Published online Mar 9, 2023. doi: 10.5492/wjccm.v12.i2.71
Peer-review started: November 22, 2022
First decision: December 26, 2022
Revised: January 5, 2023
Accepted: February 17, 2023
Article in press: February 17, 2023
Published online: March 9, 2023
Processing time: 104 Days and 15.4 Hours
Despite various therapies to treat sepsis, it is one of the leading causes of mortality in the intensive care unit patients globally. Knowledge about the pathophysiology of sepsis has sparked interest in extracorporeal therapies (ECT) which are intended to balance the dysregulation of the immune system by removing excessive levels of inflammatory mediators.
To review recent data on the use of ECT in sepsis and to assess their effects on various inflammatory and clinical outcomes.
In this review, an extensive English literature search was conducted from the last two decades to identify the use of ECT in sepsis. A total of 68 articles from peer-reviewed and indexed journals were selected excluding publications with only abstracts.
Results showed that ECT techniques such as high-volume hemofiltration, coupled plasma adsorption/filtration, resin or polymer adsorbers, and CytoSorb® are emerging as adjunct therapies to improve hemodynamic stability in sepsis. CytoSorb® has the most published data in regard to the use in the field of septic shock with reports on improved survival rates and lowered sequential organ failure assessment scores, lactate levels, total leucocyte count, platelet count, interleukin- IL-6, IL-10, and TNF levels.
Clinical acceptance of ECT in sepsis and septic shock is currently still limited due to a lack of large random clinical trials. In addition to patient-tailored therapies, future research developments with therapies targeting the cellular level of the immune response are expected.
Core Tip: Sepsis is one of the leading causes of mortality in critically ill patients globally. Substantial progress is made in the field of extracorporeal therapies and sepsis. CytoSorb® is emerging as an adjunct therapy to improve hemodynamic stability. This device is an International Organization for Standardization certified, European Conformité Européenne mark-approved class IIb medical device that is designed to remove excess inflammatory cytokines from the blood. There are extensive published reports of its use in the field of septic shock with improved survival rates and other improved biochemical parameters. However, clinical acceptance is still limited due to a lack of large random clinical trials.
- Citation: Mehta Y, Paul R, Ansari AS, Banerjee T, Gunaydin S, Nassiri AA, Pappalardo F, Premužić V, Sathe P, Singh V, Vela ER. Extracorporeal blood purification strategies in sepsis and septic shock: An insight into recent advancements. World J Crit Care Med 2023; 12(2): 71-88
- URL: https://www.wjgnet.com/2220-3141/full/v12/i2/71.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i2.71
Sepsis is a global major life-threatening syndrome causing multiple organ dysfunction syndrome (MODS)[1]. The World Health Organization described the global estimate of sepsis morbidity and mortality[2] in 2017, as 48.9 million cases with 11 million sepsis related deaths. This estimate accounts for 20% of deaths worldwide[3]. In the United States, the incidence of severe sepsis and septic shock is reported as 300 cases per 100000 individuals, costing more than 20 billion dollars per year[4]. In 2005, there were 430 cases of severe sepsis per 100000 people in Sweden. Furthermore, in clinical cohort studies involving 198 European intensive care unit (ICU), the incidence of sepsis is 11.8% in Australia and New Zealand, 14.6% in France, 27.1% in the United Kingdom, and 30% in the SOAP study. Sepsis has steadily increased in most developed countries over the last several decades[5,6].
The definition of sepsis has evolved over the years and is currently defined as a life- threatening organ dysfunction caused by a dysregulated immune response of the host to infection[1]. Over stimulation of the immune response leads to a cytokine storm, which may lead to septic shock, capillary leakage, and microcirculatory disturbances finally resulting in MODS. The dysregulated reaction, however, may also lead to a protracted phase of immunoparalysis, contributing to the risk of secondary, hospital acquired infections[7].
Conventional therapies for sepsis mainly focus on fluid resuscitation, source control measures and antimicrobial administration within 1 h of recognition[8]. New therapeutic strategies aim to restore the immune balance by eliminating/ deactivating inflammatory mediators[7,9]. Extracorporeal therapies (ECT), otherwise known as blood purification therapies target attenuation of the immune response by reducing the circulating levels of cytokines and triggers that potentiate the response (endotoxins, pathogen associated molecular patterns – (PAMPs), damage associated molecular patterns (DAMPs), and leukocytes), thereby trying to achieve immune balance/homeostasis[7].
ECT is a blood purification technique in which blood and its components are removed from the body, circulated in the EC circuit and treated with various technologies before being readministered to the patient[10]. Different ECTs include; hemofiltration, hemoperfusion, intermittent or continuous high volume hemofiltration (HVHF), hemadsorption and plasmapheresis[11].
The concept of ECT is based on the objective of nonspecific clearance of inflammatory mediators and/or toxins, attenuating the overwhelming systemic expression of inflammatory mediators in the early phase of sepsis[12]. As per the ‘cytokine peak concentration’ hypothesis, eliminating the peak cytokine concentration during the early stage of sepsis can halt the inflammatory cascade, thereby limiting the organ damage and decreasing the incidence of MODS[13,14].
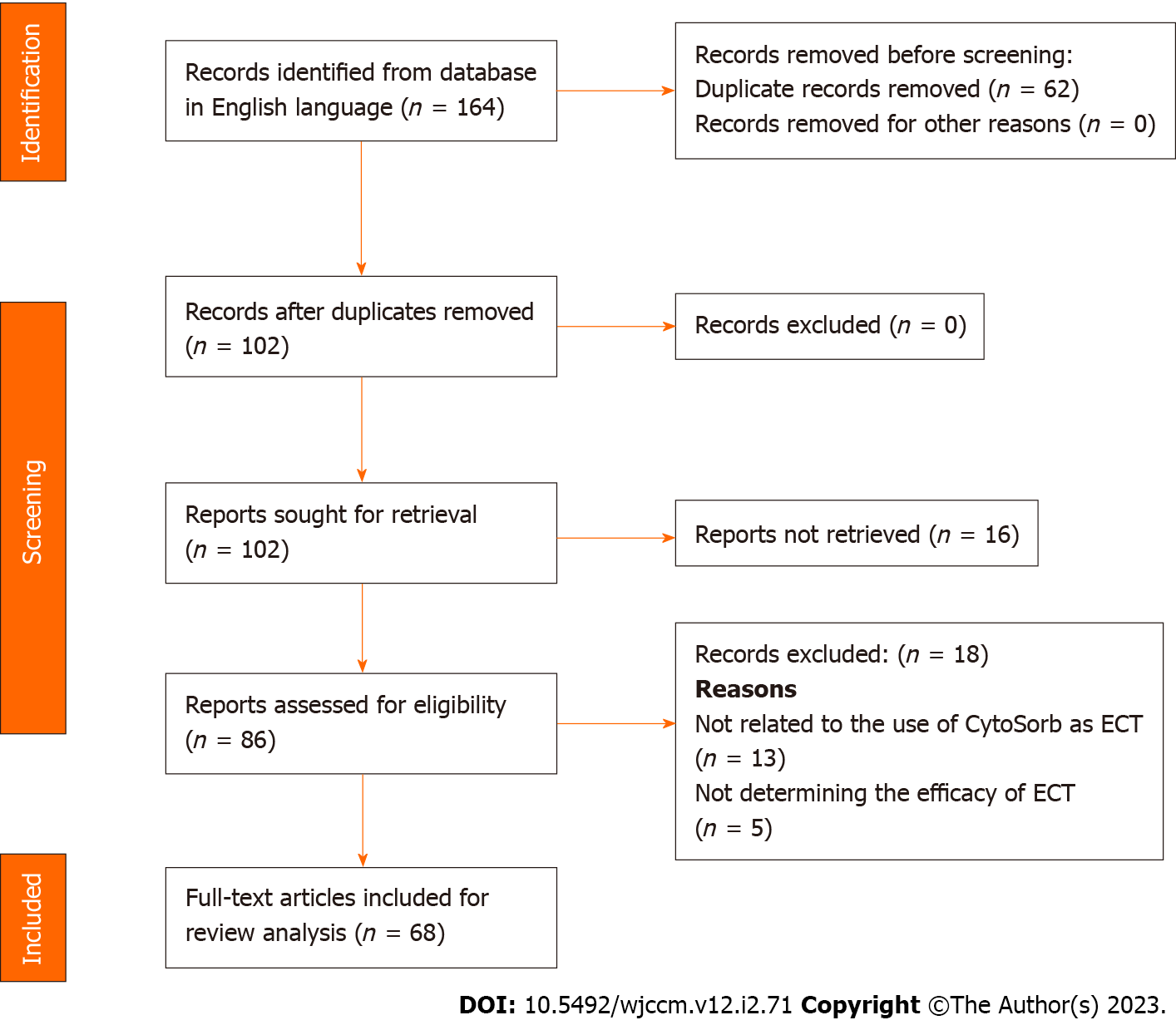
An extensive literature search was conducted for articles published in last two decades that provided information on the use of ECT in sepsis, using the key words “sepsis”, “septic shock”, “extracorporeal therapy”, “blood purification”, and “CytoSorb®”, that were in PubMed, MEDLINE, Cochrane Library, or Science Direct databases and with the filters “humans”, “English language”, “full text articles” (review articles, case reports, randomized controlled trials (RCTs) applied. Only articles published in peer-reviewed and indexed journals from 2002-2021 were selected; abstracts were excluded. The PRISMA diagram for inclusion and exclusion of articles is presented in Figure 1.
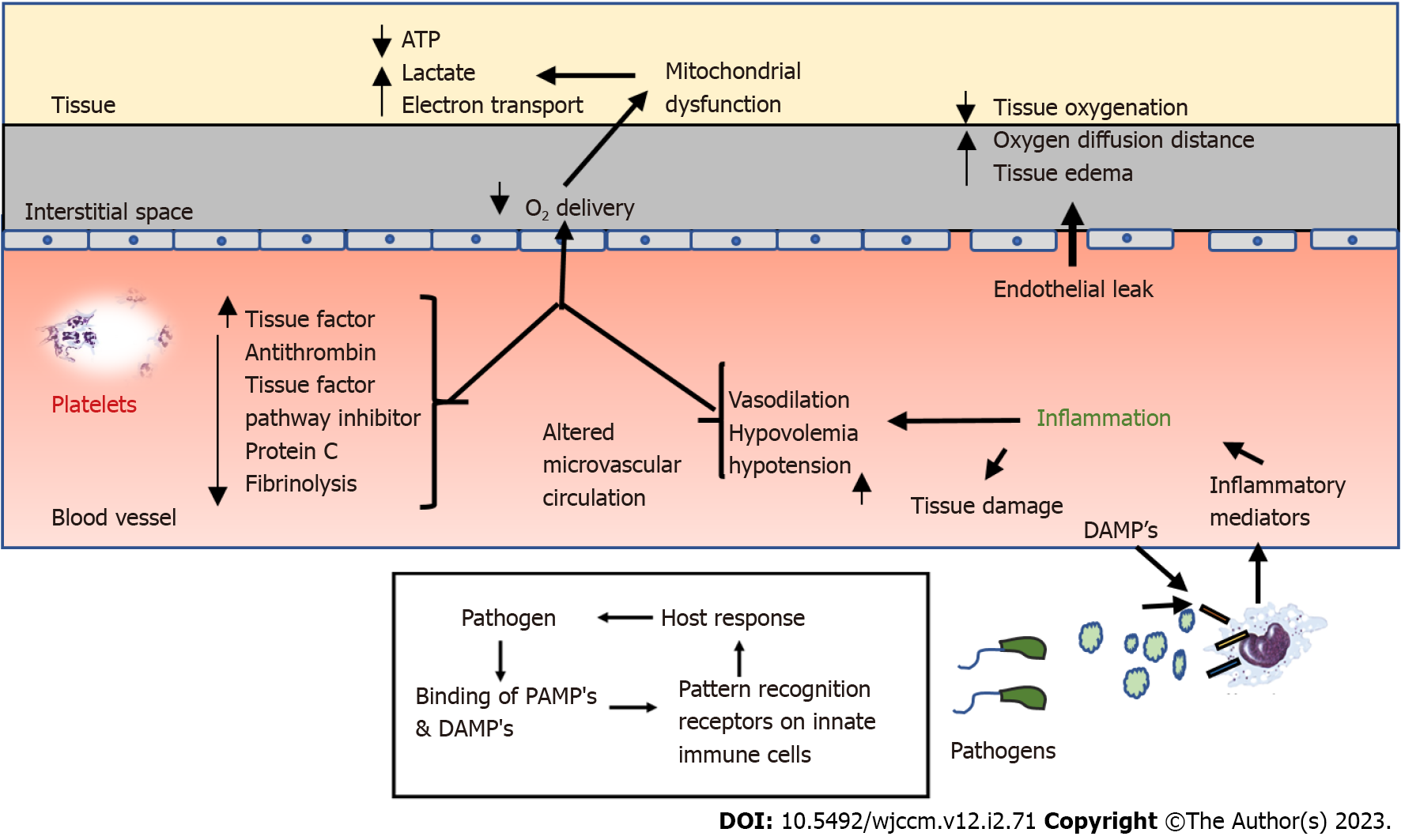
Sepsis is a multi-layered disruption of the host immune balance. Its pathophysiology involves a complex interplay between the host and the infectious agent[15]. The first step in this process, is activation of the innate immune system (macrophages, monocytes, neutrophils and natural killer cells) which occur as a result of the binding of PAMPs and DAMPs such as adenosine triphosphate and mitochondrial DNA, to the specific pattern recognition receptors present on the immune cells, which include toll like receptors, C-type leptin receptors and nucleotide binding oligomerization domain like receptors[16]. This results in intracellular signal transduction and activation of pro-inflammatory cytokines, such as interleukin - IL1, IL6, IL12, IL18 and tumor necrosis factor alpha (TNF-α)[17]. Subsequently, cytokines cause activation of leukocytes, complement system, coagulation pathways, tissue factor production, chemokine expression and overexpression of endothelial adhesion molecules[15,16]. Following this negative feedback, a compensatory anti-inflammatory response syndrome (CARS) is initiated, which down regulates the components of the adaptive immune system[17]. Upregulation of both pro- and anti-inflammatory cytokines marks the early stage of sepsis[18]. A poorly regulated systemic inflammatory response syndrome (SIRS) and CARS can lead to a mixed antagonistic response syndrome leading to progressive tissue damage and potentially causing MODS[15,19].
Coagulopathy in sepsis occurs as a result of simultaneous activation of inflammatory and hemostatic pathways. It is thought to be driven by the release of tissue factor from damaged endothelial cells, leading to systemic activation of the coagulation cascade[20]. Activation of this cascade results in thrombin production, platelet activation and formation of fibrin clots leading to perfusion defects[16,21]. In addition to this, procoagulant effects are further potentiated by suppression of natural anticoagulants such as protein C, anti-thrombin, and thrombomodulin along with tissue plasminogen activator, leading to microvascular coagulation and ultimately MODS[21,22]. Pathophysiology of sepsis is detailed[15,16,23] in Figure 2.
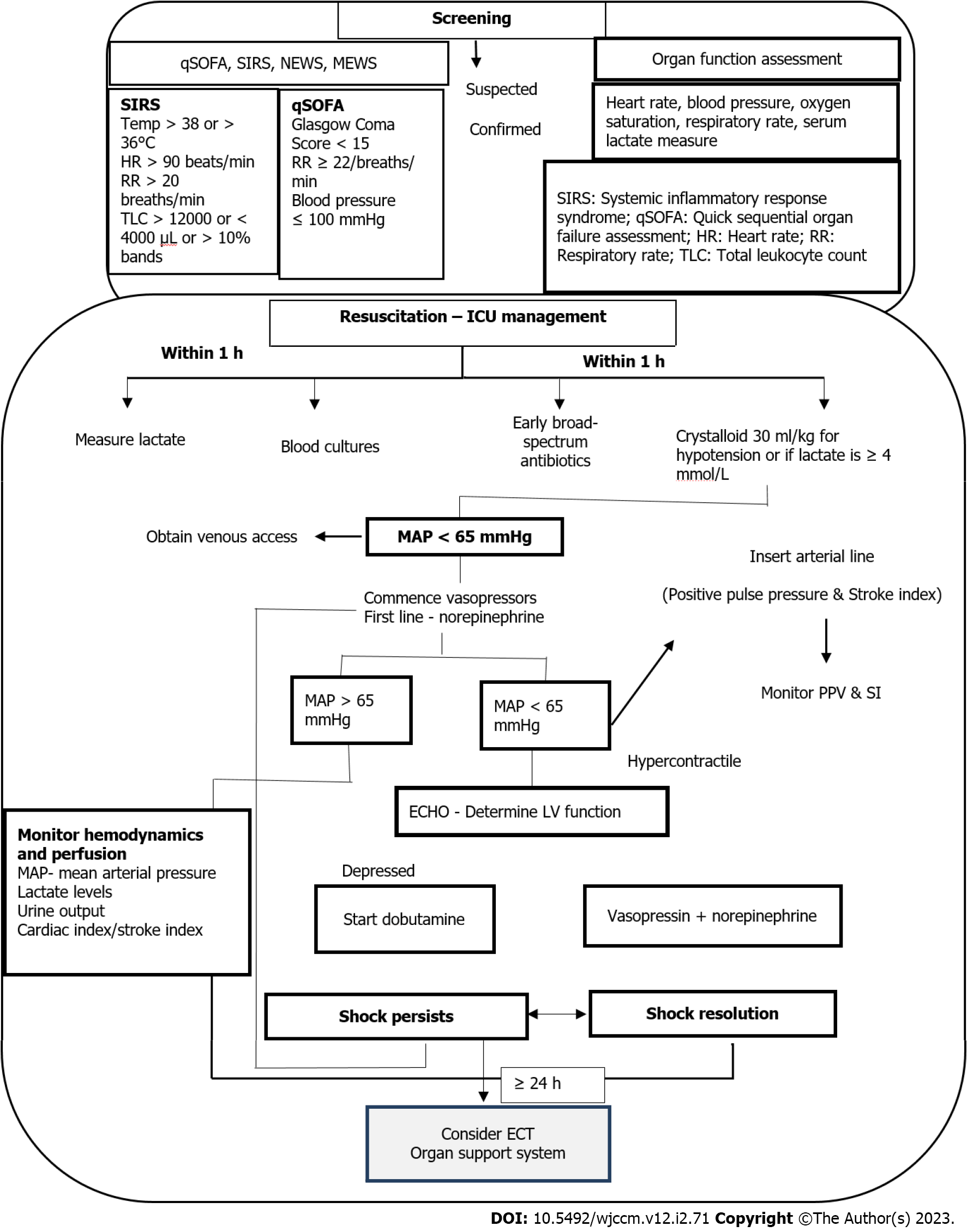
Sepsis is a medical emergency and measures taken in the initial hours after its recognition have a significant impact on the outcomes, including survival. In 2018, the Surviving Sepsis Campaign (SSC) guidelines introduced an “Hour-1-Bundle”, replacing the previous recommendation of 3- and 6-hour bundles. The ‘Hour-1 Bundle’ consists of 5 clinical interventions, which prompt immediate initiation of sepsis management and fluid resuscitation measures[24]. Management of sepsis including screening and ICU standards of care is presented in Figure 3[7,16,25,26].
Blood purification therapies: MODS caused due to an excessive release of cytokines and inflammatory mediators is a major cause of ICU morbidity and mortality in sepsis[27]. Blood purification therapies (BPTs) are the strategies proposed to restore the immune balance by eliminating or deactivating the inflammatory mediators and originates as an off-shoot of renal replacement therapy (RRT). Various approaches have been identified to maximize the effect of RRT, which include HVHF, high cut-off membranes (HCO), hemadsorption techniques alone or in combination and coupled plasma filtration adsorption (CPFA)[9,15,27]. Studies determining the efficacy of different modalities in cytokine and endotoxin removal are presented in Table 1[28-33].
| Ref. | Study type | Population | Modality | Intervention | Outcomes |
| Tapia et al[28], 2012 | Prospective cohort study | 31 severe septic shock patients | HVHF, Cytokine removal | HVHF – single short term – 6 h at 40 mL/kg/h | 25/31 responded to HVHF. Decrease in NE dose and improvement in hemodynamic, metabolic and respiratory parameters were significantly improved by 4 h |
| Joannes-Boyau et al[29], 2013 | Prospective, randomized, open multicentre trial | 137 septic shock patients (AKI < 24 h) | HVHF, Cytokine removal | HVHF – 70 mL/kg/h vs standard volume hemofiltration at 35 mL/kg/h | No difference in hemodynamic stability, severity scores, 28-d mortality, length of stay and vasopressor free days |
| Livigni et al[30], 2014 | Prospective, randomized, multicentre parallel group trial | 192 septic shock patients | CPFA, Cytokine & endotoxin removal | Conventional therapy (n = 93) vs CPFA (n = 91) | Decreased mortality in patients receiving high dose of CPFA. No difference in length of ICU stay and new organ failures in 30 d |
| Atan et al[31], 2018 | Randomized controlled trial | 76 critically ill patients with AKI | CVVH -HCOCytokine removal | CVVH-HCO (n = 38) – cut off point 100 kDa vs CVVH -Std (n = 38) – cut off point 30 kDa | No difference was observed in mortality, duration of hemofiltration, norepinephrine dose, serum albumin levels and filter life |
| Dellinger et al[32], 2018 | Randomized, multicentre trial | 449 septic shock patients | Polymyxin B hemoperfusion; Endotoxin removal | Polymyxin B hemoperfusion + Standard therapy vs Sham hemoperfusion + Standard therapy | No significant difference in 28 d mortality in overall population or in patients with MODS score of > 9 |
| Kaçar et al[33], 2020 | Prospective observational study | 23 septic shock patients with AKI | HA 330 Cytokine removal | HA 330 hemoperfusion + CVVH for 2 h once daily for 3 d | Increase in pH was observed after 1st application HA330 hemoperfusion; CRP and PCT levels decreased significantly after 2nd application |
Extracorporeal BPTs such as hemodialysis, have been used traditionally to replace renal functions in critically-ill patients. Knowledge of solute and water transport through physico-chemical mechanisms in dialysis forms the basis of extracorporeal (continuous) renal replacement techniques (CRRT) and ECT. Observations of recovering ICU septic patients treated with RRT sparked the idea of tilizing ECT in sepsis[34,35]. Different theories have been postulated to explain the effect of blood purification in restoring hemodynamic stability. Peak concentration hypothesis suggests that eliminating the peaks of cytokine blood concentrations during the early phase of sepsis could halt the inflammatory cascade, resulting in improved immune-dysregulation[14,36]. Variations in interstitial and tissue concentrations of inflammatory mediators cannot be explained by this theory. To combat the failure of peak concentration theory, a new dynamic hypothesis “threshold immunomodulation” was developed by Honore and Matson, which correlated the removal of inflammatory mediators from the blood compartment to changes in interstitial and tissue mediator levels. According to this new theory, inflammatory mediators are gradually taken from interstitium and tissues after removal from the blood compartment until a threshold is reached, at which the inflammatory cascade comes to a halt preventing further organ damage. However, it is difficult to correctly determine this threshold as changes in inflammatory mediators in the interstitium and tissues might not be reflected accurately by changes in the blood compartment in different BPT[37]. To find out how blood purification affects the passage of mediators and cytokines from the tissue and interstitium into the blood compartment, a new hypothesis i.e., “mediator delivery” hypothesis was proposed by Di Carlo and Alexander. This hypothesis suggested that use of high replacement volumes, (around 20 to 40 -fold increase in lymphatic volumes) might displace the inflammatory mediators in the blood compartment from where these could be removed during the blood purification process. Thus, high replacement volumes enhance the lymphatic transport between the blood compartments and tissue/interstitium[38]. However, Honore et al[37] developed a fourth cytotoxic hypothesis to explain the relationship between different compartments. This theory explained that removal of inflammatory mediators from central circulatory system required assistance of active transportation along with passive one. Peng et al[39] proposed a cytokinetic theory which suggested that the BPT restores immune function by regulating monocytes, neutrophils and lymphocytes at the cellular level. Many studies have reported that polymyxin B hemoadsorption could increase the expression of leukocyte surface markers such as HLA-DR making hemoadsorption a ‘re-programming system’ for the leukocytes. Another unique element proposed in this theory is that the concentration gradient from plasma to infected tissues can be restored by removing mediators from the plasma in systemic inflammation. This concentration gradient has notable effects on leukocyte trafficking and bacterial clearance[11,36,39].
Mechanisms involved in extracorporeal blood purification are either diffusion, convection or adsorption. With the diffusion process, the solute is transported through a semi permeable membrane down/across its concentration gradient, whereas in convection, solute transport happens as part of solvent drag, and ultrafiltration is driven by a transmembrane pressure gradient. In hemadsorption, blood is passed through sorbents which attract the solutes to adhere to them (adsorb), through a series of hydrophobic and ionic interactions[27,40]. Solute clearance by diffusion depends on molecular weight (MW), membrane permeability, dialysate flow and surface area. Various EC blood purification techniques are described in Figure 3[15,40,41].
It has been postulated that sepsis induced organ injury can be mitigated by curtailing the inflammatory cascade. This could be achieved by disrupting the peak of inflammatory mediators[13]. BPT used for cytokine removal are the convection therapies [CRRT, HVHF, HCO, adsorption therapies (Polymixin B, CytoSorb® (hemadsorption)] and combination therapies[7,15].
HVHF: HVHF is defined as continuous hemofiltration at a rate of 50-70 mL/kg/h for 24 h or 100-120 mL/kg/h intermittently for 4-8 h followed by conventional renal dose hemofiltration[42]. Circulating inflammatory mediators are water insoluble with a MW of < 60 kDa (kilodaltons), and can thus be effectively removed from the plasma via the convection method. Additionally, these membranes have adsorptive properties which further enhance molecular clearance[12]. Recent meta-analysis studies have observed improvements in hemodynamic variables and reduced mortality in critically ill patients with HVHF therapy[28,43]. However, HVHF has also shown contradictory results with no improvement in mortality or hemodynamic variables in randomized trials[29,44-46]. Potential drawbacks of HVHF are the loss of small molecules (vitamins, nutrients, antibiotics) and large volume replacement which may increase treatment costs and the risk of electrolyte imbalance[12,47]. In order to avoid the drawbacks of HVHF, the concept of cascade hemofiltration was introduced which allows selective removal of middle weight molecules. It includes two hemofilters with different cut off values incorporated into a single EC unit, through which only middle molecular weight molecules are filtered and the lower molecular weight molecules are reinfused into the blood circuit. However, in the study conducted by Quenot et al[48] cascade hemofiltration failed to provide any beneficial effects in comparison to standard care.
Coupled plasma filtration and adsorption: In this technology, plasma is separated from the blood with the help of a high cut off filter and then passed through a sorbent cartridge for adsorption of cytokines and endotoxins. The filtrate plasma is then redirected to the dialyzer to combine with blood and used in RRT[7,49]. Several studies evaluating CPFA in sepsis and septic shock patients resulted in hemod
CytoSorb® hemoadsorber: Hemoadsorption is a technique where the sorbents contained in cartridges are placed in direct contact with the blood via an EC circuit, removing toxins and inflammatory mediators[12,36]. The rationale of using adsorption therapy is to restore the (proinflammatory and anti-inflammatory) immune balance[52].
Features: CytoSorb®(CytoSorbent, New Jersey, United States) hemoadsorption device is an International Organization for Standardization certified, European CE mark approved class IIb medical device, made up of biocompatible as well as hemocomaptible polystyrene divinylbenzene copolymer beads, designed to remove excess inflammatory cytokines from the blood (IL-1B, IL-6,8,10, TNFα monomer, TNFα trimer, IFN γ)[15,53,54]. It has a surface area of > 45000 m2, so in principle has a far greater capacity for adsorption than with dialyzers/hemofilters and provides size-selective removal of hydrophobic subtstances with a molecular cut-off size of 60kDa, thus resulting in adsorption of both pro and anti-inflammatory mediators, toxins and drugs. However, endotoxins are an exception, as their MW is 100kDa[7,11]. CytoSorb® is compatible with both citrate anticoagulation and systemic heparin, and the duration of therapy is up to 24 h/sessions/d for 2-7 consecutive days depending on the clinical situation with blood flow ranging between 150-700 mL/min[55]. CytoSorb® also eliminates proteins (myoglobin, free hemoglobin dimer, ferritin, free hemoglobin tetramer), metabolites (bilirubin and bile acids), PAMPs (aflatoxin, Staph. aureus hemolysin, Staph. aureus toxic shock toxin, Strept. pyogenes exotoxin, Clostr. perfringens toxin and Shiga-like toxin), DAMPs (C5A, S100 and HMGB-1), which may in part be responsible for the dysregulated inflammatory response[7,53,54]. Due to the size-selectivity substances such as immunoglobulins, albumin and coagulation factors are not adsorbed in a significant manner by CytoSorb® as shown in studies[56,57] CytoSorb® can be used as a standalone therapy on cardiopulmonary bypass (CPB), or with CRRT and ECMO. CytoSorb® is approved for hemoperfusion/ hemadsorption and for intraoperative use in CPB surgery for removal of P2Y12-Inhibitor like Ticagrelor and/or the factor Xa-Inhibitor, Rivaroxaban[7,53,54].
Clinical evidence: Various clinical publications support the use of CytoSorb® in septic shock patients and have shown promising results prompting the need for RCTs to conclude on the benefits of blood purification with CytoSorb® in critically ill patients[58]. Brouwer et al[59] observed in their retrospective analysis on patients in septic shock requiring CRRT a significantly improved 28-d mortality by adding CytoSorb® as an adjunctive therapy, when they applied the statistical Inverse Probability Treatment Weighting method to compensate for baseline differences. In a follow-up long-term analysis of the same patient cohort, the authors concluded that the addition of CytoSorb® to CRRT improved survival from 28 d to 1 year. Lactate level > 6.0 mmol/L at the initiation of CytoSorb® therapy had a 79% positive predi
An international (130 centres from 22 countries) registry established in 2015 evaluated the use of CytoSorb® in critically-ill patients in the ‘real world’. The interim analysis reported an observed mortality of 65% in comparison to acute physiology and chronic health evaluation II (APACHE II) predicted mortality of 78%. No significant reduction was observed in SOFA. Moreover, a marked reduction in IL-6 levels was observed[52].
In a prospective single center study including 20 patients with refractory septic shock, CytoSorb® therapy led to significant reductions in norepinephrine requirements improvements in lactate clearance and resolution of shock in 65% of patients[62].
Studies conducted in India by Mehta et al[53] also reported a favourable outcome in sepsis or septic shock patients with the use of CytoSorb® therapy. A retrospective observational study showed a decrease in total leucocyte count, reduction in biomarkers such as procalcitonin (PCT) (65%), C-reactive protein (CRP) (27%), serum lactate (27%), bilirubin (43%), IL-6 (87%), IL-10 (92%) and TNF (24%) levels and decrease in SOFA scores by 16.2% post therapy. Mehta et al[53] developed a CytoSorb® Scoring (CS) system that categorized patients in < 8, 8-13 or > 13, where 8-13 scores based on 5 parameters representing 5 organ systems to determine the number of devices required for therapy. The score of 8-13 was observed as the most appropriate for initiating CytoSorb® therapy. Study results revealed that survivors had a mean score of 12, whereas non-survivors a mean score of 14.
Kogelmann et al[63] reported that the effects of hemadsorption therapy (hemodynamic stabilization and survival) using CytoSorb® was more pronounced in patients in whom therapy was started in < 24 h of sepsis onset, whereas a poor response was associated with a delay in therapy, in terms of vasopressor demand and survival. Further research is required to establish its use in treatment of sepsis[64]. CytoSorb® has shown promising results in sepsis both individually as well as an adjunct therapy by reducing SOFA scores, lactate levels, total leucocyte count, platelet count, IL-6, IL-10, TNF levels and improving survival[65-68] as presented in Tables 2[52,53,59,60-63,68,69] and 3[64,70-73].
| Ref. | Study design | Population | Intervention | Outcomes |
| Friesecke et al[62], 2017 | Prospective, single center study | 20 septic shock patients | CytoSorb hemoperfusion | Norepinephrinedose reduced after 6 and 12 h; Improved lactate clearance; SOFA scores unchanged; Shock reversal achieved in 65% of patients; 28-d survival – 45% |
| Kogelmann et al[63], 2017 | Case series | 26 septic shock patients | CytoSorb+CVVHD | Rapid hemodynamic stabilization; Reduction in Vasopressor dose by 67%; Decrease in blood lactate by 26.4%; Shock reversal in 38.5% patients; Decreased mortality than predicted by APACHE II; No adverse events reported |
| Friesecke et al[52], 2017 | International registry | 135 septic shock patients | CytoSorb hemoperfusion | Reduced observed mortality of 65% than predicted by APACHE II of 78%; Marked reduction in IL6 levels; No significant reduction in SOFA scores; Safe and well tolerated without any adverse events |
| Brouwer et al[59], 2019 | Retrospective, investigator-initiated study | 116 septic shock patients | CytoSorb +CRRT | In CytoSorb group, the mean predicted mortality rate was 74.5%, while 28 d mortality rate was 47.8%; In CRRT group, the mean predicted mortality rate was 67.9%, while 28-d mortality was 51.0%; CytoSorb group was associated with a reduced 28-d mortality in comparison to CRRT (53% vs 72.3%) |
| CRRT alone | ||||
| Brouwer et al[60], 2021 | Long term follows up | 116 septic shock patients | CytoSorb +CRRT | CytoSorb was significantly associated with long term outcome compared to CRRT |
| Retrospective cohort study | CRRT alone | |||
| Mehta et al[53], 2020 | Retrospective, observational study | 40 septic shock patients | CytoSorb hemoperfusion (Survivor group vs non survivor group) | Improvement in MAP (62.82 ± 9.73mmHg); Reduction in vasopressor dose; Reduction IL-6 levels (87%) and TNF levels (24%); Decrease in SOFA scores by 16.2% |
| Paul et al[68], 2021 | Prospective, real time, observational multicentre study | 45 septic shock patients | CytoSorb+ Standard therapy | 26 patients survived post therapy; Reduction in vasopressor dose (NE- 51.4%, Epinephrine – 69.4% and Vasopressin -13.9%); 52.3% reduction in IL-6 levels; Reduction in APACHE II and SOFA scores, 20.1 ± 2.47 and 9.04 ± 3.00 respectively |
| (Survivor vs non survivor group) | ||||
| Akil et al[69], 2020 | 20 patients with pneumogenic sepsis and ARDS | CytoSorb + Combined high flow veno-venous ECMO (CytoSorb group); ECMO therapy alone (Control group) | The 30-d mortality rate was 0% in CytoSorb group, whereas 57% was observed in control group; Significant reduction in procalcitonin and C-reactive levels were observed in CytoSorb group in comparison to control group | |
| Rugg et al[61], 2020 | Retrospective single center study | 42 septic shock patients compared to 42 matched controls | Cytosorb +RRT | Catecholamines requirements decreased to 0.26 µg/kg/min within 24 h of therapy with CytoSorb; In hospital mortality was significantly lower in CytoSorb group as compared to controls (35.7% vs 61.9%); Risk factors in CytoSorb group were high lactate levels and low thrombocyte counts proior to therapy. Lactate value of 7.5 mmol/L, predicted mortality with high specificicty (88.9%) |
| Ref. | Study type | Population | Intervention | Outcomes |
| Alharthy et al[70], 2020 | Retrospective case series | 50 COVID-19 patients with AKI, ARDS, Sepsis and hyperinflammation | CytoSorb + CRRT [Survivors (n = 35) vs non survivors (n = 15)] | Decreased SOFA score, lactate levels, ferritin, D-dimers, CRP and IL-6 levels in th survivor group after 2 ± 1 sessions of CRRT + CytoSorb |
| Mehta et al[64], 2021 | Case series | 3 critically ill COVID-19 patients | CytoSorb hemoperfusion other prescribed medications (tocilizumab, antivirals, hydroxychloroquine, azithromycin) | Significant improvement in biochemical parameters and clinical outcomes post CytoSorb therapy; Reduction in CRP levels by 91.5%, 97.4% and 55.75%, respectively; Improvement in MAP by 18%, 23% and 17% by 7th day post therapy |
| Nassiri et al[71], 2021 | Retrospective case series | 26 COVID-19 patients with ARDS | CytoSorb hemadsorption therapy | 21 patients survived; Significant decrease in NE requirement; PCT, CRP and ferritin reduced post therapy; Significant improvement in SOFA scores; Therapy was well tolerated |
| Paisey et al[72], 2021 | Retrospective case series | 15 severely ill COVID-19 patients | CytoSorb hemadsorption therapy | Adjunctive treatment with CytoSorb lead to reduction in ferritin, CRP, PCT and lactate levels |
| Song et al[73], 2021 | Multicenter observational study | 52 ICU COVID -19 patients on ECMO | ECMO + CytoSorb hemadsorption therapy | ICU mortality was 17.3% on day 30, 26.9% on day 90, and 30.8% at final follow up of 143 d; Lower baseline D-Dimer levels were observed among survivors (2.3 ± 2.5 vs 19.8 ± 32.2 µg/mL) compared to non survivors; Borderline association observed between baseline D-Dimer levels and mortality with a 32% increase in risk of death per 1 µg/mL increase |
However, other retrospective analysis did not support the above findings. Wendel Garcia et al[74] did not see differences in IL-6 or vasopressor needs in their analysis on the use of CytoSorb® in septic shock patients compared to historical control patients and even discussed an increased hazard of death associated with hemoadsorption. Similar Scharf et al[75] showed no difference in IL-6 reduction and hemodynamic stabilization, or mortality in patients with CytoSorb® treatment compared to a matched patient population.
De Wolf et al[76] in a recent meta-analysis suggested that the evidence with a low degree of certainty signified that administering CytoSorb® to critically ill patients with inflammatory conditions could even increase mortality. Adverse events were common, but they were not routinely evaluated and were also underreported. A need for high-quality RCTs to clarify mortality and adverse events related to CytoSorb® is suggested by the findings with significant uncertainty, which prevents drawing firm conclusions.
Regardless of the fact that all the included studies were not powered for mortality as an endpoint, it can also be discussed whether mortality is a reasonable endpoint for a single intervention in critically-ill patients with numerous potential causes for death.
However, considering the aspect that patient selection, timing and dosing was not always applied to the best possible manner or the current understanding respectively, might explain at least partly the contradictory results of the studies presented above. CytoSorb® should primarily be used in refractory cases where standard measures of care are not sufficient to stabilize the patient rapidly and start of the therapy should ideally be within the first 6-24 h after diagnosis of septic or vasoplegic shock. The therapy should be continued until sufficient stabilization. For this the adsorber should be replaced every 12-24 h depending on the degree of hemodynamic stabilization being observed. With regard to adequate timing, Kogelmann et al[77] evaluated a dynamic scoring system intended to support initiation of CytoSorb® in septic shock patients. The study reported that earlier treatment was associated with a better outcome. Additionally, outcomes improved if CytoSorb® was applied within 12 h after diagnosis in patients with the highest CS score > 8.
The CS still requires prospective validation and adapatibility. Nevertheless, more robust evidence is needed to better understand ideal patient selection, timing and dosing.
Novel use of CytoSorb®: CytoSorb® also has CE approval for the reduction of bilirubin and myoglobin in liver failure and severe trauma/rhabdomyolysis. It can also be used in severe acute pancreatitis and severe cardiogenic shock. Patients undergoing major aortic surgery with CytoSorb® incorporated in the CPB circuit demonstrated a promising therapeutic option for critically ill patients with multiorgan failure after cardiac surgery and may help in cytokine reduction with improved organ function[78]. In 2020, CytoSorb® was also approved for the removal for two antithrombotic drugs – ticagrelor and rivaroxaban in emergent and urgent cardiothoracic surgery, in order to reduce the risk of intra- and post-operative bleeding.
Jafron HA-330 and HA-380 adsorber: (Jafron Biomedical Co., Ltd.No.98, Technology Sixth Road, High-tech Zone, Zhuhai City, 519085, Guangdong, China).
The HA-330 (HA-380 is 15% bigger than HA-330) is a disposable hemoperfusion cartridge with an adsorbent material made up of neutral microporous resin and collodion coating. It is indicated for the removal of middle to large pathogenic substances from the blood (endogenous or exogenous), such as residual drugs, toxins and metabolic substances. It is used either as a stand alone or in combination with hemodialysis and hemoperfusion circuits. However, it is not clear if integration with ECMO is recommended or not.
HA-330 (and HA-380) have limited options for circuit configurations and a shorter treatment time up to 4 h when used in conjunction with a dialyser. Moreover, HA-330 (and HA380) have a maximum blood flow and operating time depending on the mode of operation.
Both HA-330 and HA-380 adsorbers have a storage fluid considered to be extremely acidic, with a pH of 1.8, which, even after a careful and 45-min-long rinsing procedure, remains as low as pH 3.3. A case series conducted in septic pediatric patients with cancer and other hematological disorders has confirmed the efficacy of HA-330 and HA-380. However, detailed studies in a larger population was recommended by the authors[79]. Treatment with CytoSorb®, resulted in significant removal of IL-6 in a severely ill patient population with septic shock, ARDS, and multi-organ failure in a multicenter randomised study. This, however, had no effect on normalised IL-6-plasma levels[80,81]. A comparative in-vitro study was conducted on both the CytoSorbents and Jafron hemoadsorption technologies and showed that both systems can remove cytokines from whole blood, but the CytoSorb® 300 device appears to be more effective and dynamic in this regard. Therefore, in severe septic state where quick cytokine clearance is desired, it might be the preferred device[82]. HA-330 and HA-380 have very limited published articles (far less than 50) to support its therapeutic benefits and clinical experience.
Biosky MG 350 adsorber: (Biosun Medical Technology Co. Ltd, China). The Biosky MG350 adsorber is another disposable hemoperfusion cartridge made up of microporous adsorptive resin, recommended for application in sepsis and hyperinflammation. Published literature in the English language is extremely scarce, and currently limited to one case report. Sequential use of CytoSorb® and the MG350 filter was carried out in a coronavirus disease 2019 (COVID-19) patient with severe ARDS. After initial successful CytoSorb® use, an MG350filter was used in parallel to an ECMO circuit. The combination of an antibiotic regimen and Biosky filter resulted in decreased inflammatory markers (CRP, PCT, IL-6 and IL-2). However, the patient suffered with severe respiratory failure and later died[83]. Biosky MG350 has a blood flow of 400 mL/min with an operating time of 2 h depending on the mode of operation. Compared to other adsorbers, Biosky MG350 requires a long rinsing procedure (Table 4[84-88]).
| Feature | CytoSorb 300[84,85,86] | Jafron HA-series (80, 130, 180, 230, 280, 330, 380)[87] | Biosky MG-Series[88] |
| Manufacturer | CytoSorbents™ Inc, United States | Jafron Biomedical Co., Ltd. No. 98, Technology Sixth Road, High-tech Zone, Zhuhai City, 519085, Guangdong, China | Biosun Medical Technology Co. Ltd, China |
| IFU version | October 1, 2021[87] | 11-Sep-19 | 1-Aug-18 |
| Adsorbent | Crosslinked Divinylbenzene | Neutral Macroporous Resin | Medical Neutral Macroporous Synthetic Resin |
| Coating | Polyvinylpyrollidone | Collodion | No data |
| Adsorbent Surface | > 45000 m2 | 100000m2 | No data |
| Storage fluid | Isotonic saline | Water for injection | Sterile water |
| Use time/cartridge | 24 h, Can be administered up to 7 consecutive days | Depending on mode of operation: Hemoperfusion 100-250 mL/ min; Dialysis < 320 ml/ min with use upto 4 h; CRRT 150-250 mL/min with use upto 12 h; CPB up to 700 mL/ min with use upto 2.5 h | 120-180 min, Not suggested to use more than 3 times within 24 h |
| Blood flow | 100-700 mL/min, Recommended > 150 mL/min | 100-700 mL/min | 100-400 mL/min; Highest rate is 250 mL/min |
| Pmax | 760 mmHg | 750 mmHg | 750 mmHg |
| Mode of operation covered | Hemoperfusion, Intermittent hemodialysis, CRRT, Cardiopulmonary bypass (CPB) ECMO | Hemoperfusion; Hemodialysis; CRRT; CPB | Hemoperfusion; Hemodialysis; CRRT; CPB only as comment in anticoagulation, not in setup |
| Shelf life | 3 yr | 2 yr | 2 yr |
| Safety report status | As of 2021: > 162000 treatments distributed without confirmed serious device related events | No data | No data |
Miscellaneous: Several other cartridges available for adsorption include Hemofeel (Toray, Tokyo, Japan), a polymethyl methacrylate hemofilter, and Theranova 400/500 dialysers developed by Baxter. Multiple other cartridges that have an affinity to bind to bacteria and viruses are also under investigation. The Seraph 100 Microbind Affinity blood filter (ExThera, California, United States) is an adsorbing technology which consists of non-porous heparin coated beads designed to reduce blood-borne pathogens during bloodstream infections. Hemopurifier (Aethlon Medical, California, United States) and FcMBL (Opsonix Inc, United States) is also other make that is also available[7].
Lipopolysaccharide (LPS) an endotoxin, is a component of gram-negative bacteria that induces an inflammatory response. A dysregulated host response to LPS might lead to multiple organ failure or fatal septic shock if unchecked. Endotoxin activity (EA) levels are measured on a scale of 0 to 1: low (< 0.4 units), intermediate (0.4-0.6 units), high (> 0.6 units). More than 80% of septic shock patients have intermediate or high EA levels indicating the function of endotoxin as a critical activator of the sepsis cascade. Clinical evidence for LPS is obtained from case series in critically ill patients reporting a reduction in endotoxin levels and improvement in hemodynamics with no significant adverse effects[89-91].
Polymyxin B: A polymyxin B-(PMX) immobilised fiber column (Toraymyxin: Toray, Tokyo, Japan) has been extensively used for endotoxin removal. The findings of a subsequent RCT in Europe, the EUPHAS study, which was carried out in Italy, were published in 2009, demonstrating that PMX has a significant effect on sepsis-related mortality[92]. The EUPHRATES RCT trial compared Polymyxin B hemoperfusion to a combination of sham hemoperfusion and standard therapy (n = 226) showing no significant difference in 28 d mortality among the overall population[32]. Subsequently, a post hoc analysis of the EUPHRATES trial demonstrated a significant reduction in 28 d mortality and impro
Alteco LPS adsorber: The Alteco LPS adsorber (Alteco Medical; Sweden) is an endotoxin adsorber cartridge, consisting of polyethylene plates with peptides which have a high affinity to adsorb LPS. A multicentre feasibility trial of the Alteco LPS adsorber –the ASSET trial was terminated early due to patient recruitment difficulties[95].
oXiris membrane: The oXiris filter is a modified AN69ST membrane which has an affinity to adsorb both endotoxins and cytokines. Initially, it was approved in 2009 in Europe, and in 2017 the indication was extended for patients requiring blood purification, CRRT and in conditions with excessive levels of inflammatory mediators and endotoxins[96]. It was also authorised by the FDA for emergency use for COVID-19 treatment[97]. However, its use is only indicated with the Prismaflex unit. Evidence supporting the use of oXiris comes largely from case series. In the study conducted by Shum et al[98], 6 patients with septic AKI received oXiris-CVVH and were compared to historical controls with a similar disease severity (n = 24). Results showed a significant reduction in SOFA scores by 37% after the use of oXiris-CVVH for 48 h whereas there was a 3% increase in the control group. However, there was no significant difference observed in length of ICU stay and hospital mortality. A single centred prospective study by Premužić et al[84] showed the efficacy of oXiris filters in reducing IL-6 and SOFA score severity in ICU patients. Improvement in respiratory status, chest X-ray severity score and other clinical symptoms were also reported in this study. Russell et al[85] used a hybrid purification system in fifteen critically ill sepsis patients. Treatment involved RRT with the oXiris filter and a CytoSorb® adsorbent cartridge also included in RRT system. Procalcitonin, IL6, cardiorespiratory function and endotoxins were monitored at baseline and at the completion of treatment. It was concluded that RRT with the oXiris filter and CytoSorb® cartridge were associated with improved hemodynamic stability, inflammatory response and renal function.
In an in-vitro comparison of three different blood purification devices – oXiris, polymyxin B, and CytoSorb®, oXiris showed a similar reduction in endotoxins and cytokines in comparison to polymyxin B and CytoSorb®, respectively[86]. Feri et al[99] pointed out the flaws in this in-vitro investigation, including the fact that the in-vitro comparison was carried out for two hours using 500 mL plasma solutions, pre-incubated with pathological quantities of inflammatory mediators. As stated by Feri et al[99], all the three devices (oXiris, polymyxin B, and CytoSorb®) work with whole blood and not just plasma, and the volume utilised by Malard et al[86] was very limited (500 mL), in humans the devices work with blood volume of 5 L. Furthermore, the concentration of inflammatory mediators was low, as was the duration of the experiment.
Feri et al[99] further stated that the actual application time of CytoSorb® and oXiris is 24 h and 72 h, respectively. Potential advantages and comparable results in endotoxin and cytokine clearance is limited to case series/reports, and no large, randomized trials exist thus far[96]. However, several ongoing trials have recently been completed and it is expected that oXiris may provide some new insights in the management of sepsis and septic shock. Studies showing the efficacy of oXiris in endotoxin and cytokine removal are presented in Table 5[49,96,98,100].
| Ref. | Study type | Population | Intervention | Outcomes |
| Shum et al[98], 2013 | Prospective case series with historical controls | 6 patients with septic AKI | oXiris + CVVH | Significant reduction in SOFA scores by 37% after 48 h of therapy |
| Ugurov et al[96], 2020 | Single centre case series | 15 COVID -19 patients | oXiris hemofilter | Early initiation of oXiris was associated with stable or reducing levels of IL-6,8,10 and TNFα |
| Zhang et al[49], 2021 | Case series | 5 COVID-19 patients | CRRT followed by oXiris hemofilter therapy | Reduced levels of cytokines, haemodynamic stabilization and improvement of organ function was observed with oXiris. |
| Rosalia et al[100], 2020 | Prospective cohort study | 44 COVID 19 cases | CVVH + oXiris | Reduction in CRP, ferritin, fibrinogen and other inflammatory mediators were observed |
Renal assisting device (RAD) is a cell-based therapy containing human proximal tubular cells. It was developed based on the concept that the kidney also have metabolic, immune and endocrine functions during sepsis[51]. RAD was found to be beneficial in replacing solute and water clearance along with active reabsorptive transport and metabolic functions[101]. However, its development was discontinued due to manufacturing and distribution issues. A selective cytopheretic device (SCD) is another therapeutic strategy targeting activated leucocytes. With a CRRT circuit, it results in sequestration of activated leucocytes. Evaluation of SCD was carried out in a randomized trial of 134 AKI patients. No significant differences in mortality were found between the treated (SCD) and control populations (CRRT)[102]. Molecular adsorbent recirculating system (MARS™) is another extracorporeal system which supports the liver by removing albumin-bound toxins from the blood. Short-term benefits of MARS have been evaluated in 3 prospective randomized studies showing improvement in survival rates of patients with hepato-renal syndrome and hepatic encephalopathy[103].
Based on SSC guidelines, evidence of ECT efficacy is evolving and has a sound rationale based on our current understanding of sepsis pathophysiology. Overall, however, hard evidence based on prospective RCTs is still scarce. As with every therapy proper patient selection, timing and dosing is crucial for therapeutic success. ECT has to be seen as an adjunctive therapy aiming at restoring homeostasis in hyperinflammatory conditions. In the light of the critically-ill patients with numerous co-morbidities usually treated with ECT in multi-nodal approach, one should not target mortality as the primary endpoint of such trials, but rather consider the improvements in organ dysfunction. Additionally, the challenge of patient heterogeneity usually mentioned in many of these trials and coming from the fact that sepsis is a syndrome rather than a specific disease, has to be taken into consideration for trial planning, too.
In clinical practice, timing of ECT is still often delayed as doctors see it too much as a final rescue therapy. So better guidance in regard to patient selection, timing and dosing has to compiled and provided to the user at the bedside. Importantly and with regard to the different ECT systems available in the market, it has to be stated that clinical results, but particularly safety relevant aspects, are not transferable between various hemoadsorption products due to technical differences[104].
Hemodynamic improvements, length of ICU stay and decreasing mortality were among the frequently studied end-points in most of the studies that have evaluated different ECT modalities. Further sepsis trials should target patient populations as homogeneous as possible and therefore focus on patient pheno- and endotypes including biomarker-based approaches to try to obtain more consistent outcomes of the therapy, thereby increasing the understanding of optimal therapy management and reducing the possibility of conflicting results.
Substantial progress has been made in the field of ECT therapies and sepsis. Among the presented technologies in this review, CytoSorb® seems to currently represent the most investigated and clinically established procedure. However, more robust evidence is still needed. Additionally, the achievement of beneficial clinical effects of these adjunct modalities in routine use requires identification of the right patient, right timing and right dose. Therefore, high quality RCTs are needed to provide definitive answers for these questions and also to facilitate individualised ECT treatments of critically ill patients.
Sepsis is one of the main causes of mortality in patients in critical care units worldwide, despite the fact that it can be treated with a variety of medications. Extracorporeal treatments (ECT), which aim to balance the dysregulation of the immune system by eliminating high quantities of inflammatory mediators, have drawn attention as a result of knowledge about the biology of sepsis.
The biology of sepsis has brought attention to extracorporeal therapies (ECT), which try to regulate immune system dysregulation by removing large amounts of inflammatory mediators.
To analyze new research on ECT use in sepsis and evaluate its impact on key inflammatory and clinical outcomes.
To find the usage of ECT in sepsis, a thorough search of the English literature from the previous two decades was done for this review. The selection process excluded publications that had only abstracts and resulted in a total of 68 articles from peer-reviewed and indexed journals.
The findings demonstrated the emergence of ECT approaches such as high-volume hemofiltration, coupled plasma adsorption/filtration, resin or polymer adsorbers, and CytoSorb® as adjuvant therapy to enhance hemodynamic stability in sepsis. With findings on increased survival rates and decreased sequential organ failure assessment scores, lactate levels, total leucocyte count, platelet count, interleukin-IL-6, IL-10, and TNF levels, CytoSorb® has the most published evidence in relation to its usage in the field of septic shock.
The absence of significant random clinical trials currently limits the clinical adoption of ECT in sepsis and septic shock. Future research breakthroughs with treatments aiming at the cellular level of the immune response are anticipated, in addition to patient-tailored medicines.
To achieve more consistent treatment outcomes, future clinical trials involving patients with sepsis should be as homogeneous as feasible and focus on patient phenotypes and endotypes, including biomarker-based techniques. This will not only increase our grasp of how to handle proper therapy, but it will also lessen the possibility of inconsistency.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT, Brazil; Wang HD, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17223] [Article Influence: 1913.7] [Reference Citation Analysis (2)] |
| 2. | Organization WH. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions [Internet]. World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/334216. |
| 3. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4143] [Article Influence: 828.6] [Reference Citation Analysis (4)] |
| 4. | Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb-. [PubMed] |
| 5. | Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1778] [Cited by in RCA: 1909] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 6. | Álvaro-Meca A, Jiménez-Sousa MA, Micheloud D, Sánchez-Lopez A, Heredia-Rodríguez M, Tamayo E, Resino S; Group of Biomedical Research in Critical Care Medicine (BioCritic). Epidemiological trends of sepsis in the twenty-first century (2000-2013): an analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr. 2018;16:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Govil D, Kumar GP. Extracorporeal Therapy in Sepsis. Indian J Crit Care Med. 2020;24:S117-S121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 2293] [Article Influence: 573.3] [Reference Citation Analysis (0)] |
| 9. | Ankawi G, Neri M, Zhang J, Breglia A, Ricci Z, Ronco C. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Crit Care. 2018;22:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Bellomo R, Honoré PM, Matson J, Ronco C, Winchester J. Extracorporeal blood treatment (EBT) methods in SIRS/Sepsis. Int J Artif Organs. 2005;28:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Honore PM, Hoste E, Molnár Z, Jacobs R, Joannes-Boyau O, Malbrain MLNG, Forni LG. Cytokine removal in human septic shock: Where are we and where are we going? Ann Intensive Care. 2019;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Shum HP, Yan WW, Chan TM. Extracorporeal blood purification for sepsis. Hong Kong Med J. 2016;22:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ronco C, Tetta C, Mariano F, Wratten ML, Bonello M, Bordoni V, Cardona X, Inguaggiato P, Pilotto L, d'Intini V, Bellomo R. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003;27:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Ronco C, Bonello M, Bordoni V, Ricci Z, D'Intini V, Bellomo R, Levin NW. Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif. 2004;22:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Jarczak D, Kluge S, Nierhaus A. Sepsis-Pathophysiology and Therapeutic Concepts. Front Med (Lausanne). 2021;8:628302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 16. | Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:2050312119835043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 17. | Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1016] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 18. | Tamayo E, Fernández A, Almansa R, Carrasco E, Heredia M, Lajo C, Goncalves L, Gómez-Herreras JI, de Lejarazu RO, Bermejo-Martin JF. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw. 2011;22:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | McCance KL, Huether SE. Pathophysiology: The biologic basis for disease in adults and children. 8th ed. Amsterdam: Elsevier Health Sciences 2019. 1720 p. |
| 20. | Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 21. | Gotur DB. Sepsis Diagnosis and Management. J Med Sci Heal. 2017;3:1-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 701] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 23. | László I, Trásy D, Molnár Z, Fazakas J. Sepsis: From Pathophysiology to Individualized Patient Care. J Immunol Res. 2015;2015:510436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 688] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 25. | Marik PE. Surviving sepsis: going beyond the guidelines. Ann Intensive Care. 2011;1:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Marik PE. Early management of severe sepsis: concepts and controversies. Chest. 2014;145:1407-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Daza JL, Ferro MCC, Cardenas AD, Daza L, Rey E, Jong J de, Galindo J, Gutiérrez G, Puello L, de la Cruz Y. Multiple-Organ Extracorporeal Support Therapies in Critically Ill Patients. Open J Nephrol. 2021;11:281-93. [DOI] [Full Text] |
| 28. | Tapia P, Chinchón E, Morales D, Stehberg J, Simon F. Effectiveness of short-term 6-hour high-volume hemofiltration during refractory severe septic shock. J Trauma Acute Care Surg. 2012;72:1228-1237; discussion 1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Joannes-Boyau O, Honoré PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Rozé H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013;39:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 30. | Livigni S, Bertolini G, Rossi C, Ferrari F, Giardino M, Pozzato M, Remuzzi G; GiViTI: Gruppo Italiano per la Valutazione degli Interventi in Terapia Intensiva (Italian Group for the Evaluation of Interventions in Intensive Care Medicine) is an independent collaboration network of Italian Intensive Care units. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open. 2014;4:e003536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Atan R, Peck L, Prowle J, Licari E, Eastwood GM, Storr M, Goehl H, Bellomo R. A Double-Blind Randomized Controlled Trial of High Cutoff Versus Standard Hemofiltration in Critically Ill Patients With Acute Kidney Injury. Crit Care Med. 2018;46:e988-e994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, Palevsky PM, Weisberg LS, Schorr CA, Trzeciak S, Walker PM; EUPHRATES Trial Investigators. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA. 2018;320:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 33. | Kaçar CK, Uzundere O, Kandemir D, Yektaş A. Efficacy of HA330 Hemoperfusion Adsorbent in Patients Followed in the Intensive Care Unit for Septic Shock and Acute Kidney Injury and Treated with Continuous Venovenous Hemodiafiltration as Renal Replacement Therapy. Blood Purif. 2020;49:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Ronco C, Ghezzi PM, Brendolan A, Crepaldi C, La Greca G. The haemodialysis system: basic mechanisms of water and solute transport in extracorporeal renal replacement therapies. Nephrol Dial Transplant. 1998;13 Suppl 6:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Ronco C, Ricci Z, Husain-Syed F. From Multiple Organ Support Therapy to Extracorporeal Organ Support in Critically Ill Patients. Blood Purif. 2019;48:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Rimmelé T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Honore PM, Jacobs R, Joannes-Boyau O, Boer W, De Waele E, Van Gorp V, De Regt J, Spapen HD. Moving from a cytotoxic to a cytokinic approach in the blood purification labyrinth: have we finally found Ariadne's thread? Mol Med. 2012;18:1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Di Carlo JV, Alexander SR. Hemofiltration for cytokine-driven illnesses: the mediator delivery hypothesis. Int J Artif Organs. 2005;28:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Peng Z, Singbartl K, Simon P, Rimmelé T, Bishop J, Clermont G, Kellum JA. Blood purification in sepsis: a new paradigm. Contrib Nephrol. 2010;165:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Lim CCW, Tan HK. An introduction to extracorporeal blood purification in critical illness. Proc Singapore Healthc. 2012;21:109-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Bonavia A, Groff A, Karamchandani K, Singbartl K. Clinical Utility of Extracorporeal Cytokine Hemoadsorption Therapy: A Literature Review. Blood Purif. 2018;46:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Honoré PM, Jacobs R, Boer W, Joannes-Boyau O, De Regt J, De Waele E, Van Gorp V, Collin V, Spapen HD. New insights regarding rationale, therapeutic target and dose of hemofiltration and hybrid therapies in septic acute kidney injury. Blood Purif. 2012;33:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Ratanarat R, Brendolan A, Piccinni P, Dan M, Salvatori G, Ricci Z, Ronco C. Pulse high-volume haemofiltration for treatment of severe sepsis: effects on hemodynamics and survival. Crit Care. 2005;9:R294-R302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Junhai Z, Beibei C, Jing Y, Li L. Effect of High-Volume Hemofiltration in Critically Ill Patients: A Systematic Review and Meta-Analysis. Med Sci Monit. 2019;25:3964-3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Clark E, Molnar AO, Joannes-Boyau O, Honoré PM, Sikora L, Bagshaw SM. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care. 2014;18:R7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Lehner GF, Wiedermann CJ, Joannidis M. High-volume hemofiltration in critically ill patients: a systematic review and meta-analysis. Minerva Anestesiol. 2014;80:595-609. [PubMed] |
| 47. | Monard C, Rimmelé T, Ronco C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019;47 Suppl 3:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 48. | Quenot JP, Binquet C, Vinsonneau C, Barbar SD, Vinault S, Deckert V, Lemaire S, Hassain AA, Bruyère R, Souweine B, Lagrost L, Adrie C. Very high volume hemofiltration with the Cascade system in septic shock patients. Intensive Care Med. 2015;41:2111-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Zhang L, Feng Y, Fu P. Blood purification for sepsis: an overview. Precis Clin Med. 2021;4:45-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Ronco C, Brendolan A, Lonnemann G, Bellomo R, Piccinni P, Digito A, Dan M, Irone M, La Greca G, Inguaggiato P, Maggiore U, De Nitti C, Wratten ML, Ricci Z, Tetta C. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30:1250-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Hassan J, Cader RA, Kong NC, Mohd M, Rahman AR, Hod R. Coupled Plasma Filtration Adsorption (CPFA) plus Continuous Veno-Venous Haemofiltration (CVVH) versus CVVH alone as an adjunctive therapy in the treatment of sepsis. EXCLI J. 2013;12:681-692. [PubMed] |
| 52. | Friesecke S, Träger K, Schittek GA, Molnar Z, Bach F, Kogelmann K, Bogdanski R, Weyland A, Nierhaus A, Nestler F, Olboeter D, Tomescu D, Jacob D, Haake H, Grigoryev E, Nitsch M, Baumann A, Quintel M, Schott M, Kielstein JT, Meier-Hellmann A, Born F, Schumacher U, Singer M, Kellum J, Brunkhorst FM. International registry on the use of the CytoSorb® adsorber in ICU patients : Study protocol and preliminary results. Med Klin Intensivmed Notfmed. 2019;114:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Mehta Y, Mehta C, Kumar A, George JV, Gupta A, Nanda S, Kochhar G, Raizada A. Experience with hemoadsorption (CytoSorb®) in the management of septic shock patients. World J Crit Care Med. 2020;9:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | CytoSorb®. The Adsorber-CytoSorbents Europe GmbH [Internet]. Available from: https://CytoSorb-therapy.com/en/the-adsorber/. |
| 55. | Ankawi G, Xie Y, Yang B, Xie P, Ronco C. What Have We Learned about the Use of Cytosorb Adsorption Columns? Blood Purif. 2019;48:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 56. | Poli EC, Alberio L, Bauer-Doerries A, Marcucci C, Roumy A, Kirsch M, De Stefano E, Liaudet L, Schneider AG. Cytokine clearance with CytoSorb® during cardiac surgery: a pilot randomized controlled trial. Crit Care. 2019;23:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 57. | Bernardi MH, Rinoesl H, Dragosits K, Ristl R, Hoffelner F, Opfermann P, Lamm C, Preißing F, Wiedemann D, Hiesmayr MJ, Spittler A. Effect of hemoadsorption during cardiopulmonary bypass surgery - a blinded, randomized, controlled pilot study using a novel adsorbent. Crit Care. 2016;20:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 58. | Calabrò MG, Febres D, Recca G, Lembo R, Fominskiy E, Scandroglio AM, Zangrillo A, Pappalardo F. Blood Purification With CytoSorb in Critically Ill Patients: Single-Center Preliminary Experience. Artif Organs. 2019;43:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 59. | Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 2019;23:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 60. | Brouwer WP, Duran S, Ince C. Improved Survival beyond 28 Days up to 1 Year after CytoSorb Treatment for Refractory Septic Shock: A Propensity-Weighted Retrospective Survival Analysis. Blood Purif. 2021;50:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Rugg C, Klose R, Hornung R, Innerhofer N, Bachler M, Schmid S, Fries D, Ströhle M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective 'Genetic' Matched Analysis. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 62. | Friesecke S, Stecher SS, Gross S, Felix SB, Nierhaus A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs. 2017;20:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 63. | Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 64. | Mehta Y, Dixit SB, Zirpe K, Sud R, Gopal PB, Koul PA, Mishra VK, Ansari AS, Chamle VS. Therapeutic Approaches in Modulating the Inflammatory and Immunological Response in Patients With Sepsis, Acute Respiratory Distress Syndrome, and Pancreatitis: An Expert Opinion Review. Cureus. 2021;13:e18393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Singh YP, Chhabra SC, Lashkari K, Taneja A, Garg A, Chandra A, Chhabra M, Singh GP, Jain S. Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb(®)) in the management of septic shock: A retrospective observational study. Int J Artif Organs. 2020;43:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Hetz H, Berger R, Recknagel P, Steltzer H. Septic shock secondary to β-hemolytic streptococcus-induced necrotizing fasciitis treated with a novel cytokine adsorption therapy. Int J Artif Organs. 2014;37:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 68. | Paul R, Sathe P, Kumar S, Prasad S, Aleem M, Sakhalvalkar P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb(®)) in patients with sepsis and septic shock. World J Crit Care Med. 2021;10:22-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 69. | Akil A, Ziegeler S, Reichelt J, Rehers S, Abdalla O, Semik M, Fischer S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac Cardiovasc Surg. 2021;69:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Alharthy A, Faqihi F, Memish ZA, Balhamar A, Nasim N, Shahzad A, Tamim H, Alqahtani SA, Brindley PG, Karakitsos D. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artif Organs. 2021;45:E101-E112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 71. | Nassiri AA, Hakemi MS, Miri MM, Shahrami R, Koomleh AA, Sabaghian T. Blood purification with CytoSorb in critically ill COVID-19 patients: A case series of 26 patients. Artif Organs. 2021;45:1338-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Paisey C, Patvardhan C, Mackay M, Vuylsteke A, Bhagra SK. Continuous hemadsorption with cytokine adsorber for severe COVID-19: A case series of 15 patients. Int J Artif Organs. 2021;44:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Song T, Hayanga J, Durham L, Garrison L, McCarthy P, Barksdale A, Smith D, Bartlett R, Jaros M, Nelson P, Molnar Z, Deliargyris E, Moazami N. CytoSorb Therapy in COVID-19 (CTC) Patients Requiring Extracorporeal Membrane Oxygenation: A Multicenter, Retrospective Registry. Front Med (Lausanne). 2021;8:773461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Wendel Garcia PD, Hilty MP, Held U, Kleinert EM, Maggiorini M. Cytokine adsorption in severe, refractory septic shock. Intensive Care Med. 2021;47:1334-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 75. | Scharf C, Schroeder I, Paal M, Winkels M, Irlbeck M, Zoller M, Liebchen U. Can the cytokine adsorber CytoSorb(®) help to mitigate cytokine storm and reduce mortality in critically ill patients? Ann Intensive Care. 2021;11:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 76. | De Wolf AM, Van den Berg BW, Hoffman HJ, Van Zundert AA. Pulmonary dysfunction during one-lung ventilation caused by HLA-specific antibodies against leukocytes. Anesth Analg. 1987;66:463-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Kogelmann K, Hübner T, Schwameis F, Drüner M, Scheller M, Jarczak D. First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Singh A, Mehta Y, Trehan N. Bilirubin Removal Using CytoSorb Filter in a Cardiac Surgical Patient. J Cardiothorac Vasc Anesth. 2019;33:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Sazonov V, Abylkassov R, Tobylbayeva Z, Saparov A, Mironova O, Poddighe D. Case Series: Efficacy and Safety of Hemoadsorption With HA-330 Adsorber in Septic Pediatric Patients With Cancer. Front Pediatr. 2021;9:672260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Schädler D, Porzelius C, Jörres A, Marx G, Meier-Hellmann A, Putensen C, Quintel M, Spies C, Engel C, Weiler N, Kuhlmann M. A multicenter randomized controlled study of an extracorporeal cytokine hemoadsorption device in septic patients. Crit Care. 2013;17:P62. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Schädler D, Pausch C, Heise D, Meier-Hellmann A, Brederlau J, Weiler N, Marx G, Putensen C, Spies C, Jörres A, Quintel M, Engel C, Kellum JA, Kuhlmann MK. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS One. 2017;12:e0187015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 82. | Nierhaus A, Morales J, Wendt D, Scheier J, Gutzler D, Jarczak D, Born F, Hagl C, Deliargyris E, Mehta Y. Comparison of the CytoSorb(®) 300 mL and Jafron HA380 hemoadsorption devices: an in vitro study. Minim Invasive Ther Allied Technol. 2022;31:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Mezger M, Eitel I, Ensminger S, Pogorzalek D, Huang Z, Graf T. Sequential Use of Hemadsorption Using CytoSorb® and Biosky® Filter-Technology in A COVID-19 Patient Suffering from Severe ARDS. Arch Clin Med Case Reports. 2020;04:969-977. [DOI] [Full Text] |
| 84. | Premužić V, Babel J, Gardijan D, Lapić I, Gabelica R, Ostojić Z, Lozić M, Pavliša G, Hrabak M, Knežević J, Rogić D, Mihaljević S. Extracorporeal blood purification is associated with improvement in biochemical and clinical variables in the critically-ill COVID-19 patients. Ther Apher Dial. 2022;26:316-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Russell JA. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. 2019;45:1503-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 86. | Malard B, Lambert C, Kellum JA. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med Exp. 2018;6:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 87. | CytoSorbents. Biosky MG-Series CytoSorb 300-Comparison of Products. 2020. |
| 88. | CytoSorbents. JAFRON HA-Series and CytoSorb 300. 2020. |
| 89. | Adamik B, Zielinski S, Smiechowicz J, Kübler A. Endotoxin Elimination in Patients with Septic Shock: An Observation Study. Arch Immunol Ther Exp (Warsz). 2015;63:475-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Ala-Kokko TI, Laurila J, Koskenkari J. A new endotoxin adsorber in septic shock: observational case series. Blood Purif. 2011;32:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Monti G, Bottiroli M, Pizzilli G, Minnini M, Terzi V, Vecchi I, Gesu G, Brioschi P, Vesconi S, Casella G. Endotoxin activity level and septic shock: a possible role for specific anti-endotoxin therapy? Contrib Nephrol. 2010;167:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 93. | Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018;44:2205-2212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 94. | Antonelli M, Ronco C. Polymyxin B hemoperfusion in sepsis: growing body of evidence and occasional conflicting results. Blood Purif. 2015;39:I-II. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Lipcsey M, Tenhunen J, Sjölin J, Frithiof R, Bendel S, Flaatten H, Kawati R, Kuitunen A, Tønnessen TI, Rubertsson S. Abdominal Septic Shock - Endotoxin Adsorption Treatment (ASSET) - endotoxin removal in abdominal and urogenital septic shock with the Alteco® LPS Adsorber: study protocol for a double-blinded, randomized placebo-controlled trial. Trials. 2016;17:587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Ugurov P, Popevski D, Gramosli T, Neziri D, Vuckova D, Gjorgon M, Stoicovski E, Marinkovic S, Veljanovska-Kiridjievska L, Ignevska K, Mehandziska S, Ambarkova E, Mitrev Z, Rosalia RA. Early Initiation of Extracorporeal Blood Purification Using the AN69ST (oXiris(®)) Hemofilter as a Treatment Modality for COVID-19 Patients: a Single-Centre Case Series. Braz J Cardiovasc Surg. 2022;37:35-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Baxter. Baxter obtains U. S. FDA emergency use authorization for Oxiris blood purification filter for COVID-19 treatment. 2020 Available at: FOR IMMEDIATE RELEASE (baxter.com). Available from: https://www.baxter.com/baxter-newsroom/baxter-obtains-us-fda-emergency-use-authorization-oxiris-blood-purification-filter. |
| 98. | Shum HP, Chan KC, Kwan MC, Yan WW. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med J. 2013;19:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Feri M. "In vitro comparison of the adsorption of inflammatory mediators by blood purification devices": a misleading article for clinical practice? Intensive Care Med Exp. 2019;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Rosalia RA, Ugurov P, Neziri D, Despotovska S, Kostoska E, Veljanovska-Kiridjievska L, Kuzmanov D, Trifunovski A, Popevski D, Villa G, Mitrev Z. Extracorporeal Blood Purification in Moderate and Severe COVID-19 Patients: A Prospective Cohort Study. Blood Purif. 2022;51:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Issa N, Messer J, Paganini EP. Renal assist device and treatment of sepsis-induced acute kidney injury in intensive care units. Contrib Nephrol. 2007;156:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Tumlin JA, Galphin CM, Tolwani AJ, Chan MR, Vijayan A, Finkel K, Szamosfalvi B, Dev D, DaSilva JR, Astor BC, Yevzlin AS, Humes HD; SCD Investigator Group. A Multi-Center, Randomized, Controlled, Pivotal Study to Assess the Safety and Efficacy of a Selective Cytopheretic Device in Patients with Acute Kidney Injury. PLoS One. 2015;10:e0132482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Saliba F. The Molecular Adsorbent Recirculating System (MARS) in the intensive care unit: a rescue therapy for patients with hepatic failure. Crit Care. 2006;10:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Krenn CG, Steltzer H. [Hemoadsorption for blood purification-incomparability of clinically available procedures]. Med Klin Intensivmed Notfmed. 2021;116:449-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |