Published online Dec 9, 2024. doi: 10.5492/wjccm.v13.i4.97149
Revised: September 24, 2024
Accepted: October 10, 2024
Published online: December 9, 2024
Processing time: 160 Days and 7.7 Hours
Cerebral autoregulation (CA) is the mechanism that maintains stable cerebral blood flow (CBF) despite fluctuations in systemic blood pressure, crucial for brain homeostasis. Recent evidence highlights distinct regional variations in CA between the anterior (carotid) and posterior (vertebrobasilar) circulations. Non-invasive neuromonitoring techniques, such as transcranial Doppler, transfer function analysis, and near-infrared spectroscopy, facilitate the dynamic assess
Core Tip: Cerebral autoregulation (CA) maintains stable cerebral blood flow (CBF) despite systemic blood pressure changes. Recent evidence highlights regional CA variations between anterior and posterior circulations. Non-invasive neuromonitoring techniques like transcranial Doppler, transfer function analysis, and near-infrared spectroscopy facilitate dynamic CBF assessment. Studies show robust autoregulation in the anterior circulation but lower capacity in the posterior circulation. Impaired CA in the posterior circulation, especially during acute brain injuries, may result from decreased sympathetic innervation, endothelial dysfunction, increased metabolic demands, and impaired blood-brain barrier integrity. Understanding these mechanisms is vital for improving cerebrovascular disorder management.
- Citation: Srichawla BS, Garcia-Dominguez MA. Regional dynamic cerebral autoregulation across anterior and posterior circulatory territories: A detailed exploration and its clinical implications. World J Crit Care Med 2024; 13(4): 97149
- URL: https://www.wjgnet.com/2220-3141/full/v13/i4/97149.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i4.97149
Cerebral autoregulation (CA), the inherent property of the cerebral vasculature to maintain relatively constant cerebral blood flow (CBF) following fluctuations in cerebral perfusion pressure (CPP), and is crucial to maintaining normal brain function[1]. This dynamic process ensures that the brain receives an adequate blood supply to meet its metabolic demands while preventing hyperperfusion or hypoperfusion-induced damage[2]. However, emerging evidence suggests that CA may vary in different circulatory territories within the brain, with implications for neurological function and clinical outcomes.
Recent studies have indicated differential cerebral autoregulatory mechanisms between the anterior and posterior circulatory territories of the brain[3]. The anterior circulation, supplied primarily by the internal carotid arteries and their branches, encompasses critical regions such as the frontal, parietal, and temporal lobes, as well as deep structures including the basal ganglia and internal capsule. On the contrary, the posterior circulation, which is primarily served by the vertebral and basilar arteries, supplies vital structures such as the brainstem, cerebellum, and posterior cerebral hemispheres[2]. Understanding the nuanced differences in autoregulatory behavior between these different circulatory territories is essential to elucidate the pathophysiology of various neurological conditions and guide clinical management strategies[4]. Furthermore, such knowledge may have profound implications for the development of targeted therapeutic interventions aimed at optimizing cerebral perfusion and mitigating the risk of ischemic or hemorrhagic brain injury[5]. Studying CA clinically is crucial because it directly impacts the management of patients with neurological conditions such as stroke, traumatic brain injury (TBI), and subarachnoid hemorrhage. Variations in autoregulatory function between different regions of the brain can influence clinical outcomes, particularly in conditions affecting the posterior circulation, where critical structures like the brainstem and cerebellum are highly vulnerable to dysregulated blood flow.
In this manuscript, we undertake a detailed exploration of regional CA across anterior and posterior circulatory territories. Drawing on a comprehensive review of existing literature, we seek to identify the underlying physiological principles governing regional variations in autoregulatory function and their clinical relevance. By synthesizing current evidence and identifying key research gaps, our objective is to provide valuable information on the intricate interplay between cerebral hemodynamics, energetics, and circulatory anatomy.
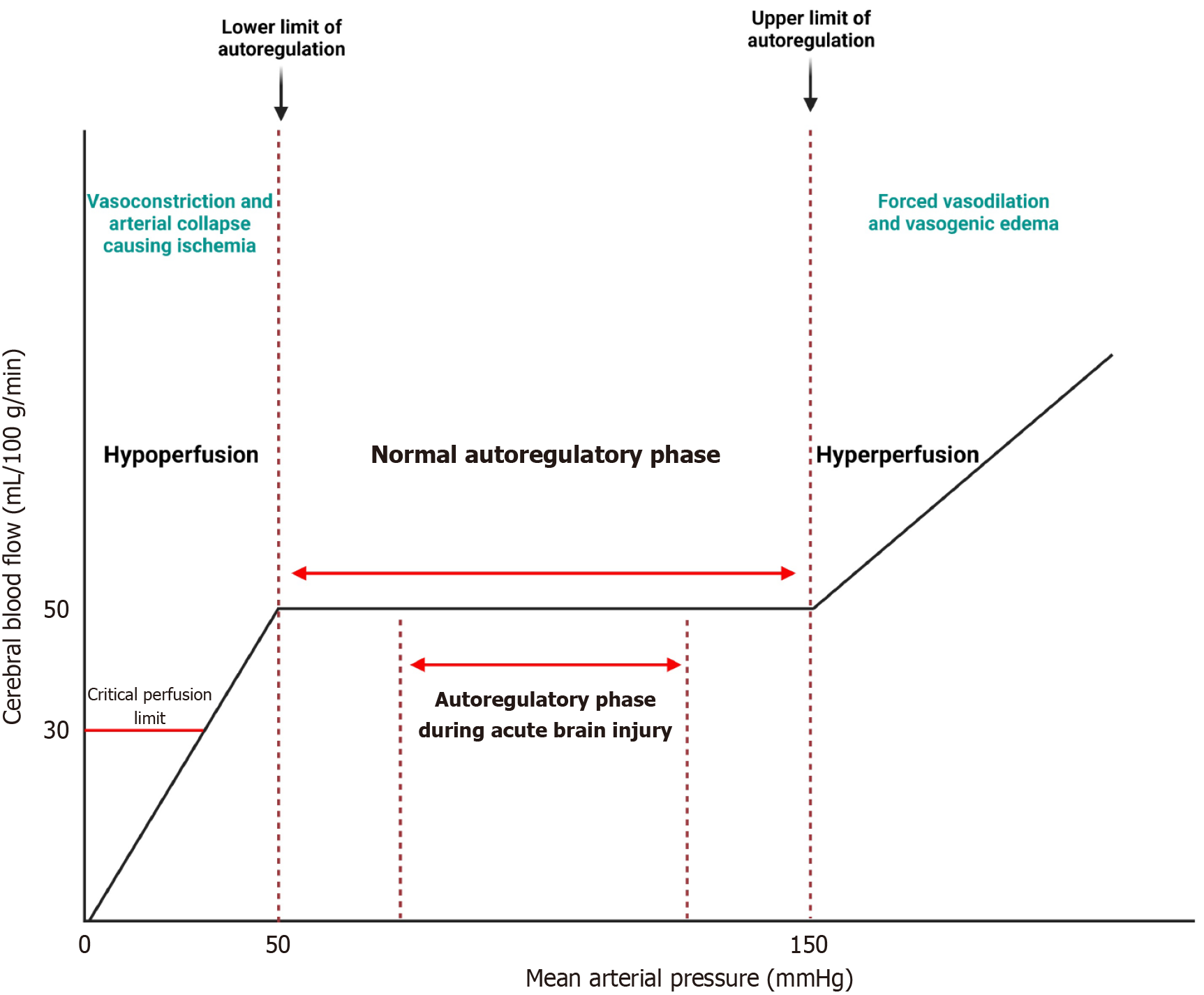
Lassen's curve, proposed by the Danish physiologist Niels A. Lassen, is a graphical representation of the relationship between CBF and CPP. It represents the autoregulatory capacity of the cerebral vasculature over a range of CPP values. In the classic representation, Lassen's curve shows a sigmoidal shape, indicating that CBF remains relatively constant within a certain range of CPP, but begins to decrease at lower CPP values and plateau at higher CPP values[6] (Figure 1).
Although Lassen's curve has been instrumental in understanding CA, it is not without its criticisms. The autoregulatory curve assumes a linear relationship between CPP and CBF within the autoregulatory range[7]. However, autoregulatory mechanisms often exhibit non-linear behavior, especially under extreme physiological conditions or in the presence of cerebrovascular disease. Lassen's curve does not account for the temporal dynamics of CA, including the time constants associated with vascular responses to changes in CPP. Dynamic models, such as transfer function analysis, provide a more complete understanding of autoregulatory dynamics over a range of frequencies[5]. Despite these criticisms, Lassen's curve remains a valuable conceptual tool for illustrating the principles of CA. However, its limitations underscore the need for more sophisticated modeling approaches and dynamic assessments of autoregulatory function in clinical practice.
CA is maintained through the intricate interplay of several physiological mechanisms, including myogenic, metabolic, and neurogenic factors.
The myogenic mechanism is based on the inherent contractility of cerebral arterioles in response to changes in intravascular pressure. When systemic blood pressure (BP) increases, arteriolar smooth muscle contracts, reducing vessel diameter and limiting blood flow to the cerebral microcirculation. In contrast, a decrease in systemic BP causes vasodilation, allowing increased CBF to maintain adequate perfusion[8].
Metabolic factors, such as changes in tissue O2 tension, CO2 levels, and pH, also influence CA. Increased metabolic activity, as seen during neuronal activation, leads to local vasodilation to meet the increased demand for oxygen and nutrients. In contrast, the accumulation of metabolic by-products, such as CO2, triggers vasodilation to improve cerebral perfusion and facilitate waste removal[9].
Neurogenic factors, including sympathetic and parasympathetic innervation, play a role in modulating cerebral vascular tone. Sympathetic stimulation typically induces vasoconstriction, while parasympathetic activity promotes vasodilation. These autonomic influences help regulate CBF in response to physiological and environmental stimuli[10].
Understanding the principles of CA is essential for the management of various neurological conditions, including TBI, stroke, and neurodegenerative diseases. Impairments in autoregulatory function can predispose individuals to cerebral ischemia or edema, exacerbating neurological injury. Throughout the years a patient oriented CPP-protocol ‘CPPOPT’ has been proposed to optimize individual CA across various acute brain injuries. Steiner et al[11] was the first to introduce the term CPPOPT in 2002[11]. CPPOPT represents the ideal range of CPP in which autoregulation is most effective in main
The pressure reactivity index (PRx) is a valuable metric in neurocritical care that provides information on the dynamic relationship between intracranial pressure (ICP) and CPP. Lang et al[12] introduced the PRx to assess CA and is often used in neurocritical care scenarios[12]. PRx is calculated as the moving correlation coefficient between slow waves of ICP and CPP and reflects the ability of the cerebrovascular system to autoregulate in response to changes in BP. In neurocritical care, PRx serves as a surrogate marker for CA, allowing clinicians to assess the brain's ability to maintain stable CBF during a range of BP fluctuations. A positive PRx value indicates impaired autoregulation, since increases in ICP are associated with decreases in CPP, suggesting a passive response of the cerebral vasculature to changes in perfusion pressure[13]. On the contrary, a negative PRx value suggests intact autoregulation, with increases in ICP accompanied by compensatory increases in CPP to maintain stable CBF[14].
In Petersen et al's study[15], the authors investigated the efficacy of personalized autoregulation-based BP targets compared to static systolic BP thresholds in patients undergoing endovascular thrombectomy for acute ischemic stroke. Using tissue oxygenation derived from NIRS to measure autoregulatory function, personalized limits of autoregulation were successfully computed for the 90 patients. The study found that the percentage of time with mean arterial pressure (MAP) exceeding the upper limit of autoregulation was significantly associated with worse 90-day outcomes, particularly in patients with hemorrhagic transformation. Although there was a trend toward worse outcomes with increasing time above fixed systolic BP thresholds, the effect sizes were smaller compared to the personalized approach. The study concludes that deviation from the BP targets based on personalized autoregulation can increase the risk of further brain injury and poor functional outcomes, suggesting that this approach may present a better strategy for hemodynamic management in patients with acute ischemic stroke compared to the classical approach of maintaining systolic BP below a predetermined value[15].
Svedung Wettervik et al[16] conducted an observational study aiming to investigate the combined effect of insult intensity and duration, specifically focusing on ICP, PRx, CPP, and CPPopt on clinical outcomes in pediatric TBI. The study included 61 pediatric patients with severe TBI between 2007 and 2018, with at least 12 hours of ICP data in the first 10 days after injury. The results indicated that brief episodes of ICP > 25 mmHg and longer episodes (20 minutes) of ICP between 20-25 mmHg were associated with unfavorable results. Similarly, higher PRx values, especially for longer durations, were correlated with worse outcomes. A transition was observed from favorable to unfavorable outcomes for CPP < 50 mmHg, with no association found for high CPP values. Furthermore, deviations from CPPopt below -10 mmHg were associated with worse outcomes, suggesting a potential role for autoregulatory-oriented management in pediatric TBI[16].
Various mathematical approaches have been employed to characterize CA, including linear and non-linear models, which aim to capture the complex interactions between physiological parameters governing cerebrovascular dynamics. Linear transfer function analysis is a commonly used method to assess CA by analyzing the frequency-dependent relationship between CPP and CBF fluctuations. The transfer function H(f) represents the transfer of pressure fluctuations to CBF fluctuations at different frequencies, typically in the range of 0.03-0.15 Hz, corresponding to the low-frequency range of autoregulatory responses[17].
The transfer function is defined as: H(f) = P(f)/CBF(f), where P(f) and CBF(f) represent the fourier transformations of the CPP and CBF signals, respectively. Gain and phase change of the transfer function provide information on the magnitude and timing of CBF responses to changes in CPP.
Nonlinear models offer a more comprehensive representation of dynamic CA by capturing the nonlinearities inherent in cerebrovascular regulation. One such approach is the autoregulatory index (ARI), which quantifies the relationship between CPP and CBF over a range of pressures.
The ARI is calculated as: ARI = CBF/CPP.
ARI values close to zero indicate intact autoregulation, while deviations from zero suggest impaired autoregulatory function.
The relationship between CBF, CPP, and cerebrovascular resistance (CVR) can be described by the following equations: CBF = CPP/CVR; CVR = (MAP-ICP)/CBF.
These equations highlight the dependence of CBF on CPP and CVR, emphasizing the importance of maintaining adequate perfusion pressure to ensure sufficient blood flow to the brain.
The posterior circulation of the brain is primarily supplied by the vertebral arteries, which ascend through the cervical vertebrae and join to form the basilar artery at the base of the brainstem. This system feeds vital structures such as the occipital lobes, cerebellum, brainstem, and part of the thalamus. The vertebral arteries give branches such as the posterior inferior cerebellar artery, while the basilar artery gives rise to branches including the anterior inferior cerebellar arteries, pontine arteries, and superior cerebellar arteries, culminating in the posterior cerebral arteries that supply the posterior cerebral hemispheres. Pathologies in this circulation, such as brain stem strokes or cerebellar infarctions, significantly impact motor control and vital functions, underscoring the clinical importance of understanding the unique autoregulatory dynamics of these territories[18,19].
The cerebral collateral circulation, particularly the anterior communicating artery (ACoA) and the posterior commu
The ACoA connects the left and right anterior cerebral arteries, forming a vital component of the Circle of Willis. It allows crossflow between the hemispheres, which is particularly important in cases of unilateral stenosis or occlusion of the internal carotid artery (ICA). Studies have shown that the presence of patent ACoA significantly enhances the compensatory capacity of the cerebral circulation, maintaining adequate blood flow to the affected hemisphere. In a study by Hartkamp et al[20], patients with functional ACoA demonstrated better cerebral hemodynamics and less severe symptoms during carotid artery occlusion compared to those without functional ACoA. The PCoA connects the posterior cerebral artery (PCA) with the internal carotid artery, facilitating collateral flow between the anterior and posterior circulations. Its role becomes critical in conditions such as basilar artery or bilateral vertebral artery stenosis. Research indicates that a patent PCoA can significantly improve dynamic CA by providing an alternative route of blood flow to the posterior circulation[20]. A study by Schomer et al[21] demonstrated that people with a well-developed PCoA exhibited better outcomes in terms of maintaining cerebral perfusion in the presence of vertebrobasilar insufficiency[21].
Guo et al[22] investigated the influence of ACoA and PCoA on dCA in patients with different types of stenosis. The researchers evaluated dCA using transfer function analysis in 51 patients with ischemic stroke with specific collateral anatomies, divided into two groups based on results of digital subtraction angiography: Group 1 with severe stenosis/occlusion in the basal and/or bilateral vertebral arteries (subdivided by the presence of bilateral PCoAs) and Group 2 with severe stenosis/occlusion in the unilateral internal carotid artery (subdivided by the presence of ACoA and/or PCoAs). The results showed that patients in Group 1 with patent PCoA had significantly higher values of PCA phase difference, indicating better autoregulation, while in Group 2, those with patent ACoA had higher MCA phase difference values. This suggests that the presence of ACoA and PCoA contributes significantly to compensating for impaired dCA, highlighting their crucial role in maintaining stable CBF under stenotic conditions[22].
Srichawla et al[23] reported the case of hemorrhagic posterior reversible encephalopathy syndrome (PRES) secondary to chemotherapy. The hemorrhage was present contralateral to a fetal-type PCA (fPCA)[23]. A fPCA has been implicated as an increased risk of stroke and ischemic events[24,25]. Emmert et al[26] examined cerebrovascular reserve in 385 healthy participants. Cerebrovascular reserve was decreased ipsilaterally to the fPCA[26]. Taking these results into account, the presence of a fPCA can influence local CA and can play a role in diseases such as PRES and reversible cerebral vasoconstriction syndrome, which have been traditionally understood as loss of CA within the posterior circulation.
The anterior circulation, which includes the internal carotid arteries and their branches, receives its sympathetic innervation primarily from the superior cervical ganglion. Nerve fibers follow the carotid artery into the skull, forming a network around the vessel known as the carotid plexus. These sympathetic nerves modulate vascular tone by controlling vascular smooth muscle contraction, thus influencing the diameter of the arteries and blood flow to the anterior brain regions such as the frontal, parietal and temporal lobes[27]. On the contrary, the posterior circulation, supplied by the vertebral and basilar arteries, receives sympathetic innervation that is less studied, but believed to be similarly sourced from the superior cervical ganglion, with nerve fibers accompanying the arteries through the foramina in the cervical vertebrae and forming the vertebral plexus. These fibers provide autonomic control over blood flow to critical areas such as the cerebellum, brainstem, and occipital lobes[28].
Differences in sympathetic innervation between the anterior and posterior circulations suggest that autoregulatory mechanisms could also differ between these territories. For example, the anterior regions of the brain, richly innervated, may exhibit a more robust autonomic response to changes in BP, potentially leading to more pronounced vasoconstriction or vasodilation in response to fluctuations in systemic BP. This is supported by studies like those of Hardebo and Owman[29], who demonstrated that the density of sympathetic innervation is higher in the anterior circulation, particularly around the circle of Willis[29]. On the contrary, the posterior circulation might have a different threshold or sensitivity to sympathetic stimulation, which can be crucial in clinical scenarios. For example, the posterior brain regions are critical for maintaining basic life functions controlled by the brainstem and cerebellum. Dysregulation of sympathetic control in these regions could lead to significant clinical manifestations, such as vertebrobasilar insufficiency or stroke of the posterior circulation, especially under stress or significant hemodynamic changes[30].
The parasympathetic nervous system, primarily through the vagus nerve, plays a significant role in modulating CBF, particularly under resting conditions. Unlike broadly distributed sympathetic innervation, parasympathetic effects on the cerebral circulation are more nuanced and less understood, especially regarding differences between the anterior and posterior circulatory territories. The anterior circulation receives some degree of parasympathetic innervation from the sphenopalatine and otic ganglia. These ganglia release neurotransmitters such as acetylcholine and vasoactive intestinal peptides, which can induce vasodilation of the cerebral arteries. The effect of parasympathetic activation in the anterior circulation is generally considered to promote blood flow during conditions of increased neuronal activity, facilitating neurovascular coupling (NVC) and metabolic regulation[31].
Unlike the anterior circulation, the parasympathetic innervation of the posterior circulation is less understood. It is proposed to be mediated indirectly through local reflex arcs within the brainstem, sensitive to changes in BP and chemical stimuli (e.g., nucleus of the tractus solitarii). This innervation is vital to maintain autonomic functions controlled by the brainstem and cerebellum, affecting the overall hemodynamic stability of these regions[32,33]. Understanding the distinct parasympathetic pathways and their functional implications is crucial to developing targeted therapeutic strategies that address specific cerebral territories, particularly in managing conditions such as vertebrobasilar insufficiency and posterior circulation ischemic events.
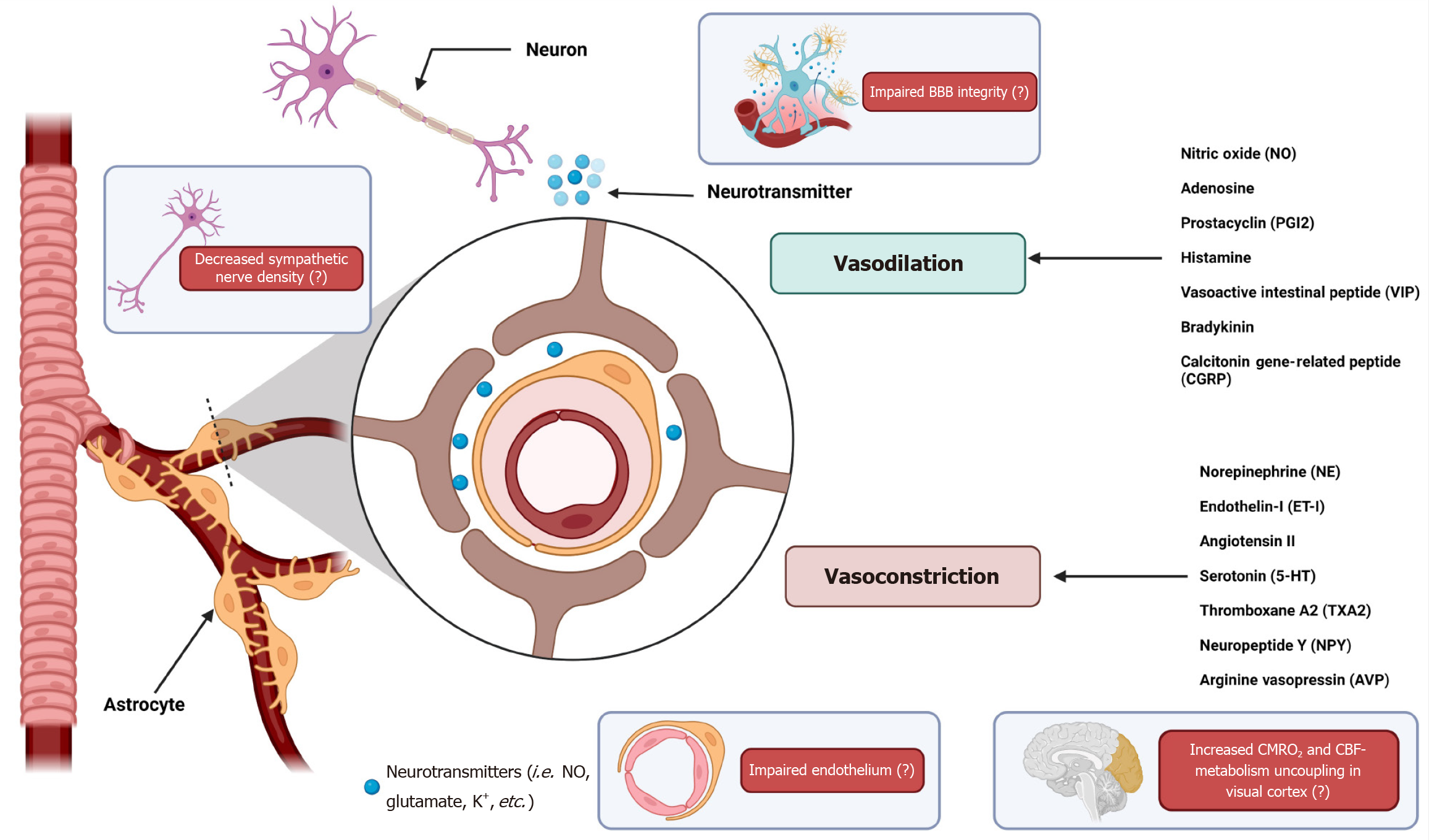
NVC refers to the dynamic relationship between neuronal activity and subsequent changes in CBF. This intricate process ensures that the active regions of the brain receive adequate oxygen and nutrients to meet their metabolic demands. NVC may have a close relationship with CA.
NVC involves multiple cell types, including neurons, astrocytes, and vascular endothelial cells, which work together to regulate blood flow. When neurons are activated, they release neurotransmitters that increase the activity of adjacent astrocytes. Astrocytes then release vasoactive substances such as NO, arachidonic acid metabolites and potassium ions, which act on nearby blood vessels to induce vasodilation and increase local blood flow[34]. This response is crucial for rapidly delivering glucose and oxygen to active brain regions. The NVC process can be divided into three primary phases: Initiation, propagation, and resolution. During the initiation of the therapy, neuronal activity leads to the release of signaling molecules that trigger a cascade of events involving astrocytes and endothelial cells. Propagation involves the spread of these signals through the vascular network, resulting in coordinated vasodilation and increased blood flow. Resolution occurs when signaling molecules are cleared and blood vessels return to their baseline state[35].
Impaired NVC is associated with various neurological disorders, including stroke, TBI, and neurodegenerative diseases. For example, in stroke patients, disruptions in NVC can lead to inadequate blood supply to affected brain regions, exacerbating tissue damage and affecting recovery[36]. Similarly, in conditions such as Alzheimer's disease, reduced NVC may contribute to cognitive decline by altering the delivery of nutrients to metabolically active neurons[37]. Recent studies have highlighted the importance of preserving NVC in the management of cerebrovascular disorders. Therapeutic strategies aimed at improving NVC, such as pharmacological agents that promote vasodilation or interventions that support endothelial function, have shown promise in improving outcomes in patients with impaired CA[38]. Additionally, non-invasive imaging techniques, such as functional magnetic resonance imaging and NIRS, are valuable tools for assessing NVC and guiding treatment decisions in clinical settings[39] (Figure 2).
Cerebral energetics, the study of energy consumption and metabolism in the brain, reveals significant regional differences between the anterior and posterior circulatory territories. These differences are driven by the distinct functional specializations of these brain regions and their corresponding metabolic demands. Nakagawa et al[40] investigated the efficiency of dynamic CA in the PCA compared to the middle cerebral artery (MCA) and the effect of cerebral vasodilation due to visual stimulus on these characteristics. The study continuously measured blood flow velocity (BFV) in PCA and MCA and MAP in 45 healthy volunteers with eyes open and an additional 20 subjects with eyes closed and open. Autoregulation was assessed using gains in transfer function across low, high, and cardiac frequency ranges.
The results showed that with eyes open, the gains were significantly higher in the PCA than in the MCA at low (0.03-0.07 Hz) and high (0.07-0.15 Hz) frequencies. Visual stimulation (eyes open) increased BFV and reduced CVR in PCA but not in MCA. This vasodilation was associated with an increase in gain in the low frequency autoregulatory range in the PCA, while the MCA gain remained unchanged. These findings suggest that the previously reported impaired autoregulation in PCA is likely due to visual activation-induced metabolic vasodilation rather than an inherent difference in autoregulation capacity between PCA and MCA[40].
Chen et al[41] investigated NVC and dynamic CA in PCA among 60 healthy volunteers, identifying no sex differences in these functions. They found that the PCA response to visual stimulation through eyes-open tasks resulted in significantly higher CBF velocity and cerebrovascular conductance index compared to the eyes-closed rest phase. Furthermore, while the gain in PCA during autoregulation was consistently lower than that observed in the MCA at all frequencies, the phase response was similar in both cerebrovascular territories. This study confirms the robust activation of NVC during visual tasks and suggests equivalent functions of dynamic CA and NVC in the PCA between sexes[9].
Chen et al[41] explored the impact of cerebral venous sinus thrombosis (CVST) on dynamic CA and NVC in the MCA and PCA during silent reading. The study involved 60 CVST patients and 30 controls, using TCD to measure CBFV and related parameters during visual tasks. The results indicated that the CVST group exhibited impaired dynamic CA in the PCA, particularly in the very low frequency phase, compared to controls. Furthermore, the CVST group showed reduced cerebrovascular conductance and a visual evoked flow response during NVC, suggesting that CVST negatively affects cerebral hemodynamic stability during visual stimulation[41].
Labrecque et al[42] investigated dynamic CA and cerebrovascular reactivity to carbon dioxide (CVRco2) in the MCA and PCA in 11 young endurance-trained women. Using a multimodal approach, including sit-to-stand tests and TFA of BP oscillations, the study found no significant differences between MCA and PCA in reduction in CBFV, onset of regulatory response, or rate of regulation during initial orthostatic stress. Although the TFA gain and phase showed location and frequency effects, the normalized gain was similar between the arteries. Absolute CVRco2 did not differ significantly between MCA and PCA, but relative CVRco2 was 39% lower in MCA. These results suggest that the cerebral pressure-flow relationship is comparable between MCA and PCA in young women, with potential sex-specific differences in CVRco2 that require further investigation[42].
PRES is a clinical and radiological syndrome characterized by headache, encephalopathy, visual impairment, and seizures. It is associated with a range of conditions including severe hypertension, eclampsia, autoimmune diseases, and immunosuppressants. PRES predominantly affects the posterior circulation of the brain, particularly the parieto-occipital regions. The pathophysiology of PRES is closely related to failure of CA, especially in the posterior circulation. Under normal conditions, CA maintains stable CBF despite fluctuations in systemic BP. However, in PRES, this autoregulatory mechanism fails, leading to hyperperfusion and vasogenic edema. Several hypotheses and mechanisms have been proposed to explain this regional vulnerability.
As mentioned above, the posterior cerebral circulation has less sympathetic innervation compared to the anterior circulation, which plays a crucial role in maintaining vascular tone and protecting against rapid increases in BP. The relative paucity of sympathetic nerves in the posterior circulation makes it more susceptible to autoregulatory failure during hypertensive episodes[43]. Additionally, the endothelium of the posterior circulation is more prone to dysfunction due to systemic insults such as hypertension, eclampsia, or cytotoxic drugs. This endothelial injury disrupts the blood-brain barrier and alters autoregulation, leading to vasogenic edema. The vulnerability of the posterior circulation to endothelial dysfunction is also involved in migraine pathophysiology[44]. Furthermore, the posterior regions of the brain have a less robust blood-brain barrier compared to the anterior circulation, making areas such as the parieto-occipital regions more susceptible to breakdown when exposed to high BP or toxic agents, resulting in fluid leakage and edema[45]. The high metabolic demands of the posterior brain regions, such as the occipital lobes involved in visual processing, further strain autoregulatory mechanisms during acute hypertensive crises or toxic insults, leading to a higher propensity to autoregulatory failure and subsequent edema[46].
Future research on regional CA should focus on several key areas to improve our understanding and improve the clinical management of neurological conditions. Large-scale, multicenter studies are needed to validate and expand on the findings related to differential autoregulatory mechanisms in the posterior circulation. These studies should incorporate advanced imaging techniques and dynamic evaluations of cerebrovascular reactivity to provide a more comprehensive understanding of autoregulation under various physiological and pathological conditions. The development and refinement of noninvasive monitoring tools, such as TCD, diffuse correlation spectroscopy, and NIRS are essential for real-time assessment of CA in clinical settings. These tools will enable clinicians to dynamically monitor and adjust therapeutic strategies, improving the management of acute brain injuries and chronic cerebrovascular conditions. The relationship between resting and active cerebral metabolism and O2 consumption and its effect on CBF and autoregulation is yet to be fully understood. Differences in endothelial function and blood-brain barrier capacity in regional CA are also further areas of research[47]. Additionally, sex-specific differences in CA and cerebrovascular reactivity warrant further investigation.
CA is a vital process that ensures stable CBF despite fluctuations in CPP. This mechanism is crucial for maintaining brain function and preventing damage from hyperperfusion or hypoperfusion. Our review underscores the significant differences in the autoregulatory mechanisms between the anterior and posterior circulatory territories of the brain. The susceptibility of the posterior circulation to autoregulatory failure, as seen in conditions like PRES, highlights the need for targeted therapeutic interventions. The unique challenges in managing CA in conditions such as TBI and subarachnoid hemorrhage further emphasize the need for precise, region-specific approaches. Several mechanisms contribute to these regional differences, including variations in sympathetic and parasympathetic innervation, CBF-metabolism coupling, endothelial function, and integrity of the blood-brain barrier. The relative lack of sympathetic innervation of the posterior circulation, combined with its higher metabolic demands and less robust integrity of the blood-brain barrier, may make it particularly vulnerable to autoregulatory dysfunction during systemic insults. Understanding these regional differences in autoregulation can help tailor therapeutic interventions to maintain optimal cerebral perfusion and prevent ischemic or hemorrhagic damage, ultimately improving patient outcomes in critical care settings. Moreover, advancing knowledge of CA can guide the development of novel monitoring techniques and therapeutic strategies that are specific to the affected circulatory territory. Advancing our understanding of regional CA is crucial for improving the diagnosis, prognosis, and management of various cerebrovascular disorders.
| 1. | Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1102] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 2. | Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592:841-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 627] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 3. | Reinhard M, Roth M, Müller T, Guschlbauer B, Timmer J, Czosnyka M, Hetzel A. Effect of carotid endarterectomy or stenting on impairment of dynamic cerebral autoregulation. Stroke. 2004;35:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology. 2015;122:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008;8:42-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Lassewn NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1324] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 7. | Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856-H1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 (Pt 1):199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 546] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 9. | Yoshihara M, Bandoh K, Marmarou A. Cerebrovascular carbon dioxide reactivity assessed by intracranial pressure dynamics in severely head injured patients. J Neurosurg. 1995;82:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985). 2006;100:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 557] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 12. | Lang EW, Kasprowicz M, Smielewski P, Santos E, Pickard J, Czosnyka M. Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. J Neurosurg. 2015;122:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Velle F, Lewén A, Howells T, Hånell A, Nilsson P, Enblad P. The effects of cerebral pressure autoregulation status and CPP levels on cerebral metabolism in pediatric traumatic brain injury. Acta Neurochir (Wien). 2024;166:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 14. | Svedung Wettervik T, Beqiri E, Hånell A, Bögli SY, Placek M, Donnelly J, Guilfoyle MR, Helmy A, Lavinio A, Hutchinson PJ, Smielewski P. Visualization of Cerebral Pressure Autoregulatory Insults in Traumatic Brain Injury. Crit Care Med. 2024;52:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, Schindler JL, Falcone GJ, Gilmore EJ, Jasne AS, Cord B, Hebert RM, Johnson M, Matouk CC, Sheth KN. Fixed Compared With Autoregulation-Oriented BP Thresholds After Mechanical Thrombectomy for Ischemic Stroke. Stroke. 2020;51:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Svedung Wettervik T, Velle F, Hånell A, Howells T, Nilsson P, Lewén A, Enblad P. ICP, PRx, CPP, and ∆CPPopt in pediatric traumatic brain injury: the combined effect of insult intensity and duration on outcome. Childs Nerv Syst. 2023;39:2459-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Panerai RB, Brassard P, Burma JS, Castro P, Claassen JA, van Lieshout JJ, Liu J, Lucas SJ, Minhas JS, Mitsis GD, Nogueira RC, Ogoh S, Payne SJ, Rickards CA, Robertson AD, Rodrigues GD, Smirl JD, Simpson DM; Cerebrovascular Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: A CARNet white paper 2022 update. J Cereb Blood Flow Metab. 2023;43:3-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 18. | Kawashima M, Rhoton AL Jr, Tanriover N, Ulm AJ, Yasuda A, Fujii K. Microsurgical anatomy of cerebral revascularization. Part II: posterior circulation. J Neurosurg. 2005;102:132-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Liu J, Tseng BY, Khan MA, Tarumi T, Hill C, Mirshams N, Hodics TM, Hynan LS, Zhang R. Individual variability of cerebral autoregulation, posterior cerebral circulation and white matter hyperintensity. J Physiol. 2016;594:3141-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999;30:2671-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994;330:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 221] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Guo ZN, Sun X, Liu J, Sun H, Zhao Y, Ma H, Xu B, Wang Z, Li C, Yan X, Zhou H, Zhang P, Jin H, Yang Y. The Impact of Variational Primary Collaterals on Cerebral Autoregulation. Front Physiol. 2018;9:759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Srichawla BS, Presti K, Kipkorir V, Berrios Morales I. Chemotherapy-associated hemorrhagic posterior reversible encephalopathy syndrome (PRES) with considerations for circle of Willis variants on cerebral blood flow and autoregulation: A case report. Medicine (Baltimore). 2024;103:e37250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Lochner P, Golaszewski S, Caleri F, Ladurner G, Tezzon F, Zuccoli G, Nardone R. Posterior circulation ischemia in patients with fetal-type circle of Willis and hypoplastic vertebrobasilar system. Neurol Sci. 2011;32:1143-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Arjal RK, Zhu T, Zhou Y. The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin Imaging. 2014;38:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Emmert K, Zöller D, Preti MG, Van De Ville D, Giannakopoulos P, Haller S. Influence of Vascular Variant of the Posterior Cerebral Artery (PCA) on Cerebral Blood Flow, Vascular Response to CO2 and Static Functional Connectivity. PLoS One. 2016;11:e0161121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010;41:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233-H241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 474] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Hardebo JE, Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann Neurol. 1980;8:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 165] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Sándor P. Nervous control of the cerebrovascular system: doubts and facts. Neurochem Int. 1999;35:237-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Phillips AA, Chan FH, Zheng MM, Krassioukov AV, Ainslie PN. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016;36:647-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 32. | Spencer SE, Sawyer WB, Wada H, Platt KB, Loewy AD. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 1990;534:149-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Nakai M, Tamaki K, Ogata J, Matsui Y, Maeda M. Parasympathetic cerebrovasodilator center of the facial nerve. Circ Res. 1993;72:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2007] [Cited by in RCA: 1762] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 35. | Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73-e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Terborg C, Gora F, Weiller C, Röther J. Reduced vasomotor reactivity in cerebral microangiopathy : a study with near-infrared spectroscopy and transcranial Doppler sonography. Stroke. 2000;31:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1612] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 38. | Madsen PL, Holm S, Herning M, Lassen NA. Average blood flow and oxygen uptake in the human brain during resting wakefulness: a critical appraisal of the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1993;13:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Obrig H, Villringer A. Beyond the visible--imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 417] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Nakagawa K, Serrador JM, Larose SL, Moslehi F, Lipsitz LA, Sorond FA. Autoregulation in the posterior circulation is altered by the metabolic state of the visual cortex. Stroke. 2009;40:2062-2067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Chen S, Chen H, Duan J, Cui L, Liu R, Xing Y. Impaired Dynamic Cerebral Autoregulation in Patients With Cerebral Venous Sinus Thrombosis: Evaluation Using Transcranial Doppler and Silent Reading Stimulation. Ultrasound Med Biol. 2023;49:2221-2226. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Labrecque L, Drapeau A, Rahimaly K, Imhoff S, Brassard P. Dynamic cerebral autoregulation and cerebrovascular carbon dioxide reactivity in middle and posterior cerebral arteries in young endurance-trained women. J Appl Physiol (1985). 2021;130:1724-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 714] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 44. | Perko D, Pretnar-Oblak J, Šabovič M, Zaletel M, Žvan B. Associations between cerebral and systemic endothelial function in migraine patients: a post-hoc study. BMC Neurol. 2011;11:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Fittro K, Dizon R. Understanding posterior reversible encephalopathy syndrome. JAAPA. 2018;31:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 717] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 47. | Venkat P, Chopp M, Chen J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J. 2016;57:223-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |