Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.88540
Peer-review started: October 1, 2023
First decision: November 23, 2023
Revised: December 4, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: March 9, 2024
Processing time: 156 Days and 1.2 Hours
Thrombocytopenia is common in patients with sepsis and septic shock.
To analyse the decrease in the number of platelets for predicting bloodstream infection in patients with sepsis and septic shock in the intensive care unit.
A retrospective analysis of patients admitted with sepsis and septic shock in Xingtai People Hospital was revisited. Patient population characteristics and laboratory data were collected for analysis.
The study group consisted of 85 (39%) inpatients with bloodstream infection, and the control group consisted of 133 (61%) with negative results or contamination. The percentage decline in platelet counts (PPCs) in patients positive for pathogens [57.1 (41.3-74.6)] was distinctly higher than that in the control group [18.2 (5.1–43.1)] (P < 0.001), whereas the PPCs were not significantly different among those with gram-positive bacteraemia, gram-negative bacteraemia, and fungal infection. Using receiver operating characteristic curves, the area under the curve of the platelet drop rate was 0.839 (95%CI: 0.783-0.895).
The percentage decline in platelet counts is sensitive in predicting bloodstream infection in patients with sepsis and septic shock. However, it cannot identify gram-positive bacteraemia, gram-negative bacteraemia, and fungal infection.
Core Tip: Thrombocytopenia is common in sepsis and septic shock, but there are few reports on the diagnostic value of thrombocytopenia in bloodstream infection. Our results found that the rate of platelet drop but not the lowest platelet count has a high predictive ability for bloodstream infection in patients with sepsis or septic shock. However, it cannot identify gram-positive bacteraemia, gram-negative bacteraemia, and fungal infection. Dynamic detection of platelet counts appears to be an early alert for the clinician in identifying the site of infection and evaluating serious infection. This will guide the performance of blood cultures and the use of empirical antibiotics.
- Citation: Li X, Wang S, Ma J, Bai SG, Fu SZ. Predictive value of thrombocytopenia for bloodstream infection in patients with sepsis and septic shock. World J Crit Care Med 2024; 13(1): 88540
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/88540.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.88540
Bloodstream infection (BSI) is a life-threatening condition caused by the presence of microorganisms, generally bacteria or fungi, in blood. The ability to diagnose BSI early can have a significant impact on patient outcomes. Platelets constantly roam the vascular system and play an active role in pathogen capture. Platelets can kill bacterial pathogens directly via microbicidal proteins, known as thrombocidins[1]. Platelets are able to release cytokines, recruit leukocytes, interact with bacteria and the endothelium, and promote microthrombi formation[2,3]. Either a relative or an absolute decrease in the platelet number is often seen in patients who most likely develop sepsis and septic shock. However, few reports have documented the relationship between a drop in platelet counts and BSI. The aims of this study were to determine the diagnostic ability of the percentage decline of platelet counts (PPCs) for predicting the presence of BSI and evaluating the cut-off point for detecting BSI.
We conducted a retrospective cohort study at Xingtai People’s Hospital, Hebei Province, China, which has 2200 beds serving local residents. Adult patients (age ≥ 18 years) who were admitted to the intensive care unit (ICU) with a diagnosis of sepsis or septic shock and stayed at least 3 d in the ICU were included in the study. The exclusion criteria included haematologic disease, acute bleeding, history of platelet disorders, cirrhosis, and use of chemotherapy (in the last 30 d prior to admission). The following variables were collected from the electronic medical records: patient population characteristics (age, sex); underlying disease (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, cardiovascular disease, cerebrovascular disease); laboratory data (aetiology, daily platelet counts, white blood cell count, neutrophil count, haemoglobin, C-reactive protein, procalcitonin, blood urea nitrogen, serum creatinine, alanine aminotransferase, aspartate aminotransferase, serum bilirubin, serum albumin, fibrinogen, D-dimer, prothrombin time, activated partial thromboplastin time); source of infection; primary diagnosis, mechanical ventilation, requirement for renal replacement therapy; and Acute Physiology and Chronic Health Evaluation II score (APACHE-II score). This study was a retrospective clinical data analysis and patients did not undergo invasive procedures.
According to the sepsis 3 guidelines[4], sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. The clinical criteria for sepsis include suspected or documented infection and an acute increase in two or more Sequential Organ Failure Assessment points as a proxy for organ dysfunction. Septic shock was defined by the clinical criteria of sepsis and vasopressor therapy needed to elevate mean arterial pressure ≥ 65 mmHg and lactate > 2 mmol/L (18 mg/dL) despite adequate fluid resuscitation. Blood cultures were drawn from the patients within 1 h after ascertaining the patient had sepsis or septic shock. BSI was defined as one or more bacterial species in blood samples. Bloodstream infection caused by coagulase-negative staphylococci was determined after careful evaluation by the doctor according to the clinical manifestations and treatment effect. Negative specimen culture was defined as negative culture for 5 d. Daily platelet counts were recorded from the day the blood cultures were taken. If the platelet counts were performed twice or more within 24 h, we recorded the lowest count for analysis. The rate of the drop in platelets was calculated by the formula (Platelet1− Plateletlow)/Platelet1, where Platelet1 is the Platelet value at the time of drawing blood cultures, and Plateletlow is the lowest platelet value within the following 3 d.
During the study period, a total of 218 patients with sepsis and septic shock were enrolled. Of these, 85 had positive blood cultures, and 133 had negative cultures or contamination. Demographic, clinical and laboratory characteristics are presented in Tables 1 and 2. The median age was 63 years, and 122 (56%) patients were male. There was no difference in age, sex, underlying comorbidities (hypertension, diabetes, cardiovascular disease, chronic obstructive pulmonary disease), APACHE II score, mechanical ventilation, renal replacement therapy, or 28-d mortality between patients with positive blood cultures and those with negative blood cultures or contamination. However, patients with cerebrovascular disease had fewer positive blood cultures (P = 0.044). Regarding the primary source of infection, respiratory tract infections were the most common infections in patients in the ICU, which were detected in 101 patients (46.3%). Patients with positive blood cultures were more often admitted with hepatobiliary and urinary infections and less often with respiratory tract infections (Table 1).
| Characteristic | All patients (n =218) | Blood culture results | P value | |
| Negative or contamination (n = 133) | Positive with pathogen (n = 85) | |||
| Age (yr) | 63 (53–73) | 64 (53–73) | 62 (52–70) | 0.253 |
| Sex (male/female) | 218 (122/96) | 133 (81/52) | 85 (41/44) | 0.066 |
| Comorbidities | ||||
| Hypertension | 79 (36.2) | 50 (37.6) | 29 (34.1) | 0.603 |
| Diabetes | 49 (22.5) | 29 (21.8) | 20 (23.5) | 0.766 |
| Cardiovascular disease | 23 (10.6) | 17 (12.8) | 6 (7.1) | 0.18 |
| COPD | 16 (7.3) | 13 (9.8) | 3 (3.5) | 0.085 |
| Cerebrovascular disease | 34 (15.6) | 26 (19.5) | 8 (9.4) | 0.044 |
| Primary diagnosis for cultures | ||||
