Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.90746
Peer-review started: December 13, 2023
First decision: January 15, 2024
Revised: January 19, 2024
Accepted: February 20, 2024
Article in press: February 20, 2024
Published online: March 9, 2024
Processing time: 83 Days and 3.5 Hours
The discovery and utilization of volatile anesthetics has significantly transformed surgical practices since their inception in the mid-19th century. Recently, a paradigm shift is observed as volatile anesthetics extend beyond traditional confines of the operating theatres, finding diverse applications in intensive care settings. In the dynamic landscape of intensive care, volatile anesthetics emerge as a promising avenue for addressing complex sedation requirements, managing refractory lung pathologies including acute respiratory distress syndrome and status asthmaticus, conditions of high sedative requirements including burns, high opioid or alcohol use and neurological conditions such as status epilepticus. Volatile anesthetics can be administered through either inhaled route via anesthetic machines/devices or through extracorporeal membrane oxygenation circuitry, providing intensivists with multiple options to tailor therapy. Further
Core Tip: This paper sets to explore the transformative impact of volatile anesthetics on surgical practices and their expanding role into intensive care settings. In this paradigm shift, volatile anesthetics prove a promising therapy modality with diverse applications in the critically ill patient population. From addressing intricate sedation needs to managing refractory seizure conditions, volatile anesthetics are a useful addition to intensivists’ toolkits.
- Citation: Wieruszewski ED, ElSaban M, Wieruszewski PM, Smischney NJ. Inhaled volatile anesthetics in the intensive care unit. World J Crit Care Med 2024; 13(1): 90746
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/90746.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.90746
The discovery and application of volatile anesthetics has revolutionized surgical practices, with some of the earliest applications dating back to the mid-19th century when ether and chloroform were first utilized[1,2]. Since then, the use of inhaled anesthetics has evolved to be a fundamental component of anesthetic applications worldwide. Traditionally confined to the realm of operating theatres, volatile anesthetics are starting to carve niche uses among intensive care practices (Table 1).
| MAC (%) | Blood:Gas at 37oC | Boiling point (oC) | Odor | Metabolism (%) | Cardiovascular effects | Central nervous system effects | |
| Halothane | 0.75 | 2.4 | 122 | Organic solvent | 15-20 | Decrease CO, decrease HR | Decrease CPP, increase CBF |
| Isoflurane | 1.15 | 1.4 | 48 | Ethereal/pungent | 0.2 | Decrease CO, increase HR, decrease SVR | Decrease CPP, increase CBF |
| Desflurane | 6.0 | 0.4 | 23 | Ethereal/pungent | 0.02 | Increase HR, decrease SVR | Decrease CPP, increase CBF |
| Sevoflurane | 2.0 | 0.68 | 59 | Organic solvent | 5 | Decrease SVR | Decrease CPP, increase CBF. Can induce epileptiform EEG |
In the dynamic landscape of intensive care, the utilization of volatile anesthetics is emerging as a promising avenue for various applications. These include addressing complex sedation requirements, managing refractory lung pathologies such as acute respiratory distress syndrome (ARDS) and status asthmaticus, employing them in conjunction with mechanical circulatory support, and managing neurological pathologies like status epilepticus (Table 2)[3-7]. The unique pharmacokinetic profiles of volatile anesthetics, characterized by rapid onset and offset, make them particularly well-suited for the intricate balance required in intensive care units[8]. Furthermore, their ability to achieve precise titration empowers clinicians to tailor management with heightened precision and thereby mitigating the risks associated with traditional modalities of sedation such as benzodiazepines[9,10].
| Indication | Agents studied | Advantages | Disadvantages |
| Short-term postoperative | Desflurane, isoflurane, sevoflurane | Quick awakening; Faster extubation; Titratability; Minimal drug interactions; Minimal metabolism; Provides analgesia | No benefit on ICU length of stay; Reduces blood pressure |
| Prolonged sedation during mechanical ventilation | Isoflurane, sevoflurane | Faster return to spontaneous breathing; Titratability; Minimal drug interactions; Minimal metabolism; Provides analgesia | Special equipment required in ICU; Reduces blood pressure |
| Status asthmaticus | Isoflurane, sevoflurane | Bronchodilation | Reduces blood pressure |
| Status epilepticus | Isoflurane, desflurane | Sustained EEG burst suppression | May increase intracranial pressure through cerebral vasodilation |
| ARDS | Isoflurane, sevoflurane | Lung protective; Anti-inflammatory | Special equipment required in ICU; Reduces blood pressure |
| COVID-19 | Isoflurane, sevoflurane | Decreased sedative, NMBA requirements | Special equipment required in ICU; Reduces blood pressure |
| Other high sedative requirements (burn, alcohol or opioid use at baseline) | Isoflurane, sevoflurane | Decreased inflammation in burns; Decreased sedative requirements | Not proven in literature, hypothesis generating at this time |
This paper delves into the application of inhaled volatile anesthetics in intensive care units (ICUs) by exploring their pharmacological characteristics, administration modalities, listing various applications, and providing a comprehensive review of the evidence and potential future advances in this field.
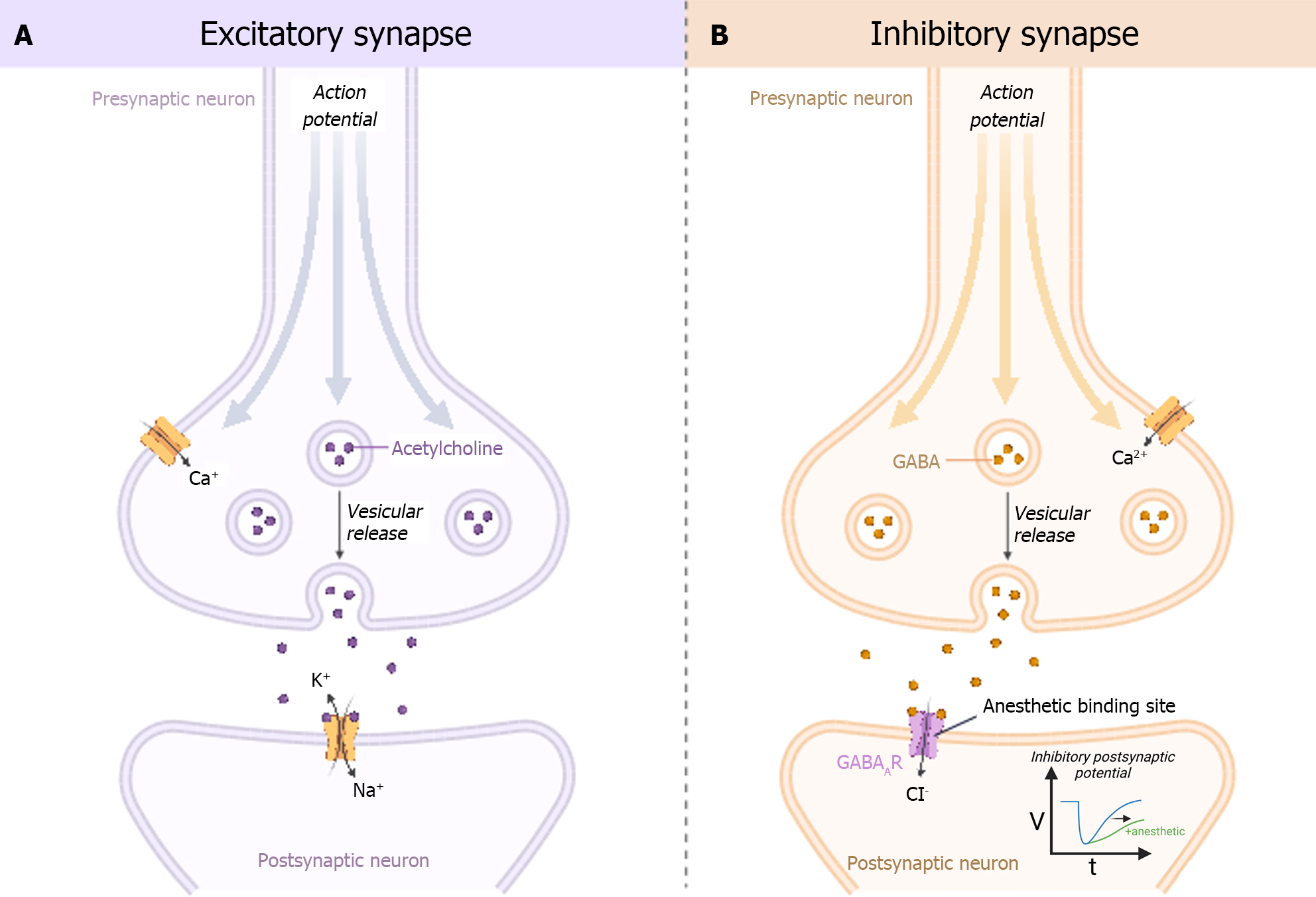
Over a century ago, scientists proposed the Meyer-Overton rule, which states that the potency of an anesthetic is linearly correlated to its oil/water partition coefficient. The downstream or indirect effects of this theory postulate that volatile anesthetics disrupt the lipid bilayer of cell membranes, according to their potency, thereby inducing conformational changes to proteins in the membrane resulting in its anesthetic effect. However, numerous examples (short chain 1-alkanols, perfluorinated alkanes, perfluoroalkyl methanols) have been described that seem to contradict this theory, suggesting alternative mechanisms by which volatile anesthetics exert their effect. Although data are sparse, some theorize that volatile anesthetics bind to specific ligand-gated ion channels within the cell membrane exerting a more direct effect. Others theorize that the anesthetic effects of volatile anesthetics are the result of disrupted lateral stresses in the lipid bilayer (lateral pressure profile) that are mechanistically linked to altered protein conformational equilibria[11]. Given the aforementioned evidence and admitting a degree of uncertainty, in general, volatile anesthetics appear to exert their effects through the central nervous system by augmenting signals to chloride and potassium channels through γ-Aminobutyric acid (GABA) receptors, and attenuating excitatory neurotransmission pathways through glutamate, acetylcholine, nicotinic, serotonin, and N-methyl-D-aspartic acid (NMDA) receptors (Figure 1)[12].
In general, there are two main methods of delivery of volatile anesthetics to ICU patients. One option includes the use of an anesthesia machine that allows for similar ventilator modes of ICU ventilators yet allows for rebreathing and scavenging of volatile anesthetics. Case reports have described the use of anesthesia machines used in the operating theatre that have been transported to the ICU for administration of volatile anesthesia in patients with acute respiratory failure such as from status asthmaticus[13,14].
The second option is to use a device, such as the Anesthesia Conserving Device (AnaConDa), placed at the y-connector of the breathing circuit that allows for heated humidification, vaporization of anesthetic, and reflection of volatile gas to allow for rebreathing, minimizing the total requirement overall[15]. This system requires a medication pump to administer the liquid anesthetic, a gas scavenging system attached to the ICU ventilator, and a gas monitor to measure the end-tidal concentration of volatile anesthetic. The MIRUS™ (TIM, Koblenz, Germany) device is a newer system that also uses a reflector and allows administration of isoflurane and sevoflurane, but unlike the AnaConDa, it also allows for administration of desflurane[16]. Additionally, the MIRUS™ system also has a feature for adaptive regulation of end-tidal anesthetic concentration.
Extracorporeal membrane oxygenation (ECMO) is a form of temporary life supportive therapy that provides a bridge to recovery, transplantation, or durable ventricular support in patients with medically refractory cardiac and/or respiratory failure. There is evolving evidence supporting a so-called ‘awake ECMO’ wherein sedation is minimized, allowing for participation in physical rehabilitation to promote recovery, particularly in the pre-transplantation setting[17]. However, sedation may still be necessary to facilitate safe ECMO flow, promote comfort, and prevent excessive respiratory effort in certain circumstances which may lead to patient self-induced lung injury, as was increasingly seen during the coronavirus infectious disease (COVID)-19 pandemic[18]. The utilization of ultra-protective ventilator settings during ECMO support poses challenges to traditional inhalational anesthetic delivery through orotracheal tubes due to excessively low minute ventilation[17].
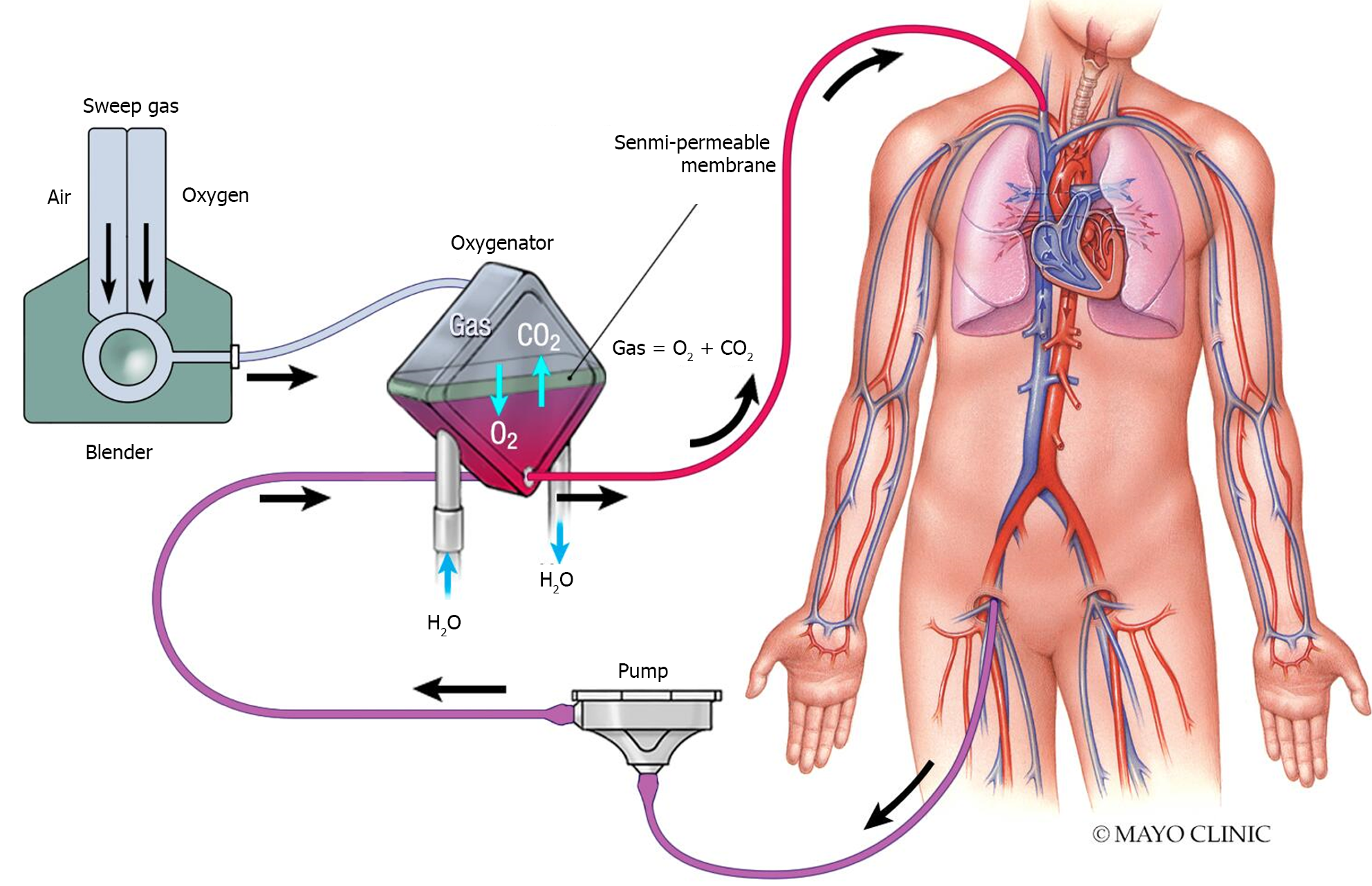
During ECMO, circulating blood is oxygenated and decarboxylated by a membrane that is composed of hollow fibers. A sweep gas (typically blended oxygen) passes through these fibers to facilitate gas exchange (Figure 2). Case reports have described successful administration of sevoflurane (using the AnaConDa device)[19] and isoflurane (using an isoflurane vaporizer)[20] through direct insertion in the ECMO sweep gas airline, between the blender and the membrane oxygenator. The sweep gas flow rate can be used to calculate the anesthetic consumption and estimate the effective concentration[21]. While oxygenators constructed of polymethylpentene fibers are becoming increasingly popular in ECMO for their longer-term durability and reducing the ‘plasma leakage’ phenomenon, their non-porous (diffusion-based) surface appears to limit the transfer of volatile anesthetics[22,23]. Microporous polypropylene oxygenators may be preferred if delivering volatile anesthetics through the membrane during ECMO support. Modifying the ECMO circuity to deliver anesthetic gas requires careful considerations including a collection system for gas from the membrane gas outlet and any gas from the native expiration of the ventilator[19].
Malignant hyperthermia (MH) is a rare but serious adverse effect of all inhaled anesthetics, except nitrous oxide, as well as depolarizing neuromuscular blockers, such as succinylcholine, whereby the skeletal muscles exhibit a hypermetabolic state[24]. It occurs seldom, less than once in the professional lifetime of an anesthesiologist (approximately 1 in every 250000 anesthetic exposures). Although rare, it can be a serious and deadly complication of volatile anesthetics, fortunately however, it is readily treatable when recognized. Despite evidence of a genetic link, it can also occur in individuals that don't have a genetic predisposition (no documented family history).
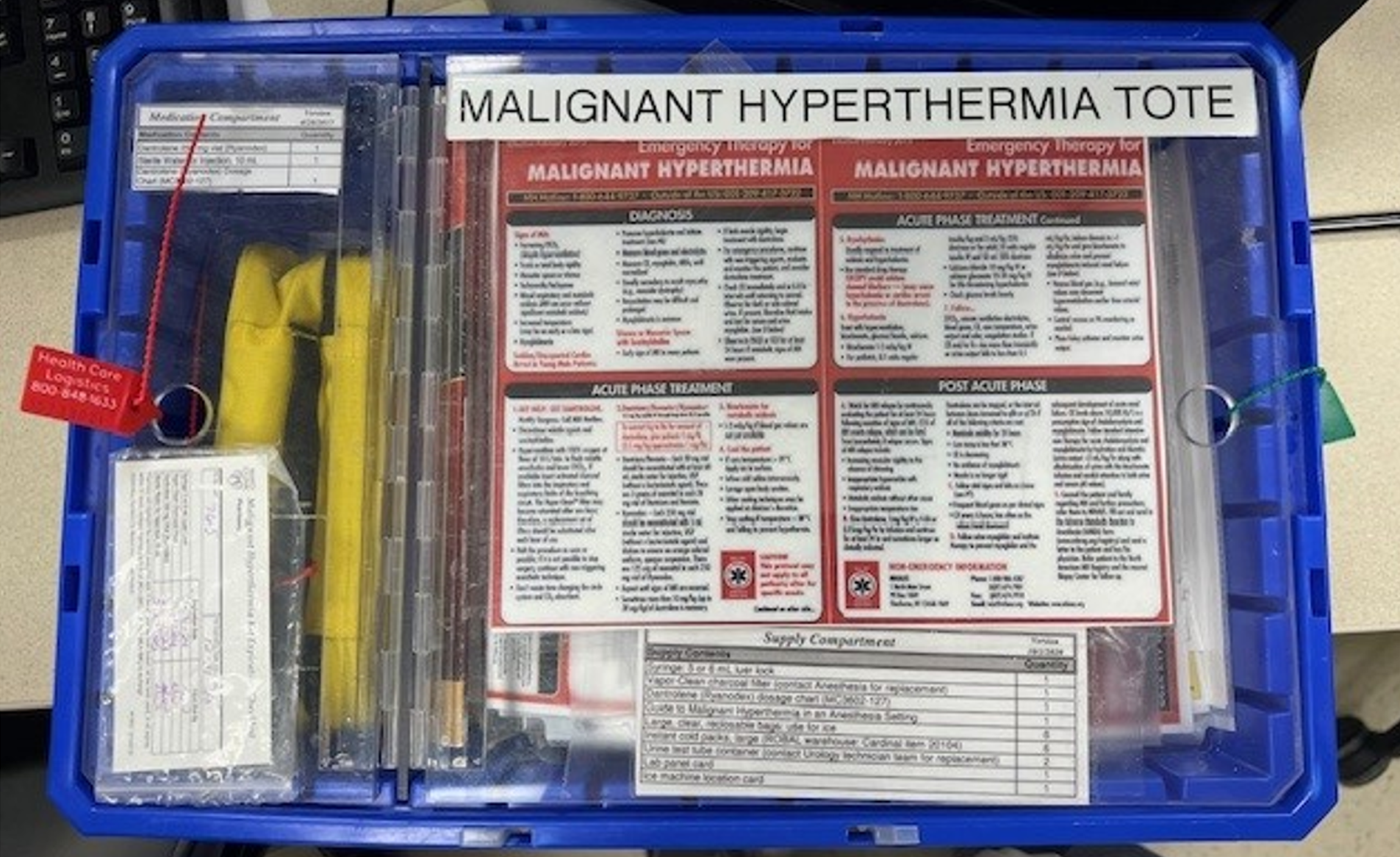
The features of MH are non-specific and include a rapidly increasing carbon dioxide level despite increased minute ventilation (usually the first sign), high fever (≥ 103°F), muscle rigidity, tachycardia, arrhythmias, hypotension, and a new unexplained lactic acidosis[24]. MH can be readily treated by stopping the offending agent, administering dantrolene, and providing supportive care[24]. Having an emergency treatment plan and pre-prepared kits available in the ICU is critical as it is an environment that may be less familiar with MH (Figure 3). There is an MH hotline to call in case of an emergency or questions, +1-800-644-9737.
Traditionally, intravenous-based anesthesia has been the mainstay for providing sedation in the ICU. With the development of specific devices to safely deliver volatile anesthetics, there has been growing interest in applying them in the ICU, without the need for traditional anesthesia machines that are used in operating theatres. In a general ICU population requiring mechanical ventilation, randomization to isoflurane using the AnaConDa device resulted in less opioid consumption, more frequent day 1 spontaneous breathing, and faster awakening with shorter time to extubation when compared to propofol[25]. A meta-analysis of 13 trials assessing volatile anesthesia in a variety of ICU clinical conditions found shorter awakening times and time to extubation, but no differences in length of stay[26]. The following sections will discuss specific conditions encountered in ICU practice that may be of particular interest for use of volatile anesthesia.
Although volatile anesthesia is commonly deployed throughout the operating theatres, continuation into the early postoperative setting following surgery has been a growing area of interest (Table 3). There have been a few trials of brief postoperative volatile anesthesia following non-cardiac operations[3,27,28], and several following cardiac operations that utilize cardiopulmonary bypass[29-34]. In general, use of volatile anesthetics upon arrival to the ICU for brief (few hours) sedation until appropriate for extubation, appears to allow for quicker awakening and faster time to extubation compared to propofol-based sedation (Table 3). Despite this, no study has demonstrated that this benefit results in any differences in ICU length of stay.
| Ref. | Treatment | Surgeries | Sedation duration | Time to awakening/extubation | Other outcomes |
| Non-cardiac surgery | |||||
| Bellgardt et al[3], 2019, Randomized trial | Isoflurane with MIRUS™ (n = 10) | Major surgery (aortic, pancreatic, esophagectomy, spinal fusion, hyperthermic intraperitoneal chemotherapy, necrotizing fasciitis) | 17.9 (16.6–20.6) h | NR | Isoflurane had longest awakening times followed by sevoflurane, with desflurane the shortest (open eyes, follow verbal commands, extubation, tell birthday). Desflurane was most expensive followed by sevoflurane, with isoflurane the cheapest (per hour) |
| Sevoflurane with MIRUS™ (n = 10) | 16.5 (10.4–37.4) h | NR | |||
| Desflurane with MIRUS™ (n = 10) | 18.8 (14.1–33.8) h | NR | |||
| Jung et al[27], 2020, Prospective interventional | Sevoflurane with AnaConDa (n = 25) | Head and neck surgery with tracheostomy | 771 ± 338.4 min | NR | Sevoflurane required less continuous opioid. Similar vasopressor use and length of stay |
| Propofol (n = 24) | 1508 ± 2074.7 min | NR | |||
| Romagnoli et al[28], 2017, Prospective interventional | Sevoflurane with MIRUS™ (n = 62) | Laparoscopic and robotic-assisted noncardiac | 3.33 (2.33–5.75) h | 4 (2.2–5) min (awakening after drug cessation) | No adverse effects. Pollution < 1 ppm at all timepoints assessed |
| Cardiac surgery | |||||
| Hellström et al[29], 2011, Randomized trial | Sevoflurane with AnaConDa (n = 50) | Elective or subacute coronary artery bypass grafting using cardiopulmonary bypass | 176 min | NR | Sevoflurane had less intense increase in troponin at 12 h. Similar hemodynamics and length of stay |
| Propofol (n = 50) | 221 min | NR | |||
| Jerath et al[30], 2015, Randomized trial | Isoflurane or sevoflurane with AnaConDa (n = 67) | Elective coronary artery bypass grafting using cardiopulmonary bypass | NR | 182 (140–255) min (extubation after ICU arrival) | No adverse effects. Similar hemodynamics and lengths of stay |
| Propofol (n = 74) | NR | 292 (210–420) min (extubation after ICU arrival) | |||
| Röhm et al[31], 2008, Randomized trial | Sevoflurane with AnaConDa (n = 35) | Elective coronary artery bypass grafting using cardiopulmonary bypass | 8.1 ± 3.5 h | 9.0 ± 4.0 h (extubation after ICU arrival) | Sevoflurane had faster times of recovery after sedation cessation (eye opening, following commands, hand grip, and extubation). Similar ICU length of stay, sevoflurane with lower hospital length of stay |
| Propofol (n = 35) | 8.4 ± 4.2 h | 12.5 ± 5.8 h (extubation after ICU arrival) | |||
| Soro et al[32], 2012, Randomized trial | Sevoflurane with AnaConDa (n = 36) | Elective coronary artery bypass grafting using cardiopulmonary bypass | NR | NR | No differences in postoperative cardiac biomarkers, hemodynamics, or lengths of stay |
| Propofol (n = 37) | NR | NR | |||
| Steurer et al[33], 2012, Randomized trial | Sevoflurane with AnaConDa (n = 46) | Valve replacement with cardiopulmonary bypass | At least 4 h | NR | Sevoflurane had lower troponin T and creatine kinase concentrations on postoperative day 1 |
| Propofol (n = 56) | At least 4 h | NR | |||
| Wąsowicz et al[34], 2018, Randomized trial | Isoflurane (n = 30) or sevoflurane (n = 30) with AnaConDa | Elective or urgent coronary artery bypass grafting using cardiopulmonary bypass | NR | 172.1 ± 175.5 min (extubation after ICU arrival) | No difference in postoperative troponin values or ICU or hospital length of stay |
| Propofol (n = 67) | NR | 219.6 ± 104.9 min (extubation after ICU arrival) | |||
In patients undergoing cardiac surgery (mainly coronary artery bypass grafting) with use of cardiopulmonary bypass, some trials suggest a myocardial protective effect with postoperative volatile anesthesia[29,33], while others have shown no differences[32,34]. Furthermore, hemodynamics and use of vasoactive agents were also similar between volatile anesthetic and propofol groups.
The Society of Critical Care Medicine Pain Agitation and Delirium 2018 guidelines make no recommendations for the use of inhaled anesthetics for prolonged sedation due to lack of randomized control trial evidence[35]. There are several advantages to utilizing inhaled anesthetics for prolonged sedation during mechanical ventilation in the ICU, including associated opioid-sparing effects, increased time spent at goal sedation targets, decreased time to extubation, and ease of titration when compared to continuous intravenous sedation (Table 2)[36,37].
A 2016 retrospective cohort study by Bellgardt et al[38] compared surgical ICU patients receiving ventilation and sedation for at least 96 h via continuous infusion propofol and midazolam with or without inhaled isoflurane with an AnaConDa delivery device within 72 h of an initial intubation event. A Ramsay sedation score of 2-4 was targeted for all patients and the primary outcome of interest was inpatient mortality. One hundred twenty-eight patients were included in the intravenous sedation group, and 72 patients were in the intravenous plus inhaled anesthetic group over a 6-year time frame. Isoflurane utilization was associated with decreased in hospital mortality 40% vs 63%, adjusted odds ratio 0.39 (95%CI, 0.22-0.71, P = 0.002). While this study has many limitations, including its retrospective nature and mixed surgical population, it raises questions of the benefits of inhaled anesthetic use in the ICU for prolonged sedation[38].
A 2022 meta-analysis of 15 studies, including 1520 patients, compared inhaled vs intravenous anesthetics and their effect on patient outcomes. This study revealed similar mortality rates with inhaled anesthetics vs intravenous sedation but the used of inhaled anesthetics was associated with decreased duration of ventilation (P = 0.03), time to awakening (P = 0.04) and cardiac troponin levels (P < 0.001). These outcomes remained true in the subpopulations examined, including surgical vs medical ICU patients, as well as those treated with propofol vs other continuous intravenous sedatives[5].
Status asthmaticus is defined as life-threatening bronchospasm refractory to treatment that has significant morbidity and mortality implications. Importantly, 21% of patients with status asthmaticus requiring mechanical ventilation die[39]. In a retrospective report of 30-year experience of management of status asthmaticus, 61.2% of patients required intubation and experienced an overall mortality of 0.4%[40]. Aside from the algorithmic managements of status asthmaticus that include hallmark use of short acting inhaled beta-agonists, systemic steroids, anticholinergics, and magnesium[41,42]. Advanced therapies such as venovenous ECMO and inhaled volatile anesthetics have been trialed and reported in case reports/series[43,44]. Volatile anesthetics are proposed to facilitate bronchorelaxation through direct relaxation of airway bronchial smooth muscles[45], beta-2-adrenergic stimulation, inhibition of inflammatory markers such as tumor necrosis factor alpha, transforming growth factor beta, and vascular endothelial growth factor[46], and inhibition of vagal-mediated reflexes. Volatile anesthetics may be delivered via direct inhalation, during mechanical ventilation, or through ECMO circuits as previously described[13,14,45-48]. Limitations to volatile anesthetic use in this context include limited availability of resources such as anesthesia machines outside the operating theatres, sufficient technology to integrate existing anesthetic conserving systems seamlessly and safely to ECMO circuitry, and trained personnel availability for the duration of treatment.
The majority of data for treatment of status asthmaticus with inhaled volatile anesthetics is in the pediatric population, although the concepts of mechanism and outcomes may be extrapolated to adults, evidence is lacking[41,49]. In a 2015 case series of three pediatric patients treated with volatile anesthetics showed use of isoflurane in addition to intubation and standard care for asthma resulted in safe use for 3-17 d, resulting in improved arterial carbon dioxide and tidal volumes. The only adverse effect reported was mild hypotension in one patient[41]. A recent retrospective, descriptive cohort of 45 pediatric ICU patients receiving isoflurane with or without ECMO showed improved arterial carbon dioxide levels and acidosis within four hours of anesthetic initiation[50].
Refractory status epilepticus is a severe subset of status epilepticus (continuous clinical/electroencephalogram-based seizure activity or recurrent seizures without recovery) that continues despite first and second line therapies[51]. Often prolonged sedation with barbiturates, propofol, and benzodiazepines is needed to break through this refractory condition. Volatile anesthetics pose as an option in the management of status epilepticus particularly in refractory states. Volatile agents apply their anticonvulsant properties through promotion of the inhibitory GABA-a pathways and inhibition of the excitatory NMDA pathways (Figure 1)[52,53]. In addition, with prolonged seizures NMDA receptors are upregulated resulting in a vicious cycle that potentiates glutamate-mediated excitotoxicity[54]. Volatile anesthetic gases counteract that through their cerebral protective properties with inhibition of this glutamate mediated NMDA excitotoxicity. In particular, isoflurane and desflurane have been shown to possess NMDA inhibitive properties.
A case report by Zhumadilov et al[55] reveals a remarkable response to isoflurane for the management of refractory status epilepticus. Reviews and case series showing efficacy of their use in pediatric and adult populations have also been reported[56-58]. With regards to which agents can be used, in general the majority of inhaled anesthetics result in electroencephalographic burst suppression with the best evidence surrounding isoflurane use. In particular, enflurane should be avoided as it lowers the seizure threshold and can induce seizure activity[59]. The epileptogenicity of sevoflurane has been scrutinized with conflicting evidence, where some studies reveal increased epileptiform discharges, and animal studies also reveal some epileptogenic effects[60-62].
ARDS is a pulmonary disorder defined by non-cardiogenic pulmonary edema, resulting in severe hypoxemia and is treated with protective mechanical ventilation and often requires deep levels of sedation to promote ventilation synchrony and prevent patient self-induced lung inury[63,64]. In 2020 with the COVID-19 pandemic, patients in ICUs across the world developed ARDS and had extremely high sedative requirements, and often required neuromuscular blocking agent administration to allow for lung protective ventilation[65,66].
Inhaled halogenated anesthetics have a potential multimodal benefit in ARDS, as they have opioid or other intravenous sedative agent sparing effects as well as potential for lung protective effects. Halogenated anesthetics in animal models have been shown to preserve epithelial tight junction integrity and decrease capillary leak, therefore decreasing direct acute injury to alveoli which is a hallmark effect of ARDS[67,68].
Several small case series have been published, showing promising effects of isoflurane and sevoflurane, with or without intravenous sedation, specifically in patients who were unable to reach optimal sedation goals on intravenous sedative agents alone[69-71]. This decrease in intravenous sedation use was critical at the peak of COVID-19 due to widespread drug shortages of commonly used sedative agents and continuous intravenous opioids. The use of inhaled anesthetics is attractive for their ability to spare the use of these agents in these challenging settings[72].
In the largest study to date of this population, Coupet and colleagues conducted a retrospective cohort study of 196 patients with COVID-19 ARDS from 10 European and United States centers; 85 patients received intravenous sedation only, 111 received intravenous and inhaled sedation[4]. Patients receiving inhaled sedation were administered it for a median of 5 d and it was most commonly sevoflurane. The primary outcome of interest was ventilation free days through 28 d. After propensity matching and multivariable adjustment, there were no differences in ventilation-free days between the two groups, although both groups had a median of 0 ventilation-free days at 28 d, highlighting the severity of illness in this cohort[4].
Similar to COVID-19 ARDS, several patient populations have extraordinarily high anesthetic requirements and would benefit from the opioid and sedative sparing effects of inhaled anesthetics, including those with burns, alcohol use, or high opioid use at baseline. Patients with significant alcohol, benzodiazepine or opioid tolerance at baseline have altered neurotransmitter sensitivity and typically require higher doses of sedatives to achieve sedation goals[73]. In small retrospective studies, isoflurane and sevoflurane have been shown to decrease opioid, propofol, and neuromuscular blockade requirements in patients with ARDS[69-71]. This makes inhaled anesthetics an attractive adjunctive agent in these patient populations.
Burn patients experience pharmacokinetic changes, systemic inflammation, and increased volume of distribution which alters drug metabolism and further complicates the ability to manage the severe pain they experience[74,75]. Sevoflurane has anti-inflammatory properties, wherein it down regulates interleukin-8; this is hypothesized to be beneficial in the highly inflammatory state post-burn. A study of 12 mechanically ventilated burn patients receiving sevoflurane for procedural sedation, such as dressing changes (2-4 h periods of sedation), compared to non-burn controls has been conducted to assess the pharmacokinetics in this population[76]. The authors concluded that use of sevoflurane in this population is safe but has altered metabolism and prolonged clearance. More studies are needed to assess the clinical utility of sevoflurane for pain management in this population[6].
Although positive data are emerging leading to enthusiasm for use of volatile anesthesia in the ICU, barriers remain[77]. Staff education and development of safe devices and technology will be paramount to continued successful implementation of volatile anesthetics in the ICU. In addition, further development of technology, such that devices can be safely adapted to unique scenarios, such as integration with ECMO circuitry, is needed. And lastly, high quality studies assessing volatile anesthesia and intravenous anesthesia in the ICU, across various clinical conditions are needed.
Currently, there are two parallel phase 3, multicenter, randomized, controlled, open-label, assessor blinded trials on-going in the United States to evaluate the efficacy and safety of inhaled isoflurane delivered via the Sedaconda anesthetic conserving device-S compared to intravenous propofol for sedation of mechanically ventilated intensive care unit adult patients (NCT05312385, NCT05327296). Additional ongoing trials include a multicenter, randomized, controlled, open-label trial in France evaluating the frequency of occurrence of delirium of intravenous propofol compared to inhaled sedation with isoflurane (NCT04341350) and another multicenter, randomized, controlled, open-label trial in Canada evaluating the effects on ventilatory parameters and survival between intravenous sedation and inhaled sedation with either isoflurane or sevoflurane (NCT04415060).
In conclusion, evidence is accumulating suggesting benefits of employing volatile anesthetics for patients in the ICU with indications ranging from ARDS to status epilepticus. While volatile anesthetics are widely utilized in operating theatres worldwide, their underutilization in the ICU persists, potentially influenced by a multitude of structural or medical considerations and lingering uncertainties on quality of evidence supporting benefits. Of note, technological developments are changing the landscape, whereby the simplification of volatile anesthetic handling poses a possible avenue for broader implementation in ICU settings, particularly for conditions refractory to traditional intravenous modalities.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao L, United States S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Duncum BM. The development of inhalation anaesthesia. London: Royal Soc. of Med. Press. |
| 2. | Nunn JF. Nunn's applied respiratory physiology. 6th ed. Edinburgh Philadelphia: Elsevier Butterworth Heinemann. |
| 3. | Bellgardt M, Georgevici AI, Klutzny M, Drees D, Meiser A, Gude P, Vogelsang H, Weber TP, Herzog-Niescery J. Use of MIRUS™ for MAC-driven application of isoflurane, sevoflurane, and desflurane in postoperative ICU patients: a randomized controlled trial. Ann Intensive Care. 2019;9:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 4. | Coupet R, Schläpfer M, Neff TA, Boucher P, Bailly P, Bellgardt M, Badenes R, Carbonell J, Becher T, Varillon C, Morand D, Blondonnet R, Constantin JM, Pereira B, O'Gara B, Jabaudon M; ISCA Study Group. Inhaled Sedation in Patients with COVID-19-Related Acute Respiratory Distress Syndrome: An International Retrospective Study. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Likhvantsev V, Landoni G, Ermokhina N, Yadgarov M, Berikashvili L, Kadantseva K, Grebenchikov O, Okhinko L, Kuzovlev A. Halogenated anesthetics vs intravenous hypnotics for short and long term sedation in the intensive care unit: A meta-analysis. Med Intensiva (Engl Ed). 2023;47:267-279. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Perbet S, Bourdeaux D, Lenoire A, Biboulet C, Pereira B, Sadoune M, Plaud B, Launay JM, Bazin JE, Sautou V, Mebazaa A, Houze P, Constantin JM, Legrand M; PRONOBURN group. Sevoflurane for procedural sedation in critically ill patients: A pharmacokinetic comparative study between burn and non-burn patients. Anaesth Crit Care Pain Med. 2018;37:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Mikkelsen ME, Woo YJ, Sager JS, Fuchs BD, Christie JD. Outcomes using extracorporeal life support for adult respiratory failure due to status asthmaticus. ASAIO J. 2009;55:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Gropper MA (ed). Miller's anesthesia. Ninth edition. Philadelphia, PA: Elsevier. |
| 9. | De Bels D, Bousbiat I, Perriens E, Blackman S, Honoré PM. Sedation for adult ICU patients: A narrative review including a retrospective study of our own data. Saudi J Anaesth. 2023;17:223-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 381] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 11. | Cantor RS. Breaking the Meyer-Overton rule: predicted effects of varying stiffness and interfacial activity on the intrinsic potency of anesthetics. Biophys J. 2001;80:2284-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, Cai M, Shi L, Dong H, Xiong L. Neuroprotective gases--fantasy or reality for clinical use? Prog Neurobiol. 2014;115:210-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Keenan LM, Hoffman TL. Refractory Status Asthmaticus: Treatment With Sevoflurane. Fed Pract. 2019;36:476-479. [PubMed] |
| 14. | LaGrew JE, Olsen KR, Frantz A. Volatile anaesthetic for treatment of respiratory failure from status asthmaticus requiring extracorporeal membrane oxygenation. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Hendrickx J, Poelaert J, De Wolf A. Sedation with inhaled agents in the ICU: what are we waiting for? J Clin Monit Comput. 2018;32:593-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane--the Mirus™. Anaesthesia. 2014;69:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Wieruszewski PM, Ortoleva JP, Cormican DS, Seelhammer TG. Extracorporeal Membrane Oxygenation in Acute Respiratory Failure. Pulm Ther. 2023;9:109-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 18. | Elabbadi A, Urbina T, Berti E, Contou D, Plantefève G, Soulier Q, Milon A, Carteaux G, Voiriot G, Fartoukh M, Gibelin A. Spontaneous pneumomediastinum: a surrogate of P-SILI in critically ill COVID-19 patients. Crit Care. 2022;26:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Iwasaki Y, Shiga T, Hoshi N, Irimada D, Saito H, Konno D, Saito K, Yamauchi M. Sevoflurane administration from extracorporeal membrane oxygenation via the AnaConDa device for a patient with COVID-19: A breakthrough solution for the shortage of intravenous anesthetics. Heart Lung. 2022;56:70-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Gill B, Bartock JL, Damuth E, Puri N, Green A. Case report: Isoflurane therapy in a case of status asthmaticus requiring extracorporeal membrane oxygenation. Front Med (Lausanne). 2022;9:1051468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Biro P. Calculation of volatile anaesthetics consumption from agent concentration and fresh gas flow. Acta Anaesthesiol Scand. 2014;58:968-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Wiesenack C, Wiesner G, Keyl C, Gruber M, Philipp A, Ritzka M, Prasser C, Taeger K. In vivo uptake and elimination of isoflurane by different membrane oxygenators during cardiopulmonary bypass. Anesthesiology. 2002;97:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Philipp A, Wiesenack C, Behr R, Schmid FX, Birnbaum DE. High risk of intraoperative awareness during cardiopulmonary bypass with isoflurane administration via diffusion membrane oxygenators. Perfusion. 2002;17:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Meiser A, Volk T, Wallenborn J, Guenther U, Becher T, Bracht H, Schwarzkopf K, Knafelj R, Faltlhauser A, Thal SC, Soukup J, Kellner P, Drüner M, Vogelsang H, Bellgardt M, Sackey P; Sedaconda study group. Inhaled isoflurane via the anaesthetic conserving device versus propofol for sedation of invasively ventilated patients in intensive care units in Germany and Slovenia: an open-label, phase 3, randomised controlled, non-inferiority trial. Lancet Respir Med. 2021;9:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 26. | Kim HY, Lee JE, Kim HY, Kim J. Volatile sedation in the intensive care unit: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e8976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Jung S, Na S, Kim HB, Joo HJ, Kim J. Inhalation sedation for postoperative patients in the intensive care unit: initial sevoflurane concentration and comparison of opioid use with propofol sedation. Acute Crit Care. 2020;35:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Romagnoli S, Chelazzi C, Villa G, Zagli G, Benvenuti F, Mancinelli P, Arcangeli G, Dugheri S, Bonari A, Tofani L, Belardinelli A, De Gaudio AR. The New MIRUS System for Short-Term Sedation in Postsurgical ICU Patients. Crit Care Med. 2017;45:e925-e931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Hellström J, Öwall A, Bergström J, Sackey PV. Cardiac outcome after sevoflurane versus propofol sedation following coronary bypass surgery: a pilot study. Acta Anaesthesiol Scand. 2011;55:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Jerath A, Beattie SW, Chandy T, Karski J, Djaiani G, Rao V, Yau T, Wasowicz M; Perioperative Anesthesia Clinical Trials Group. Volatile-based short-term sedation in cardiac surgical patients: a prospective randomized controlled trial. Crit Care Med. 2015;43:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Röhm KD, Wolf MW, Schöllhorn T, Schellhaass A, Boldt J, Piper SN. Short-term sevoflurane sedation using the Anaesthetic Conserving Device after cardiothoracic surgery. Intensive Care Med. 2008;34:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Soro M, Gallego L, Silva V, Ballester MT, Lloréns J, Alvariño A, García-Perez ML, Pastor E, Aguilar G, Martí FJ, Carratala A, Belda FJ. Cardioprotective effect of sevoflurane and propofol during anaesthesia and the postoperative period in coronary bypass graft surgery: a double-blind randomised study. Eur J Anaesthesiol. 2012;29:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Steurer MP, Steurer MA, Baulig W, Piegeler T, Schläpfer M, Spahn DR, Falk V, Dreessen P, Theusinger OM, Schmid ER, Schwartz D, Neff TA, Beck-Schimmer B. Late pharmacologic conditioning with volatile anesthetics after cardiac surgery. Crit Care. 2012;16:R191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Wąsowicz M, Jerath A, Luksun W, Sharma V, Mitsakakis N, Meineri M, Katznelson R, Yau T, Rao V, Beattie WS. Comparison of propofol-based versus volatile-based anaesthesia and postoperative sedation in cardiac surgical patients: a prospective, randomized, study. Anaesthesiol Intensive Ther. 2018;50:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46:e825-e873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 2156] [Article Influence: 359.3] [Reference Citation Analysis (0)] |
| 36. | Jerath A, Parotto M, Wasowicz M, Ferguson ND. Volatile Anesthetics. Is a New Player Emerging in Critical Care Sedation? Am J Respir Crit Care Med. 2016;193:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Manatpon P, Kofke WA. Toxicity of inhaled agents after prolonged administration. J Clin Monit Comput. 2018;32:651-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Bellgardt M, Bomberg H, Herzog-Niescery J, Dasch B, Vogelsang H, Weber TP, Steinfort C, Uhl W, Wagenpfeil S, Volk T, Meiser A. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients: Retrospective analysis. Eur J Anaesthesiol. 2016;33:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Afessa B, Morales I, Cury JD. Clinical course and outcome of patients admitted to an ICU for status asthmaticus. Chest. 2001;120:1616-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Peters JI, Stupka JE, Singh H, Rossrucker J, Angel LF, Melo J, Levine SM. Status asthmaticus in the medical intensive care unit: a 30-year experience. Respir Med. 2012;106:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Carrié S, Anderson TA. Volatile anesthetics for status asthmaticus in pediatric patients: a comprehensive review and case series. Paediatr Anaesth. 2015;25:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Pollart SM, Compton RM, Elward KS. Management of acute asthma exacerbations. Am Fam Physician. 2011;84:40-47. [PubMed] |
| 43. | Corbridge TC, Hall JB. The assessment and management of adults with status asthmaticus. Am J Respir Crit Care Med. 1995;151:1296-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Hirshman CA, Edelstein G, Peetz S, Wayne R, Downes H. Mechanism of action of inhalational anesthesia on airways. Anesthesiology. 1982;56:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Burburan SM, Silva JD, Abreu SC, Samary CS, Guimarães IH, Xisto DG, Morales MM, Rocco PR. Effects of inhalational anaesthetics in experimental allergic asthma. Anaesthesia. 2014;69:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Bierman MI, Brown M, Muren O, Keenan RL, Glauser FL. Prolonged isoflurane anesthesia in status asthmaticus. Crit Care Med. 1986;14:832-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97:698-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Mondoñedo JR, McNeil JS, Amin SD, Herrmann J, Simon BA, Kaczka DW. Volatile Anesthetics and the Treatment of Severe Bronchospasm: A Concept of Targeted Delivery. Drug Discov Today Dis Models. 2015;15:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Kolli S, Opolka C, Westbrook A, Gillespie S, Mason C, Truitt B, Kamat P, Fitzpatrick A, Grunwell JR. Outcomes of children with life-threatening status asthmaticus requiring isoflurane therapy and extracorporeal life support. J Asthma. 2023;60:1926-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 51. | Jagoda A, Riggio S. Refractory status epilepticus in adults. Ann Emerg Med. 1993;22:1337-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1300] [Cited by in RCA: 1265] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 53. | Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 545] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 54. | Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav. 2005;7 Suppl 3:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Zhumadilov A, Gilman CP, Viderman D. Management of super-refractory status epilepticus with isoflurane and hypothermia. Front Neurol. 2014;5:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Kofke WA, Snider MT, Young RS, Ramer JC. Prolonged low flow isoflurane anesthesia for status epilepticus. Anesthesiology. 1985;62:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Kofke WA, Young RS, Davis P, Woelfel SK, Gray L, Johnson D, Gelb A, Meeke R, Warner DS, Pearson KS. Isoflurane for refractory status epilepticus: a clinical series. Anesthesiology. 1989;71:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 99] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M. Modern inhalational anesthetics for refractory status epilepticus. Can J Neurol Sci. 2015;42:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Wilson MT, Sleigh JW, Steyn-Ross DA, Steyn-Ross ML. General anesthetic-induced seizures can be explained by a mean-field model of cortical dynamics. Anesthesiology. 2006;104:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Julliac B, Cotillon P, Guehl D, Richez B, Sztark F. Target-controlled induction with 2.5% sevoflurane does not avoid the risk of electroencephalographic abnormalities. Ann Fr Anesth Reanim. 2013;32:e143-e148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Iijima T, Nakamura Z, Iwao Y, Sankawa H. The epileptogenic properties of the volatile anesthetics sevoflurane and isoflurane in patients with epilepsy. Anesth Analg. 2000;91:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 4296] [Article Influence: 330.5] [Reference Citation Analysis (0)] |
| 64. | Villar J, Szakmany T, Grasselli G, Camporota L. Redefining ARDS: a paradigm shift. Crit Care. 2023;27:416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 65. | Flinspach AN, Booke H, Zacharowski K, Balaban Ü, Herrmann E, Adam EH. High sedation needs of critically ill COVID-19 ARDS patients-A monocentric observational study. PLoS One. 2021;16:e0253778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Stephens RJ, Evans EM, Pajor MJ, Pappal RD, Egan HM, Wei M, Hayes H, Morris JA, Becker N, Roberts BW, Kollef MH, Mohr NM, Fuller BM. A dual-center cohort study on the association between early deep sedation and clinical outcomes in mechanically ventilated patients during the COVID-19 pandemic: The COVID-SED study. Crit Care. 2022;26:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Englert JA, Macias AA, Amador-Munoz D, Pinilla Vera M, Isabelle C, Guan J, Magaoay B, Suarez Velandia M, Coronata A, Lee A, Fredenburgh LE, Culley DJ, Crosby G, Baron RM. Isoflurane Ameliorates Acute Lung Injury by Preserving Epithelial Tight Junction Integrity. Anesthesiology. 2015;123:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Blondonnet R, Paquette B, Audard J, Guler R, Roman FX, Zhai R, Belville C, Blanchon L, Godet T, Futier E, Bazin JE, Constantin JM, Sapin V, Jabaudon M. Halogenated Agent Delivery in Porcine Model of Acute Respiratory Distress Syndrome via an Intensive Care Unit Type Device. J Vis Exp. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 69. | Coppola S, Cenci S, Cozzolino M, Chiumello D. Sevoflurane sedation and nephrogenic diabetes insipidus in patients affected with severe acute respiratory syndrome coronavirus 2. Eur J Anaesthesiol. 2021;38:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | Flinspach AN, Zacharowski K, Ioanna D, Adam EH. Volatile Isoflurane in Critically Ill Coronavirus Disease 2019 Patients-A Case Series and Systematic Review. Crit Care Explor. 2020;2:e0256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Kermad A, Speltz J, Danziger G, Mertke T, Bals R, Volk T, Lepper PM, Meiser A. Comparison of isoflurane and propofol sedation in critically ill COVID-19 patients-a retrospective chart review. J Anesth. 2021;35:625-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Ferrière N, Bodenes L, Bailly P, L'Her E. Shortage of anesthetics: Think of inhaled sedation! J Crit Care. 2021;63:104-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Shingina A, Ou G, Takach O, Svarta S, Kwok R, Tong J, Donaldson K, Lam E, Enns R. Identification of factors associated with sedation tolerance in 5000 patients undergoing outpatient colonoscopy: Canadian tertiary center experience. World J Gastrointest Endosc. 2016;8:770-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Stapelberg F. Challenges in anaesthesia and pain management for burn injuries. Anaesth Intensive Care. 2020;48:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Gregoretti C, Decaroli D, Piacevoli Q, Mistretta A, Barzaghi N, Luxardo N, Tosetti I, Tedeschi L, Burbi L, Navalesi P, Azzeri F. Analgo-sedation of patients with burns outside the operating room. Drugs. 2008;68:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Jabaudon M, Boucher P, Imhoff E, Chabanne R, Faure JS, Roszyk L, Thibault S, Blondonnet R, Clairefond G, Guérin R, Perbet S, Cayot S, Godet T, Pereira B, Sapin V, Bazin JE, Futier E, Constantin JM. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am J Respir Crit Care Med. 2017;195:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 77. | Olsby JH, Dihle A, Hofsø K, Steindal SA. Intensive care nurses' experiences using volatile anaesthetics in the intensive care unit: An exploratory study. Intensive Crit Care Nurs. 2022;70:103220. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Hudson AE, Herold KF, Hemmings HC. Pharmacology of Inhaled Anesthetics. In: Pharmacology and Physiology for Anesthesia. Elsevier, 2019: 217-240. |
| 79. | Cascorbi HF, Blake DA, Helrich M. Differences in the biotransformation of halothane in man. Anesthesiology. 1970;32:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Malan TP Jr, DiNardo JA, Isner RJ, Frink EJ Jr, Goldberg M, Fenster PE, Brown EA, Depa R, Hammond LC, Mata H. Cardiovascular effects of sevoflurane compared with those of isoflurane in volunteers. Anesthesiology. 1995;83:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 111] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Corbett TH. Pharmacology and toxicology of halogenated anesthetics. Adv Pharmacol Chemother. 1979;16:195-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |