Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.89026
Peer-review started: October 18, 2023
First decision: November 23, 2023
Revised: December 5, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: March 9, 2024
Processing time: 138 Days and 13.7 Hours
Septic shock is a severe form of sepsis characterised by deterioration in circulatory and cellular-metabolic parameters. Despite standard therapy, the outcomes are poor. Newer adjuvant therapy, such as CytoSorb® extracorporeal haemoadsorption device, has been investigated and shown promising outcome. However, there is a lack of some guidance to make clinical decisions on the use of CytoSorb® haemoadsorption as an adjuvant therapy in septic shock in Indian Setting. Therefore, this expert consensus was formulated.
To formulate/establish specific consensus statements on the use of CytoSorb® haemoadsorption treatment based on the best available evidence and contextualised to the Indian scenario.
We performed a comprehensive literature on CytoSorb® haemoadsorption in sepsis, septic shock in PubMed selecting papers published between January 2011 and March 2023 2021 in English language. The statements for a consensus document were developed based on the summarised literature analysis and identification of knowledge gaps. Using a modified Delphi approach combining evidence appraisal and expert opinion, the following topics related to CytoSorb® in septic shock were addressed: need for adjuvant therapy, initiation timeline, need for Interleukin -6 levels, duration of therapy, change of adsorbers, safety, prerequisite condition, efficacy endpoints and management flowchart. Eleven expert members from critical care, emergency medicine, and the intensive care participated and voted on nine statements and one open-ended question.
Eleven expert members from critical care, emergency medicine, and the intensive care participated and voted on nine statements and one open-ended question. All 11 experts in the consensus group (100%) participated in the first, second and third round of voting. After three iterative voting rounds and adapting two statements, consensus was achieved on nine statements out of nine statements. The consensus expert panel also recognised the necessity to form an association or society that can keep a registry regarding the use of CytoSorb® for all indications in the open-ended question (Q10) focusing on “future recommendations for CytoSorb® therapy”.
This Indian perspective consensus statement supports and provides guidance on the use of CytoSorb® haemoadsorption as an adjuvant treatment in patients with septic shock to achieve optimal outcomes.
Core Tip: This evidence-based expert consensus statement gives information/clarity on the key areas of knowledge gaps of CytoSorb® therapy: need for adjuvant therapy, initiation timeline, need for Interleukin -6 levels, duration of therapy, change of adsorbers, safety, prerequisite condition, efficacy endpoints, and (therapy) management flowchart. This expert consensus statements provides general physicians, emergency care physicians, anaesthetist, and intensivists with current information regarding the use of CytoSorb® haemoadsorption as an adjuvant treatment in patients with refractory septic shock.
- Citation: Mehta Y, Ansari AS, Mandal AK, Chatterjee D, Sharma GS, Sathe P, Umraniya PV, Paul R, Gupta S, Singh V, Singh YP. Systematic review with expert consensus on use of extracorporeal hemoadsorption in septic shock: An Indian perspective. World J Crit Care Med 2024; 13(1): 89026
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/89026.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.89026
Sepsis is described as potentially fatal organ dysfunction induced by an unbalanced host response to infection[1]. Septic shock, on the other hand, is a subset of sepsis in which the underlying circulatory and cellular metabolic abnormalities are severe enough to significantly increase mortality[1]. Sepsis and Septic shock are leading health related issues. The global incidence of sepsis is estimated to be 489 million and sepsis related deaths to be 110 million worldwide, with higher burden in developing countries[2]. India has a higher death rate from sepsis than other South Asian countries[2]. It is estimated that sepsis death rate in India is 213 per 100000 population[2].
The pathophysiology is multifaceted, with both pathogenic and host factors pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) playing a significant part in its progression and subsequent outcome[2,3]. However, the diversity of septic shock requires to accurately characterise individuals, which makes clinical intervention challenging[3,4]. The backbone of treatment remains appropriate and timely antibiotic therapy, source control, if necessary, IV fluids and titrated vasopressors[5]. However, when these treatment efforts fail to improve the patients' condition in a subset of patients, adjuvant therapies are usually explored to enhance outcomes[5-7].
Despite clinical research efforts and the development of sepsis management guide-lines over the last few decades, the potential to improve the outcome of the condition tends to be limited[8]. Newer adjuvant therapies, such as the targeted elimination of pathogen-associated toxins and mediators by specific adsorption, are gaining recognition[6,7,9]. The use of an extracorporeal haemoadsorption device called CytoSorb® (Cyto-Sorbents corp, New Jersey, United States) for cytokine adsorption is one of the more recent adjuvants. It contains specially designed polymer beads with a large adsorption surface and an adsorption spectrum up to around 60 kDa. It is a high flow, low resistance cytokine adsorbent[7]. CytoSorb extracorporeal haemoadsorption therapy tends to restore the balance of the immune response to infection by eliminating the triggers for the response and the excessive cytokines produced, with the target of achieving immunological homeostasis in patients with severe cytokinemia, including septic shock[4].
Although, there is a substantial amount of clinical data from case series and prospective/retrospective research[10-12] that supports the likelihood of improving treatment outcomes with CytoSorb® hemoadsorption in septic shock, the limited evidence from randomised clinical trials[7] makes it difficult to endorse or adopt in management guide-lines. Furthermore, published evidence on proper patient selection, timing and dosing of CytoSorb® therapy is still scarce. So, there is lack of a consensus guidance to make clinical decisions on the use of CytoSorb® haemoadsorption as an adjuvant in the management of septic shock. Our aim/objectives were to formulate/establish specific consensus statements on the use of CytoSorb® haemoadsorption treatment based on the best available evidence and contextualised to the Indian scenario. Firstly, this Indian consensus provides statements on the use of haemoadsorption as an adjuvant therapy in patients with sepsis. This expert consensus statements provides general physicians, emergency care physicians, anaesthetists, and intensivists with current information regarding the use of haemoadsorption as an adjuvant treatment in patients with refractory septic shock. Secondly, this Indian perspective consensus statement supports use of haemoadsorption as an adjuvant treatment in patients with septic shock and provides guidance to achieve better outcomes. Thirdly, it may also contribute to the optimization of refractory septic shock treatment in India.
This consensus statement was intended for a target audience of healthcare professionals/clinicians representing/working in the intensive care units/critical care units and emergency departments.
Members of the scientific panel conducted a comprehensive literature review on the use of CytoSorb® haemoadsorption in patients with sepsis, septic shock, or who were critically ill in PubMed selecting papers published between January 2011 and March 2023 2021 in English language.The following keywords and terms were use (("cytosorb"[All Fields] OR "cytosorbents"[All Fields] OR "hemoadsorption"[All Fields] OR (("extracorporal"[All Fields] OR "extracorporally"[All Fields] OR "extracorporeal"[All Fields] OR "extracorporeally"[All Fields]) AND ("blood purif"[Journal] OR ("blood"[All Fields] AND "purification"[All Fields]) OR "blood purification"[All Fields]))) AND (("shock"[MeSH Terms] OR "shock"[All Fields] OR "shocked"[All Fields] OR "shocking"[All Fields] OR "shocks"[All Fields]) AND ("sepsis"[MeSH Terms] OR "sepsis"[All Fields]) AND "septic"[All Fields] AND ("therapeutics"[MeSH Terms] OR "therapeutics"[All Fields] OR "therapies"[All Fields] OR "therapy"[MeSH Subheading] OR "therapy"[All Fields] OR "therapy s"[All Fields] OR "therapys"[All Fields]))) AND ((fha[Filter]) AND (2011/1/1:2023/3/30[pdat])).
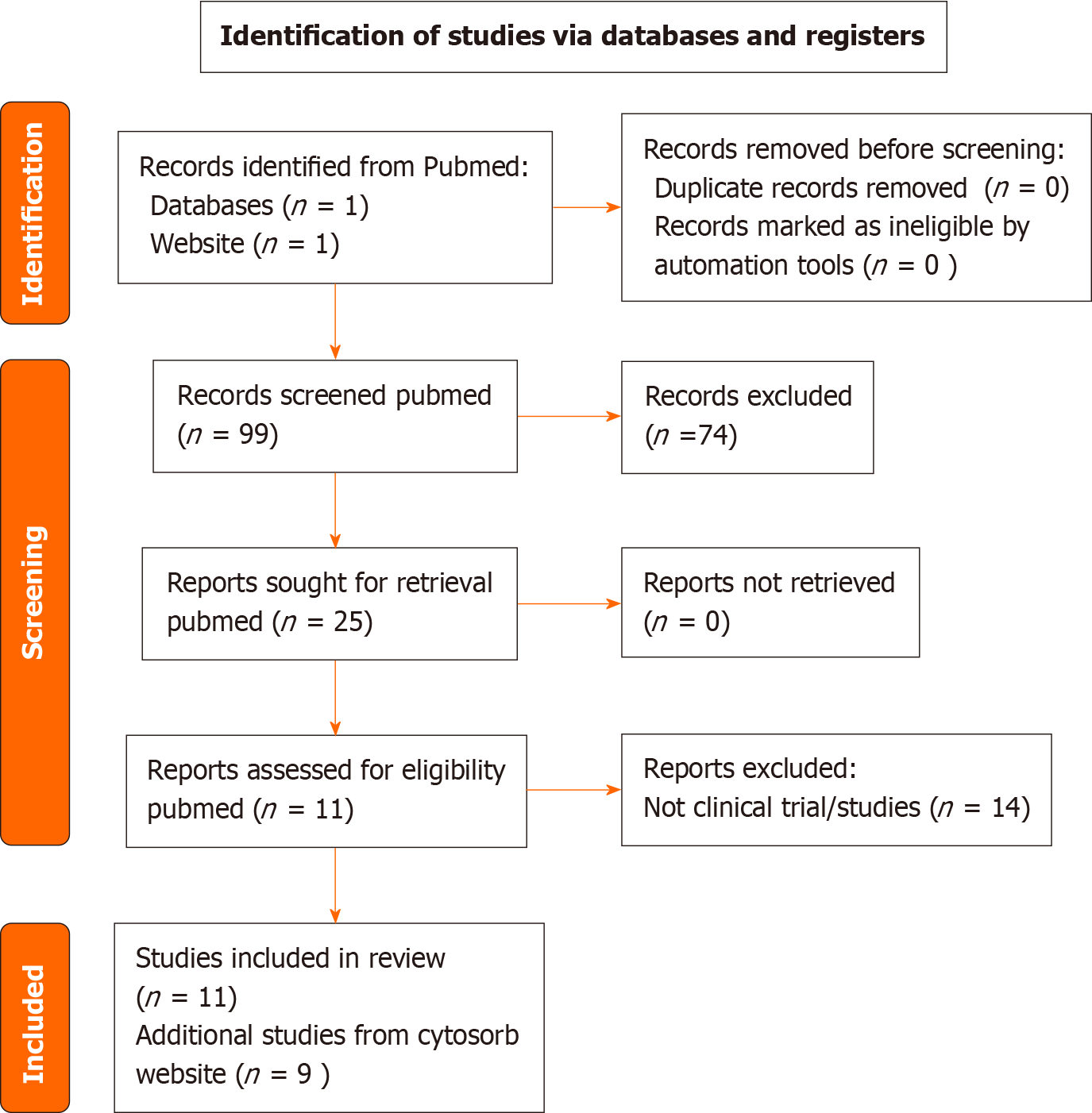
The results of a PubMed and Medline database search using suitable Mesh and search keywords yielded a reference list of CytoSorb® publications. A total of 99 papers were identified with no duplicates, and, as a first step, no papers were excluded for other reasons (PRISMA flow diagram reported in Figure 1). As a second step, we excluded papers that were not pertinent to any of the following criteria: (1) Cytosorb and Sepsis/septic shock; (2) Clinical studies/ trials of Cytosorb; and (3) Literature review or systematic reviews of extracorporeal hemoadsorption. According to the selection criteria, out of the 99 results of PubMed research assessed for eligibility, 25 studies were included, out of which 11 clinical trials of Cytosorb were included in final analysis from Pubmed as evidence. In addition, few cross references and 11 references from Cytosorb Product information website was included.