| Respiratory | 101 (46.3) | 81 (60.9) | 20 (23.5) | < 0.001 |
| Intestinal | 50 (22.9) | 28 (21.1) | 22 (25.9) | 0.408 |
| Urogenital | 33 (15.1) | 12 (9.0) | 21 (24.7) | 0.002 |
| Hepatobiliary | 21 (9.6) | 6 (4.5) | 15 (17.6) | 0.001 |
| Skin/soft tissue | 5 (2.3) | 3 (2.3) | 2 (2.4) | 1.0 |
| Other | 8 (3.7) | 3 (2.3) | 5 (5.9) | 0.308 |
| APACHE II | 20 (14-25) | 19 (14-25) | 20 (14-27) | 0.533 |
| Mechanical ventilation (yes/no) | 218 (156/62) | 133 (100/33) | 85 (56/29) | 0.137 |
| Renal replacement (yes/no) | 218 (29/189) | 133 (13/120) | 85 (16/69) | 0.055 |
| 28-d mortality | 81 (37.2) | 46 (34.6) | 35 (41.2) | 0.326 |
| Characteristic | All patients (n = 218) | Blood culture results | P value | |
| Negative or contamination (n = 133) | Positive with pathogen (n = 85) | |||
| PCT (ng/mL) | 6.9 (1.1-32.1) | 2.85 (0.4-16.6) | 16.8 (4.1-112.3) | < 0.001 |
| CRP (mg/mL) | 113.3 (47.9-174.3) | 99.4 (41.1-168.3) | 128.1 (68.5-189.7) | 0.047 |
| WBC (× 109/L) | 12.3 (8.1–17.8) | 11.7 (8.8–16.8) | 13.1 (7.5–21.7) | 0.404 |
| Neutrophils (%) | 90.1 (82.5-93.5) | 88.9 (83.4-92.3) | 92.2 (82.3-94.7) | 0.029 |
| Haemoglobin (g/L) | 109.6 ± 23.8 | 109.9 ± 24 | 109.2 ± 23.6 | 0.824 |
| HBA1C | 5.9 (5.4-7.2) | 6.0 (5.6-7.1) | 5.8 (5.3-8.6) | 0.7 |
| PT (s) | 14.4 (12.7-16.7) | 13.9 (12.7-15.7) | 15.5 (12.7-17.5) | 0.012 |
| APTT (s) | 34.5 (30.2-41.5) | 33.3 (29.6-38.0) | 37.2 (30.2-44.8) | 0.005 |
| Fib (g/L) | 3.94 (3.0-4.97) | 3.95 (3.19-5.26) | 3.93 (2.85-4.87) | 0.344 |
| D-dimer (μg/mL) | 6.37 (2.62-12.34) | 5.33 (2.19-10.94) | 8.06 (3.93-13.81) | 0.02 |
| Alanine aminotransferase (U/L) | 31.8 (18–61.2) | 24.2 (14.8–47.5) | 43.4 (23.1–113.9) | < 0.001 |
| Aspartate aminotransferase (U/L) | 46.2 (29.6–87.1) | 37.9 (25.8–70) | 67.2 (35.2–170.4) | < 0.001 |
| Total bilirubin (μmol/L) | 15.6 (9.1–29.1) | 13.5 (8.9–23.7) | 20.6 (10.7–49) | 0.009 |
| Serum albumin (g/L) | 28.8 (24.4-31.7) | 28 (24.2-32.2) | 29.4 (25-31.5) | 0.444 |
| BUN (mmol/L) | 9.9 (6.0–15.3) | 9.2 (5.9–14.2) | 10.8 (6.2–15.9) | 0.147 |
| Cr (μmol/L) | 99.6 (67.3–180) | 89.1 (62.4–146) | 134.5 (84.7–221) | 0.001 |
At admission, patients with positive blood cultures had higher levels of procalcitonin, neutrophils, and C-reactive protein but not white blood cell counts than those with negative cultures or contamination (Table 2). Marked differences were also found in the prothrombin time, activated partial thromboplastin time, D-dimer, total bilirubin, alanine aminotransferase, aspartate aminotransferase, and creatinine levels. These indicators were significantly higher in patients with positive blood cultures than in those with negative cultures or contamination. No significant differences were found for fibrinogen, urea nitrogen, serum albumin, haemoglobin, or glycosylated haemoglobin (P > 0.05).
Among the 85 bacteraemia episodes, 24 were caused by gram-positive bacteria, 59 by gram-negative bacteria and 2 by fungi. The most commonly isolated bacterial species were Escherichia coli (n = 36) and Klebsiella pneumoniae (n = 15), which accounted for 60% of blood infections (Table 3).
| Microorganism | No. (%) isolated from blood cultures |
| Gram-positive bacteria | 24 (28.2) |
| Staphylococcus aureus | 6 (7.1) |
| Staphylococcus epidermidis | 3 (3.5) |
| Staphylococcus haemolyticus | 2 (2.4) |
| Staphylococcus hominis | 2 (2.4) |
| Staphylococcus caprae | 1 (1.2) |
| Enterococcus faecium | 5 (5.9) |
| Streptococcus pyogenes | 3 (3.5) |
| Streptococcus viridans | 1 (1.2) |
| Enterococcus gallinarum | 1 (1.2) |
| Gram-negative bacteria | 59 (69.4) |
| Escherichia coli | 36 (42.3) |
| Klebsiella pneumoniae | 15 (17.6) |
| Pseudomonas aeruginosa | 3 (3.5) |
| Aeromonas sobria | 2 (2.4) |
| Enterobacter cloacae | 1 (1.2) |
| Klebsiella oxytoca | 1 (1.2) |
| Acinetobacter lwoffii | 1 (1.2) |
| Fungus | 2 (2.4) |
| Candida albicans | 2 (2.4) |
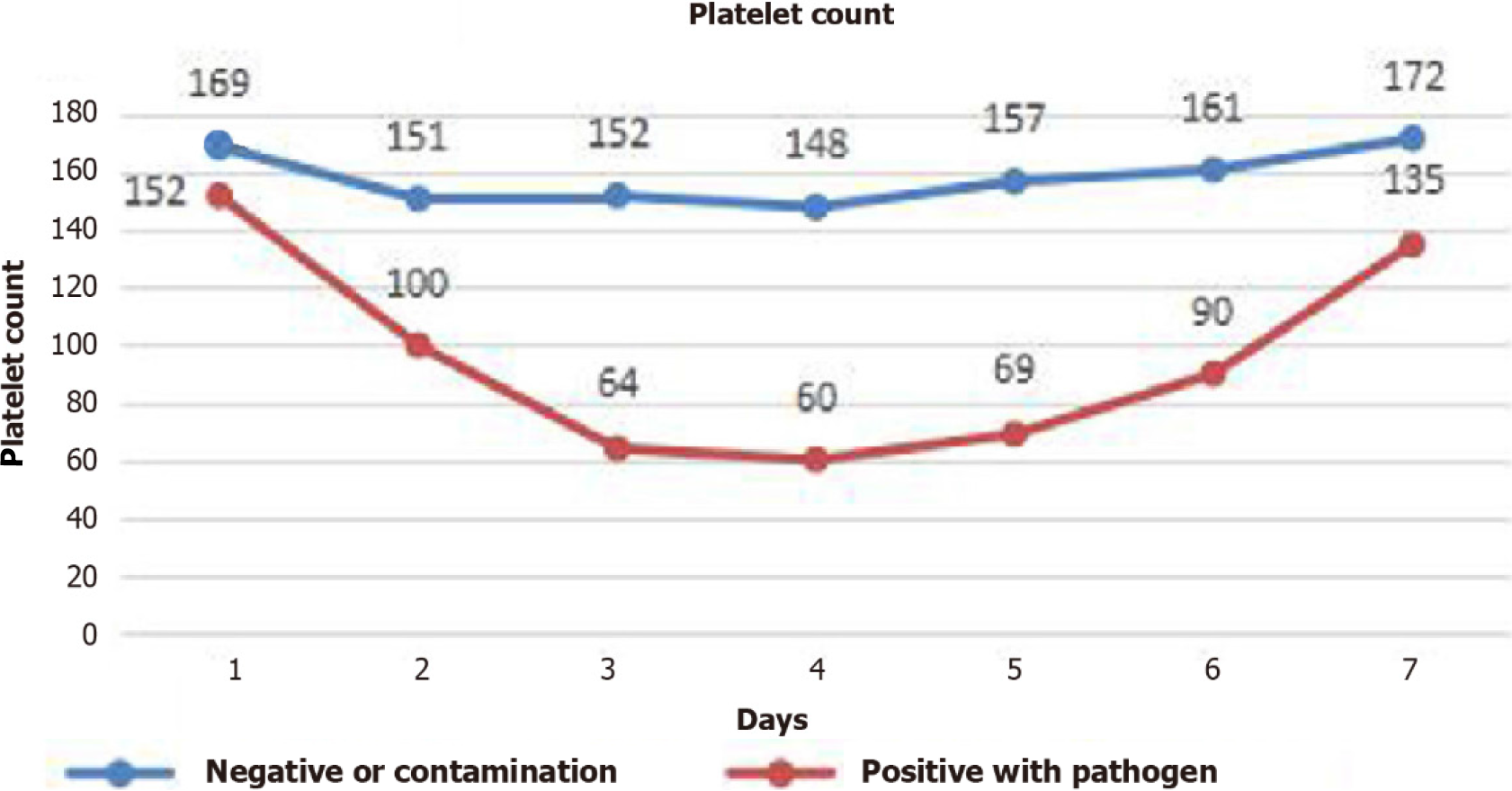
The daily platelet count over time was recorded as the median (25th, 75th percentile) (Figure 1). The median platelet count dropped to a nadir of 60 (range, 30-128) × 109/L in the group positive for pathogens and 148 (range, 73-200) × 109/L in the group negative for pathogens or contamination on the fourth day after admission to the ICU and subsequently increased. The platelet count did not differ between the two groups on the first day. From Day 2 to Day 7, the platelet count in the pathogen-positive group was significantly lower than that in the control group (P < 0.05).
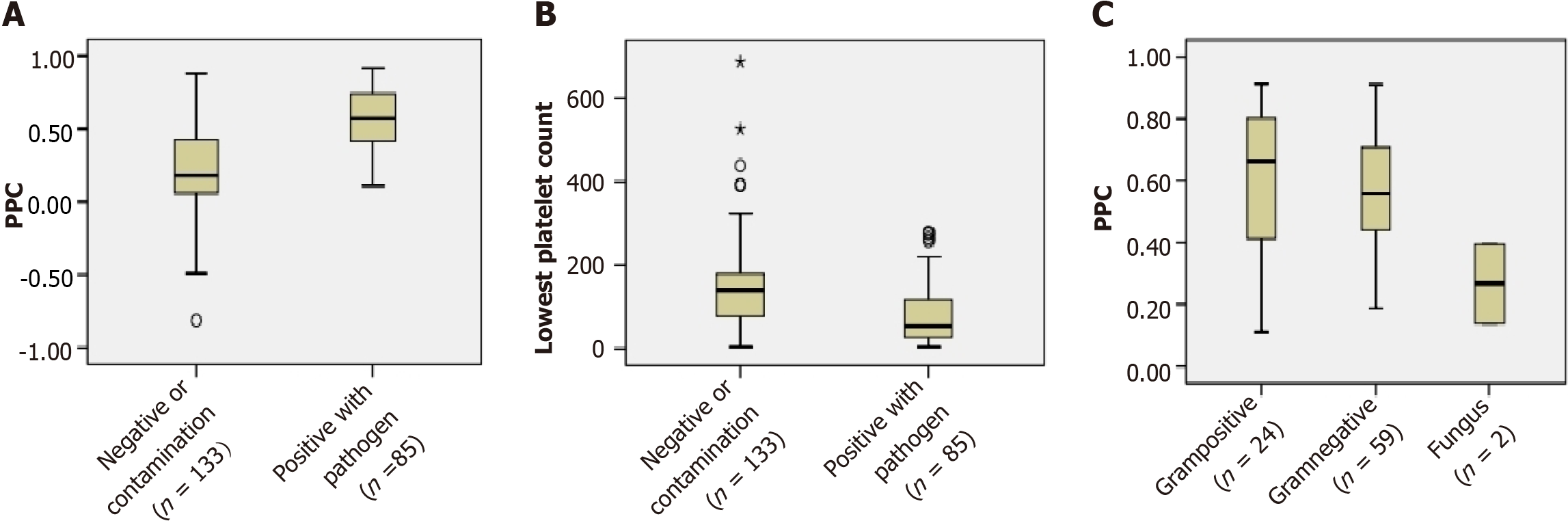
In the present study, the percentage decline in PPC in patients who were positive for pathogens [57.1 (41.3-74.6)] was distinctly higher than that in patients who were negative or had contamination [18.2 (5.1–43.1)] (P < 0.001). There were also significant differences in the lowest platelet count between the patients who were positive for pathogens [54 (27-119)] and those who were negative or contaminated [140 (77-182)] (P < 0.001). However, in the subgroup of positive with pathogens, the PPCs were not significantly different among the gram-positive bacteraemia, gram-negative bacteraemia, and fungi groups (P > 0.05) (Figure 2).
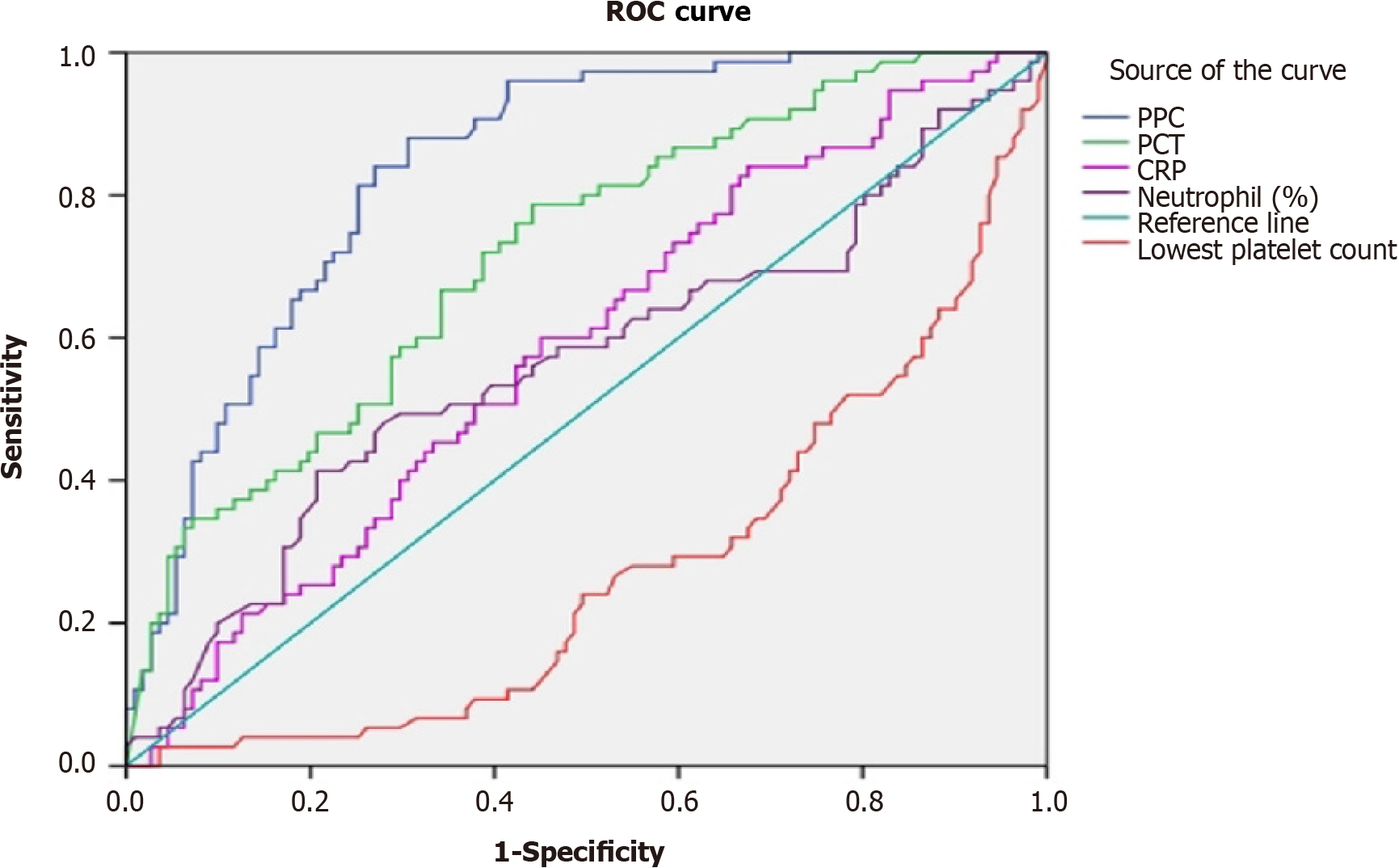
Receiver operating characteristic (ROC) curves were created for PPCs to predict BSI (Figure 3). The areas under the ROC curves (AUCs) were calculated to evaluate the biomarkers (PPC, procalcitonin, lowest platelet count, C-reactive protein, and neutrophil percentage) to determine the presence of bacteraemia. PPC had high diagnostic utility for predicting BSI. Its predictive ability was greater than that of procalcitonin; the AUC of PPC was 0.839 (95%CI: 0.783-0.895). Additionally, that of procalcitonin was 0.718 (95%CI: 0.644-0.791), whereas C-reactive protein and neutrophil percentage did not detect BSI (P > 0.05). Using the lowest platelet count, the area under the ROC curve was 0.274 (95%CI: 0.201-0.347), showing a low, not significant accuracy for BSI diagnosis. At a cut-off point of 35%, the sensitivity and specificity of PPC were 0.84 and 0.73, respectively, and the Youden index was 0.57. At cut-off points of 50% and 60%, the sensitivity was reduced to 0.63 and 0.44, respectively, but yielded high specificities of 0.82 and 0.90.
Early recognition of BSI and establishing early treatment are important for patients with infection. In this retrospective cohort, we demonstrated that the ratio of platelet drop was independently associated with BSI. This is the first study to investigate the association between these parameters. The study included 218 sepsis and sepsis shock patients, and their demographic variables and clinical and laboratory characteristics are described. Patients with BSI were associated with the severity of sepsis and sepsis shock, as indicated by higher inflammatory biomarkers (procalcitonin, C-reactive protein, neutrophils), higher percentage decline of platelet counts, liver and kidney function injury, and coagulation disorder (prothrombin time, activated partial thromboplastin time, D-dimer), compared to patients who did not have a BSI. It has been reported that bacteraemia is an independent risk factor for nosocomial infection-related mortality[5]; however, in our study, 28-d mortality was not significantly different between bacteraemia and non-bacteraemia patients. This could be due to respiratory failure caused by a respiratory infection, which was detected in 46.3% of patients in the ICU in our study, it was the main cause of death and these patients had a low incidence of BSI. We recorded the daily platelet count and found that the median duration of thrombocytopenia occurred on Day 4 after admission to the ICU (Figure 1), which is in accordance with the results of previous research[6,7].
In our study, the most common organism isolated was Escherichia coli, with Klebsiella pneumoniae being the second most common pathogen in blood infections. This is consistent with a previous finding showing that Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae and Streptococcus pneumoniae were the most commonly isolated organisms in community-acquired BSIs[8]. In recent years, it has been reported that respiratory tract, urinary tract, and intra-abdominal infections are the main sources of sepsis and sepsis shock[9,10], and gram-negative bacteraemia has a higher frequency in the ICU[11]. Similarly, our study also showed that respiratory tract infection was the main reason for admission to the ICU but the patients had a lower rate of BSIs. However, the urogenital and hepatobiliary tract have a higher incidence of BSIs in ICU patients.
Our results confirm that the rate of platelet drop but not the lowest platelet count has a high predictive ability for BSI. It has been reported that procalcitonin levels are a good biomarker for bacterial infections, and procalcitonin has been introduced into clinical use[12,13]. Similarly, our study supports this option. Comparing other inflammatory markers, the diagnostic utility of PPC (AUC of 0.839) was significantly higher than that of procalcitonin (AUC of 0.718), C-reactive protein (AUC of 0.583) and neutrophils (AUC of 0.564). A cut-off point of 35% for PPC achieved a sensitivity of 84% and a specificity of 73%, whereas a cut-off point of 50% was correlated with a sensitivity of 62.7% and a specificity of 82%. A cut-off point of 60% reduced the sensitivity to 44%, but the specificity reached 90.1%. Therefore, clinicians should consider BSIs in sepsis and sepsis shock patients with a rapid drop in platelet count.
Thrombocytopenia is very common in patients with sepsis and sepsis shock, and there are several putative mechanisms, as stated below. First, the interactions between bacteria and platelets cause the consumption of platelets. Bacteria can bind to platelets via receptors either directly or indirectly, suggesting that they may induce aggregation, which has been described for Streptococcus sanguinis, S. epidermidis, or S. pneumoniae infections[14]. Preclinical findings from murine models suggested that platelets bind to adherent neutrophils through Toll-like receptor 4 and form neutrophil extracellular traps (NETs). NETs have the greatest capacity for bacterial trapping and ensnare bacteria within the vasculature[15]. In addition to containing pathogens, human and murine platelets can exert direct microbicidal activity, such as releasing platelet microbicidal proteins to kill pathogens[16,17]. Second, bacterial infections cause damage to the vascular endothelial lining and the release of inflammatory factors, accelerating adhesion, removal and immune-mediated destruction of platelets. Third, bacterial infections cause marrow depression, decreasing the production of platelets.
Our study has the following limitations: (1) We only recorded platelet changes within 7 d after admission to the ICU in sepsis and sepsis shock patients and did not consider changes in platelets in patients with secondary infection during ICU hospitalization, which may affect mortality; (2) In our study, the median time of the platelet count dropping to a nadir was on Day 4. However, the platelet counts were very low in some patients when they came to the hospital, and their platelets dropped to the lowest value on Day 2 after admission, which affected the ratio of platelet decline; and (3) We used culture-based methods as the gold standard for the diagnosis of BSI, and the initiation of empirical antimicrobial therapy in some patients significantly reduced the sensitivity of blood cultures. Future studies should determine if there is a drop in platelet count in experimental animals with BSI.
In conclusion, the percentage decline in platelet counts is sensitive in predicting BSI in patients with sepsis and sepsis shock. However, it cannot identify gram-positive bacteraemia, gram-negative bacteraemia, and fungal infection. Dynamic detection of platelet counts appears to be an early alert for the clinician in identifying the site of infection and evaluating serious infection. This will guide the performance of blood cultures and the use of empirical antibiotics.
Either a relative or an absolute decrease in the platelet number is often seen in patients who most likely develop sepsis and septic shock. However, few reports have documented the relationship between a drop in platelet counts and bloodstream infection (BSI).
To determine whether decreased platelet counts are an early alert in identifying the site of infection and evaluating serious infection.
The aims of this study were to determine the diagnostic ability of the percentage decline of platelet counts (PPC) for predicting the presence of BSI and evaluating the cut-off point for detecting BSI.
A retrospective analysis of patients admitted with sepsis and septic shock in Xingtai People Hospital was revisited. Patient population characteristics and laboratory data were collected for analysis.
The percentage decline in platelet counts in patients positive for pathogens [57.1 (41.3-74.6)] was distinctly higher than that in the control group [18.2 (5.1–43.1)] (P < 0.001), whereas the PPC was not significantly different among patients with gram-positive bacteraemia, gram-negative bacteraemia, and fungal infection. Using receiver operating characteristic curves, the area under the curve of the platelet drop rate was 0.839 (95%CI: 0.783-0.895).
The percentage decline in platelet counts is sensitive in predicting blood stream infection in patients with sepsis and septic shock. However, it cannot identify gram-positive bacteraemia, gram-negative bacteraemia, and fungal infection.
Future studies should determine whether there is a drop in platelet count in experimental animals with BSI and clarify the underlying mechanism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moldovan CA, Romania S-Editor: Liu JH L-Editor: Webster JR P-Editor: Zhao YQ
| 1. | Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1332] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 2. | Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34:5-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Shannon O. Platelet interaction with bacterial toxins and secreted products. Platelets. 2015;26:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Napolitano LM. Sepsis 2018: Definitions and Guideline Changes. Surg Infect (Larchmt). 2018;19:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 5. | Mortensen VH, Søgaard M, Mygind LH, Wolkewitz M, Kristensen B, Schønheyder HC. Incidence and mortality of hospital-acquired bacteraemia: a population-based cohort study applying a multi-state model approach. Clin Microbiol Infect. 2022;28:879.e9-879.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. 2013;1:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Timsit JF, Ruppé E, Barbier F, Tabah A, Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46:266-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 9. | García-Rodríguez JF, Mariño-Callejo A. The factors associated with the trend in incidence of Bacteraemia and associated mortality over 30 years. BMC Infect Dis. 2023;23:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 10. | Rosales J, Ireland M, Gonzalez-Gallo K, Wisler J, Jalilvand A. Characterization of Mortality by Sepsis Source in Patients Admitted to the Surgical Intensive Care Unit. J Surg Res. 2023;283:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Ombelet S, Kpossou G, Kotchare C, Agbobli E, Sogbo F, Massou F, Lagrou K, Barbé B, Affolabi D, Jacobs J. Blood culture surveillance in a secondary care hospital in Benin: epidemiology of bloodstream infection pathogens and antimicrobial resistance. BMC Infect Dis. 2022;22:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J Cell Biochem. 2019;120:5852-5859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 13. | Lee JH, Kim SH, Jang JH, Park JH, Jo KM, No TH, Jang HJ, Lee HK. Clinical usefulness of biomarkers for diagnosis and prediction of prognosis in sepsis and septic shock. Medicine (Baltimore). 2022;101:e31895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O. Platelets and infections - complex interactions with bacteria. Front Immunol. 2015;6:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1807] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 16. | Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WH, Lindemann S, Seizer P, Yost CC, Zimmerman GA, Weyrich AS. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Shannon O. The role of platelets in sepsis. Res Pract Thromb Haemost. 2021;5:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |