Published online Jun 9, 2023. doi: 10.5492/wjccm.v12.i3.165
Peer-review started: December 23, 2022
First decision: January 31, 2023
Revised: February 3, 2023
Accepted: March 24, 2023
Article in press: March 24, 2023
Published online: June 9, 2023
Processing time: 167 Days and 0.4 Hours
Ventilator-associated pneumonia (VAP) is defined as pneumonia that occurs two calendar days following endotracheal intubation or after that. It is the most common infection encountered among intubated patients. VAP incidence showed wide variability between countries.
To define the VAP incidence in the intensive care unit (ICU) in the central gove
The research was a prospective cross-sectional observational study over six months from November 2019 to June 2020. It included adult and adolescent patients (> 14 years old) admitted to the ICU and required intubation and mechanical ventilation. VAP was diagnosed when it occurred after 48 h after endotracheal intubation using the clinical pulmonary infection score, which considers the clinical, laboratory, microbiological, and radiographic evidence.
The total number of adult patients admitted to the ICU who required intubation and mechanical ventilation during the study period was 155. Forty-six patients developed VAP during their ICU stay (29.7%). The calculated VAP rate was 22.14 events per 1000 ventilator days during the study period, with a mean age of 52 years ± 20. Most VAP cases had late-onset VAP with a mean number of ICU days before the development of VAP of 9.96 ± 6.55. Gram-negative contributed to most VAP cases in our unit, with multidrug-resistant Acinetobacter being the most identified pathogen.
The reported VAP rate in our ICU was relatively high compared to the international benchmark, which should trigger a vital action plan for reinforcing the implementation of the VAP prevention bundle.
Core Tip: Ventilator-associated pneumonia (VAP) is the most common infection among intubated patients. Early-onset VAP is usually caused by sensitive pathogens, while multidrug-resistant bacteria usually cause late-onset. Early, appropriate, and empirical antibiotics therapy for VAP is crucial to decreasing mortality risk. The VAP rate in Bahrain is relatively high compared to the international rates, which should trigger a vital action plan for reinforcing the implementation of the VAP prevention bundle. Gram-negative bacteria were the most common organisms that cause VAP in the current study, where Acinetobacter baumannii was the most common organism, followed by Klebsiella pneumoniae and Pseudomonas aeruginosa. Knowing the prevalent organisms helps choose the appropriate antibiotics until culture and sensitivity become available.
- Citation: Hassan ME, Al-Khawaja SA, Saeed NK, Al-Khawaja SA, Al-Awainati M, Radhi SSY, Alsaffar MH, Al-Beltagi M. Causative bacteria of ventilator-associated pneumonia in intensive care unit in Bahrain: Prevalence and antibiotics susceptibility pattern. World J Crit Care Med 2023; 12(3): 165-175
- URL: https://www.wjgnet.com/2220-3141/full/v12/i3/165.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i3.165
Ventilator-associated pneumonia (VAP) is defined as pneumonia that occurs two calendar days following endotracheal intubation or after that. It is the most common infection encountered among intubated patients[1]. It occurs in 10%-30% of mechanically ventilated patients[2-4]. The VAP incidence showed wide variability, ranging from 10 to 41.7 per 1000 ventilator days in developing countries[5], while the rate is much lower in developed countries ranging between 1.2 and 8.5 per 1000 ventilator days[6].
Early-onset VAP is defined as pneumonia that occurs within four days of endotracheal intubation. It is usually attributed to sensitive pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and methicillin-sensitive Staphylococcus aureus. In contrast, late-onset VAP emerges after four days of intubation. It is caused by multidrug-resistant (MDR) bacteria such as methicillin-resistant S. aureus (MRSA), Acinetobacter, Pseudomonas aeruginosa, and extended-spectrum beta-lactamase-producing bacteria. VAP caused by fungal and viral pathogens has a low contribution and tend to occur among immunocompromised host[5].
The risk for VAP is most significant during the first five days after intubation (3%), with the mean duration between intubation and development of VAP being 3.3 d. This risk declines to 2%/d between days 5 to 10 of ventilation and 1%/d after that[7]. Many previous studies showed that the related mortality for VAP ranges between 33%–50%, but this rate fluctuates and depends heavily on the underlying medical illness[5]. The diagnosis of VAP in the intensive care unit (ICU) remains challenging due to the absence of universally accepted gold-standard diagnostic criteria for VAP[8]. The clinical pulmonary infection score (CPIS) is one of the best diagnostic tools considering the clinical, physiological, microbiological, and radiographic evidence to allow a numerical value to predict the presence or absence of VAP[9,10].
Early, appropriate, and empirical antibiotics therapy for VAP is crucial as any delay in initiating proper antibiotics may increase the mortality risk with VAP. Consequently, selecting the appropriate regimen should be guided by the updated local antibiogram for each hospital and ICU. This study aimed to define VAP incidence in the ICU at Salmaniya Medical Complex, Bahrain's leading tertiary care government hospital. The study also reviewed the risk factors and the predominant pathogens that cause VAP, which helps choose the appropriate empiric antimicrobial therapy for VAP-related sepsis in adult ICU.
The study was a prospective, observational, cross-sectional study conducted at the adult ICU in Salmaniya Medical Complex from November 2019 to June 2020 to determine the microbiological profile of adult patients with VAP and evaluate the magnitude of MDR microbes among those patients. We used patients who needed mechanical ventilation and did not develop VAP as a control group. The Research and Ethics Committee at Salmaniya Medical Complex, Ministry of Health, Kingdom of Bahrain, approved the study. We did not collect consent, as the study was observational, without exposure to any personal data.
We did not determine a preset sample size as we included all the patients admitted to the adult ICU during the study periods (November 2019-June 2020) when they met the inclusion criteria. We included adult and adolescent patients (> 14 years old) who were admitted to the ICU and required intubation and mechanical ventilation.
VAP was diagnosed when it occurs after 48 h after endotracheal intubation and mechanical ventilation based on the scoring system by using the CPIS, considering the clinical, laboratory, microbiological, and radiographic evidence to allow a numerical value to predict the presence or absence of VAP[7,11-13] as summarized in Table 1. We considered the VAP as early-onset when it occurred in the first four days following intubation and late-onset after the fourth day of intubation.
| Assessed parameter | Result | Score |
| Temperature (°Celsius) | 36.5–38.4 °C | 0 |
| 38.5–38.9 °C | 1 | |
| ≤ 36 or ≥ 39 °C | 2 | |
| Leukocytes in blood (cells/mm3) | 4000–11000/mm3 | 0 |
| < 4000 or > 11000/mm3 | 1 | |
| ≥ 500 band cells | 2 | |
| Tracheal secretions (subjective visual scale) | None | 0 |
| Mild/non-purulent | 1 | |
| Purulent | 2 | |
| Radiographic findings (on chest radiography, excluding CHF and ARDS) | No infiltrate | 0 |
| Diff use/patchy infiltrate | 1 | |
| Localized infiltrate | 2 | |
| Culture results (endotracheal aspirate) | No or mild growth | 0 |
| Moderate or florid growth | 1 | |
| Moderate or florid growth and pathogen consistent with gram stain | 2 | |
| Oxygenation status | > 240 or ARDS | 1 |
| ≤ 240 and absence of ARDS | 2 |
The laboratory parameters (leukocyte count and microbial profile) were obtained daily from the patient's laboratory data and documented in the study datasheets. The treating clinical teams assessed the radiological finding and oxygenation status. Temperature documentation and assessment of tracheal secretions were obtained from the assigned nurses' notes, which are part of their daily assessment of intubated patients. All culture reports were reviewed by the medical microbiologist and infectious diseases (ID) consultant. A summative score was calculated for each patient enrolled in the study. The scores range between 0 and 12, with a score of ≥ 6 showing a good correlation with the presence of VAP.
The microbial profile of endotracheal specimens isolated from the enrolled patients was identified as part of CPIS diagnostic criteria. Positive cultures (aerobic, anaerobic, and/or fungal) were further analyzed by full antibiotics sensitivity pattern with identification of MDR according to the standard definition of the Clinical Laboratory Standards Institute[14]. The medical microbiologist and the ID consultants reviewed all microbial data.
VAP rate was calculated as a percentage of patients who developed VAP out of all intubated patients in the ICU during the study period.
VAP rate per 1000 ventilator days was also calculated according to the centers for disease control and prevention surveillance formula[15] by dividing the VAP cases (defined by CPIS ≥ 6) over the patient-ventilator days during the same period and multiplying by 1000.
To analyze the predisposing factors for VAP and the risk of complications, we evaluated the following variables among all enrolled patients and compared between the two groups: The VAP patients (case group) and non-VAP patients (control group) such as age, gender, presence of comorbidities, source of admission, and the number of ICU days before intubation, the outcome including the mortality, development of complication and the need for tracheostomy.
We performed a descriptive analysis, expressing the categorical variables in numbers and percentages and the quantitative variables in means and standard deviations. We compared the categorical variables as appropriate, using the χ2 test or Fisher's exact test (when the expected n is less than 5). In addition, we used the t-test or Mann-Whitney U test to compare continuous variables. Statistical significance was established at 95% (P < 0.05). All statistical analyses were performed using Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY).
The investigation followed the latest version of the Declaration of Helsinki and was approved by the Secondary Research Committee of Salmaniya Medical Complex, Ministry of Health, Kingdom of Bahrain. We did not get consent from the patients as it was a descriptive non-interventional study without disclosure of any patients' data.
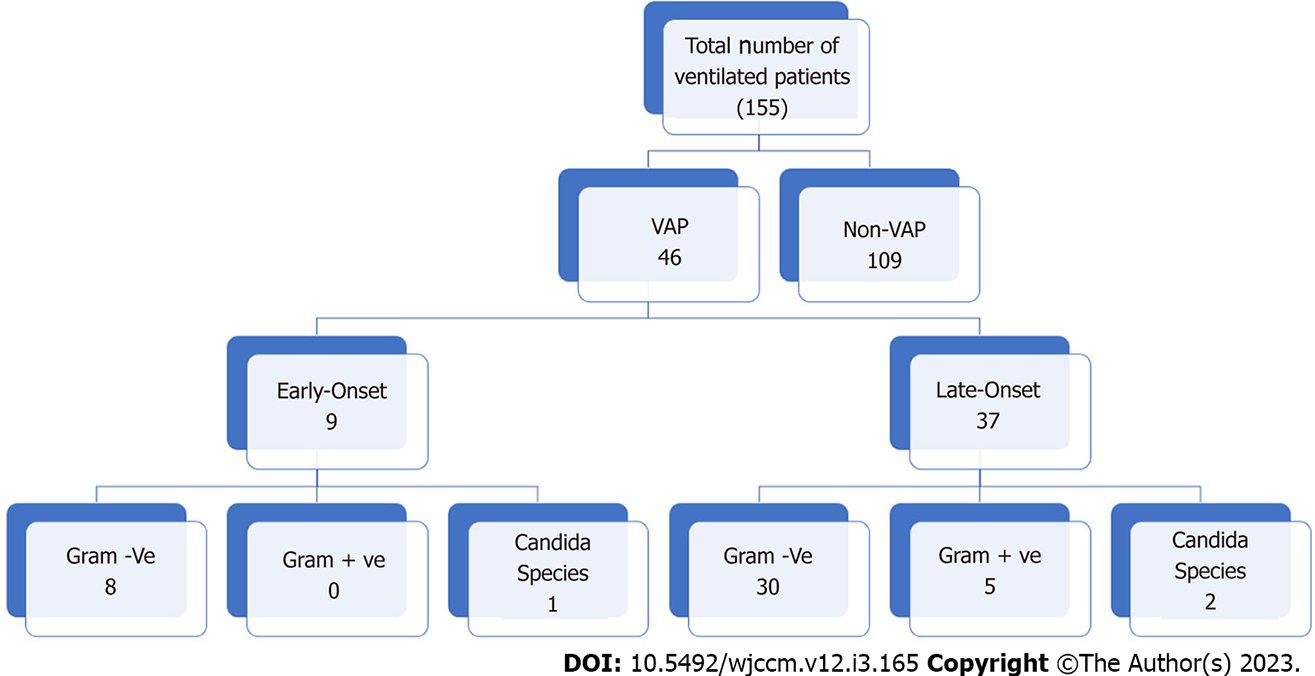
Figure 1 shows the flow chart of the study. The total number of adult patients admitted to the ICU who required intubation and mechanical ventilation during the study period was 155. Forty-six patients developed VAP during their ICU stay (29.7%), with a VAP rate of 22.14 events per 1000 ventilator days. The mean age of patients who developed VAP was 52 ± 20 (range 27–88 years.), and 32 were male (69.6%). The mean number of ICU days before VAP development was 9.96 ± 6.55 d. Most VAP cases were late-onset, with a mean time interval between intubation and VAP diagnosis of 11.37 ± 6.67 d.
Thirty-seven cases (80.4%) were late-onset VAP that was developed after 96 h from intubation, while 9 cases (19.6%) were early-onset VAP (developed within initial 96 d post-intubation). The most common comorbidities among VAP cases were diabetes mellitus and hypertension (19 patients, 41.3 % of VAP cases). Other identified comorbid conditions include chronic kidney disease (7, 15.2%), ischemic heart disease (6, 13%), and neurological disorder (5, 10.9%). Less identified comorbidities included malignancy (4, 8.7%), chronic liver disease (3, 6.5%), and sickle cell disease (3, 6.5%).
Table 2 showed no statistically significant difference between VAP and non-VAP groups regarding age, sex, and the number of hospital or ICU stays before intubation. Hypertension was the only significant risk factor for VAP acquisition among ICU intubated patients regarding the underlying comorbidities. Complications differ significantly among the VAP and non-VAP groups, where the duration of mechanical ventilation and the length of ICU stay were significantly higher in the VAP group (P = 0.009, < 0.001, respectively).
| Risk factors and complications | VAP group, | Non-VAP group, | P value | |
| Age, mean ± SD | 52.74 ± 20.42 | 61.45 ± 65.05 | > 0.5 | |
| Sex, males% | 32 (69.57) | 73 (66.97) | > 0.5 | |
| Number of hospital days before intubation, mean ± SD | 5.39 ± 8.11 | 3.38 ± 6.37 | > 0.5 | |
| Number of ICU days before intubation, mean ± SD | 0.52 ± 1.94 | 0.47 ± 2.78 | > 0.5 | |
| Presence of comorbidities | Diabetes mellitus | 19 (41.30) | 54 (49.54) | > 0.5 |
| Hypertension | 19 (41.30) | 67 (61.47) | > 0.5 | |
| Chronic kidney disease | 7 (15.22) | 28 (25.69) | > 0.5 | |
| Ischemic heart disease | 6 (13.04) | 25 (22.94) | > 0.5 | |
| Neurological disorder | 5 (10.87) | 9 (8.26) | > 0.5 | |
| Malignancy | 4 (8.70) | 3 (2.75) | > 0.5 | |
| Liver disease | 3 (6.52) | 8 (7.34) | > 0.5 | |
| Sickle cell disease | 3 (6.52) | 3 (2.75) | > 0.5 | |
| Hospital course | Length of ICU stay, mean ± SD | 21.41 ± 11.89 | 11.01 ± 10.38 | < 0.001a |
| Duration of mechanical ventilation, mean ± SD | 16.67 ± 8.70 | 12.03 ± 10.53 | 0.009a | |
| Extubation | 18 (39.13) | 79 (72.48) | < 0.001a | |
| Need of re-intubation | 12 (26.09) | 11 (10.09) | 0.014a | |
| Tracheostomy | 14 (30.43) | 9 (8.26) | < 0.001a | |
| Potential complication | Septic shock | 28 (60.87) | 31 (28.44) | < 0.001a |
| ARDS | 15 (32.61) | 12 (11.01) | 0.002a | |
| Acute kidney injury | 24 (52.17) | 36 (33.03) | 0.031a | |
| Pneumothorax | 1 (2.17) | 2 (1.83) | > 0.5 | |
| Mortality, deaths | 14 (30.43) | 21 (19.27) | > 0.5 | |
The rate of septic shock, acute respiratory distress syndrome (ARDS), and acute kidney injury was significantly higher in the VAP group than in the non-VAP group. On the other hand, the rate of pneumothorax did not show a significant difference between both groups. Notably, the extubation failure and the rate of tracheostomy and reintubation among extubated patients were significantly higher among the VAP group than in non-VAP groups. The overall ICU mortality rate was 22.6% (35/155) in all mechanically ventilated patients. However, there was no significant difference in mortality between both groups.
Table 3 shows the most common organisms isolated from patients with VAP. The total number of isolates was 46. Twenty percent of the isolates were from early-onset VAP, while 80% were from late-onset VAP. About 58.7% of the total isolates showed MDR. In early-onset VAP, gram-negative bacteria formed 89% of the total isolates (75% were MDR), followed by candida (11%). No gram-positive isolates were detected. Acinetobacter baumannii was the most common isolated gram-negative bacteria (50%); all were MDR. Klebsiella pneumoniae was the second most common gram-negative bacteria (37.5%) isolated; two-thirds were MDR. In late-onset VAP, gram-negative bacteria formed 81% of the total isolates (70% were MDR), followed by gram-positive (13.5%) and candida (5.4%). Acinetobacter baumannii was the most common isolated gram-negative bacteria (50%); 93% were MDR. Klebsiella pneumoniae was the second most common gram-negative bacteria (16.6%) isolated; all were MDR. Pseudonomas aeruginosa was detected in 8% of late-VAP, 33% of them were MDR. We detected only one MRSA isolate in the samples collected from VAP (20% of all five gram-positive isolates). We also detected candida in 2 cases with late-onset VAP (5.4%).
| Organism | Total | Early-onset VAP (Number 9) | Late-onset VAP (Number 37) | ||
| Number (of total) | MDR | Number (of total) | MDR | ||
| Total gram negative | 38 (82.6) | 8 (21) | 6 (75) | 30 (79) | 21 (70) |
| Acinetobacter baumannii | 19 (50) | 4 (21) | 4 (100) | 15 (79) | 14 (93) |
| Klebsiella pneumoniae | 8 (21.1) | 3 (37.5) | 2 (66.6) | 5 (62.5) | 5 (100) |
| Pseudomonas aeruginosa | 3 (9) | 0 | 0 | 3 (100) | 1 (33.3) |
| Stenotrophomonas maltophilia | 5 (13.1) | 0 | 0 | 5 (100) | 0 |
| Enterobacter asburiae | 1 (2.6) | 1 (100) | 0 | 0 | 0 |
| E coli | 1 (2.6) | 0 | 0 | 1 | 1 ESBL (100) |
| Hemophilus influenzae | 1 (2.6) | 0 | 0 | 1 (100) | 0 |
| Total gram positive | 5 (11) | 0 | 0 | 5 (100) | |
| Staph aureus | 4 (80) | 0 | 0 | 4 (100) | 0 |
| MRSA | 1 (20) | 0 | 0 | 1 (100) | 0 |
| 0 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | |
| Fungal infection | |||||
| Candida species | 3 (100) | 1 (33.3) | 0 | 2 (66.6) | 0 |
| Total microorganisms | 46 (100) | 9 (19.6) | 6 (13) | 37 (80.4) | |
Tables 4 and 5 showed the antibiotic susceptibility among the bacterial isolates from patients with VAP. Acinetobacter baumannii, which was the most common organism isolated from VAP, had 100% susceptibility to Colistin, 37% susceptibility to Trimethoprim/sulfamethoxazole, Gentamicin, and Amikacin, 21% susceptibility to Tigecycline, low susceptibility (5%) to Ciprofloxacin, Piperacillin-Tazobactam, Cefepime, Meropenem, Imipenem, and Ertapenem, and resistance to Levofloxacin. Klebsiella pneumonia, which was the second most common organism isolated from VAP, had 87.5% susceptibility to Tigecycline, 62.5% susceptibility to Colistin, 50% susceptibility to Trimethoprim/sulfamethoxazole, 37% susceptibility to Gentamicin, and Amikacin, 12.5% susceptibility to Levofloxacin, Cefepime, Meropenem, Imipenem, Ertapenem, Amoxiclav, Cefuroxime, and Ceftriaxone. However, it showed complete resistance to Ceftazidime and Piperacillin-Tazobactam. Stenotrophomonas maltophilia had 100% susceptibility to Trimethoprim/sulfamethoxazole, Levofloxacin, and Minocycline. It had 20% susceptibility to Ceftazidime. At the same time, Pseudomonas aeruginosa had 100% susceptibility to Ceftazidime, Piperacillin-Tazobactam, Cefepime, Colistin, Gentamicin, Amikacin, and Ciprofloxacin, 66.6% susceptibility to Meropenem, Imipenem, and Ertapenem. MRSA was 100% susceptible to Erythromycin, Clindamycin, Tetracycline, and Vancomycin.
| Antimicrobial agent | MSSA, n = 4 | MRSA, n = 1 |
| Penicillin | 0/4 = 0 (0) | 0/1 = 0 (0) |
| Oxacillin | 4 (100) | 0/1 = 0 (0) |
| Erythromycin | 4 (100) | 1 (100) |
| Clindamycin | 4 (100) | 1 (100) |
| Tetracycline | 4 (100) | 1 (100) |
| Vancomycin | 4 (100) | 1 (100) |
| Antimicrobial agent | Acinetobacter baumannii | Klebsiella pneumoniae | Stenotrophomonas maltophilia | Pseudomonas aeruginosa |
| Number of organisms | 19 (50) | 8 (42) | 5 (26) | 3 (16) |
| Ceftazidime | 1 (5.2) | 0 | 1 (20) | 3 (100) |
| Trimethoprim/sulfamethoxazole | 7 (37) | 4 (50) | 5 (100) | - |
| Levofloxacin | 0 | 1 (12.5) | 5 (100) | - |
| Minocycline | - | - | 5 (100) | - |
| Piperacillin-tazobactam | 1 (5.2) | 0 | - | 3 (100) |
| Cefepime | 1 (5.2) | 1 (12.5) | - | 3 (100) |
| Meropenem | 1 (5.2) | 1 (12.5) | - | 2 (66.6) |
| Imipenem | 1 (5.2) | 1 (12.5) | - | 2 (66.6) |
| Ertapenem | 1 (5.2) | 1 (12.5) | - | 2 (66.6) |
| Colistin | 19 (100) | 5 (62.5) | - | 3 (100) |
| Gentamicin | 7 (37) | 3 (37.5) | - | 3 (100) |
| Amikacin | 7 (37) | 3 (37.5) | - | 3 (100) |
| Ciprofloxacin | 1 (5.2) | 1 (12.5) | - | 3 (100) |
| Tigecycline | 4 (21) | 7 (87.5) | - | - |
| Ampicillin | - | - | - | - |
| Amoxiclav | - | 1 (12.5) | - | - |
| Cefuroxime | - | 1 (12.5) | - | - |
| Ceftriaxone | - | 1 (12.5) | - | - |
The Incidence of VAP among intubated patients in the current study was 29.7%; this figure is comparable to the incidence reported by other investigators in the developing region (15%–58%)[16,17]. The calculated VAP rate was 22.14 events per 1000 ventilator days which is high compared to the international standards[18] and to the rate reported by neighboring countries of the Gulf Cooperation Council, which reported a VAP rate of 4.8 per 1000 ventilator days[19]; but our rate was comparable to most data reported by other developing countries[20,21].
Such high incidence should trigger a vital action plan to reinforce healthcare workers' adherence to the recommended preventive VAP bundle.
The current study showed that age and gender were not essential risk factors for VAP development. This agrees with a recently published study by Zubair et al[22] in 2018, which demonstrated that age or gender was not a significant risk factor in developing VAP[22]. However, this finding contradicts many previous studies that defined age and gender as important independent risk factors in developing VAP[23-26].
The current study agrees with other previously published studies that VAP development significantly increases the need for re-intubation and tracheostomy and the risk of systemic complications such as septic shock, ARDS, and acute kidney injury, in addition to increasing the duration of mechanical ventilation and length of ICU stay in patients admitted to ICU[27]. Nevertheless, VAP was not a significant risk factor for the increased mortality rate among intubated patients. This finding agreed with other previously published studies, which noted that the mortality risk was not significantly high in VAP presence[27,28]. In the current study, gram-negative bacteria were the most common organisms that cause VAP, whereas Acinetobacter baumannii was the most common organism (50% of all VAP cases). This finding agrees with Ben Lakhal et al29], who had 53% of their cases caused by Acinetobacter baumannii[29]. Staph aureus was the causative organism in 11% of all recorded VAP cases in our study, all isolated from late-onset VAP. However, this rate is much lower than in previous studies such as Jones[30] and Chi et al[31], who found that Staphylococcus aureus was the most common VAP-causing organism, followed by the gram-negative organism[30,31]. The increased prevalence of gram-negative over gram-positive organisms may indicate the changing pattern of the nosocomial infection's microbial profile, including VAP in our region. Unfortunately, we did not have previous studies in our country to compare.
In the current study, Acinetobacter baumannii was the most common organism isolated from patients with VAP, with a rate of 44.4% in patients with early-onset VAP (93% MDR) and 40.5% in patients with late-onset VAP (100% MDR). Acinetobacter baumannii is an opportunistic pathogen with a high incidence among immunocompromised individuals, particularly those who have experienced a prolonged hospital stay. It is commonly associated with high humidity, colonizing the skin, and isolated in high numbers from infected individuals' respiratory and oropharynx secretions[32]. Previous studies showed that Acinetobacter baumannii is prevalent and even endemic in many Middle East and North African countries. A study from Tunisia showed that Acinetobacter baumannii caused 45% of ICU-related infections with an MDR-resistance rate of 39% during an epidemic from 2004 to 2005[33]. Another study from Saudi Arabia showed that Acinetobacter baumannii was the most common organism isolated from late-onset VAP, causing 26.65% of cases[34]. In our Acinetobacter baumannii isolates, the overall MDR rate was 95% (100% and 93% in early-onset and late-onset VAP, respectively). Our institute considers Acinetobacter baumannii a "red alert" human pathogen due to the high rate of MDR with almost resistance to all antibiotics except for Colistin. It becomes a cause for serious concern regarding nosocomially acquired infections[35].
Klebsiella pneumoniae was the second most common organism isolated from our patients, with a rate of 17.4% throughout the study with an MDR rate of 87.5%. We detected Klebsiella pneumoniae in 33.3% of patients with early-onset VAP (66.6% MDR) and 13.5 % in patients with late-onset VAP (100% MDR). Our results agree with the work from Iran by Bozorgmehr et al[36], which showed that Klebsiella pneumoniae was the second most common organism isolated from 29.82% of the patients with VAP after Acinetobacter baumannii[36]. Our results also agree with the finding observed from a Thailand study that found that Klebsiella pneumoniae was the second most common organism isolated from 17.3% of the patients with VAP after Acinetobacter baumannii[37]. However, a study from Egypt in 2020 showed that Klebsiella spp was the most frequently isolated microorganism, followed by Pseudomonas aeruginosa and Acinetobacter baumannii[38].
The guidelines for initial empiric antimicrobial therapy for VAP are highly dependent on the type of causative pathogen and the time of diagnosis. Knowing the prevalent organisms helps choose the appropriate antibiotics until culture and sensitivity are available. However, the development of rapid identification technologies and phenotypic methods would significantly help the proper choice to improve the treatment outcomes for VAP. As many hospitals may lack rapid identification technologies, knowing the most common bacterial types causing VAP and their antibiogram may help physicians make quick decisions in VAP management.
As the study was a single center-based study in the adult population, this may hinder us from generalizing the data to other public or private hospital settings and the pediatric population. However, despite the study's limitation, it can provide valuable data concerning the incidence rates and the prevalence of VAP in Bahrain, reflecting the rest of the Arabian Gulf region's status.
VAP is a common serious complication among intubated patients in our ICU; our VAP rate is relatively high compared to the international benchmark, which should trigger a vital action plan for reinforcing the implementation of the VAP prevention bundle. Gram-negative bacteria were the most common organisms that cause VAP in the current study, where Acinetobacter baumannii was the most common organism, followed by Klebsiella pneumoniae and Pseudomonas aeruginosa. Knowing the prevalent organisms helps choose the appropriate antibiotics until culture and sensitivity become available.
Ventilator-associated pneumonia (VAP) is the most common infection encountered among intubated patients, occurring in 10%-30% of mechanically ventilated patients.
The lack of data from the Kingdom of Bahrain stimulated us to investigate VAP incidence, risk factors, and microbial profiles in the central hospital in the kingdom.
We aimed to define VAP incidence in the intensive care unit (ICU) at Salmaniya Medical Complex and review the risk factors and the predominant pathogens that cause VAP to choose the appropriate empiric antimicrobial therapy for VAP-related sepsis in adult ICU.
The study was a prospective, observational, cross-sectional study done between November 2019 to June 2020 to determine the microbiological profile in adult patients with VAP and evaluate the magnitude of multidrug-resistant (MDR) microbes among those patients. We used patients who needed mechanical ventilation and did not develop VAP as a control group. We included adult and adolescent patients (> 14 years old) who were admitted to the ICU and required intubation and mechanical ventilation.
The incidence of VAP was 29.7% during the study period, with a calculated VAP rate of 22.14 events per 1000 ventilator days and a mean age of 52 years ± 20. Most VAP cases had late-onset VAP with a mean number of ICU days before the development of VAP of 9.96 ± 6.55. Gram-negative contributed to most VAP cases in our unit, with MDR Acinetobacter being the most identified pathogen.
The VAP rate in our ICU was relatively high compared to the international benchmark.
The high VAP rate in our hospital triggered us to initiate a vital action plan to reinforce the implementation of the VAP prevention bundle.
We thank the anonymous referees for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ewers A, Austria; Karim HMR, India S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 2. | Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 4733] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 3. | Horan TC, Gayness RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. Philadelphia: Lippincott Williams and Wilkins; 2004. p. 1659-1702. |
| 4. | Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2799] [Cited by in RCA: 2789] [Article Influence: 253.5] [Reference Citation Analysis (0)] |
| 5. | American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4266] [Cited by in RCA: 4252] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 6. | Indulski JA, Krajewski JA, Majka JA, Dutkiewicz T. [Chemical safety (III)--its prospects in Poland]. Med Pr. 1990;41:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 8. | Anthony B, Marin K, Hilary B. A Prospective Analysis of the National Healthcare Safety Network (NHSN) Surveillance Algorithm for Ventilator-Associated Events (VAEs). Chest. 2013;144 Suppl:562A. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51 Suppl 1:S131-S135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Johanson WG Jr, Pierce AK, Sanford JP, Thomas GD. Nosocomial respiratory infections with gram-negative bacilli. The significance of colonization of the respiratory tract. Ann Intern Med. 1972;77:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 657] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 704] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19:637-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100.Wayne, PA: Clinical and Laboratory Standards Institute; 2018. |
| 15. | Center of Disease Prevention & Control. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event. Jan, 2023. [cited 23 February 2023]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf. |
| 16. | Morehead RS, Pinto SJ. Ventilator-associated pneumonia. Arch Intern Med. 2000;160:1926-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Osman S, Al Talhi YM, AlDabbagh M, Baksh M, Osman M, Azzam M. The incidence of ventilator-associated pneumonia (VAP) in a tertiary-care center: Comparison between pre- and post-VAP prevention bundle. J Infect Public Health. 2020;13:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | El-Saed A, Balkhy HH, Weber DJ. Benchmarking local healthcare-associated infections: available benchmarks and interpretation challenges. J Infect Public Health. 2013;6:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | El-Saed A, Al-Jardani A, Althaqafi A, Alansari H, Alsalman J, Al Maskari Z, El Gammal A, Al Nasser W, Al-Abri SS, Balkhy HH. Ventilator-associated pneumonia rates in critical care units in 3 Arabian Gulf countries: A 6-year surveillance study. Am J Infect Control. 2016;44:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Rodrigues DO, Cezário RC, Filho PP. Ventilator-associated pneumonia caused by multidrug- resistant Pseudomonas aeruginosa vs. other microorganisms at an adult clinical-surgical intensive care unit in a Brazilian University Hospital: Risk factors and outcomes. Int J Med Med Sci. 1:432-437. [DOI] [Full Text] |
| 21. | Leblebicioglu H, Erben N, Rosenthal VD, Atasay B, Erbay A, Unal S, Senol G, Willke A, Özgültekin A, Altin N, Bakir M, Oncul O, Ersöz G, Ozdemir D, Yalcin AN, Özdemir H, Yıldızdaş D, Koksal I, Aygun C, Sirmatel F, Sener A, Tuna N, Akan ÖA, Turgut H, Demiroz AP, Kendirli T, Alp E, Uzun C, Ulusoy S, Arman D. International Nosocomial Infection Control Consortium (INICC) national report on device-associated infection rates in 19 cities of Turkey, data summary for 2003-2012. Ann Clin Microbiol Antimicrob. 2014;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Zubair S, Ali H, Raza SF, Warind JA, Beg AE, Bushra R. Assessment of Frequency and Transience Rate for Ventilator-Associated Pneumonia (VAP) in Geriatric Patients in Tertiary Care Settings of Karachi, Pakistan. J Coll Physicians Surg Pak. 2018;28:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Ding C, Zhang Y, Yang Z, Wang J, Jin A, Wang W, Chen R, Zhan S. Incidence, temporal trend and factors associated with ventilator-associated pneumonia in mainland China: a systematic review and meta-analysis. BMC Infect Dis. 2017;17:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Di Y, Fu S. Risk factors for ventilator-associated pneumonia among patients undergoing major oncological surgery for head and neck cancer. Front Med. 2017;11:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Bornstain C, Azoulay E, De Lassence A, Cohen Y, Costa MA, Mourvillier B, Descorps-Declere A, Garrouste-Orgeas M, Thuong M, Schlemmer B, Timsit JF; Outcomerea Study Group. Sedation, sucralfate, and antibiotic use are potential means for protection against early-onset ventilator-associated pneumonia. Clin Infect Dis. 2004;38:1401-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Forel JM, Voillet F, Pulina D, Gacouin A, Perrin G, Barrau K, Jaber S, Arnal JM, Fathallah M, Auquier P, Roch A, Azoulay E, Papazian L. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care. 2012;16:R65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Othman AA, Abdelazim MS. Ventilator–associated pneumonia in adult intensive care unit prevalence and complication. The Egyptian Journal of critical care medicine. 2017;5:61-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Tejerina E, Frutos-Vivar F, Restrepo MI, Anzueto A, Abroug F, Palizas F, González M, D'Empaire G, Apezteguía C, Esteban A; Internacional Mechanical Ventilation Study Group. Incidence, risk factors, and outcome of ventilator-associated pneumonia. J Crit Care. 2006;21:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Ben Lakhal H, M'Rad A, Naas T, Brahmi N. Antimicrobial Susceptibility among Pathogens Isolated in Early- versus Late-Onset Ventilator-Associated Pneumonia. Infect Dis Rep. 2021;13:401-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51 Suppl 1:S81-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 459] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 31. | Chi SY, Kim TO, Park CW, Yu JY, Lee B, Lee HS, Kim YI, Lim SC, Kwon YS. Bacterial pathogens of ventilator associated pneumonia in a tertiary referral hospital. Tuberc Respir Dis (Seoul). 2012;73:32-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Howard A, O'Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 33. | Brahmi N, Beji O, Abidi N, Kouraichi N, Blel Y, El Ghord H, Thabet H, Amamou M. Epidemiology and risk factors for colonization and infection by Acinetobacter baumannii in an ICU in Tunisia, where this pathogen is endemic. J Infect Chemother. 2007;13:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | El-Saed A, Balkhy HH, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int J Infect Dis. 2013;17:e696-e701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2140] [Cited by in RCA: 2396] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 36. | Bozorgmehr R, Bahrani V, Fatemi A. Ventilator-Associated Pneumonia and Its Responsible Germs; an Epidemiological Study. Emerg (Tehran). 2017;5:e26. [PubMed] |
| 37. | Chittawatanarat K, Jaipakdee W, Chotirosniramit N, Chandacham K, Jirapongcharoenlap T. Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a Northern Thai tertiary-care university based general surgical intensive care unit. Infect Drug Resist. 2014;7:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Farag AM, Tawfick MM, Abozeed MY, Shaban EA, Abo-Shadi MA. Microbiological profile of ventilator-associated pneumonia among intensive care unit patients in tertiary Egyptian hospitals. J Infect Dev Ctries. 2020;14:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |