh M, Rezaeifar P, Zununi Vahed S, Safiri S, Ardalan M, Ansarin K. The early start of hemoperfusion decreases the mortality rate among severe COVID-19 patients: A preliminary study. Hemodial Int. 2022;26:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Surasit K, Srisawat N. The Efficacy of Early Additional Hemoperfusion Therapy for Severe COVID-19 Patients: A Prospective Cohort Study. Blood Purif. 2022;51:879-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Abbasi S, Naderi Z, Amra B, Atapour A, Dadkhahi SA, Eslami MJ, Hajian MR, Hashemi M, Hashemi ST, Iraj B, Khorvash F, Madadi S, Pour HM, Mansourian M, Rezvani M, Sami R, Soltaninejad F, Shahidi S, Vahdat S, Zamani Z, Moeinzadeh F. Hemoperfusion in patients with severe COVID-19 respiratory failure, lifesaving or not? J Res Med Sci. 2021;26:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Soleimani A, Taba SMM, Hasibi Taheri S, Loghman AH, Shayestehpour M. The effect of hemoperfusion on the outcome, clinical and laboratory findings of patients with severe COVID-19: a retrospective study. New Microbes New Infect. 2021;44:100937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Hashemian SM, Shafigh N, Afzal G, Jamaati H, Tabarsi P, Marjani M, Malekmohammad M, Mortazavi SM, Khoundabi B, Mansouri D, Moniri A, Hajifathali A, Roshandel E, Mortaz E, Adcock IM. Plasmapheresis reduces cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Pulmonology. 2021;27:486-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | De Rosa S, Cutuli SL, Ferrer R, Antonelli M, Ronco C; COVID-19 EUPHAS2 Collaborative Group. Polymyxin B hemoperfusion in coronavirus disease 2019 patients with endotoxic shock: Case series from EUPHAS2 registry. Artif Organs. 2021;45:E187-E194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Katagiri D, Ishikane M, Asai Y, Izumi S, Takasaki J, Katsuoka H, Kondo I, Ide S, Nakamura K, Nakamoto T, Nomoto H, Akiyama Y, Miyazato Y, Suzuki T, Kinoshita N, Ogawa T, Togano T, Suzuki M, Hashimoto M, Sakamoto K, Kusaba Y, Katsuno T, Fukaya T, Hojo M, Sugiyama M, Mizokami M, Okamoto T, Kimura A, Noiri E, Ohmagari N, Hinoshita F, Sugiyama H. Direct hemoperfusion using a polymyxin B-immobilized polystyrene column for COVID-19. J Clin Apher. 2021;36:313-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Guo J, Xia H, Wang S, Yu L, Zhang H, Chen J, Shi D, Chen Y, Zhang Y, Xu K, Xu X, Sheng J, Qiu Y, Li L. The Artificial-Liver Blood-Purification System Can Effectively Improve Hypercytokinemia for COVID-19. Front Immunol. 2020;11:586073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, Frech F, Müller S, Kuhl M, Benk C, Maier S, Trummer G, Flügler A, Krüger K, Sekandarzad A, Stachon P, Zotzmann V, Bode C, Biever PM, Staudacher D, Wengenmayer T, Graf E, Duerschmied D. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med. 2021;9:755-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 19. | Barriga-Moreno AP, Lozano-Sanchez M, Barón-Alvarez RA, Cordoba JP, Aroca-Martinez G, Dianda D, Gonzalez-Torres H, Musso CG. Mortality Rate and Acute Kidney Injury Prevalence Reduction in COVID-19 Critical Patients Treated with Hemoperfusion. Indian J Nephrol. 2024;34:56-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Hayanga JWA, Song T, Durham L, Garrison L, Smith D, Molnar Z, Scheier J, Deliargyris EN, Moazami N. Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: the CytoSorb therapy in COVID-19 (CTC) registry. Crit Care. 2023;27:243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Chiewroongroj S, Ratanarat R, Naorungroj T, Teeratakulpisarn N, Theeragul S. Efficacy of additional hemoperfusion in hospitalized patients with severe to critical COVID-19 disease. Sci Rep. 2024;14:17651. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Alavi Darazam I, Kazempour M, Pourhoseingholi MA, Hatami F, Rabiei MM, Javandoust Gharehbagh F, Amirdosara M, Hajiesmaeili M, Shabani M, Shokouhi S, Lotfollahi L, Mardani M, Haghighi-Morad M, Nassiri AA, Rangraz D, Falahaty H, Syami H, Irannejad Y, Fallah M, Zangi M, Shafigh N. Efficacy of Hemoperfusion in Severe and Critical Cases of COVID-19. Blood Purif. 2023;52:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Peng JY, Li L, Zhao X, Ding F, Hou X, Peng Z. Hemoperfusion with CytoSorb® in Critically Ill COVID-19 Patients. Blood Purif. 2022;51:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Alharthy A, Faqihi F, Memish ZA, Balhamar A, Nasim N, Shahzad A, Tamim H, Alqahtani SA, Brindley PG, Karakitsos D. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artif Organs. 2021;45:E101-E112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Villa G, Romagnoli S, De Rosa S, Greco M, Resta M, Pomarè Montin D, Prato F, Patera F, Ferrari F, Rotondo G, Ronco C. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: a pilot study. Crit Care. 2020;24:605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Ugurov P, Popevski D, Gramosli T, Neziri D, Vuckova D, Gjorgon M, Stoicovski E, Marinkovic S, Veljanovska-Kiridjievska L, Ignevska K, Mehandziska S, Ambarkova E, Mitrev Z, Rosalia RA. Early Initiation of Extracorporeal Blood Purification Using the AN69ST (oXiris(®)) Hemofilter as a Treatment Modality for COVID-19 Patients: a Single-Centre Case Series. Braz J Cardiovasc Surg. 2022;37:35-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Dai X, Zhang Y, Yu L, Jiang YA, Chen L, Chen Y, Li M, Gao C, Shang J, Xiang S, Li Y, Li J, Zhou C, Zhou X, Chen N, Liu Y, Liu J, Zhang Y, Chen X, Zhu D, Gao H, Tang L, Zhu M, Li L. Effect of artificial liver blood purification treatment on the survival of critical ill COVID-19 patients. Artif Organs. 2021;45:762-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Asgharpour M, Mehdinezhad H, Bayani M, Zavareh MSH, Hamidi SH, Akbari R, Ghadimi R, Bijani A, Mouodi S. Effectiveness of extracorporeal blood purification (hemoadsorption) in patients with severe coronavirus disease 2019 (COVID-19). BMC Nephrol. 2020;21:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Darban M, Yarmohamadi M, Mohammadkhani MM, Jazaeri SM. Outcome and Complications of Hemoperfusion in Patients with COVID-19 in Intensive Care Unit: A Cross-Sectional Study. Cardiovasc Hematol Agents Med Chem. 2023;21:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Uysal S, Merter M, Uysal A, Akbulut A. Effects of cytokine hemadsorption as salvage therapy on common laboratory parameters in patients with life-threatening COVID-19. Transfus Apher Sci. 2023;62:103701. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | van Zyl C, Badenhorst M, Hanekom S, Heine M. Unravelling 'low-resource settings': a systematic scoping review with qualitative content analysis. BMJ Glob Health. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 32. | Ricci Z, Romagnoli S, Reis T, Bellomo R, Ronco C. Hemoperfusion in the intensive care unit. Intensive Care Med. 2022;48:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 33. | Terayama T, Yamakawa K, Umemura Y, Aihara M, Fujimi S. Polymyxin B Hemoperfusion for Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. Surg Infect (Larchmt). 2017;18:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Chang T, Tu YK, Lee CT, Chao A, Huang CH, Wang MJ, Yeh YC. Effects of Polymyxin B Hemoperfusion on Mortality in Patients With Severe Sepsis and Septic Shock: A Systemic Review, Meta-Analysis Update, and Disease Severity Subgroup Meta-Analysis. Crit Care Med. 2017;45:e858-e864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Fujimori K, Tarasawa K, Fushimi K. Effectiveness of polymyxin B hemoperfusion for sepsis depends on the baseline SOFA score: a nationwide observational study. Ann Intensive Care. 2021;11:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Olson SR, Murphree CR, Zonies D, Meyer AD, Mccarty OJT, Deloughery TG, Shatzel JJ. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021;67:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 37. | Malard B, Lambert C, Kellum JA. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med Exp. 2018;6:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 38. | Li C, Zhang J, Yang P, Wang R, Chen T, Li L. The role of polymyxin B-immobilized hemoperfusion in reducing mortality and enhancing hemodynamics in patients with sepsis and septic shock: A systematic review and meta-analysis. Heliyon. 2024;10:e33735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Damianaki A, Stambolliu E, Alexakou Z, Petras D. Expanding the potential therapeutic options of hemoperfusion in the era of improved sorbent biocompatibility. Kidney Res Clin Pract. 2023;42:298-311. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |