Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.90176
Peer-review started: November 26, 2023
First decision: December 23, 2023
Revised: December 28, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 9, 2024
Processing time: 100 Days and 4.2 Hours
Critical care medicine in the 21st century has witnessed remarkable advancements that have significantly improved patient outcomes in intensive care units (ICUs). This abstract provides a concise summary of the latest developments in critical care, highlighting key areas of innovation. Recent advancements in critical care include Precision Medicine: Tailoring treatments based on individual patient characteristics, genomics, and biomarkers to enhance the effectiveness of thera
Core Tip: In 1959, the first modern critical care unit, led by Dr. Peter Safar, emerged at the University of Pittsburgh, as detailed in the American Thoracic Society Journal. Critical care medicine has since expanded from traditional hospital settings to include emergency departments, ambulances, and even aircraft. The advent of the 21st century has ushered in notable advancements, including enhanced ventilation and organ support systems such as extra-corporeal membrane oxygenations, or even integrating telemedicine to extend critical care expertise to remote regions. In this dynamic environment, staying abreast of innovations such as artificial intelligence, precision medicine, nanotechnology in sepsis management, and inventive infection control strategies is crucial for reshaping our intensive care units. Our goal, amid these advances, is to provide a comprehensive overview of 21st-century progress in critical care, offering succinct insights on various specific topics in critical care medicine.
- Citation: Padte S, Samala Venkata V, Mehta P, Tawfeeq S, Kashyap R, Surani S. 21st century critical care medicine: An overview. World J Crit Care Med 2024; 13(1): 90176
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/90176.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.90176
Critical care medicine has been defined by multiple entities. According to the American College of Physicians, it is defined as clinical care consisting of the diagnosis and treatment of a wide variety of clinical problems representing the extremes of human disease[1]. Villar et al[2] defined it as medicine that has the capacity to reverse near-fatal disease states, providing temporary support to the vital organ system while the patient recovers from the underlying disease processes.
Foundations of critical care medicine were laid in the mid-20th century with the initiation of mechanical ventilation, and continuous monitoring of physiological parameters while providing care to the critically ill[3]. Since the advent of the 21st century, there have been significant advances in this field leading to better outcomes for critically ill patients[4-6]. These advances include better ventilation strategies to prevent lung injury, the initiation of advanced life support systems such as Extra Corporeal Membrane Oxygenation (ECMO), and better management of sepsis syndromes with earlier interventions among others. With the incorporation of technology such as telemedicine into critical care, it has been made possible to provide necessary critical care expertise in rural areas who otherwise would not have access to it. In this ever-evolving landscape, contemporary critical care practitioners are confronted with the formidable task of keeping abreast of the latest advancements and innovations in their field, amidst the vast array of research articles and educational initiatives available. In addition to these challenges and advancements, the integration of artificial intelligence (AI) and the emergence of precision medicine has begun to reshape the intensive care unit (ICU) landscape[7].
Our aim, considering these developments, is to provide a comprehensive overview of progress made in the 21st century in the multidisciplinary field of critical care. By offering concise descriptions of select articles categorized by specific advances, our goal is to provide fundamental insights into the cutting-edge strides made within this domain. Ultimately, we hope to inspire our readers to delve into the original articles for a deeper understanding of the evolving landscape of critical care. The flow diagram of included studies is listed in Figure 1.
The beginning of the 21st century marked the completion of the Human Genome Project in 2003 and ushered in the hope that medical care could be tailored to an individual using their DNA[8]. However, it wasn’t until 2009 that the term “precision medicine” was first coined[9]. This was closely followed by the emergent growth of P medicine – Personalized, Precision, Preventive, Predictive, Pharmacotherapeutic, and Patient Participatory Medicine[10]. The term, broadly constructed, describes an approach to disease prevention and treatment that exploits the multiple distinct characteristics of individuals[11]. Despite multiple definitions existing, the Institute of Precision Medicine defined it succinctly “Precision medicine is targeted, individualized care that is tailored to each patient based on his or her specific genetic profile and medical history.” The advances in precision medicine have their roots in oncology but recent developments have seen them make their way into critical care medicine[12].
Genomics and precision medicine go hand in hand as they focus on the individual’s unique heritable characteristics. Recent interest in genomics has gradually increased, with multiple genome-wide association studies and large-scale projects gaining relevance. The International Hap-Map Project, the Precision Medicine Initiative (PMI) in the United States, and the 10000 Genome Project in Great Britain reflect that perhaps the key to precision medicine lies in our genes[11,13].
Recent advances have been hopeful. A retrospective study on 167 trauma patients showed that when a set of 63 genes were incorporated into the genomic score, they appeared to perform better in predicting outcomes than the Injury Severity Score and the Acute Physiology and Chronic Health Evaluation II (APACHE-II) (VSV1) metric[14-16]. Gene expression analyses have also been successful in distinguishing the molecularly defined subtypes of sepsis and even estimating their responses to various treatment modalities, such as corticosteroid therapy[11]. Efforts to simplify genetic signatures have led to the identification of an 11-gene signature that can be used to distinguish sepsis from other non-infectious states. Similarly, a two-gene signature was used to classify pediatric infections as either viral or bacterial in origin[11]. A multi-pronged approach to identifying the Single Nucleotide Polymorphisms responsible for the development of acute respiratory distress syndrome (ARDS) from pulmonary or extra-pulmonary causes directed us toward the role of FAAH and the POPDC3 gene[8]. Despite the ever-advancing research, the clinical application of these studies remains uncertain.
Biomarkers in critical care play the all-important role of bringing precision medicine from research papers to the intensive care unit[17]. The value of biomarkers in the ICU lies in their ability to predict the prognosis or even assess the efficacy of a treatment line. There has been much excitement surrounding them, with multiple new biomarkers being studied for the two prototypical ICU Illnesses - ARDS and Sepsis[11].
Serum markers have shown reasonable success in classifying ARDS based on the etiology (direct vs. indirect lung injury)[11,18]. The ARMA Trial showed that elevated levels of Von Willebrand (VWf) protein, soluble tumor necrosis factor receptors 1 and 2 (sTNFr), plasminogen activator inhibitor-1 (PAI-1) and plasma surfactant protein-D (SP-D) have all been independently associated with worse outcomes in ARDS[19]. A higher baseline plasma soluble receptor for advanced glycation end products was linked to an increased risk of 90-d mortality, according to a meta-analysis of eight studies[19]. Similarly, higher tumor necrosis factor-α and Interleukin 1-β levels in Broncho-Alveolar Lavage Fluid have been associated with the development of ARDS in at-risk patients[8].
Around 180 biomarkers have been studied in sepsis and septic shock[8]. For the early detection of sepsis, the following five biomarkers were shown to have a sensitivity and specificity of over 90%: Interferon-induced protein (IP)-10, Group II phospholipase (PLA2-II), CD64, and CD11b. PLA2 and CD64 are the only ones that have demonstrated encouraging outcomes in adult patients[8]. In recent years, procalcitonin has also become a biomarker that is increasingly popular in differentiating between bacterial infection and sepsis for better diagnostic accuracy[17]. Additional predictive biomarkers for higher mortality in sepsis include angiopoietin-1 and angiopoietin-2, plasma cell-free host DNA, and IL-6 and IL-8[19]. Multi-marker panels are also under investigation, aiming to provide a more comprehensive understanding of complex conditions like sepsis[20-22]. While biomarkers hold great potential, their widespread clinical use still faces challenges and requires further research to fully harness their diagnostic and prognostic capabilities.
As per the Centers for Medicare and Medicaid Services, telemedicine is defined as two-way real-time interactive communication between a patient and a physician or practitioner at a distant site through telecommunications equipment that includes audio and visual equipment[23]. Telemedicine is the diagnosis and treatment of patients over long distances using communication technologies[24]. In the critical care setting/Intensive care unit, critical care services offered via telemedicine are known as Tele-ICU or Tele-Critical Care[17]. Even though evidence regarding mortality benefits of 24 × 7 intensivist coverage (both day and nighttime) in the ICU reports mixed results, studies indicate improved mortality rates with high-intensity ICU coverage (mandatory consultation with an intensivist or transfer of care to an intensivist-led team) when compared to low-intensity coverage (optional consultation with the intensivist)[25-27].
Unfortunately, the worsening shortage of intensivists in the United States prevents hospitals nationwide from providing full-time intensivist coverage in the ICU. Tele-ICU has emerged as an alternative model to provide critical intensivist services to hospitals[28-30]. Patients on continuous monitors can be reviewed by remote critical care clinicians, enabling the early identification of emerging health issues[30]. Clinicians can then communicate with on-site care teams to initiate necessary interventions promptly. In addition, the application of tele-critical care has been shown to reduce hospital transfers and improve patient outcomes in sepsis[31].
With the advancement of communicative technologies in the 1970s, early consultative telemedicine critical care services were used unsuccessfully in 1982 by Grundy et al[32]. As technology continued to improve, in 2000 Rosenfeld et al[29] at Johns Hopkins conducted a 16-wk trial of telemedicine on ICU patients and reported improved patient mortality rates. They concluded that telemedicine critical care services can be offered with improved patient outcomes.
Since these studies were done and with the advancement of technological services, Tele-ICU use has grown across the United States. Kahn et al[33] looked at data from the Centers for Medicare and Medicaid Services between 2003-2010, they reported that the number of hospitals using Tele-ICU increased from 16 (0.4% of the total) to 213 (4.6% of the total) and the number of beds covered by telemedicine increased from 598 (0.9%) to 5799 (7.9%).
Over the past two decades, multiple studies have been done looking at outcomes with Tele-ICU. Even though initial studies looking at a few hundred patients show mixed results in terms of mortality, recent multiple larger studies indicate improved outcomes including ICU mortality and length of stay. A meta-analysis done by Young et al[34] in 2011 and Chen et al[35] in 2017 that looked at 41374 and 192265 patients respectively showed improved ICU mortality and length of stay with Tele-ICU. Similarly, a review done by Mackintosh showed improved patient mortality with Tele-ICU[36].
The major barrier with Tele-ICU is the costs ($50000-$100000 per bed)[37]. Despite this, as the physician shortage is expected to worsen, Tele-ICU shows promising results as a potential alternative to provide much-needed critical care services in hospitals around the country.
AI constitutes the set of algorithms that enable machines to reason and execute tasks that include problem-solving, object and word recognition, state inference, and decision-making[38]. It expands upon Machine Learning via Artificial Neural Networks by utilizing tensors for "Deep Learning" (DL). As opposed to a sole clinician, DL can efficiently process multiple inputs, programming complex non-linear relationships to generate individualized prediction models[39]. In 1981, logistic regression was applied to validate the APACHE score, signifying the first use of AI in critical care medicine to support clinical decisions[40].
Featuring two super learner prediction models, SL1 and SL2, Pirracchio et al[41] introduced a novel mortality prediction algorithm for ICU patients in 2015. Compared to the Sequential Organ Failure Assessment (SOFA) and Simplified Acute Physiology Score II (SAPS II) scores, it displayed greater efficacy in predicting early mortality[42]. In addition, the United States Food and Drug Administration has authorized the clinical application of certain healthcare products, such as image analysis software (e.g., DeepRhythmAI[43] by Medicalgorithmics SA) and basic cardiopulmonary function monitoring software (e.g., IRNF App by Apple Inc. and Air Next by NuvoAir AB)[40]. Jiao et al[44] developed an AI system for predicting the prognosis of coronavirus disease 2019 (COVID-19) patients based on chest X-rays. The model exhibited significantly better prognostic performance compared to conventional severity scores in both internal and external testing. AI-based tools for rapid COVID-19 diagnostics have been developed, including those by Mount Sinai Health System and the University of Minnesota, in collaboration with Epic Systems and M Health Fairview[45]. Software predictors, like COViage[46] and the CLEWICU System[47], identify high-risk patients for clinical events such as intubation, respiratory failure, and low blood pressure. Additionally, adherence to AI-specific reporting guidelines like SPIRIT-AI[48], CONSORT-AI, and HUMANE can enhance AI application development and quality[49].
AI-guided tools for sepsis have shown promise as well. Earlier detection via sepsis surveillance algorithm has been a pioneering advancement in the 21st century[50]. Automation of sepsis clinical data abstraction via computable phenotype is crucial in sepsis care and research[51]. A reinforcement learning model improved mortality when clinical decisions aligned with the model's suggestions[52]. Predicting changes in urine output after fluid administration, an essential end-organ perfusion indicator, achieved an area under the curve of 0.86 using a gradient-boosting algorithm[52,53]. AI-guided POCUS can even enhance inter-operator reliability[54]. Machine learning holds the potential for enhancing sepsis management and volume responsiveness prediction in the ICU in this new century.
In a complex ICU setting with challenging staff and resource management, AI can enhance disease prognostication for informed decision-making[45]. Elhazmi et al[55] utilized a decision tree to predict COVID-19 patients' 28-d ICU mortality. It proved useful for identifying individuals at high risk. Leveraging a cohort of 289351 COVID-19 patients, Lazzarini et al[56] introduced the "Gradient Boosting Decision Tree" machine learning model that predicts severe COVID-19 cases, demonstrating its superior performance compared to older models and even human experts in terms of precision and recall.
Language learning models like Chat-GPT/GPT-4 have the potential to assist clinical judgment under intensivist supervision, improving clinical decision support optimization, as well as in clinical research in critical care[57]. Its applications extend to ECMO management, education, weaning, and decision-making[57]. Furthermore, it could be applied to other commonly used ICU medical equipment such as ventilators, defibrillators, electrocardiograms, or critical care radiology workflow[58]. While the model displays a capacity to learn and adapt, it has certain limitations in terms of medical expertise[45].
Challenges in applying machine learning (ML) in intensive care include poor data quality, ethical and legal concerns, and the absence of specific educational programs[59]. Most ML algorithms lack external validation, and real-world performance issues, like the EPIC sepsis-alleviation system, highlight practical hurdles. A vast majority of ML studies in intensive care are retrospective, with limited evaluation in clinical practice[45]. Inclusive datasets are crucial for AI model applicability, but transparency and legal guidelines are vital for trust and accountability[52]. Despite challenges, AI is poised to grow in its role in critical care in the future.
Extra corporal membrane oxygenation: The ECMO is a device that temporarily replaces the heart and lung function via gas exchange through an extracorporeal circuit, while the patient is being resuscitated from underlying cardiopulmonary pathology[60]. In addition to providing respiratory and circulatory support, one of the main goals of ECMO is to prevent ventilator-induced lung injury which is a major cause of mortality in this population[61]. This feature of ECMO is thought to be more important in improving outcomes than providing oxygenation support[60,61].
ECMO technology was developed in the 1960s and used on humans for the first time in the 1970s[62,63]. Due to the advancement of technology, new extracorporeal circuit design, and better ventilation strategies, observational studies started reporting promising results in terms of survival outcomes[64,65]. In 2009 multicenter randomized controlled trial (RCT) done by Peek et al[66] reported improved outcomes with ECMO compared to conventional management. Even though the results of the Conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR) trial[66] were controversial, these studies led to renowned interest in ECMO. This along with continuing development in technology led to an increase in the use of ECMO worldwide[67,68]. As of 2015, more than 78000 cases have been reported to the Extracorporeal Life Support Organization (ELSO) and the number of hospitals reporting to ELSO has been steadily rising[68]. Meta-analysis of RCT done by Munshi et al[69] in 2019 published in the Lancet showed reduced 60-d mortality when compared to mechanical ventilation. With recent studies showing strong evidence in favor of ECMO, even after cardiopulmonary resuscitation and with ongoing advances in technology ECMO will likely be the standard of care in the future, if not already for ICU patients who meet the ECMO support initiation criteria[70].
Dialysis, an external machine that can serve as renal replacement therapy, was initially conceptualized in 1854 by Thomas Graham and was first used in patients in 1945 by Dr. William Koff. Since then with advances in technology and techniques, dialysis has become the standard of care for renal replacement in patients with renal failure[71]. Traditional dialysis is performed intermittently, typically a few days a week. In 1977 Peter Kramer performed the first continuous arteriovenous hemofiltration (CAVH), with the main advantage being hemodynamic stability even in critically ill patients (VSV3)[72,73]. With technological advancements, CAVH has paved the way for development of present-day continuous veno-venous hemofiltration (CVVH).
In addition, over a 48-h period CVVH leads to a higher net fluid removal compared to regular dialysis, so it has shown to be beneficial in critically ill patients requiring large-volume fluid resuscitation[74]. Another advantage of CVVH would be in patients with cerebral edema, ischemic heart disease (IHD) may lead to hypotension and consequent compensatory cerebral vasodilation. Also, the rapid removal of urea with IHD can lead to water shifting into the cellular space[74,75]. Despite the ambiguity of survival benefit with CVVH compared to IHD. There are clear advantages with CVVH compared to IHD in specific critically ill patients and it's been used widely in intensive care units since the beginning of the 21st century.
In the ICU, 19.2% of patients develop infections, compared to 5.2% in other hospital wards[76]. On any given day, about one in 31 hospital patients has at least one healthcare-associated infection[77]. Mucosal disruption, immune suppression, invasive devices, surgery, antibiotic use, and encounters with multidrug-resistant pathogens are all contributing factors.
Effective prevention measures including universal gloving, gowns, masks, and hand hygiene have been used since the inception of critical care, but they can be time-consuming[78]. Electronic monitoring tools for hand hygiene, like Elec
In the past two decades, healthcare worker (HCW) vaccination against nosocomial infections has gained prominence[79]. Since July 2007, the Joint Commission has mandated annual influenza vaccination programs in healthcare facilities. Pertussis vaccination for HCWs was recommended due to the rising incidence. Similarly, COVID-19 vaccines became mandatory to curb the pandemic since the year 2020[81]. Biocontainment facilities have become essential for managing infectious diseases like the Ebola virus while minimizing transmission risks in the 21st century[82].
Viana Martins et al[83] made a crucial development in the COVID-19 fight by highlighting the efficacy of UV-C devices in inactivating the airborne severe acute respiratory syndrome coronavirus. The BETR Disinfection Study[84] from 2016 influenced healthcare facilities to adopt UV-C as a standard method for multidrug-resistant infections. However, Rock et al[85] found UV-C disinfection did not reduce certain infections in immunocompromised patients. Nonetheless, UV-C disinfection remains crucial in preventing other healthcare-associated infections. Novel technologies that enhance environmental decontamination, like hydrogen peroxide vapor, ultraviolet light, self-disinfecting surfaces, antimicrobial textiles, and sporicidal disinfectants, effectively combat contamination and minimize infection risks in the 21st century and are expected to advance further[79].
With the increasing use of electronic health record systems in the past two decades, electronic orders and reminders have become essential tools for curbing Catheter-Associated Urinary Tract Infections[80]. They prompt appropriate urinary catheter use and prompt removal, significantly reducing infection rates. Decision-support tools, such as electronic nudges and hard stops, steer clinicians away from choices that may heighten HAI risk. These tools are invaluable in the era of rapid infectious disease spread.
Digital polymerase chain reaction has emerged as a promising diagnostic technology in the past 20 years. It aids in the early identification of microbial genes, quantifies microbial burden, and explores host responses, making it a valuable tool in diagnosing and differentiating conditions like sepsis from systemic inflammatory response syndrome (SIRS)[86]. Moreover, predictive modeling using machine learning is revolutionizing infection prevention. Recent studies demonstrate its potential in assessing patients' risk of central line-associated bloodstream infections, ventilator-associated pneumonia, C. difficile infections, and catheter-associated urinary tract infections. Some models outperform traditional regression methods[79]. More testing and validation are underway to incorporate these new advances into practice.
Mechanical ventilation (MV) is a lifesaving intervention that provides temporary oxygenation and ventilation for patients with respiratory failure until the underlying pathology is treated. It consists of invasive and non-invasive MV. Invasive MV consists of an endotracheal tube placed in the patient's trachea through the mouth or nose. This tube is connected to a ventilator which delivers a set amount of oxygen and a number of breaths per minute[87]. Non-invasive ventilation (NIV) consists of a machine that delivers a set amount of oxygen and ventilatory breaths via an external mask[87].
The 21st-century ventilators have the option of various ventilation modes[88]. Volume limited, Pressure Limited, and Pressure Support (PSV) are the three main modes of ventilation. In volume limited, inspiration ends after a set inspiratory volume, in pressure limited inspiration ends after a set inspiratory pressure. Each mode has its advantages and can be used in specific patient situations. With PSV ventilation mode, once a breath is triggered by the patient, inspiratory pressure is delivered until the inspiratory flow decreases to a predetermined percentage of its peak value.
Enhancing ventilation strategies for ARDS patients is one of the more significant advances in the field of critical care over the past two decades and it has led to improved patient outcomes. The landmark ARDSnet trial in 2000 demonstrated that lower tidal volume ventilation (6 mL/kg) in ARDS patients leads to lower mortality and an increased number of days without ventilator use[89]. This was subsequently supported by multiple studies which reported similar results[90-92].
As of the year 2023, low tidal volume ventilation (LTV) should be the standard of care for ARDS patients around the world. Despite the existing evidence, studies suggest that low tidal volume ventilation in ARDS patients is underutilized. In a multicenter observational study of ARDS patients around the United States showed that LTV was utilized in only 30% of the cases. Awareness needs to be raised and there needs to be a standardized institutional protocol to improve compliance with LTV[92].
Numerous studies conducted at the onset of the 21st century have confirmed the efficacy of NIV in COPD exacerbation[93,94]. As per the latest American Thoracic Society/European Respiratory Journal guidelines in the year 2017, NIV is indicated in specific patients with acute respiratory failure secondary to COPD exacerbation and cardiogenic pulmonary edema[95].
Sepsis, a life-threatening organ dysfunction from an infection-triggered host response, presents a rising global challenge. Incidence has steadily increased since 1991, causing about 49 million sepsis cases and 11 million deaths in 2017[96]. Despite therapeutic progress, septic patients face high in-hospital mortality, comprising 20% of all global deaths. The Surviving Sepsis Campaign (SSC) started at the dawn of 21st century in the year 2003, uniting medical societies across the globe to create guidelines for reducing sepsis mortality[97]. Conventional therapies center on fluids, source control, and antimicrobials. With the initiation of Surviving Sepsis Campaign, indicators like central venous pressure and central venous oxygen saturation were added to the definition to help with the diagnosis[97,98]. These guidelines have been continuously updated, with the latest in 2021[99]. The evolving care bundles with shorter time frames (from 3-6-h bundles to 1-h bundles) for sepsis, emphasizing rapid recognition and resuscitation, place emergency physicians in a critical role[98,100]. The third re-definition in 2016 was done to improve the specificity of the old definition of Sepsis[101]. In this latest definition terms like SIRS, Severe Sepsis were removed. Sepsis-related quick SOFA (qSOFA) was introduced[102] as per the latest update. Screening tools like SIRS, MEWS, NEWS, and qSOFA help identify septic patients[103]. Below are the main advances in various aspects of sepsis management over the past two decades.
Since 2004, sepsis management guidelines recommended the initiation of antibiotic therapy within one hour of the presentation of patients with severe sepsis and septic shock in the ICU[104]. Multiple large studies have demonstrated increased mortality with each hour of delay in antibiotic administration in this population[105,106].
As per the latest SSC guidelines, in patients with sepsis or septic shock therapy initiation covering all likely pathogens and antibiotic coverage, once the pathogen is identified has been a cornerstone management in this century[102]. In the current standard of care, most patients with sepsis shock receive anti-pseudomonal antibiotic coverage[107] as part of empiric antibiotic therapy[100].
Based on Rivers et al[108], SSC had initially recommended fluid bolus administration as an integral component of sepsis management[104,108]. After this over the past two decades, multiple large trials[109-112] and studies have shown variable results in outcomes between EGDT and usual therapy. Taking all the existing evidence into consideration, SSC guidelines currently recommend 30 mL/kg bolus as part of resuscitation from sepsis-induced hypoperfusion.
Even though guidelines do not recommend a specific type of intravenous fluid in sepsis, evidence indicates improved mortality with the use of balanced crystalloids such as lactated ringer, and plasmalyte compared to normal saline[113]. Additionally, albumin inclusion during resuscitation has no impact on sepsis or septic shock mortality[114].
Innovative approaches in the 21st century target immune balance through extracorporeal therapies (ECT) or blood purification for sepsis care[115]. ECT aims to reduce inflammatory mediators and toxins, achieving immune balance and homeostasis. Removing peak cytokine levels in early sepsis, per the 'cytokine peak concentration' hypothesis, can limit organ damage and multi-organ dysfunction syndrome. ECT methods include convection therapies (CRRT, HVHF, HCO), adsorption therapies (e.g., Polymixin B, CytoSorb®), and combination therapies[115]. The Molecular Adsorbent Recirculating System (MARS™) supports the liver by removing albumin-bound toxins, showing short-term benefits in hepato-renal syndrome and hepatic encephalopathy[116]. "Externally modulated electric-based devices" sense and target endotoxins with antibiotics to curb inflammation[117]. A lipopolysaccharide (LPS) neutralizing cartridge detects and neutralizes LPS, combining antibiotic and anti-inflammatory effects.
Recent advances in critical care technology have introduced hemodynamic monitoring tools like pulse contour analysis, which calculates cardiac output from arterial line data. Various invasive and noninvasive markers, including end-tidal CO2, inferior vena cava collapsibility index, and point-of-care ultrasound (POCUS), are evaluated for sepsis[118]. POCUS offers rapid LV and RV function assessments, even remotely. Venous Doppler waveform analysis and the venous excess ultrasound score help predict venous congestion severity. However, POCUS alone can't replace a comprehensive cardiovascular assessment. It is being considered alongside clinical parameters.
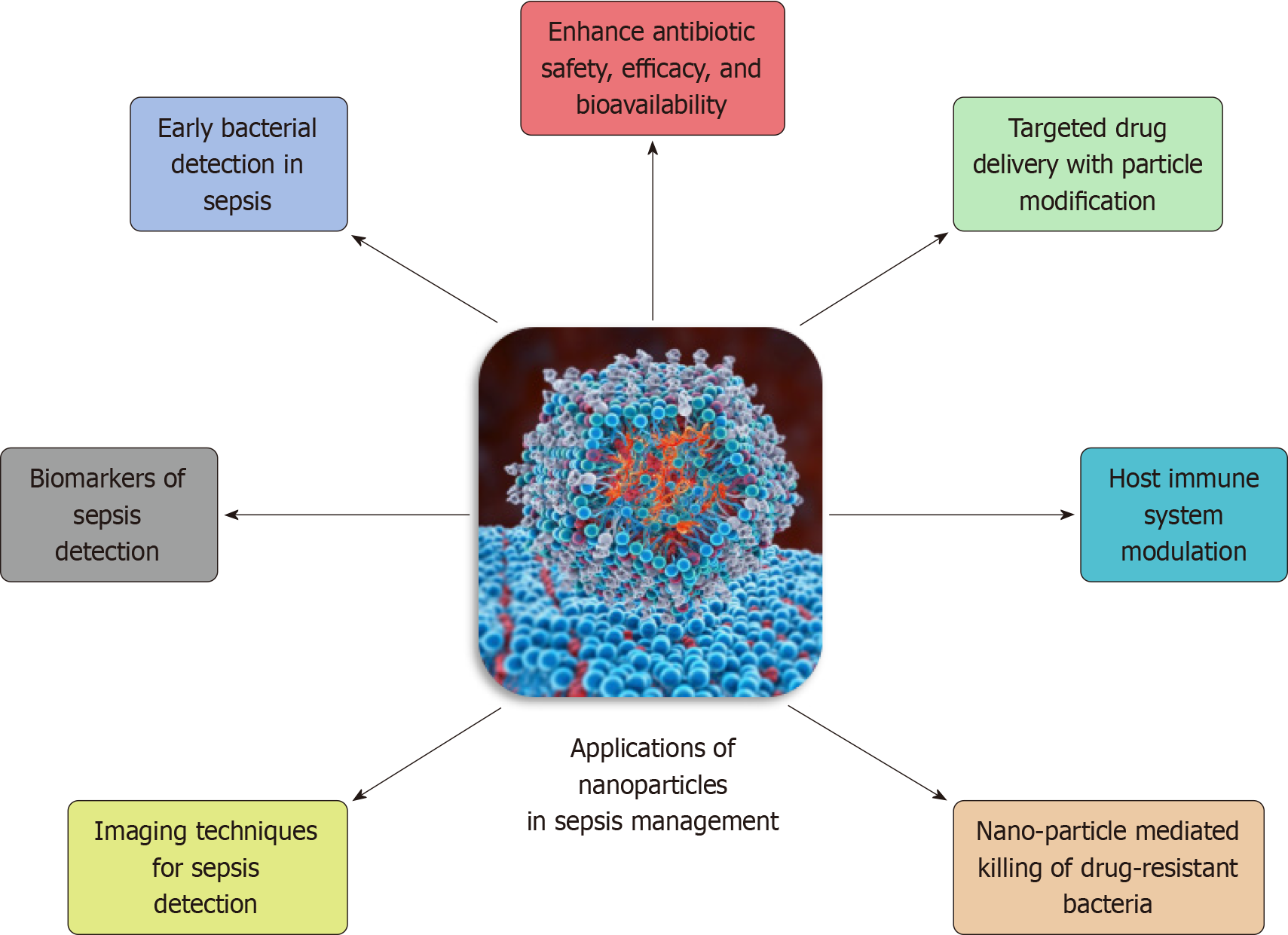
Recently, nanomaterial strategies have offered a more versatile tool for sepsis management. They serve as inherent therapeutics or nanocarriers for precise agent delivery. These formulations possess antibacterial, anti-inflammatory, immunomodulatory, and anti-oxidative effects, providing a multifunctional treatment against sepsis[119]. Cell membrane-derived biomimetic nanoplatforms trap and neutralize toxins. Nano-delivery systems customize agent kinetics and enhance targeted distribution, reducing peripheral exposure and toxicity[120]. Clinical trials for sepsis treatment include amikacin-loaded lipid nanocrystals, Resatorvid emulsion, Pegylated filgrastim, Spi-Argent®, Arikayce®, and more[117]. Figure 2 outlines the various applications of nanoparticle technology in sepsis management[120].
Several innovations have occurred in the 21st century towards a more patient-centered approach in ICU care, emphasizing communication, engagement, and individualized support for both patients and their families[121]. The primary focus is on 'Family-centered rounds', which involve including family members in daily rounds. It has enhanced communication, transparency, and shared decision-making between healthcare providers and the patient's family, fostering a collaborative care approach and improving outcomes[122]. Secondly, the ‘Tele-ICU services’ enable timely interventions, facilitate communication with off-site specialists, and provide an additional layer of support, particularly in underserved or rural areas or resource-limited countries[123].
Thirdly, the use of ‘Patient and family education technologies’ has empowered patients and their families with information about medical conditions, treatment options, and post-discharge care, promoting a more informed and engaged healthcare experience[124]. Additionally, ‘Virtual visitation programs’ have addressed challenges related to physical visitation, particularly during the COVID-19 pandemic, by allowing patients to connect with their loved ones virtually, positively influencing emotional well-being. Lastly, the ‘Patient-controlled comfort measures’ have integration of customizable environmental and comfort controls. This allows patients to personalize their immediate surroundings, influencing factors like lighting and noise levels, contributing to a more comfortable and healing-oriented environment including pain and sedation control[125].
In conclusion, the landscape of critical care medicine in the 21st century has been marked by remarkable advancements, underscoring a commitment to enhancing patient outcomes in ICUs. The summarized developments, ranging from precision medicine and telemedicine to AI and advanced life support systems, showcase the dynamic evolution of critical care practices. These innovations not only demonstrate a keen focus on individualized patient care but also reflect a broader integration of technology and research into clinical decision-making processes. The emphasis on infection control, ventilation strategies, and sepsis management underscores a commitment to combating emerging challenges in critical care.
Furthermore, the transformative shift towards patient-centered care highlights a holistic approach, recognizing the importance of addressing not only the medical needs but also the psychological and emotional well-being of patients. As the field continues to evolve, the promise of even more innovative solutions is on the horizon, poised to meet the complex and ever-evolving challenges of modern medicine in the critical care setting. These advancements collectively contribute to a future where the synergy of technology, research, and patient-centered approaches not only improves the quality of care but also saves lives in ICUs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Critical Care Medicine.
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Wan XH, China S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Critical Care Medicine The Discipline. Critical Care Medicine. Available from: https://www.acponline.org/about-acp/about-internal-medicine/subspecialties-of-internal-medicine/critical-care-medicine. |
| 2. | Villar J, Méndez S, Slutsky AS. Critical care medicine in the 21st century: from CPR to PCR. Crit Care. 2001;5:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, Baron RM, Bauer M, Buchman TG, Calfee CS, Dos Santos CC, Giamarellos-Bourboulis EJ, Gordon AC, Kellum JA, Knight JC, Leligdowicz A, McAuley DF, McLean AS, Menon DK, Meyer NJ, Moldawer LL, Reddy K, Reilly JP, Russell JA, Sevransky JE, Seymour CW, Shapiro NI, Singer M, Summers C, Sweeney TE, Thompson BT, van der Poll T, Venkatesh B, Walley KR, Walsh TS, Ware LB, Wong HR, Zador ZE, Marshall JC. Redefining critical illness. Nat Med. 2022;28:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 211] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 4. | Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med. 2017;195:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Valencia Morales DJ, Bansal V, Heavner SF, Castro JC, Sharma M, Tekin A, Bogojevic M, Zec S, Sharma N, Cartin-Ceba R, Nanchal RS, Sanghavi DK, La Nou AT, Khan SA, Belden KA, Chen JT, Melamed RR, Sayed IA, Reilkoff RA, Herasevich V, Domecq Garces JP, Walkey AJ, Boman K, Kumar VK, Kashyap R. Validation of automated data abstraction for SCCM discovery VIRUS COVID-19 registry: practical EHR export pathways (VIRUS-PEEP). Front Med (Lausanne). 2023;10:1089087. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Kovacevic P, Dragic S, Kovacevic T, Momcicevic D, Festic E, Kashyap R, Niven AS, Dong Y, Gajic O. Impact of weekly case-based tele-education on quality of care in a limited resource medical intensive care unit. Crit Care. 2019;23:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Lal A, Pinevich Y, Gajic O, Herasevich V, Pickering B. Artificial intelligence and computer simulation models in critical illness. World J Crit Care Med. 2020;9:13-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Ahasic AM, Christiani DC. Personalized Critical Care Medicine: How Far Away Are We? Semin Respir Crit Care Med. 2015;36:809-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 9. | Christensen CM, Grossman JH, Hwang J. The Technological Enablers of Disruption. In: The Innovator's Prescription: A Disruptive Solution for Health Care. New York, NY: McGraw-Hill Education, 2018. Available from: https://accessmedicine.mhmedical.com/content.aspx?aid=1150325300. |
| 10. | Sugeir S, Naylor S. Critical Care and Personalized or Precision Medicine: Who needs whom? J Crit Care. 2018;43:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Maslove DM, Lamontagne F, Marshall JC, Heyland DK. A path to precision in the ICU. Crit Care. 2017;21:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (2)] |
| 12. | Shankar-Hari M, Summers C, Baillie K. In Pursuit of Precision Medicine in the Critically Ill. In: Vincent J-L, editor. Annual Update in Intensive Care and Emergency Medicine 2018. Cham: Springer International Publishing, 2018: 649-658. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (3)] |
| 13. | Kessler C. Genomics and Precision Medicine: Implications for Critical Care. AACN Adv Crit Care. 2018;29:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Dehouche N. The injury severity score: an operations perspective. BMC Med Res Methodol. 2022;22:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Cuenca AG, Gentile LF, Lopez MC, Ungaro R, Liu H, Xiao W, Seok J, Mindrinos MN, Ang D, Baslanti TO, Bihorac A, Efron PA, Cuschieri J, Warren HS, Tompkins RG, Maier RV, Baker HV, Moldawer LL; Inflammation and Host Response to Injury Collaborative Research Program. Development of a genomic metric that can be rapidly used to predict clinical outcome in severely injured trauma patients. Crit Care Med. 2013;41:1175-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | N R, Narayanaswamy S, Hegde S. Clinical Study of a New Modified Early Warning Scoring System for Rapidly Evaluating Shock in Adults. Cureus. 2023;15:e38224. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Bauer PR, Kashyap R, League SC, Park JG, Block DR, Baumann NA, Algeciras-Schimnich A, Jenkins SM, Smith CY, Gajic O, Abraham RS. Diagnostic accuracy and clinical relevance of an inflammatory biomarker panel for sepsis in adult critically ill patients. Diagn Microbiol Infect Dis. 2016;84:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Guervilly C, Parzy G, Papazian L. Acute respiratory distress syndrome phenotyping and latent class analysis, first steps toward precision medicine in critical care illness? J Thorac Dis. 2019;11:S303-S306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 19. | Sarma A, Calfee CS, Ware LB. Biomarkers and Precision Medicine: State of the Art. Crit Care Clin. 2020;36:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA; NHLBI ARDS Clinical Trials Network. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA, May AK, Ware LB. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | American Medical Association. Telehealth resource center: Definitions. 2021. Available from: https://www.ama-assn.org/practice-management/digital/telehealth-resource-center-definitions. |
| 24. | Jin MX, Kim SY, Miller LJ, Behari G, Correa R. Telemedicine: Current Impact on the Future. Cureus. 2020;12:e9891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Wilcox ME, Chong CA, Niven DJ, Rubenfeld GD, Rowan KM, Wunsch H, Fan E. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med. 2013;41:2253-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 27. | Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 1070] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 28. | Krell K. Critical care workforce. Crit Care Med. 2008;36:1350-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Rosenfeld BA, Dorman T, Breslow MJ, Pronovost P, Jenckes M, Zhang N, Anderson G, Rubin H. Intensive care unit telemedicine: alternate paradigm for providing continuous intensivist care. Crit Care Med. 2000;28:3925-3931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Murias G, Sales B, Garcia-Esquirol O, Blanch L. Telemedicine in critical care. Open Respir Med J. 2009;3:10-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Al-Omari A, Al Mutair A, Al Ammary M, Aljamaan F. A Multicenter Case-Historical Control Study on Short-Term Outcomes of Tele-Intensive Care Unit. Telemed J E Health. 2020;26:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Grundy BL, Jones PK, Lovitt A. Telemedicine in critical care: problems in design, implementation, and assessment. Crit Care Med. 1982;10:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Kahn JM, Cicero BD, Wallace DJ, Iwashyna TJ. Adoption of ICU telemedicine in the United States. Crit Care Med. 2014;42:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Young LB, Chan PS, Lu X, Nallamothu BK, Sasson C, Cram PM. Impact of telemedicine intensive care unit coverage on patient outcomes: a systematic review and meta-analysis. Arch Intern Med. 2011;171:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Chen J, Sun D, Yang W, Liu M, Zhang S, Peng J, Ren C. Clinical and Economic Outcomes of Telemedicine Programs in the Intensive Care Unit: A Systematic Review and Meta-Analysis. J Intensive Care Med. 2018;33:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Mackintosh N, Terblanche M, Maharaj R, Xyrichis A, Franklin K, Keddie J, Larkins E, Maslen A, Skinner J, Newman S, De Sousa Magalhaes JH, Sandall J. Telemedicine with clinical decision support for critical care: a systematic review. Syst Rev. 2016;5:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Subramanian S, Pamplin JC, Hravnak M, Hielsberg C, Riker R, Rincon F, Laudanski K, Adzhigirey LA, Moughrabieh MA, Winterbottom FA, Herasevich V. Tele-Critical Care: An Update From the Society of Critical Care Medicine Tele-ICU Committee. Crit Care Med. 2020;48:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Hashimoto DA, Witkowski E, Gao L, Meireles O, Rosman G. Artificial Intelligence in Anesthesiology: Current Techniques, Clinical Applications, and Limitations. Anesthesiology. 2020;132:379-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 39. | Datta R, Singh S. Artificial intelligence in critical care: Its about time! Med J Armed Forces India. 2021;77:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Cui X, Chang Y, Yang C, Cong Z, Wang B, Leng Y. Development and Trends in Artificial Intelligence in Critical Care Medicine: A Bibliometric Analysis of Related Research over the Period of 2010-2021. J Pers Med. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Pirracchio R, Petersen ML, Carone M, Rigon MR, Chevret S, van der Laan MJ. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): a population-based study. Lancet Respir Med. 2015;3:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 42. | Magunia H, Lederer S, Verbuecheln R, Gilot BJ, Koeppen M, Haeberle HA, Mirakaj V, Hofmann P, Marx G, Bickenbach J, Nohe B, Lay M, Spies C, Edel A, Schiefenhövel F, Rahmel T, Putensen C, Sellmann T, Koch T, Brandenburger T, Kindgen-Milles D, Brenner T, Berger M, Zacharowski K, Adam E, Posch M, Moerer O, Scheer CS, Sedding D, Weigand MA, Fichtner F, Nau C, Prätsch F, Wiesmann T, Koch C, Schneider G, Lahmer T, Straub A, Meiser A, Weiss M, Jungwirth B, Wappler F, Meybohm P, Herrmann J, Malek N, Kohlbacher O, Biergans S, Rosenberger P. Machine learning identifies ICU outcome predictors in a multicenter COVID-19 cohort. Crit Care. 2021;25:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | DeepRhythmAI. Available from: https://www.medicalgorithmics.com/current-reports/51-2022-system-drai-deeprhythmai-registration-by-us-food-and-drug-administration-fda/. |
| 44. | Jiao Z, Choi JW, Halsey K, Tran TML, Hsieh B, Wang D, Eweje F, Wang R, Chang K, Wu J, Collins SA, Yi TY, Delworth AT, Liu T, Healey TT, Lu S, Wang J, Feng X, Atalay MK, Yang L, Feldman M, Zhang PJL, Liao WH, Fan Y, Bai HX. Prognostication of patients with COVID-19 using artificial intelligence based on chest x-rays and clinical data: a retrospective study. Lancet Digit Health. 2021;3:e286-e294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 45. | Kołodziejczak MM, Sierakowska K, Tkachenko Y, Kowalski P. Artificial Intelligence in the Intensive Care Unit: Present and Future in the COVID-19 Era. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | COViage. Available from: https://www.isolaceinc.com/deviceoverview. |
| 47. | CLEWICU - Instructions for Use. 2020. Available from: https://www.fda.gov/media/138372/download. |
| 48. | Ibrahim H, Liu X, Rivera SC, Moher D, Chan AW, Sydes MR, Calvert MJ, Denniston AK. Reporting guidelines for clinical trials of artificial intelligence interventions: the SPIRIT-AI and CONSORT-AI guidelines. Trials. 2021;22:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 49. | du Toit C, Tran TQB, Deo N, Aryal S, Lip S, Sykes R, Manandhar I, Sionakidis A, Stevenson L, Pattnaik H, Alsanosi S, Kassi M, Le N, Rostron M, Nichol S, Aman A, Nawaz F, Mehta D, Tummala R, McCallum L, Reddy S, Visweswaran S, Kashyap R, Joe B, Padmanabhan S. Survey and Evaluation of Hypertension Machine Learning Research. J Am Heart Assoc. 2023;12:e027896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 50. | Harrison AM, Thongprayoon C, Kashyap R, Chute CG, Gajic O, Pickering BW, Herasevich V. Developing the surveillance algorithm for detection of failure to recognize and treat severe sepsis. Mayo Clin Proc. 2015;90:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Dhungana P, Serafim LP, Ruiz AL, Bruns D, Weister TJ, Smischney NJ, Kashyap R. Machine learning in data abstraction: A computable phenotype for sepsis and septic shock diagnosis in the intensive care unit. World J Crit Care Med. 2019;8:120-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Mlodzinski E, Wardi G, Viglione C, Nemati S, Crotty Alexander L, Malhotra A. Assessing Barriers to Implementation of Machine Learning and Artificial Intelligence-Based Tools in Critical Care: Web-Based Survey Study. JMIR Perioper Med. 2023;6:e41056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Mlodzinski E, Stone DJ, Celi LA. Machine Learning for Pulmonary and Critical Care Medicine: A Narrative Review. Pulm Ther. 2020;6:67-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Lau YH, See KC. Point-of-care ultrasound for critically-ill patients: A mini-review of key diagnostic features and protocols. World J Crit Care Med. 2022;11:70-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (7)] |
| 55. | Elhazmi A, Al-Omari A, Sallam H, Mufti HN, Rabie AA, Alshahrani M, Mady A, Alghamdi A, Altalaq A, Azzam MH, Sindi A, Kharaba A, Al-Aseri ZA, Almekhlafi GA, Tashkandi W, Alajmi SA, Faqihi F, Alharthy A, Al-Tawfiq JA, Melibari RG, Al-Hazzani W, Arabi YM. Machine learning decision tree algorithm role for predicting mortality in critically ill adult COVID-19 patients admitted to the ICU. J Infect Public Health. 2022;15:826-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Lazzarini N, Filippoupolitis A, Manzione P, Eleftherohorinou H. A machine learning model on Real World Data for predicting progression to Acute Respiratory Distress Syndrome (ARDS) among COVID-19 patients. PLoS One. 2022;17:e0271227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 57. | Lu Y, Wu H, Qi S, Cheng K. Artificial Intelligence in Intensive Care Medicine: Toward a ChatGPT/GPT-4 Way? Ann Biomed Eng. 2023;51:1898-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Grewal H, Dhillon G, Monga V, Sharma P, Buddhavarapu VS, Sidhu G, Kashyap R. Radiology Gets Chatty: The ChatGPT Saga Unfolds. Cureus. 2023;15:e40135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (2)] |
| 59. | Montomoli J, Hilty MP, Ince C. Artificial intelligence in intensive care: moving towards clinical decision support systems. Minerva Anestesiol. 2022;88:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Brodie D, Slutsky AS, Combes A. Extracorporeal Life Support for Adults With Respiratory Failure and Related Indications: A Review. JAMA. 2019;322:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 61. | Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1351] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 62. | Bartlett R, Arachichilage DJ, Chitlur M, Hui SR, Neunert C, Doyle A, Retter A, Hunt BJ, Lim HS, Saini A, Renné T, Kostousov V, Teruya J. The History of Extracorporeal Membrane Oxygenation and the Development of Extracorporeal Membrane Oxygenation Anticoagulation. Semin Thromb Hemost. 2024;50:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 63. | Northwestern Medicine. History of ECMO. Available from: https://www.nm.org/conditions-and-care-areas/pulmonary/extracorporeal-membrane-oxygenation-program/history-of-ecmo. |
| 64. | Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2740] [Cited by in RCA: 2330] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 65. | Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettilä V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1102] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 66. | Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, Killer H, Mugford M, Thalanany M, Tiruvoipati R, Truesdale A, Wilson A. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, Müller T, Windisch W. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 68. | Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, Paden ML; ELSO member centers. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 662] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 69. | Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 70. | Guru PK, Seelhammer TG, Singh TD, Sanghavi DK, Chaudhary S, Riley JB, Friedrich T, Stulak JM, Haile DT, Kashyap R, Schears GJ. Outcomes of adult patients supported by extracorporeal membrane oxygenation (ECMO) following cardiopulmonary arrest. The Mayo Clinic experience. J Card Surg. 2021;36:3528-3539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | KDIGO Clinical Practice Guideline for Acute Kidney Injury. Available from: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. |
| 72. | Bellomo R, Ronco C. Continuous haemofiltration in the intensive care unit. Crit Care. 2000;4:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Ronco C. Continuous Renal Replacement Therapy: Forty-year Anniversary. Int J Artif Organs. 2017;40:257-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Saunders H, Sanghavi DK. Continuous Renal Replacement Therapy. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2023 Available from: https://www.ncbi.nlm.nih.gov/pubmed/32310488. |
| 75. | Davenport A, Honore PM. Continuous renal replacement therapy under special conditions like sepsis, burn, cardiac failure, neurotrauma, and liver failure. Semin Dial. 2021;34:457-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, Jans B, Hopkins S, Hansen S, Lyytikäinen O, Reilly J, Deptula A, Zingg W, Plachouras D, Monnet DL; Healthcare-Associated Infections Prevalence Study Group. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 77. | Hospital Acquired Infection. 2018. Available from: https://cdc.gov/hai/data/index.html. |
| 78. | Stahmeyer JT, Lutze B, von Lengerke T, Chaberny IF, Krauth C. Hand hygiene in intensive care units: a matter of time? J Hosp Infect. 2017;95:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 79. | Safdar N, Anderson DJ, Braun BI, Carling P, Cohen S, Donskey C, Drees M, Harris A, Henderson DK, Huang SS, Juthani-Mehta M, Lautenbach E, Linkin DR, Meddings J, Miller LG, Milstone A, Morgan D, Sengupta S, Varman M, Yokoe D, Zerr DM; Research Committee of the Society for Healthcare Epidemiology of America. The evolving landscape of healthcare-associated infections: recent advances in prevention and a road map for research. Infect Control Hosp Epidemiol. 2014;35:480-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Pryor R. Latest Advancements in Infection Prevention Technology. Available from: https://www.infectioncontroltoday.com/view/latest-advancements-in-infection-prevention-technology. |
| 81. | Shah A, Kashyap R, Tosh P, Sampathkumar P, O'Horo JC. Guide to Understanding the 2019 Novel Coronavirus. Mayo Clin Proc. 2020;95:646-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 82. | Kandi V. Implementation of Hospital Infection Control Committee: A Big Step Forward Towards Improved Patient Care. |
| 83. | Viana Martins CP, Xavier CSF, Cobrado L. Disinfection methods against SARS-CoV-2: a systematic review. J Hosp Infect. 2022;119:84-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Anderson DJ, Chen LF, Weber DJ, Moehring RW, Lewis SS, Triplett PF, Blocker M, Becherer P, Schwab JC, Knelson LP, Lokhnygina Y, Rutala WA, Kanamori H, Gergen MF, Sexton DJ; CDC Prevention Epicenters Program. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet. 2017;389:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 85. | Rock C, Hsu YJ, Curless MS, Carroll KC, Ross Howard T, Carson KA, Cummings S, Anderson M, Milstone AM, Maragakis LL. Ultraviolet-C Light Evaluation as Adjunct Disinfection to Remove Multidrug-Resistant Organisms. Clin Infect Dis. 2022;75:35-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Moreno-Manuel A, Calabuig-Fariñas S, Obrador-Hevia A, Blasco A, Fernández-Díaz A, Sirera R, Camps C, Jantus-Lewintre E. dPCR application in liquid biopsies: divide and conquer. Expert Rev Mol Diagn. 2021;21:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 87. | Walter K. Mechanical Ventilation. JAMA. 2021;326:1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Kacmarek RM. The mechanical ventilator: past, present, and future. Respir Care. 2011;56:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 89. | Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8487] [Cited by in RCA: 8329] [Article Influence: 333.2] [Reference Citation Analysis (3)] |
| 90. | Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;2013:CD003844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 91. | Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 92. | Qadir N, Bartz RR, Cooter ML, Hough CL, Lanspa MJ, Banner-Goodspeed VM, Chen JT, Giovanni S, Gomaa D, Sjoding MW, Hajizadeh N, Komisarow J, Duggal A, Khanna AK, Kashyap R, Khan A, Chang SY, Tonna JE, Anderson HL 3rd, Liebler JM, Mosier JM, Morris PE, Genthon A, Louh IK, Tidswell M, Stephens RS, Esper AM, Dries DJ, Martinez A, Schreyer KE, Bender W, Tiwari A, Guru PK, Hanna S, Gong MN, Park PK; Severe ARDS: Generating Evidence (SAGE) Study Investigators; Society of Critical Care Medicine's Discovery Network. Variation in Early Management Practices in Moderate-to-Severe ARDS in the United States: The Severe ARDS: Generating Evidence Study. Chest. 2021;160:1304-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (2)] |
| 93. | Liesching T, Kwok H, Hill NS. Acute applications of noninvasive positive pressure ventilation. Chest. 2003;124:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 94. | Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J. 2008;31:874-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 95. | Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S; Navalesi P Members Of The Steering Committee; Antonelli M, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S; Raoof S Members Of The Task Force. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 845] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 96. | Sepsis Factsheet - World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/sepsis#:~:text=A%20recent%20scientific%20publication%20estimated,all%20global%20deaths%20(1). |
| 97. | Marshall JC, Dellinger RP, Levy M. The Surviving Sepsis Campaign: a history and a perspective. Surg Infect (Larchmt). 2010;11:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Srzić I, Nesek Adam V, Tunjić Pejak D. Sepsis definition: What's new in the treatment guidelines. Acta Clin Croat. 2022;61:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 99. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 2275] [Article Influence: 568.8] [Reference Citation Analysis (0)] |
| 100. | Leedahl DD, Personett HA, Gajic O, Kashyap R, Schramm GE. Predictors of mortality among bacteremic patients with septic shock receiving appropriate antimicrobial therapy. BMC Anesthesiol. 2014;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Kashyap R, Singh TD, Rayes H, O'Horo JC, Wilson G, Bauer P, Gajic O. Association of septic shock definitions and standardized mortality ratio in a contemporary cohort of critically ill patients. J Crit Care. 2019;50:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4008] [Article Influence: 501.0] [Reference Citation Analysis (0)] |
| 103. | Kashyap R, Sherani KM, Dutt T, Gnanapandithan K, Sagar M, Vallabhajosyula S, Vakil AP, Surani S. Current Utility of Sequential Organ Failure Assessment Score: A Literature Review and Future Directions. Open Respir Med J. 2021;15:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Jackson KE, Semler MW. Advances in Sepsis Care. Clin Chest Med. 2022;43:489-498. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3798] [Cited by in RCA: 3965] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 106. | Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1043] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 107. | Magill SS, Edwards JR, Beldavs ZG, Dumyati G, Janelle SJ, Kainer MA, Lynfield R, Nadle J, Neuhauser MM, Ray SM, Richards K, Rodriguez R, Thompson DL, Fridkin SK; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 281] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 108. | Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6993] [Cited by in RCA: 6389] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 109. | ProCESS Investigators; Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1611] [Cited by in RCA: 1692] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 110. | ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 111. | Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1031] [Cited by in RCA: 1076] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 112. | Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. 2011;39:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 113. | Panchal V, Sivasubramanian BP, Samala Venkata V. Crystalloid Solutions in Hospital: A Review of Existing Literature. Cureus. 2023;15:e39411. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 114. | Liu C, Li H, Peng Z, Hu B, Dong Y, Gao X, Frank RD, Kashyap R, Gajic O, Kashani KB. Inclusion of Albumin in the Initial Resuscitation of Adult Patients with Medical Sepsis or Septic Shock: a Propensity Score-Matched Analysis. Shock. 2021;56:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Mehta Y, Paul R, Ansari AS, Banerjee T, Gunaydin S, Nassiri AA, Pappalardo F, Premužić V, Sathe P, Singh V, Vela ER. Extracorporeal blood purification strategies in sepsis and septic shock: An insight into recent advancements. World J Crit Care Med. 2023;12:71-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 116. | Saliba F. The Molecular Adsorbent Recirculating System (MARS) in the intensive care unit: a rescue therapy for patients with hepatic failure. Crit Care. 2006;10:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Soni M, Handa M, Singh KK, Shukla R. Recent nanoengineered diagnostic and therapeutic advancements in management of Sepsis. J Control Release. 2022;352:931-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 118. | Zhang Y, McCurdy MT, Ludmir J. Sepsis Management in the Cardiac Intensive Care Unit. J Cardiovasc Dev Dis. 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 119. | Zhao Y, Pu M, Zhang J, Wang Y, Yan X, Yu L, He Z. Recent advancements of nanomaterial-based therapeutic strategies toward sepsis: bacterial eradication, anti-inflammation, and immunomodulation. Nanoscale. 2021;13:10726-10747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 120. | Zhou C, Liu Y, Li Y, Shi L. Recent advances and prospects in nanomaterials for bacterial sepsis management. J Mater Chem B. 2023;11:10778-10792. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Yu C, Xian Y, Jing T, Bai M, Li X, Li J, Liang H, Yu G, Zhang Z. More patient-centered care, better healthcare: the association between patient-centered care and healthcare outcomes in inpatients. Front Public Health. 2023;11:1148277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 122. | Kashyap R, Murthy S, Arteaga GM, Dong Y, Cooper L, Kovacevic T, Basavaraja C, Ren H, Qiao L, Zhang G, Sridharan K, Jin P, Wang T, Tuibeqa I, Kang A, Ravi MD, Ongun E, Gajic O, Tripathi S; SCCM Discovery CERTAINp Collaborative Investigators. Effectiveness of a Daily Rounding Checklist on Processes of Care and Outcomes in Diverse Pediatric Intensive Care Units Across the World. J Trop Pediatr. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Tripathi S, Kaur H, Kashyap R, Dong Y, Gajic O, Murthy S. A survey on the resources and practices in pediatric critical care of resource-rich and resource-limited countries. J Intensive Care. 2015;3:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 124. | Wilson ME, Krupa A, Hinds RF, Litell JM, Swetz KM, Akhoundi A, Kashyap R, Gajic O, Kashani K. A video to improve patient and surrogate understanding of cardiopulmonary resuscitation choices in the ICU: a randomized controlled trial. Crit Care Med. 2015;43:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 125. | Chlan LL, Weinert CR, Tracy MF, Skaar DJ, Gajic O, Ask J, Mandrekar J. Study protocol to test the efficacy of self-administration of dexmedetomidine sedative therapy on anxiety, delirium, and ventilator days in critically ill mechanically ventilated patients: an open-label randomized clinical trial. Trials. 2022;23:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |