INTRODUCTION
Patients with cirrhosis frequently experience acute kidney injury (AKI) during hospitalization, and it is associated with a high morbidity and mortality[1,2]. Broadly, AKI can be secondary to renal hypoperfusion (hemodynamic AKI), intrinsic parenchymal damage or urinary obstruction and a systematic evaluation must be performed in all patients in order to ascertain its cause[3]. In patients with cirrhosis, it is particularly challenging to approach hemodynamic AKI, which can be caused by hypovolemia, distributive physiology (hepato-renal syndrome), intra-abdominal hypertension (IAH) or congestive nephropathy (type 1 cardio-renal syndrome).
HEMODYNAMIC CHANGES IN CIRRHOSIS
Hemodynamic changes in patients with cirrhosis and portal hypertension make them highly susceptible to AKI from renal hypoperfusion. Parenchymal distortion due regenerative nodules and fibrosis, as well as an increase in intrahepatic vascular tone are followed by resistance in portal inflow and portal hypertension[4]. In response, activation of vasodilators (mainly nitric oxide), results in systemic vasodilation and decreased systemic vascular resistance (SVR)[5,6]. Further, arteriovenous and portosystemic shunts can also contribute to a decrease in SVR[6]. Resulting hypoperfusion is initially compensated through the renin-angiotensin-aldosterone system (RAAS) and antidiuretic hormone mediated volume expansion, as well adrenergic activation[7,8]. These mechanisms increase cardiac output through increased preload and chronotropism[7,8]. However, if the resulting cardiac output proves to be insufficient for metabolic demand, and volume expansion exceeds the limits of the Frank-Starling mechanism, high-output cardiac failure (HOHF) will occur resulting in elevated filing pressures and venous congestion. HOHF is defined as congestive heart failure with elevated filling pressures in the setting of an elevated cardiac index (≥ 4 L/min/m2)[9]. Interestingly, cirrhosis was the second most prevalent cause of HOHF, surpassed only by obesity in a large case series[9]. Moreover, it has been recognized that compromised contractile reserve is common, even in individuals with maintained left ventricular ejection fraction (LVEF). Given that longitudinal contractile function tends to be impaired before radial function loss, recent guidelines propose utilizing global longitudinal strain, with a normal value of 18% or higher) to detect myocardial contractile dysfunction in those with preserved LVEF[10]. Therefore, although renal dysfunction can be aggravated by the splanchnic vasodilation and renal vasoconstriction, as in the case of the classical hepatorenal syndrome (HRS), it is also paramount to consider that decreased renal function could also be a consequence of venous congestion in the setting of advanced diastolic disfunction or even reduced cardiac output in the setting of reduced systolic function. The term hepatocardiorenal syndrome has been proposed to encompass the intricate interplay among the liver, heart, and kidneys[11].
HRS AND GUIDELINE-BASED TREATMENT
HRS is a potentially reversible reduction in glomerular filtration rate (GFR) that occurs in patients with advanced liver disease in the absence of shock, nephrotoxic drugs, and histological changes in renal parenchyma that does not respond to diuretic withdrawal and volume expansion[12]. It is thought that HRS is the consequence of the compensatory activation of the RAAS and sympathetic nervous system, with resulting renal vasoconstriction, suppression of vasodilatory mechanisms and thus a decreased GFR[13]. This is supported by reduced renal cortical perfusion on renal arteriography[14], the presence of high levels of renin activity, as well as high levels of norepinephrine in serum of patients with HRS[15]. Furthermore, the effectivity of terlipressin in some patients with HRS supports the role of splanchnic vasodilation as a cornerstone in its pathophysiology[16]. It is worth mentioning that other pathogenic factors contributing to HRS are systemic inflammation due to bacterial translocation, with release of pathogen-associated and damage-associated molecular patterns[17]. However, their role in HRS lies beyond the scope of this review. At present, HRS is a diagnosis of exclusion, and current guidelines recommend empiric treatment with volume expansion with albumin for 48 h to rule out volume depletion in patients with cirrhosis and AKI[18]. Persistent kidney injury after volume expansion is compatible with a diagnosis of HRS and vasopressors (terlipressin or norepinephrine) are the recommended treatment[13]. However, volume expansion and vasoconstrictor therapy are not without risks. Lessons learned from the ATTIRE trial are that routine albumin infusion in patients with decompensated cirrhosis is unbeneficial and associated with an increased risk of life-threatening adverse events, including fluid overload and respiratory failure due to pulmonary edema[19]. Moreover, higher rates of pulmonary failure were found in those who received both terlipressin and albumin in the CONFIRM trial[16], probably because of increased preload and afterload[20]. Hence, it is advisable to perform a thorough evaluation of volume status, possibly utilizing point-of-care ultrasonography (POCUS) before administering intravenous fluids[21-23].
CARDIAC FUNCTION IN ADVANCED CIRRHOSIS
The heterogeneous response to treatment might be a consequence of overlooking a key element of hemodynamics in patients with cirrhosis, which is cardiac function. Initially, a decreased SVR is compensated by volume expansion which is expected to result in increased cardiac output via the Frank-Starling mechanism. However, altered cardiac function, specifically impaired relaxation, can result in less tolerance for volume expansion resulting in increased filling pressures and venous congestion. Altered myocardial relaxation is frequently observed in patients with cirrhosis and this has been referred to as “cirrhotic cardiomyopathy”[10]. Cirrhotic cardiomyopathy is characterized by impaired diastolic function[24], enlarged cardiac chambers[25], a hampered response to stress[26], and electrophysiological changes in patients with advanced liver disease and no previous heart disease[27,28]. In a study by Ruíz-del-Árbol et al[29], 37 of 80 patients with cirrhosis had left ventricular diastolic dysfunction, which was associated with lower mean arterial pressures, higher Model for End-Stage Liver Disease scores, and greater risk of HRS-AKI and mortality. In 2019, the Cirrhotic Cardiomyopathy Consortium proposed echocardiographic criteria to define cirrhotic cardiomyopathy[10]. Under these criteria, the prevalence of cirrhotic cardiomyopathy has been reported to be as high as 60%[30].
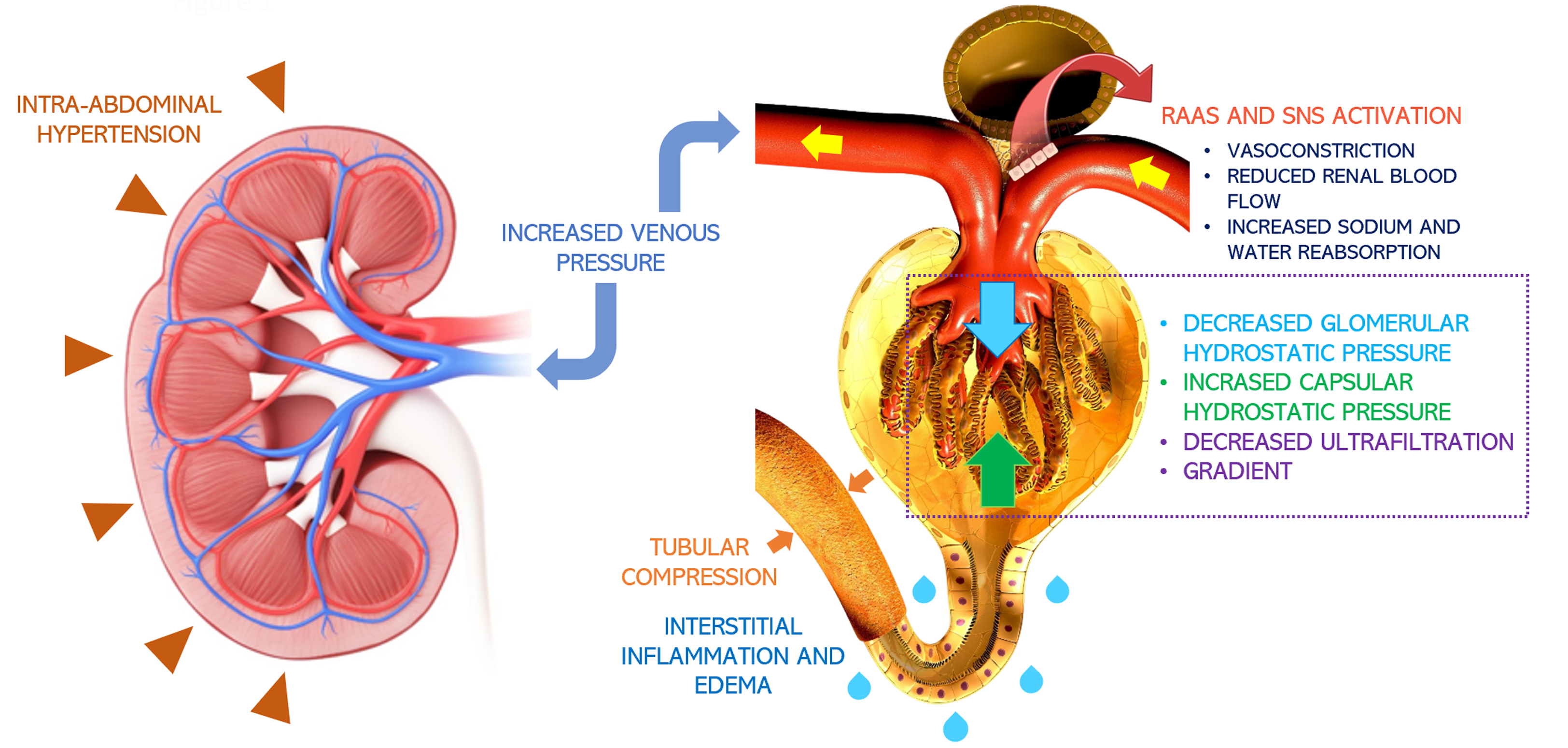
Failing to consider cardiac function in the setting of AKI in cirrhosis and treating with a “one-size-fits-all” approach could be detrimental. Increasing preload on an already surpassed Frank-Starling mechanism leads to further congestion, with no benefit on cardiac output or renal perfusion. Furthermore, the use of vasoconstrictors on patients with low contractility would increase the afterload on an already strained heart, which would further compromise cardiac output. In fact, we now know that venous congestion is the main hemodynamic determinant of renal dysfunction in patients with heart failure (Figure 1)[31]. AKI in the setting of severe venous congestion is likely to improve by achieving decongestion through diuresis, ultrafiltration and, possibly inotropic drugs in the setting of reduced contractility. Thus, it is imperative to perform an accurate hemodynamic evaluation in patients with cirrhosis and AKI to tailor their management. This approach is supported by a retrospective study by Pelayo et al[32], where 127 patients with cirrhosis and a diagnosis of HRS underwent a right heart catheterization, where 62% of patients were found with elevated pulmonary wedge pressures consistent with cardiorenal syndrome physiology[32]. These patients were treated with diuretics instead of volume expansion achieving adequate response.
Figure 1 Pathophysiology of congestive nephropathy: Congestion-induced acute renal dysfunction is mediated by retrograde transmission of central venous pressure to the kidneys, leading to development of interstitial edema, inflammation, and activation of the renin-angiotensin-aldosterone system and sympathetic nervous system[66].
This further results in global cessation of glomerular filtration. Intra-abdominal hypertension adds to the problem by simulating a tamponade pathophysiology together with increased interstitial pressures. Citation: Argaiz ER, Romero-Gonzalez G, Rola P, Spiegel R, Haycock KH, Koratala A. Bedside Ultrasound in the Management of Cardiorenal Syndromes: An Updated Review. Cardiorenal Med 2023; 13: 372-384. Copyright© The Author(s) 2023. Published by S. Karger AG, Basel, authors’ prior work published under CC BY-NC License.
INITIAL APPROACH TO AKI IN PATIENTS WITH CIRRHOSIS USING POCUS
HRS-AKI diagnosis mandates the exclusion of other causes of AKI, including shock, nephrotoxic drugs, and intrinsic renal damage. However, other potentially correctible causes of AKI such as obstructive AKI and abdominal compartment syndrome (ACS) are sometimes overlooked. Obstructive or post-renal AKI arises from an obstruction downstream of the renal collecting system, commonly due to urinary retention, benign prostatic hyperplasia, nephrolithiasis, malignancy or even an obstructed Foley catheter. Upon ultrasonographic examination, hydronephrosis and dilation of the pelvicalyceal system in suggestive of obstruction, and promptly relieving obstruction is warranted[33]. Tension ascites should also be considered, as IAH (defined as ≥ 12 mmHg) and ACS (defined as ≥ 20 mmHg and signs of organ dysfunction) reduce renal perfusion pressure and lead to AKI[34]. A large volume ascites and a small, collapsed inferior vena cava (IVC) with minimal respiratory variation should raise the suspicion for abdominal hypertension[35,36]. After ruling out obstruction and ACS, as well as shock, nephrotoxic drugs and intrinsic kidney damage, a systematic approach to hemodynamic AKI must be performed.
CAN POCUS ASSESS HEMODYNAMICS BETTER THAN PHYSICAL EXAMINATION?
Increased awareness of the risk of the overzealous administration of intravenous fluids have shifted paradigms towards the evaluation of fluid tolerance instead of fluid responsiveness[37]. Although fluid administration in true hypovolemia is indubitably beneficial, physical exam findings consistent with hypovolemia often lack sensitivity[37]. It is also important to realize that no ultrasonographic parameter can confirm the diagnosis of hypovolemia. Low cardiac filing pressures and absence of venous congestion do not indicate hypovolemia but rather the absence of congestive heart failure. However, the presence of pulmonary and systemic venous congestion is indicative of heart failure and volume intolerance[37]. Clinical evaluation of congestion is often inaccurate. In a study by Torino et al[38], the comparison of lung ultrasound with standardized lung auscultation revealed a low sensitivity (9%) of lung crackles in detecting extravascular lung water in end-stage renal disease. Similarly, Breidthardt et al[39] showed that in patients presenting with acute heart failure to the emergency department, treating physicians were unable to correctly assess the degree of congestion through clinical jugular vein examination when compared to invasive central venous pressure monitoring. Multiple studies have illustrated how POCUS enhances the sensitivity of conventional physical examination across various clinical contexts[40,41].
EVALUATION OF CONGESTION USING POCUS
POCUS has emerged as a non-invasive, bedside diagnostic tool that, when integrated with clinical and laboratory data, offers a holistic insight into a patient's hemodynamic status. This can substantially improve decision-making regarding fluid administration or removal by obtaining qualitative and quantitative data on cardiac function, as well as pulmonary and vascular congestion[42].
Right-sided congestion
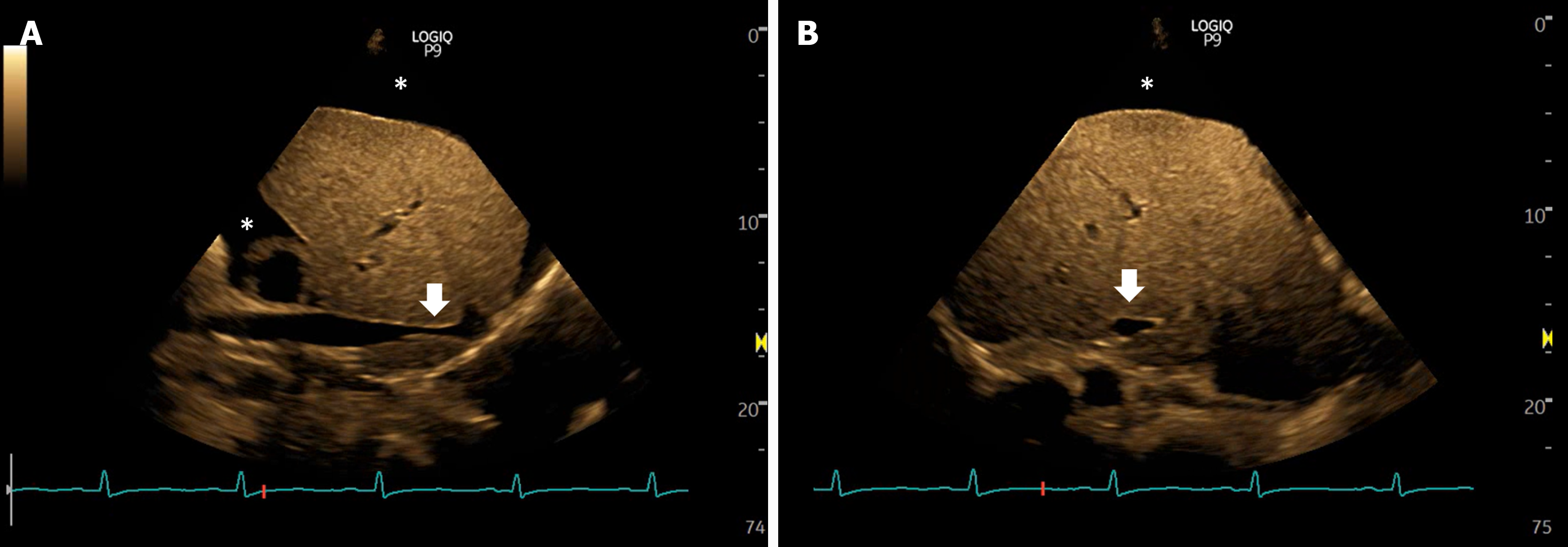
Right atrial pressure (RAP) serves as an estimation of preload, and an elevated RVP is suggestive of an absolute excess of venous return, or a relative one that surpasses cardiac output, which can then lead to congestive organ dysfunction through backward transmission. RAP can be invasively measured through catheterization or non-invasively estimated through POCUS. IVC ultrasound to assess size and respiratory variation has been widely adopted as a way to estimate RAP[43]. In a landmark study by Velez et al[44], authors performed IVC ultrasound in patients with cirrhosis and found that 21% had signs of elevated RAP (IVC diameter > 20 mm), these patients were treated with diuretics with adequate response. Despite this, it is important to recognize that IVC ultrasound in patients with cirrhosis faces important caveats. Hepatic fibrosis and the resulting stiffness can impair pressure-dependent changes in venous diameter[45]. Furthermore, IVC dilation can be secondary to factors unrelated to raised RAP, such as portosystemic collaterals draining to the IVC, constriction from an enlarged caudate lobe, or extension of hepatic vein thromboses or hepatocellular carcinoma into the IVC[46]. Importantly, IAH due to ascites can lead to IVC compression and nipping (Figure 2)[35]. A promising alternative to IVC POCUS to assess RAP is internal jugular vein (IJV) ultrasound. Previous studies in patients without cirrhosis have shown excellent correlation with RAP[43,47-49]. In patients with cirrhosis, IJV POCUS shows better correlation with RAP compared to IVC POCUS, possibly because changes in IJV diameter are independent of liver stiffness and IAH. Importantly, it can be performed even in patients with abundant ascites[50]. However, one should be cautious about caveats in IJV POCUS, including inappropriate head angle, excessive pressure on the vein with the transducer, inaccessibility due to previous thrombosis, variations in right atrial depth (as opposed to the traditional assumption of 5 cm), and the diversity of techniques in literature (e.g., respiratory variation in diameter, cross sectional area, response to Valsalva, column height, etc.).
Figure 2 Views of the inferior vena cava in a case of intra-abdominal hypertension showing narrowing or slit-like appearance of the intrahepatic portion (arrows), sometimes described as nipping.
Asterisk indicates ascites. A: Long axis views; B: Transverse views.
POCUS in the assessment of renal congestion
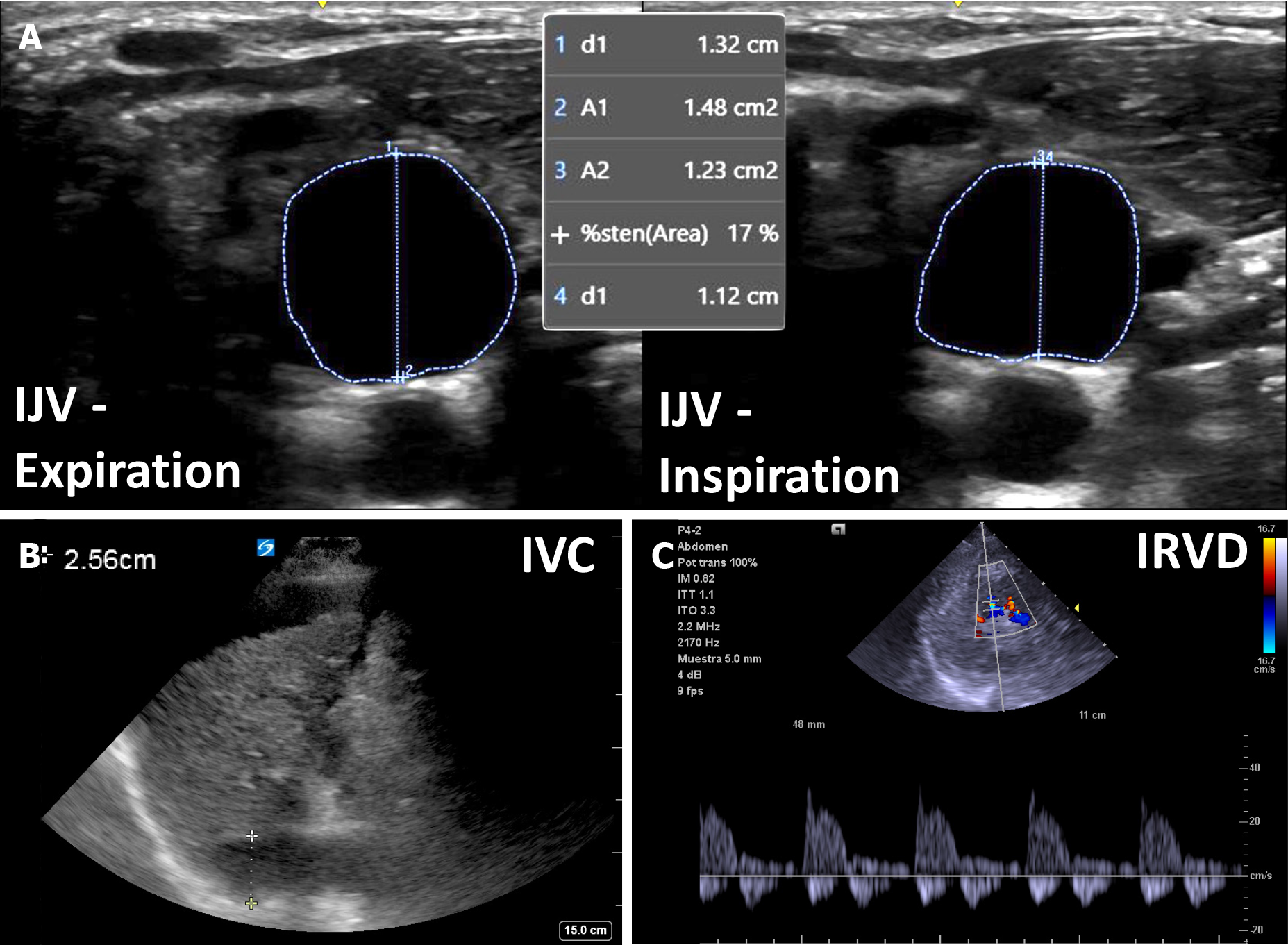
Unlike IVC/IJV POCUS, intrarenal venous doppler ultrasonography (IRVD) evaluates venous compliance of the renal vasculature. As RAP increases, increased volume and venous wall stretch lead to loss of venous compliance. This enhances distal transmission of pulsatile right atrial flow resulting in interrupted venous flow. Normal IRVD is continuous, while it becomes discontinuous with a biphasic or monophasic pattern in moderate and severe congestion, respectively[51]. Prospective studies have shown a strong independent association between congestive IRVD patterns and worse clinical outcomes in patients with heart failure[52] and pulmonary hypertension[53,54], likely mediated by congestion induced worsening renal function and diuretic resistance[55]. Altered IRVD predicts appropriate response diuretic treatment[56], and improvement in IRVD with diuretics is associated with improved clinical outcomes[57]. These data suggest that therapeutic efforts aimed at normalizing altered IRVD could be beneficial. However, intervention trials are needed. It is important to realize that no studies evaluating IRVD have been performed in patients with cirrhosis. However, it is our experience that it accurately reflects venous congestion in most cases. Hepatic and portal veins, commonly employed for assessing systemic venous congestion, may be unreliable in cirrhosis due to local structural and functional changes. Although not a universal observation[58], this area requires further study before making definitive recommendations. Figure 3 shows an example of venous congestion findings in cirrhosis.
Figure 3 An example of venous congestion in cirrhosis.
A: Plethoric internal jugular vein with less than 25% antero-posterior inspiratory collapse; B: Plethoric inferior vena cava (> 2.0 cm); C: Biphasic intra-renal venous Doppler. IJV: Internal jugular vein; IVC: Inferior vena cava; IRVD: Intra-renal venous Doppler.
Left ventricular function, cardiac output, and left-sided congestion
In the setting of venous congestion, as suggested by a plethoric IVC/IJC, cardiac function should be evaluated. Albeit formal echocardiography warrants extensive training, focused cardiac ultrasonography (FoCUS) by non-echocardiographers might be enough to discern between normal, moderate, or severe systolic dysfunction. This was shown by Melamed et al[59], where intensivists with minimal training were able to estimate left ventricular (LV) function with reasonable accuracy when compared with experienced echocardiographers. Specifically, endocardial excursion, myocardial thickening, and septal motion of the anterior leaflet of the mitral valve are useful and feasible to obtain[60]. Moreover, stroke volume can be estimated by advanced POCUS users by measuring the velocity-time integral of the LV outflow tract[61]. Information obtained by FoCUS is crucial as a hyperdynamic circulation is better addressed using vasopressors while a severely depressed systolic function is unlikely to tolerate afterload increasing drugs such as terlipressin. Most patients with cirrhosis and heart failure will present with a preserved ejection fraction and diastolic dysfunction. While assessment of diastolic dysfunction is considered an advanced POCUS application, 6-point or 8-point lung ultrasound (LUS) reliably detects pulmonary congestion through the presence of B lines[62] and this is correlated with increased left ventricular end diastolic pressure[63]. The combination of albumin and terlipressin that is frequently initiated in patients with cirrhosis and AKI creates both an increase in preload and in afterload potentially driving hydrostatic pulmonary edema[64]. Acting upon LUS findings suggestive of pulmonary edema, which often precede clinical signs and symptoms could prevent further decline in respiratory function. In addition, LUS is effective in detecting pleural effusions. In a study of 116 patients with decompensated cirrhosis, anteroposterior chest X-rays missed about 40% of pleural effusions identified by LUS. Interestingly, detecting effusions with LUS was linked to a longer hospital stay (10 d vs 5.5 d, P < 0.001) and doubled mortality (39.7% vs 20.7%, P = 0.021)[65].
PROPOSED ALGORITHM
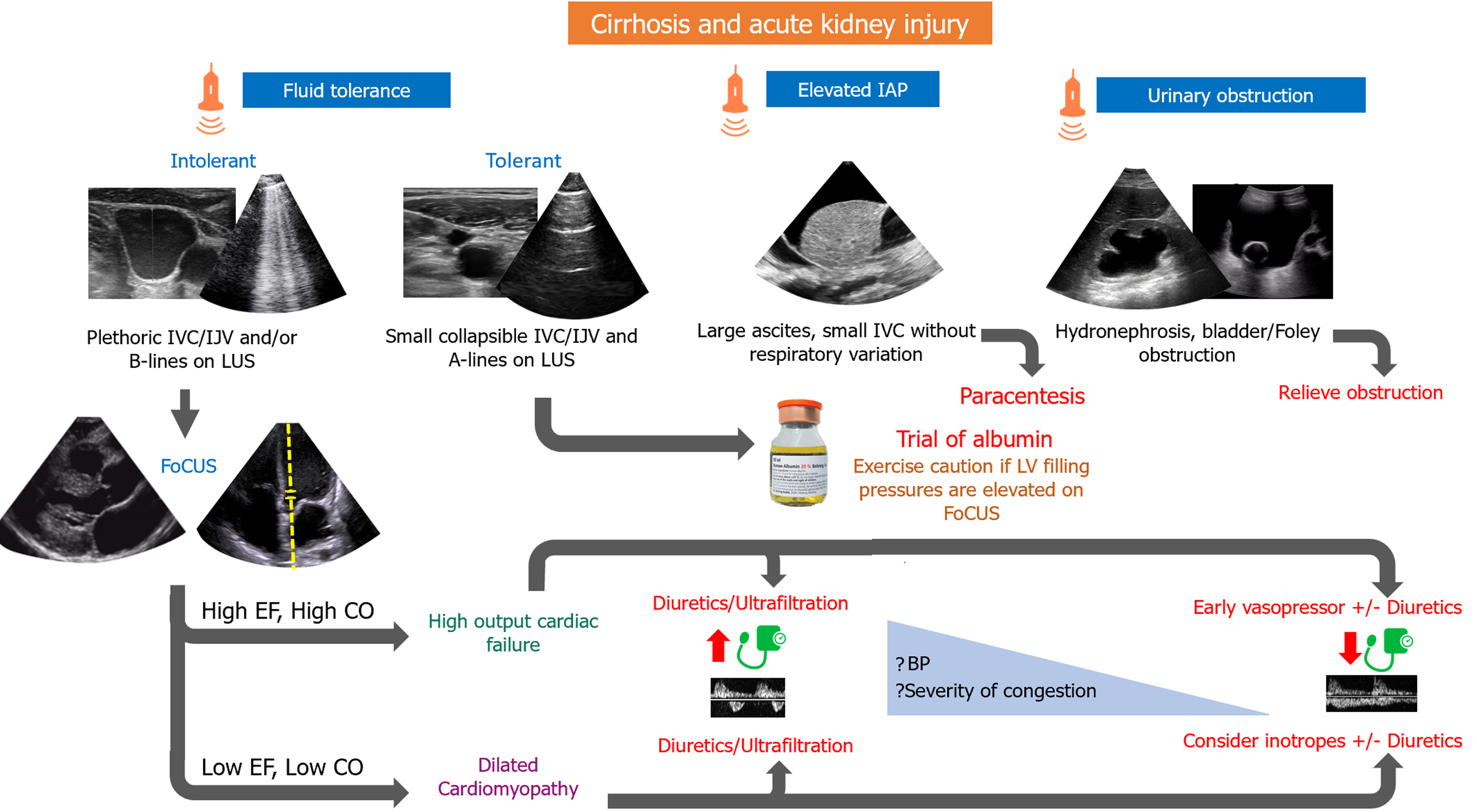
We propose that when evaluating a patient with AKI and cirrhosis, obstructive and intrinsic causes, as well as ACS, must initially be ruled out. In the case of hemodynamic AKI, fluid tolerance must be assessed through IJV/IVC POCUS, as well as LUS. If a patient is deemed fluid tolerant, an albumin trial could be a reasonable approach. In such cases, if FoCUS performed by an advanced user or a formal echocardiogram reveals Doppler stigmata of elevated left ventricular filling pressures, risks vs benefits of albumin therapy must be reweighed. On the other hand, if a patient is found to be fluid intolerant (pulmonary or venous congestion), then heart failure is diagnosed, and further cardiac evaluation should be performed. Most patients with cirrhosis and heart failure will display a hyperdynamic phenotype [high ejection fraction (EF), high cardiac output (CO)]. In these cases, decongestion should be attempted. Increased urine output and natriuresis can be achieved by means of diuretic drugs or by initiating vasopressor therapy in patients with inadequate mean arterial blood pressure. Vasopressors lead to increased renal blood flow and thus higher urine output. If congestion is severe, ultrafiltration might be needed in the presence of severe diuretic resistance (Figure 4).
Figure 4 Proposed algorithm for management of cirrhosis and acute kidney injury.
IAP: Intra-abdominal pressure; IJV: Internal jugular vein; IVC: Inferior vena cava; LUS: Lung ultrasound; EF: Ejection fraction; CO: Cardiac output; LV: Left ventricular; BP: Blood pressure; FoCUS: Focused cardiac ultrasound.
This approach does not apply to patients who present with concomitant advanced cardiomyopathy and decreased contractility (low EF, low CO) where vasopressors could lead to further decrease in cardiac output. Besides diuretics/ultrafiltration, adequate decongestion may require inodilator drugs.
CONCLUSION
The blurred line between hepatorenal and cardiorenal syndrome is often difficult to discern, and hemodynamic AKI in patients with cirrhosis warrants a systematic evaluation of a patient´s individual physiology to provide a tailored approach to treatment as opposed to an empirical one. When evaluating AKI in cirrhosis, cardiac function is crucial but is frequently disregarded. A thorough hemodynamic assessment will aid in detecting those patients that do not benefit from routine HRS-AKI treatment with albumin and vasoconstrictors, and instead benefit from decongestion or inotropic drugs. Future investigations should consider incorporating multi-organ POCUS in the evaluation of these patients, moving away from isolated organ assessments. Prospective studies are needed to validate the proposed algorithm in larger cohorts and examine the impact of POCUS-guided interventions on patient outcomes. This approach should focus on understanding the effects of incorporating POCUS on tangible outcomes such as successful decongestion, clinical improvement, duration of intensive care unit stays, and hospitalization. It is important to note that expecting a mortality benefit solely from the use of a diagnostic test (POCUS) might be overly optimistic, given the limited therapies demonstrating mortality benefit in this patient population.
Having said that, it is crucial to exercise caution, as POCUS is operator-dependent, and effective patient management relies heavily on the operator’s skill in image acquisition, accurate interpretation, and clinical integration of data in the appropriate context. As such, it is necessary to have comprehensive training in POCUS across all levels of medical education, with robust quality assessment programs in place. There is an urgent need to establish global standards in POCUS training and competency assessment, and professional societies should collaborate to form multidisciplinary expert committees for this purpose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Liu Y, China S-Editor: Che XX L-Editor: A P-Editor: Cai YX