The statements for a consensus document were developed based on the summarised literature analysis and identification of knowledge gaps. A total of nine consensus question statements focused on the use of CytoSorb® therapy in septic shock were formulated. One question was kept open-ended for discussion.
The scientific panel convened a consensus expert group of 11 members, each with more than 20 years of expertise in emergency medicine or critical care medicine. These individual experts from India's various geographical cities (Gurugram, Mumbai, Mohali, Kolkata, Delhi, Pune, Vadodara, and Hyderabad) were invited for voting and to express their expert opinion in the consensus process.
The Delphi procedure gathers a group of experts for decision making through an iterative series of questions, anonymous responses, and controlled feedback to the respondents[13]. Using a modified Delphi approach, involving combination of scientific evidence appraisal and expert opinion based on clinical experience of the consensus members, the following topics related statements to CytoSorb® in refractory septic shock were addressed to achieve consensus: need for adjuvant therapy, initiation timeline, need for Interleukin-6 levels, duration of therapy, change of adsorbers, safety, prerequisite condition, efficacy endpoints and (therapy) management flowchart.
The consensus expert members were asked to vote on all of the statements (agree/yes, disagree/no, or abstain) based on their clinical experience and scientific evidence appraisal obtained from systematic review. They were also asked to offer feedback on the content and/or phrasing of the statements, as well as to suggest any new statements they thought would be beneficial.
Consensus was reached for a particular statement when there was at least 80% agreement in the voting. Statements with no consensus (less than 80% agreement), statements with consensus but relevant remarks that resulted in paraphrasing, and additional statements suggested by experts were reformulated and presented for voting in subsequent modified Delphi rounds. To achieve a decision, maximum three modified Delphi voting rounds were held. The total number of consensuses achieved were calculated.
All 11 experts in the consensus group (100%) participated in the first, second and third round of voting and commenting for the consensus statements.
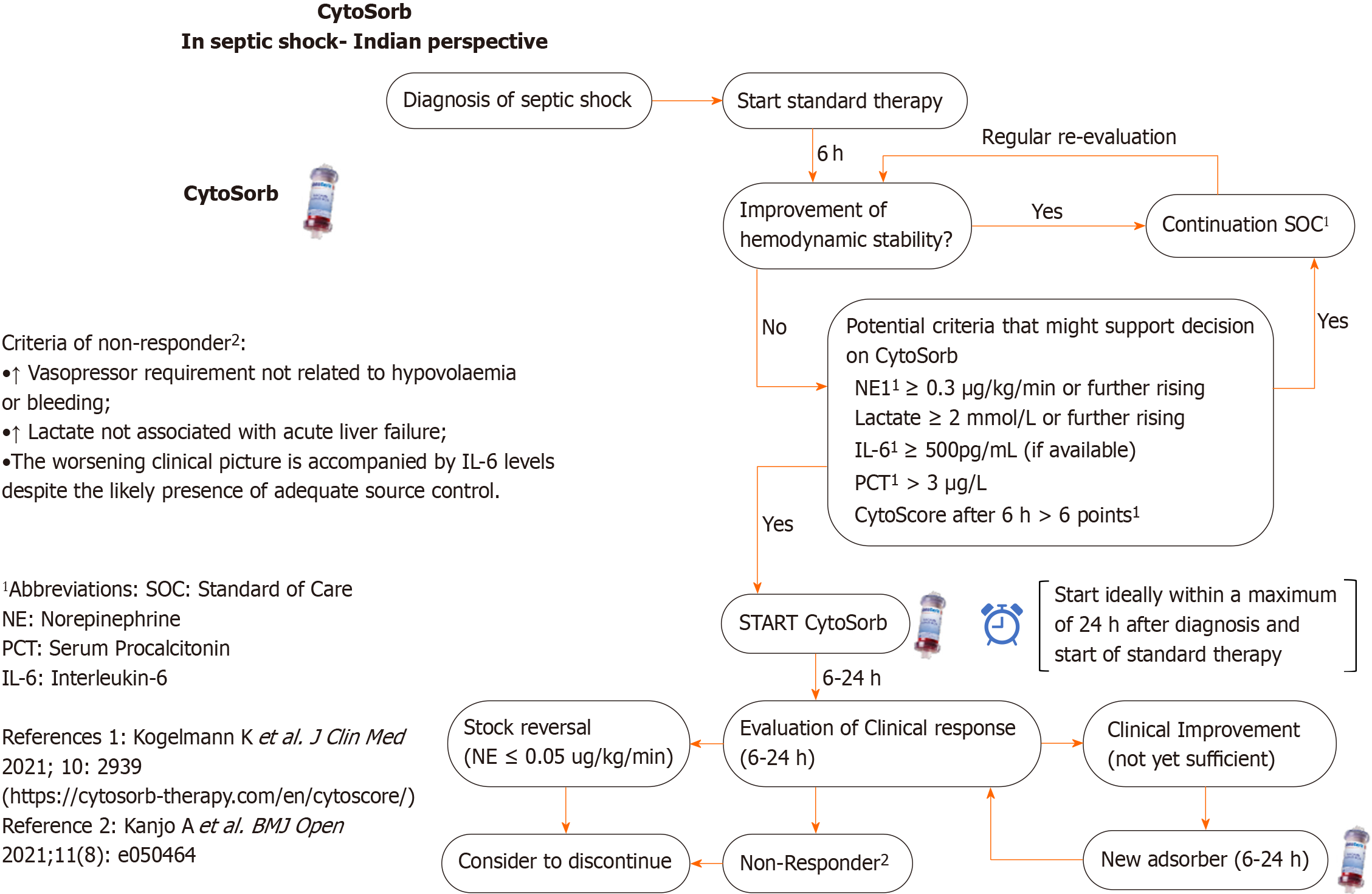
In the first round, consensus was obtained in 8 (Q1- Q8) of the 9 selected initial statements, whereas consensus was not reached in 1 statement (Q9). It was discussed and re-posted for the second round of voting and comments. Furthermore, 1 statement (Q8) with consensus had positive comments that prompted a modest revision of the phrases. This revised statement Q8 was sent out again along with Q9 for the second round of voting. The one revised statement (Q8) obtained consensus in the second round of voting. For the last statement (Q9, flowchart) agreement was reached in the third round of voting after therapy timelines were modified (Figure 2). Overall, consensus was reached in all nine out of nine statements (Table 1).
| Questions | Responses, n = 11 (%) | Consensus status - overall agreement | |
| Agreed/yes (%) | Disagreed/no (%) | ||
| Q1. Is there a need for adjuvant therapy in the management of refractory septic shock patients, when standard of care is insufficient? | 10 (90.91) | 1 (9.09) | A total of 90.91% experts agreed on the need for adjuvant therapy in the management of refractory septic shock patients, when the standard of care is insufficient. (Consensus Achieved) |
| Q2. In case of refractory septic shock cycle, CytoSorb® ideally be initiated within a maximum of 24 h after diagnosis and start of standard therapy | 11 (100) | 0 (0) | All experts (100%) agreed that in refractory septic shock cycle, CytoSorb® ideally be initiated within a maximum of 24 h after diagnosis and start of standard therapy. (Consensus Achieved) |
| Q3. IL-6 levels are not a mandatory parameter to decide on using CytoSorb® therapy in refractory septic shock patients | 10 (90.91) | 1 (9.09) | A total of 90.91% experts agreed that IL-6 levels are not a mandatory parameter to decide on using CytoSorb® therapy in refractory septic shock patients. (Consensus Achieved) |
| Q4. There are patients who may require more than one CytoSorb® adsorber to achieve sufficient haemodynamic stabilization | 10 (90.91) | 1 (9.09) | A total of 90.91% experts agreed that there are patients who may require more than one CytoSorb® adsorber to achieve sufficient haemodynamic stabilization. (Consensus Achieved) |
| Q5. If you want to continue with CytoSorb® therapy, the absorber should be changed after 6-24 h depending on the clinical course and the machine type availability | 11 (100) | 0 (0) | All experts (100%) agreed that if CytoSorb® therapy is continued, the absorber should be changed after 6-24 h depending on the clinical course and the machine type availability. (Consensus Achieved) |
| Q6. CytoSorb® therapy is generally a safe therapy | 10 (90.91) | 1 (9.09) | A total of 90.91% experts agreed that CytoSorb® is generally a safe therapy. (Consensus Achieved) |
| Q7. Sepsis-induced AKI requiring RRT is no prerequisite to initiate CytoSorb® therapy in refractory septic shock patients | 11 (100) | 0 (0) | All experts (100%) agreed that sepsis-induced AKI requiring RRT is not a prerequisite to initiate CytoSorb® therapy in refractory septic shock patients. (Consensus Achieved) |
| Q8. Evaluation of the efficacy of CytoSorb® therapy should be based on more proximal endpoints like haemodynamic stabilization, inflammatory biomarkers, and/or improvement in the organ function instead of mortality | 10 (90.91) | 1 (9.09) | A total of 90. 91% experts agreed that the evaluation of the efficacy of CytoSorb® therapy should be based on endpoints like haemodynamic stabilization, inflammatory biomarkers, and/or improvement in the organ function instead of mortality. (Consensus Achieved) |
| Q9. Do you think this flowchart can be helpful to a doctor very new to the therapy to ensure a certain level of best practice? | 11 (100) | 0 (0) | All experts (100%) agreed on the (revised) flowchart for doctor who are new to the therapy to ensure a certain level of best practice. (Consensus Achieved) |
The consensus expert panel also recognised the necessity to form an association or society that can keep a registry regarding the use of CytoSorb® for all indications in the open-ended question (Q10) focusing on “future recommendations for CytoSorb® therapy”. The potential of this treatment for treating a variety of clinical disorders and its impact on patient outcomes will be better understood with the aid of this registry.
Q1: Is there a need for adjuvant therapy in the management of refractory septic shock patients when standard of care is insufficient?
Expert panel agreement: A total of 90.91% experts agreed on the need for adjuvant therapy in the management of refractory septic shock patients. (Consensus Achieved).
Reason/scientific evidence: Standard of care in septic shock with the cornerstones of source control and fluid and catecholamine therapy is of unquestionable importance, however, not directly addressing the dysregulated immune response as a central problem. Especially in refractory patients, with no adequate response to standard therapy measures, adjuvant approaches might be needed and be able to fill this therapeutic gap. Consequently CytoSorb® haemoadsorption treatment attempts to restore the balance of the immune response to infection by eliminating some triggers for the response and the excessive cytokines produced, with the target of achieving immunological homeostasis[4,7,14]. It has the capacity to disrupt the immune response at various stages by eliminating various inflammatory mediators like PAMPs, DAMPs and cytokines from blood, thereby directly addressing the problem of the dysregulated host response.
Q2: In case of refractory septic shock cycle, CytoSorb® haemoadsorption should ideally be initiated within a maximum of 24 h after diagnosis and start of standard therapy.
Expert panel agreement: All experts (100%) agreed that in refractory septic shock, CytoSorb® should ideally be initiated within a maximum of 24 h. (Consensus Achieved).
Reason/scientific evidence: Kogelmann et al[15] presented a dynamic scoring system to support patient selection for CytoSorb® therapy in early refractory septic shock. Among other things analysis of nearly 200 patients treated with CytoSorb® in septic shock revealed that those treated within the first 24 h had a higher chance of surviving than those treated after 24 h, and for every hour of CytoSorb® haemoadsorption treatment delay, the risks of death at Day 56 increased by 1.5% (P < 0.034). These positive findings are in line with various other publications, like data from Singh et al[16] and Paul et al[17], in which CytoSorb® therapy was shown to be a safe and well tolerated rescue therapy which should be used preferably within the first 24 h after onset of septic shock. Approaches in which CytoSorb® therapy was initiated in selected refractory patients within the first 24 h of onset of septic shock or start of standard therapy respectively showed positive effects with regard to improved hemodynamic stabilization and signals for improved survival[12].
Q3: IL-6 level is not a mandatory parameter to decide on using CytoSorb® therapy in refractory septic shock patients.
Expert panel agreement: A total of 90.91% experts agreed that IL-6 level is not a mandatory parameter to decide on using CytoSorb® therapy in refractory septic shock patients. (Consensus Achieved).
Reason/scientific evidence: Although IL-6 levels are a promising target due to its involvement in the pathogenesis of septic shock, the profile of IL-6 kinetics in critically ill patients may be heterogeneous and influenced by a number of factors. Furthermore, IL-6 levels alone may not be especially predictive of the patient’s future reaction[4]. Addition-ally, from a practical perspective IL-6 levels might not be available in a timely manner in every center. Various clinical studies have shown good results with CytoSorb® therapy when patient selection was not based on IL-6 levels, but rather the clinical picture of (refractory) septic shock with elevated (and increasing) levels of vasopressor needs and other criteria[7,12,18]. In the light of all this it was decided that measuring IL-6 levels before initiating CytoSorb® treatment for refractory septic shock was NOT mandatory.
Q4: There are patients who may require more than one CytoSorb® adsorber to achieve sufficient hemodynamic stabilization.
Expert panel agreement: A total of 90.91% experts agreed that there are patients who may require more than one CytoSorb® adsorber to achieve sufficient hemodynamic stabilization (Consensus Achieved).
Reason/scientific evidence: In a systematic review and meta-analysis, Hawchar et al[10] examined the role of haemoadsorption using CytoSorb® in attaining quick haemo-dynamic stabilisation in patients with refractory vasoplegic shock. The available data demonstrated that early CytoSorb® therapy resulted in a considerable reduction in vasopressor (norepinephrine) need following treatment (median from 0.55 µg/kg/min to 0.09 microg/kg/min, P < 0.001), which indicates the important contribution of early haemoadsorption in achieving rapid haemodynamic stabilization in patients with refractory vasoplegic shock[10]. Rugg et al[12] could improve haemodynamic stabilization with only one adsorber having been used in the majority of the patients. Friesecke et al[19] on the other hand utilized a mean of 3 ± 1.5 CytoSorb® adsorbers per patient when they conducted a prospective clinical study in twenty patients with refractory septic shock. Also, in this research, CytoSorb® therapy had favorable outcomes and resulted in a considerable reduction in vasopressor (noradrenaline) needs as well as an increase in lactate clearance. Shock reversal was achieved in 65% (n = 13) of the patients[19]. So, in conclusion the number of adsorbers needed might vary from patient to patient and there are patients who may require more than one CytoSorb® adsorber to achieve sufficient haemodynamic stabilization.
Q5: If you want to continue with CytoSorb® therapy, the adsorber should be changed after 6-24 h depending on the clinical course and the machine type availability.
Expert panel agreement: All experts (100%) agreed that if CytoSorb® therapy is continued, the adsorber should be changed after 6-24 h depending on the clinical course and the machine type availability. (Consensus Achieved).
Reason/scientific evidence: According to the current instructions for use (IFU)[20], one adsorber can stay for up to 24 h on a patient. Recent experiences however suggest that some patients seem to benefit from earlier changes of the adsorber i.e., after 12 h or even earlier. Back in April 2020 the United States (US) Food and Drug Administration’s (FDA) Emergency Use Authorization had been granted for CytoSorb® extracorporeal blood purification treatment to reduce hyperinflammation in seriously ill coronavirus disease 2019 (COVID-19) patients[21]. An FDA-specific dose of 12:12:24:24 h had to be used in these patients. Hayanga et al[21] retrospectively analysed the data from a US CytoSorb® Therapy in COVID-19 (CTC) Registry. The analysis showed that CytoSorb® treatment was linked with improved survival rates in critically ill COVID-19 patients who received extracorporeal membrane oxygenation. Earlier changes might ensure an ongoing high removal capacity of the adsorber avoiding early saturation in situation with a high cytokine load for the device[22]. Therefore, a change of adsorber might be appropriate anytime between 6-24 h. It was discussed that it does not need to be changed earlier than 6 h as the device would work properly but a change should not occur later than 24 h to comply with the current IFU, also as no significant removal capacity beyond this point should be expected from the adsorber. As usual, the exact timing of adsorber changes (if applicable) would vary from patient to patient.
Q6: CytoSorb® therapy is generally a safe therapy.
Expert panel Agreement: A total of 90.91% experts agreed that CytoSorb® is generally a safe therapy. (Consensus Achieved).
It was also acknowledged that as with all other therapeutic measures even CytoSorb® has its own side effects, but it is generally a safe therapy.
Reason/scientific evidence: To date CytoSorb® therapy has been used in a wide variety of critically ill patients[23]. Features like size-selectivity and concentration dependency as well as the high biocompatibility support a favourable safety profile of the device, which was further supported by various publications[23].
Diab et al[24] conducted a multicenter randomized controlled trial of CytoSorb therapy in patients undergoing surgery for infective endo carditis (REMOVE trial). A total of 288 patients were randomly allocated to either intraoperative CytoSorb® hemoadsorption (n = 142) or control (n = 146). Apart from the effect on postoperative organ dysfunction, the trial also investigated the safety profile in the two groups, which included peri-operative complications and adverse events[24]. The trial found that the frequency and pattern of postoperative complications and adverse events (distributive shock, acute renal dysfunction, respiratory insufficiency, re-exploration for bleeding, central nervous system related, and cardiac events) were comparable in both groups, confirming the safety of this device[24].
The results of the Eleventh analysis of registry data from an International CytoSorb® Registry conducted by Hawchar et al[25] further supported the favourable safety profile of CytoSorb® therapy. Data from 1434 critically ill patients (sepsis/septic shock (65.3%), cardiac surgery perioperatively (11.9%), cardiac surgery postoperatively (4.7%), and other (18.1%) indications) from 46 centres revealed that CytoSorb® treatment related complications (cardiac, respiratory, blood, central nervous, and kidney related) were re-ported in only 2.16% (n = 31) patients, whereas the majority of patients (97.8%, n = 1403) had no reported CytoSorb® treatment-related complications[25]. They concluded that in line with all other papers published so far, regardless of the type of the study or case report, the 11th analysis of the Registry data further suggests that CytoSorb® therapy is safe[25]. So, despite acknowledging that, like any other therapeutic interventions, CytoSorb® can also have adverse effects, e.g., with regard to unwanted drug removal or complications associated with the extracorporeal circuit, the therapy was regarded as generally safe.
Q7: Sepsis-induced acute kidney injury (AKI) requiring renal replacement therapy (RRT) is no prerequisite to initiate CytoSorb® therapy in refractory septic shock patients.
Expert panel agreement: All experts (100%) agreed that sepsis-induced AKI requiring RRT is not a prerequisite to initiate CytoSorb® therapy in refractory septic shock patients. (Consensus Achieved).
Reason/scientific evidence: CytoSorb® therapy is a haemoadsorption therapy targeting small and middle-sized hydrophobic substances. This is in contrast to the classical hydrophilic targets of RRT. Circuits from renal replacement systems can be used technically for integration of the CytoSorb® adsorber, however, in principle the decision for or against CytoSorb® should be made independent of the indication and start of continuous renal replacement therapy or other extracorporeal therapies as one cannot replace the other[26].
Hawchar et al[7] conducted a prospective, randomised pilot study of CytoSorb® as a stand-alone therapy in patients with septic shock in Hungary. Twenty (n = 20) patients with septic shock of medical origin, on mechanical ventilation, norepinephrine > 10 µg/min, procalcitonin > 3 ng/mL, but no requirement for RRT were included in this proof-of-concept trial and were randomised into CytoSorb® (n = 10) and Control (n = 10) groups[7]. Over the assessed time-points, vasopressor (norepinephrine) requirements and procalcitonin levels decreased significantly in the CytoSorb® group compared to the control group (P < 0.05)[7].
If early need for RRT due to sepsis-induced AKI crises, integration of CytoSorb® into the circuit can still be easy, however waiting for an RRT indication shouldn’t delay the start of CytoSorb® when appropriate to address hyperinflammation and ongoing haemo-dynamic instability in early refractory septic shock. Therefore, sepsis-induced AKI requiring RRT was NOT seen as a prerequisite to initiate CytoSorb® therapy in these patients.
Q8: Evaluation of the efficacy of CytoSorb® therapy should be based on endpoints like haemodynamic stabilization, inflammatory biomarkers, and/or improvement in the organ function instead of mortality.
Expert panel agreement: A total of 90.91% experts agreed that the evaluation of the efficacy of CytoSorb® therapy should be based on endpoints like haemodynamic stabilization, inflammatory biomarkers, and/or improvement in the organ function instead of mortality. (Consensus Achieved).
Reason/scientific evidence: Sepsis is a syndrome and not a disease and septic shock is a disorder with a diverse phenotype. First of all, CytoSorb® therapy is not a primary therapy to treat sepsis, but only an adjunctive option to address the dysregulated immune response as an underlying problem in septic shock patients. So CytoSorb® is solely used to eliminate cytokines (and other mediators) and decrease the complications of a dysregulated host response[8]. Thus, objective assessment of CytoSorb® in septic shock is challenging. Furthermore, the reason for mortality in septic shock patients may be multifunctional and not directly attributable to the host response, which can lead to overestimation of syndrome-attributable risks[27].
Various endpoints such as haemodynamic stabilisation, improvement in organ function or inflammatory biomarkers, and survival have been recorded in studies with CytoSorb® in sepsis/septic shock[7,8,10,19]. Understanding the complexity of the syndrome, assessment of the efficacy of CytoSorb® treatment in studies should be based on the complexities of critical illness syndromes with endpoints such as haemodynamic stability, inflammatory biomarkers, and/or improvement in organ function rather than mortality.
Q9: Do you think this flowchart can be helpful to a doctor very new to the therapy to ensure a certain level of best practice?
Expert panel disagreement: initially but all experts (100%) agreed on the revised flowchart for doctors new to therapy. (Consensus Achieved).
Reasons: Based on the following discussion, the original flowchart was revised and the revised flowchart was agreed upon (see Figure 2).
Suggested modifications in original flowchart: (1) Changing the time period to change the adsorber from the 12 h specified in the chart to 6-24 h based on clinical criteria; (2) The flowchart should preferably be modified to contain three distinct pathways for patients who were significantly improving, slightly improving, and not at all improving; and (3) For the benefit of physicians with less experience in this area, it may also be necessary to mention the potential criteria for starting therapy with inclusion of the CytoScore[15] definition along with therapy flow chart.
Q10: Future recommendations for CytoSorb® therapy (Open ended discussion and not for voting).
Recommendation: To establish an association/society that can maintain a registry on the utilization of CytoSorb® in the management of different indications. This will help to get valuable real-world evidence data about the potential of this therapy in multiple clinical conditions and its effect on patient outcomes.
Septic shock occurs from a dysfunctional host response to infection, resulting in a state described as a "cytokine storm" that progresses to shock and carries the high risk of development of a multi organ dysfunction syndrome[1,28]. The standard therapy is timely resuscitation, antibiotics, and targeted vasopressors[5]. Despite standard therapy, a certain subset of individuals have poor outcomes and require adjuvant therapy[5]. To improve outcomes, various innovative adjuvant therapies have been explored. Blood purification treatments, such as high-volume continuous haemofiltration or cytokine and/or endotoxin elimination, have been proposed as one such strategy to promote immune homeostasis[4].
Sorbent technologies have recently garnered a lot of consideration. CytoSorb® based haemoadsorption is one such therapy. The CytoSorb® device is composed of biocompatible, extremely porous polymer beads[7,20,24]. The adsorber has a surface area of around 45000 m2 compared to a standard hemofilter with a surface area of 1-1.5 m2 and a molecular cut-off of approximately 60 kDa for eliminating cytokines as well as other hydrophobic substances. As a result, CytoSorb® does not adsorb endotoxin with a molecular weight of 100 kDa[4,7,20,29]. CytoSorb® has been developed and approved for treatment in patients with severe cytokinemia, but can also adsorb bilirubin, myoglobin, free haemoglobin and the antithrombotics ticagrelor and rivaroxaban during cardiopulmonary bypass[24]. Studies have revealed favorable results in patients with sepsis and septic shock, with, however, only limited evidence from randomized control trials[7,10,11,12,17,28].
In this consensus paper, an attempt was made to address the utilization and adoption of CytoSorb based haemoadsorption therapy in patients with septic shock with critical appraisal of the evidence from the current available literature. This consensus statement gives more information/clarity on the key areas of knowledge gaps of CytoSorb® therapy: Need for adjuvant therapy, initiation timeline, need for Interleukin -6 levels, duration of therapy, change of adsorbers, safety, prerequisite condition, efficacy endpoints and (therapy) management flowchart. Table 2 summarizes the consensus statement. The current consensus statements are based on existing literature data, primarily from case series, pro
| Number | Summary of consensus statements |
| 1 | There is the need for adjuvant therapy (CytoSorb® haemoadsorption) in the management of refractory septic shock patients, when the standard of care is insufficient |
| 2 | In refractory septic shock cycle, CytoSorb® ideally be initiated within a maximum of 24 h after diagnosis and start of standard therapy |
| 3 | In the initiation of CytoSorb® therapy in refractory septic shock patient, IL-6 levels are not a pre-requisite or mandatory parameter for decision making |
| 4 | In a subset of patients, more than one CytoSorb® adsorber may be required to achieve sufficient haemodynamic stabilization |
| 5 | In continuation of CytoSorb® therapy, the absorber should be changed after 6-24 h depending on the clinical course and the machine type availability |
| 6 | CytoSorb® therapy is generally a safe therapy |
| 7 | Sepsis-induced AKI requiring RRT is not a prerequisite to initiate CytoSorb® therapy in refractory septic shock patients |
| 8 | The evaluation of the efficacy of CytoSorb® therapy should be based on endpoints like haemodynamic stabilization, inflammatory biomarkers, and/or improvement in the organ function, instead of mortality |
| 9 | The (displayed, Figure 2) flowchart can be helpful to a doctor very new to the therapy to ensure a certain level of best practice |
These consensus statements are intended to offer guidance to clinicians working in the field of critical care/ emergency care, healthcare manager, healthcare organizations and patients regarding the use of CytoSorb® in septic shock.
We expect that this expert agreement will facilitate the personalized, safe, and pragmatic use of CytoSorb® haemoadsorption in septic shock patients in the critical care setting. Knowledge always lags behind evidence, and this expert consensus has shortcomings that we intend to resolve in future.
Major strengths: (1) Being the first sort of consensus statement that provides information and guidance on the use of CytoSorb® therapy in critically ill/septic shock patients in India; (2) involving a significant group of experts from various geographical cities across India with long standing experience in the field of critical care; (3) providing various articles on CytoSorb therapy (based on a systematic review) and critically appraising evidence by sharing it with all participating experts; (4) using a modified Delphi technique with open-ended (text-based) feedback from respondents and subsequent adaptation; and (5) providing of a Flowchart for the Indian market which will help doctors to optimise for the use of CytoSorb® therapy in septic shock patients.
Limitations: Although the majority of the publications critically evaluated after the systematic review were research studies, case series, and systematic reviews, there is substantially less evidence from randomised control trials.
This Indian perspective consensus statement supports and provides guidance on the use of CytoSorb® haemoadsorption as an adjuvant treatment in patients with septic shock to achieve optimal outcomes. We hope that this consensus statement will help in facilitating proper treatment initiation and maintenance of CytoSorb® haemoadsorption therapy in the management of refractory septic shock and it may also contribute to the optimization of refractory septic shock treatment in India.
Septic shock is a severe form of sepsis characterised by deterioration in circulatory and cellular-metabolic parameters. Despite standard therapy, the outcomes are poor. Newer adjuvant therapy, such as CytoSorb® extracorporeal haemoadsorption device, has been investigated and shown promising outcome.
There is a lack of some guidance to make clinical decisions on the use of CytoSorb® haemoadsorption as an adjuvant therapy in septic shock.
To formulate/establish specific consensus statements on the use of CytoSorb® haemoadsorption treatment based on the best available evidence and contextualised to the Indian scenario.
We performed a comprehensive literature on CytoSorb® haemoadsorption in sepsis, septic shock in PubMed selecting papers published between January 2011 and March 2023 2021 in English language. The statements for a consensus document were developed based on the summarised literature analysis and identification of knowledge gaps. Using a modified Delphi approach combining evidence appraisal and expert opinion, the following topics related to CytoSorb® in septic shock were addressed and consensus was formulated.
All 11 experts in the consensus group (100%) participated in the first, second and third round of voting. After three iterative voting rounds and adapting two statements, consensus was achieved on nine statements out of nine statements. The consensus expert panel also recognised the necessity to form an association or society that can keep a registry regarding the use of CytoSorb® for all indications in the open-ended question (Q10) focusing on “future recommendations for CytoSorb® therapy”.
This Indian perspective consensus statement supports and provides guidance on the use of CytoSorb® haemoadsorption as an adjuvant treatment in patients with septic shock to achieve optimal outcomes.
We expect that this expert agreement will facilitate the personalized, safe, and pragmatic use of CytoSorb® haemoadsorption in septic shock patients in the critical care setting. Knowledge always lags behind evidence, and this expert consensus has shortcomings that we intend to resolve in future.
We wish to acknowledge Dr Volker Humbert (Senior director medical strategy, CytoSorbents Europe GmbH), Dr Yashpal Jadeja (Senior Director, Medical Affairs, Biocon Biologics Limited), Dr Monika P (Medical Advisor, Medical Affairs, Biocon Biologics Limited), Mr. Chethan P (Associate Director- Marketing, Biocon Biologics Limited), Mr. Nikhil Dwivedi (Associate manager- Marketing, Biocon Biologics Limited), Mr Pradeep Yanamala (Managing Director- CytoSorbents India Pvt Ltd), Mr Sukrut Khadke (Regional Business Man-ager- CytoSorbents India Pvt Ltd) and for technical support & help in organizing this Consensus meeting.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Management
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei YC, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao YQ
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 2. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4113] [Article Influence: 822.6] [Reference Citation Analysis (4)] |
| 3. | Rimmer E, Houston BL, Kumar A, Abou-Setta AM, Friesen C, Marshall JC, Rock G, Turgeon AF, Cook DJ, Houston DS, Zarychanski R. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Honore PM, Hoste E, Molnár Z, Jacobs R, Joannes-Boyau O, Malbrain MLNG, Forni LG. Cytokine removal in human septic shock: Where are we and where are we going? Ann Intensive Care. 2019;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Nandhabalan P, Ioannou N, Meadows C, Wyncoll D. Refractory septic shock: our pragmatic approach. Crit Care. 2018;22:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | László I, Trásy D, Molnár Z, Fazakas J. Sepsis: From Pathophysiology to Individualized Patient Care. J Immunol Res. 2015;2015:510436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 8. | Mehta Y, Paul R, Rabbani R, Acharya SP, Withanaarachchi UK. Sepsis Management in Southeast Asia: A Review and Clinical Experience. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mehta Y, Singh A, Gupta A, Bhan A. Modulating the Inflammatory Response With Hemadsorption (CytoSorb) in Patients Undergoing Major Aortic Surgery. J Cardiothorac Vasc Anesth. 2021;35:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Hawchar F, Rao C, Akil A, Mehta Y, Rugg C, Scheier J, Adamson H, Deliargyris E, Molnar Z. The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Akil A, Ziegeler S, Reichelt J, Rehers S, Abdalla O, Semik M, Fischer S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac Cardiovasc Surg. 2021;69:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Rugg C, Klose R, Hornung R, Innerhofer N, Bachler M, Schmid S, Fries D, Ströhle M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective 'Genetic' Matched Analysis. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Barrett D, Heale R. What are Delphi studies? Evid Based Nurs. 2020;23:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 14. | Monard C, Rimmelé T, Ronco C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019;47:1-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kogelmann K, Hübner T, Schwameis F, Drüner M, Scheller M, Jarczak D. First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Singh YP, Chhabra SC, Lashkari K, Taneja A, Garg A, Chandra A, Chhabra M, Singh GP, Jain S. Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb(®)) in the management of septic shock: A retrospective observational study. Int J Artif Organs. 2020;43:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Paul R, Sathe P, Kumar S, Prasad S, Aleem M, Sakhalvalkar P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb(®)) in patients with sepsis and septic shock. World J Crit Care Med. 2021;10:22-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 2019;23:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Friesecke S, Stecher SS, Gross S, Felix SB, Nierhaus A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs. 2017;20:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Biocon CytoSorbents. Cytosorb [Internet]. Cited 20 October 2022. Available from: https://www.biocon.com/docs/domestic-market-pi/nephro/CYTOSORB-IFU.PDF. |
| 21. | Hayanga JWA, Song T, Durham L, Garrison L, Smith D, Molnar Z, Scheier J, Deliargyris EN, Moazami N. Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: the CytoSorb therapy in COVID-19 (CTC) registry. Crit Care. 2023;27:243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Berlot G, Samola V, Barbaresco I, Tomasini A, di Maso V, Bianco F, Gerini U. Effects of the timing and intensity of treatment on septic shock patients treated with CytoSorb(®): Clinical experience. Int J Artif Organs. 2022;45:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Cytosorb Literature Database. Instant Advanced Research Tool [Internet]. Cited 20 October 2022. Available from: https://Literature.cytosorb-therapy.com. |
| 24. | Diab M, Faerber G, Doenst T. Response by Diab et al to Letter Regarding Article, "Cytokine Hemoadsorption During Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results From a Multicenter Randomized Controlled Trial". Circulation. 2022;146:e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 25. | Hawchar F, Tomescu D, Träger K, Joskowiak D, Kogelmann K, Soukup J, Friesecke S, Jacob D, Gummert J, Faltlhauser A, Aucella F, van Tellingen M, Malbrain MLNG, Bogdanski R, Weiss G, Herbrich A, Utzolino S, Nierhaus A, Baumann A, Hartjes A, Henzler D, Grigoryev E, Fritz H, Bach F, Schröder S, Weyland A, Gottschaldt U, Menzel M, Zachariae O, Novak R, Berden J, Haake H, Quintel M, Kloesel S, Kortgen A, Stecher S, Torti P, Nestler F, Nitsch M, Olboeter D, Muck P, Findeisen M, Bitzinger D, Kraßler J, Benad M, Schott M, Schumacher U, Molnar Z, Brunkhorst FM. Hemoadsorption in the critically ill-Final results of the International CytoSorb Registry. PLoS One. 2022;17:e0274315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Napp LC, Ziegeler S, Kindgen-Milles D. Rationale of Hemoadsorption during Extracorporeal Membrane Oxygenation Support. Blood Purif. 2019;48:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Girbes ARJ, de Grooth HJ. Time to stop randomized and large pragmatic trials for intensive care medicine syndromes: the case of sepsis and acute respiratory distress syndrome. J Thorac Dis. 2020;12:S101-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Brouwer WP, Duran S, Ince C. Improved Survival beyond 28 Days up to 1 Year after CytoSorb Treatment for Refractory Septic Shock: A Propensity-Weighted Retrospective Survival Analysis. Blood Purif. 2021;50:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Ankawi G, Xie Y, Yang B, Xie P, Ronco C. What Have We Learned about the Use of Cytosorb Adsorption Columns? Blood Purif. 2019;48:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |