Published online Jul 9, 2022. doi: 10.5492/wjccm.v11.i4.298
Peer-review started: November 30, 2021
First decision: April 19, 2022
Revised: April 25, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 9, 2022
Processing time: 218 Days and 15.2 Hours
The association between hospitalization for human respiratory syncytial virus (HRSV) bronchiolitis in early childhood and subsequent asthma is well established. The long-term prognosis for non-bronchiolitis lower respiratory tract infections (LRTI) caused by viruses different from HRSV and rhinovirus, on the other hand, has received less interest.
To investigate the relationship between infant LRTI and later asthma and examine the influence of confounding factors.
The PubMed and Global Index Medicus bibliographic databases were used to search for articles published up to October 2021 for this systematic review. We included cohort studies comparing the incidence of asthma between patients with and without LRTI at ≤ 2 years regardless of the virus responsible. The meta-analysis was performed using the random effects model. Sources of heterogeneity were assessed by stratified analyses.
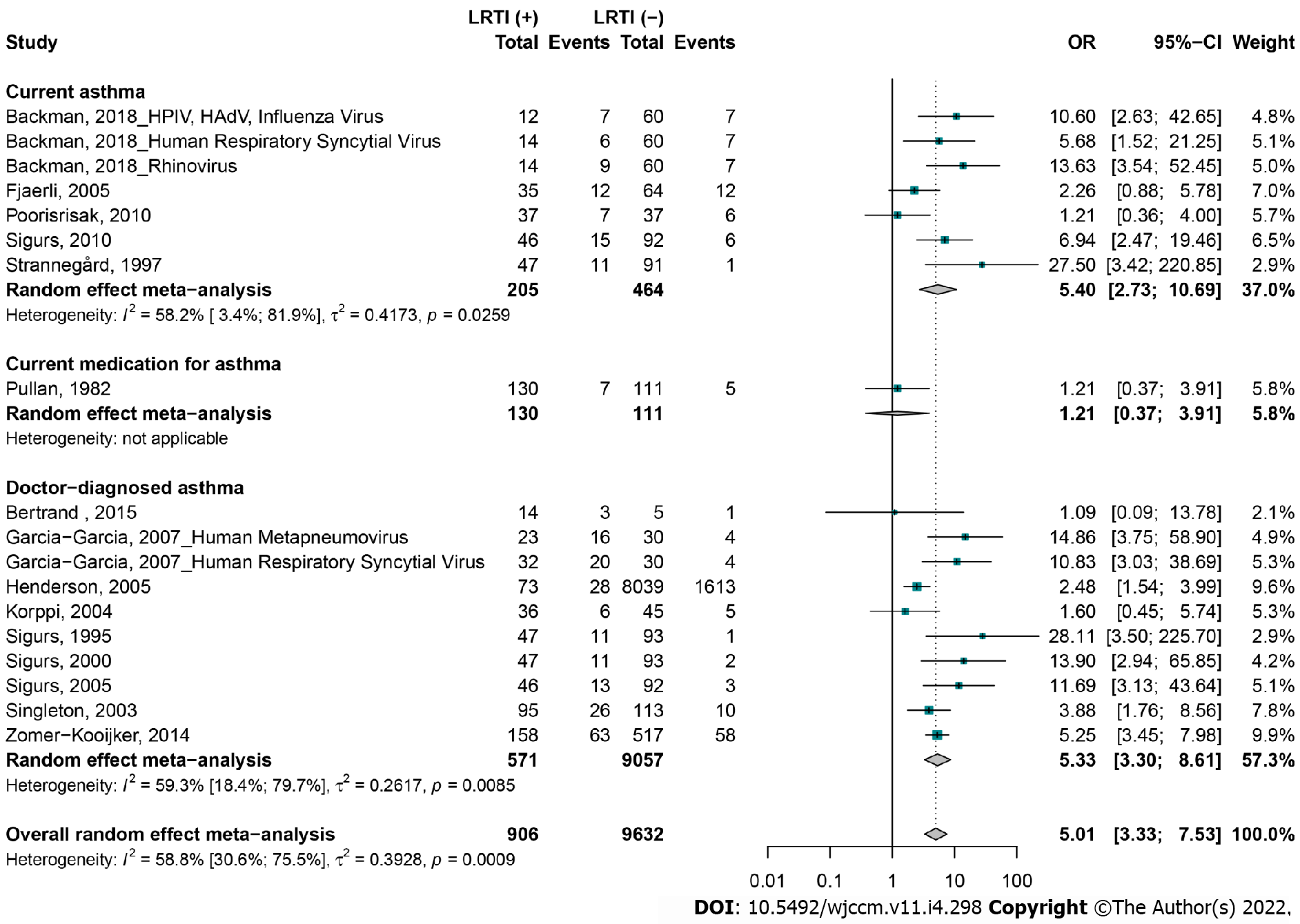
This review included 15 articles (18 unique studies) that met the inclusion criteria. LRTIs at ≤ 2 years were associated with an increased risk of subsequent asthma up to 20 years [odds ratio (OR) = 5.0, 95%CI: 3.3-7.5], with doctor-diagnosed asthma (OR = 5.3, 95%CI: 3.3-8.6), current asthma (OR = 5.4, 95%CI: 2.7-10.6), and current medication for asthma (OR = 1.2, 95%CI: 0.7-3.9). Our overall estimates were not affected by publication bias (P = 0.671), but there was significant heterogeneity [I2 = 58.8% (30.6-75.5)]. Compared to studies with hospitalized controls without LRTI, those with ambulatory controls had a significantly higher strength of association between LRTIs and subsequent asthma. The strength of the association between LRTIs and later asthma varied significantly by country and age at the time of the interview. The sensitivity analyses including only studies with similar proportions of confounding factors (gender, age at LRTI development, age at interview, gestational age, birth weight, weight, height, smoking exposure, crowding, family history of atopy, and family history of asthma) between cases and controls did not alter the overall estimates.
Regardless of the causative virus and confounding factors, viral LRTIs in children < 2 years are associated with an increased risk of developing a subsequent asthma. Parents and pediatricians should be informed of this risk.
Core Tip: The results of this meta-analysis confirmed that viral lower respiratory tract infections (LRTIs) in children < 2 years increase the risk of developing asthma later until the age of 20 years. This indicates that pediatricians and parents should be vigilant with anticipating asthma preventive measures in children with viral LRTIs in childhood.
- Citation: Kenmoe S, Atenguena Okobalemba E, Takuissu GR, Ebogo-Belobo JT, Oyono MG, Magoudjou-Pekam JN, Kame-Ngasse GI, Taya-Fokou JB, Mbongue Mikangue CA, Kenfack-Momo R, Mbaga DS, Bowo-Ngandji A, Kengne-Ndé C, Esemu SN, Njouom R, Ndip L. Association between early viral lower respiratory tract infections and subsequent asthma development. World J Crit Care Med 2022; 11(4): 298-310
- URL: https://www.wjgnet.com/2220-3141/full/v11/i4/298.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i4.298
Asthma is a major contributor to the burden of non-communicable diseases and the most common chronic respiratory disease in the world[1]. The prevalence of asthma has increased by 12.6% in 25 years (1990-2015), and asthma causes the deaths of nearly half a million people each year[1]. Asthma also represents a considerable financial burden and costs about 19 billion Euros per year in Europe[2].
Multiple factors have been involved in the development of asthma. There is evidence that respiratory viruses, particularly human respiratory syncytial virus (HRSV)[3-7], human metapneumovirus[7-12], or rhinovirus (RV)[12-22] (including mostly the recently described RV-C), were triggers for asthma and asthma exacerbation. The data also show that air pollutants were involved in the risk of developing asthma[23].
In addition, many studies have historically suggested that neonatal bronchiolitis due to HRSV, and RV recently, is a predisposing factor for asthma development later[3,5,10,24-39]. However, the involvement of other common respiratory viruses (influenza, human coronavirus, human parainfluenza virus) and non-bronchiolitis lower respiratory tract infections (LRTI) in the subsequent risk of developing asthma has not been synthesized to date.
Conflicting findings have been reported regarding the synergistic effect of early-life bronchiolitis and personal or family history of atopic sensitization or asthma, gender, maternal smoking in the onset of asthma later[6,34,40-53]. Some authors have suggested that bronchiolitis identifies children prone to developing asthma during adolescence[26,54-59]. Therefore, the causal role of early-onset bronchiolitis and the mechanisms underlying the development of subsequent asthma remain to be clarified[3,60].
Preventing or stopping the development of predictive factors would be a possible strategy for preventing asthma[61-63]. This systematic review was conducted to describe the risk of developing asthma following viral LRTI in childhood and associated factors. Our secondary objective was to evaluate the role of confounding factors of the association of neonatal LRTI and asthma during childhood using sensitivity analyses.
We registered the protocol of this systematic review in the PROSPERO with access number CRD42018116955. This review has been done in accordance with the Centre for Reviews and Dissemination guidelines[64] and presented in accordance with the PRISMA declaration[Supplemen
We included cohort studies comparing the long-term asthmatic sequelae of children with and without a history of viral LRTI in childhood. The PICOs in this study were: P, children and adults of all genders with a history of viral LRTI in childhood regardless of the virus responsible; I, LRTI at ≤ 2 years; C, children and adults of all genders with no history of viral LRTI in childhood; O, the main outcome was asthma as the long-term sequelae of LRTI in infancy. This study had no temporal, geographic, or linguistic limitations. We excluded irrelevant studies, case reports, cross-sectional studies, comments, reviews, and editorials, studies that did not report outcome of interest, articles that we did not have access to full text, studies without control groups, and studies including only high-risk subjects.
The definitions of LRTI have been adapted as described by the authors of the primary studies. Asthma has been defined by three or more episodes of bronchial obstruction. We did not take into account the differentiation of atopic asthma. In this systematic review, several categories of asthma definitions were considered, including: (1) Current doctor-diagnosed asthma; (2) Current self-reported asthma; (3) Current asthma; (4) Asthma in the last 12 mo; and (5) Asthma ever. The warning signs of asthma were considered: (1) Cough; (2) Night cough; and (3) Prolonged cough. The use of anti-asthma treatment was also taken into account: (1) Current medication for asthma; (2) Use of bronchodilators; and (3) Use of inhaled steroid. When a study had multiple defined asthma phenotypes for the same participants, we selected the phenotype according to the order of priority of asthma diagnosed by a doctor, most recent asthma, treatment for asthma, and asthma symptoms.
We searched for relevant articles in PubMed and Global Index Medicus until October 24, 2021. The search keywords are described in Supplementary Table 2. We conducted an additional manual search using Reference Citation Analysis (https://www.referencecitationanalysis.com/) by reviewing the list of references for included articles and relevant reviews on the subject.
We (JTEB and SK) have individually reviewed the titles and abstracts of the articles identified through the electronic search in the Rayyan website[66]. We evaluated the complete texts of the eligible articles after screening titles and abstracts. These two authors discussed disagreement about the inclusion or exclusion of an article to reach consent.
Two authors (JETB and SK) independently extracted all relevant data and entered into a standardized questionnaire. The disagreements were resolved by discussion between the two investigators and consultation of a third author if an agreement could not be reached (AF). The standardized questionnaire included: (1) Title; (2) First author; (3) Year of publication; (4) Time of data collection; (5) Country; (6) Participants interview period; (7) LRTI type; (8) LRTI rank; (9) LRTI period; (10) Age at LRTI; (11) Type of infection associated with the LRTI; (12) Control age; (13) Control gender; (14) Total number of cases and controls; and (15) Numbers with asthma at follow-up and numbers of confounders in case and control groups.
We (JETB and SK) independently assessed the quality of publications using the Newcastle-Ottawa scale[67]. We assessed several potential sources of bias including patient selection in the study, comparability of groups, and outcome evaluation (Supplementary Table 3). We rated the studies as “low risk of bias” and “high risk of bias” for scores of 6-9 and 0-5, respectively.
Odds ratio (OR) was used as a measure of the association between bronchiolitis potential risk factors and bronchiolitis long-term respiratory sequelae. The heterogeneity was evaluated by visual inspection of the funnel diagram, the Q test, and the I² statistic[68,69]. Heterogeneity between studies was considered significant for values of P < 0.1 and I² > 50%. The impact of the quality of the selected studies was evaluated by a sensitivity analysis omitting high risk of bias studies. Subgroup analysis was performed on the basis of the sampling approach, the countries, the age at LRTI development, the age at interview, the hospitalization status of the controls, the viruses responsible for LRTI, the type of LRTI, and the phenotype of asthma. Sensitivity analysis including only studies with the confounding factor proportions similar between cases and controls were carried out as described previously[70].
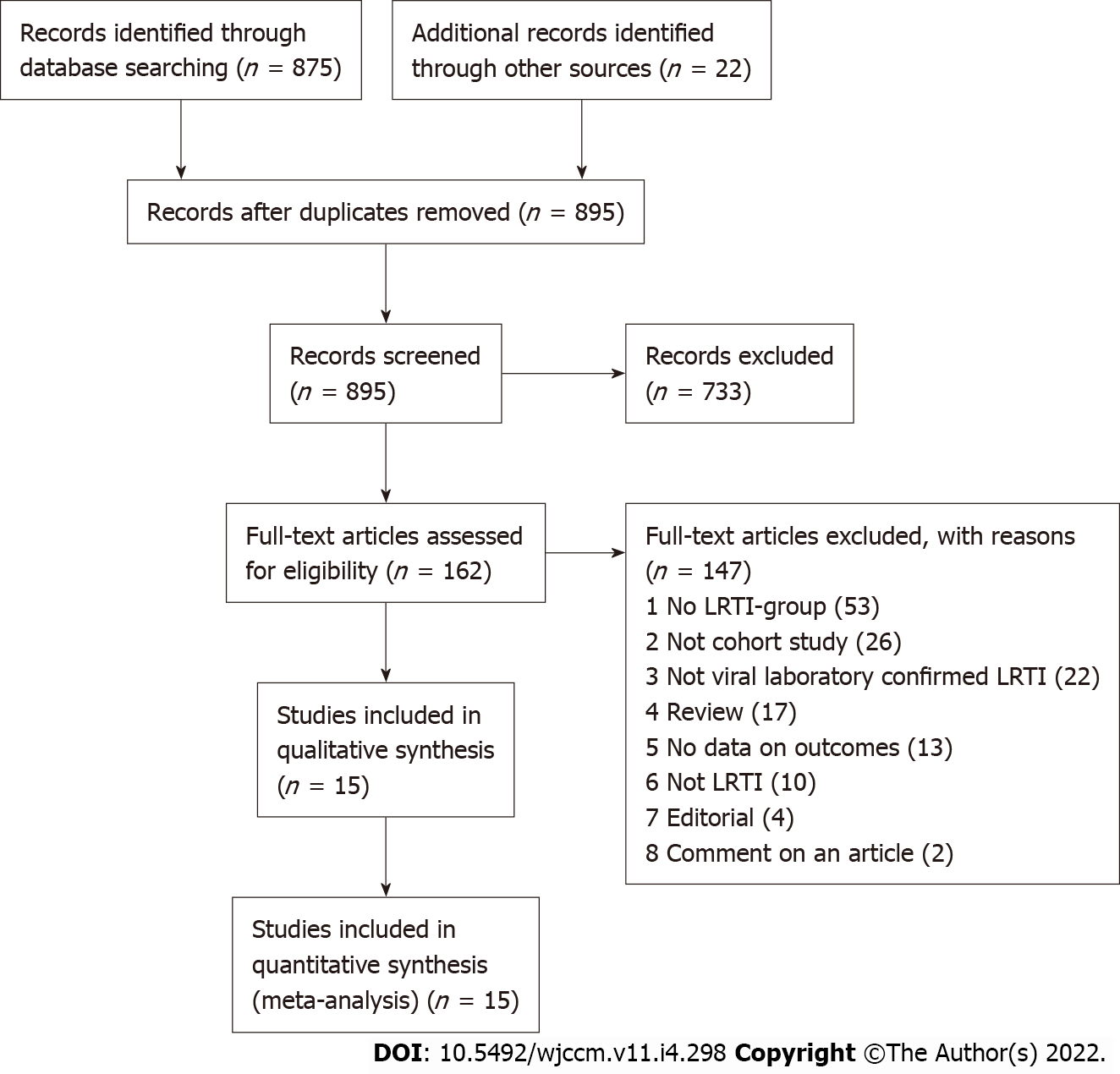
As shown in Figure 1, 875 articles were found in PubMed and Global Index Medicus. A total of 733 publications were excluded after selection according to titles and abstracts. Of the remaining 162 articles, 147 articles were eliminated for multiple reasons (no LRTI negative group, no data on asthma, wrong study design, not viral laboratory confirmed LRTI, and not LRTI, Supplementary Table 4). Based on the inclusion criteria, 15 comparative publications (18 unique studies) were finally selected for this systematic review[71-85].
The characteristics and risk of bias of the 18 unique studies are summarized in Supplementa
Compared to controls, most children in the LRTI group had subsequent asthma [OR = 5.0, 95%CI: 3.3-7.5], including doctor-diagnosed asthma (OR = 5.3, 95%CI: 3.3-8.6), current asthma (OR = 5.4, 95%CI: 2.7-10.6), and current medication for asthma (OR = 1.2, 95%CI: 0.7-3.9) (Figure 2). Sensitivity analyses including studies based on the first episode of LRTI (OR = 4.6, 95%CI: 2.6-8.1), doctor-diagnosed asthma (OR = 5.3, 95%CI: 3.3-8.6), and studies with low risk of bias (OR = 4.5, 95%CI: 2.9-7.2) showed conclusions consistent with overall analyses (Table 1). For the studies that reported confounding factors, we illustrated the definitions in Supplementary Tables 8 and 9. Qualitative confounders included gender, preterm birth, smoking exposure, crowding, family history of atopy, and family history of asthma. Quantitative confounders included age at LRTI development, age at interview, birth weight, gestational age, number of siblings, weight, and height. The association between LRTI and subsequent asthma was also maintained in all sensitivity analyses including more than two studies with confounding factor proportions similar between cases and controls, notably for male gender, weight, height, age, presence of pets in the home, family history of atopy, family history of asthma, and exposure to smoke.
| Asthma | OR (95%CI) | 95% prediction interval | Studies, n | LRTI cases, n | Controls, n | H (95%CI) | I² (95%CI) | P value, heterogeneity | P value, Egger’s test |
| Overall | 5 (3.3-7.5) | (1.2-20.3) | 18 | 906 | 9632 | 1.6 (1.2-2.0) | 58.8 (30.6-75.5) | 0.001 | 0.671 |
| Sensitivity analyses | |||||||||
| First episode of LRTI | 4.6 (2.6-8.1) | (0.8-27.1) | 11 | 725 | 9199 | 1.7 (1.3-2.4) | 67 (37.7-82.5) | 0.001 | 0.974 |
| Doctor-diagnosed asthma | 5.3 (3.3-8.6) | (1.4-19.7) | 10 | 571 | 9057 | 1.6 (1.1-2.2) | 59.3 (18.4-79.7) | 0.008 | 0.822 |
| Low risk of bias | 4.5 (2.9-7.2) | (1.1-18.2) | 14 | 732 | 1441 | 1.5 (1.1-2.0) | 54.5 (16.9-75.1) | 0.007 | 0.873 |
| Asthma in father | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Asthma in mother | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Asthma in parents | 10.6 (5.4-20.9) | (2.4-47.1) | 4 | 186 | 370 | 1 (1.0-2.6) | 0 (0-84.7) | 0.653 | 0.034 |
| Asthma in siblings | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Atopy in father | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Atopy in mother | 6.1 (4.1-8.9) | (0.5-72.6) | 3 | 213 | 577 | 1.2 (1.0-3.7) | 30.6 (0-92.8) | 0.237 | 0.358 |
| Atopy in parents | 9.1 (4.7-17.5) | (3.1-26.4) | 5 | 200 | 375 | 1.1 (1.0-2.3) | 11.2 (0-81.5) | 0.342 | 0.233 |
| Atopy in siblings | 14.9 (3.7-58.9) | NA | 1 | 23 | 30 | NA | NA | 1 | NA |
| Current allergy | 2.3 (0.9-5.8) | NA | 1 | 35 | 64 | NA | NA | 1 | NA |
| Current eczema | 2.3 (0.9-5.8) | NA | 1 | 35 | 64 | NA | NA | 1 | NA |
| Family history of asthma | 14.9 (4.9-45.4) | NA | 2 | 93 | 183 | 1 | 0 | 0.496 | NA |
| Family history of atopy | 14.9 (4.9-45.4) | NA | 2 | 93 | 183 | 1 | 0 | 0.496 | NA |
| Family smoking | 14.6 (5.9-36.2) | (0-5178.5) | 3 | 140 | 278 | 1 (1.0-3.1) | 0 (0-89.6) | 0.781 | 0.349 |
| Father smoking | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Father smoking, time of study | 1.2 (0.4-3.9) | NA | 1 | 130 | 111 | NA | NA | 1 | NA |
| Heredity for asthma | 13.9 (2.9-65.8) | NA | 1 | 47 | 93 | NA | NA | 1 | NA |
| Heredity for atopy | 13.9 (2.9-65.8) | NA | 1 | 47 | 93 | NA | NA | 1 | NA |
| History of atopic dermatitis | 1.2 (0.4-4.0) | NA | 1 | 37 | 37 | NA | NA | 1 | NA |
| Male gender | 5.3 (3.9-7.2) | (3.6-7.8) | 8 | 451 | 945 | 1.3 (1.0-2.0) | 44.3 (0-75.3) | 0.084 | 0.913 |
| Mother smoking | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Mother smoking, 10 yr before | 1.2 (0.4-3.9) | NA | 1 | 130 | 111 | NA | NA | 1 | NA |
| Parental smoking | 2.3 (0.9-5.8) | NA | 1 | 35 | 64 | NA | NA | 1 | NA |
| Pets at home | 6.5 (3.9-11.0) | (1.8-24.3) | 7 | 482 | 965 | 1.4 (1.0-2.2) | 50.8 (0-79.1) | 0.058 | 0.934 |
| Positive airway responsiveness | 1.2 (0.4-4.0) | NA | 1 | 37 | 37 | NA | NA | 1 | NA |
| Positive skin prick test | 1.2 (0.4-4.0) | NA | 1 | 37 | 37 | NA | NA | 1 | NA |
| Prematurity | 10.8 (3.0-38.7) | NA | 1 | 32 | 30 | NA | NA | 1 | NA |
| Running water | 3.9 (1.8-8.6) | NA | 1 | 95 | 113 | NA | NA | 1 | NA |
| Siblings in the house | 2.3 (0.9-5.8) | NA | 1 | 35 | 64 | NA | NA | 1 | NA |
| Single heredity for asthma | 28.1 (3.5-225.7) | NA | 1 | 47 | 93 | NA | NA | 1 | NA |
| Single heredity for atopy | 28.1 (3.5-225.7) | NA | 1 | 47 | 93 | NA | NA | 1 | NA |
| Smoke exposure | 5.1 (3.6-7.2) | (0.5-49.0) | 3 | 299 | 722 | 1 (1.0-3.1) | 0 (0-89.6) | 0.665 | 0.801 |
| Wheeze the first 5 yr of life | 1.2 (0.4-4.0) | NA | 1 | 37 | 37 | NA | NA | 1 | NA |
| Age at interview (yr) | 1.1 (0.1-13.8) | NA | 1 | 14 | 5 | NA | NA | 1 | NA |
| Age at recruitment (mo) | 12.5 (4.9-31.9) | NA | 2 | 55 | 60 | 1 | 0 | 0.741 | NA |
| Gestational age (wk) | 5.2 (3.4-8.0) | NA | 1 | 158 | 517 | NA | NA | 1 | NA |
| Height at age 6 (cm) | 5.2 (3.4-8.0) | NA | 1 | 158 | 517 | NA | NA | 1 | NA |
| Height at interview (cm) | 9.4 (4.6-19.3) | (0.1-1002.0) | 3 | 139 | 277 | 1 (1.0-3.1) | 0 (0-89.6) | 0.711 | 0.194 |
| Number of siblings | 17.9 (5.1-62.2) | NA | 2 | 94 | 186 | 1 | 0 | 0.596 | NA |
| Weight at age 6 (kg) | 5.2 (3.4-8.0) | NA | 1 | 158 | 517 | NA | NA | 1 | NA |
| Weight at interview (kg) | 14.6 (5.9-36.2) | (0-5178.5) | 3 | 140 | 278 | 1 (1.0-3.1) | 0 (0-89.6) | 0.781 | 0.349 |
The subgroup analyses are displayed in Supplementary Table 10. The strength of the association between LRTI and asthma was significantly stronger for studies with probabilistic than non-probabilistic recruitment [OR = 4.5 (3.0-6.8) vs OR = 12.5 (4.9-31.9), P = 0.048]. The strength of association between LRTI and subsequent asthma also varied significantly among countries (P < 0.001). Age at follow-up was related to the strength of the association between LRTI in childhood and the development of asthma later (P = 0.005). The association of asthma with LRTI in childhood was higher in studies with hospitalized controls (OR = 14.2, 95%CI: 6.7-30.1) compared to studies with ambulatory controls (OR = 3.9, 95%CI: 2.3-6.6) and was statistically significant (P = 0.006). Other parameters including the age of LRTI development, the virus detected in children with LRTI, the type of LRTI, and the phenotype of asthma did not significantly influence the strength of the association between LRTI and subsequent asthma.
Using visual inspection, the asymmetry distribution of the funnel graph was used to check for publication bias. We observed no publication bias by the funnel graph (Supplementary Figure 1). The P = 0.671 of the Egger regression test also indicated an absence of publication bias. We recorded a substantial heterogeneity [I2 = 58.8 (30.6-75.5)] in the overall estimates (Table 1).
We have two main results in this meta-analysis: (1) By taking into account multiple confounding factors including gender, age at LRTI development, age at interview, gestational age, birth weight, weight, height, smoking exposure, overcrowding, and family history of atopy/asthma, this meta-analysis suggests that LRTI due to several viruses in children < 2 years is significantly associated with an increased risk of asthma up to 20 years later; and (2) This increased risk of developing asthma was present regardless of the virus detected in LRTI and the type of LRTI.
Our findings are correlated with similar systematic reviews previously conducted[44,86-89]. Kneyber et al[44] reported in a quantitative analysis in 2001 the increased risk of asthma in hospitalized children for bronchiolitis episodes due to HRSV at less than 1 year compared to controls. The systematic review by Pérez-Yarza et al[88] analyzed 8 published studies from 1985 to 2006 and found a positive association between HRSV respiratory infections at less than 3 years of age and the risk of subsequent physician-diagnosed asthma development. Régnier et al[89] in 2013 showed in a review of 15 studies published from 1977 to 2012 that hospitalizations with HRSV at less than 3 years were correlated significantly with a risk of developing a parent or physician-diagnosed asthma in the 12 mo preceding follow-up. Fauroux et al[86], in a systematic review without meta-analysis conducted in 2017 on studies published between 1995 and 2015 and conducted in Western countries, also reported increased risk of developing asthma following hospitalizations due to severe HRSV LRTI registered at less than 3 years. Liu et al[87] also reported in 2017 in a review of 15 studies published between 1988 and 2017 that wheezing due to RV predisposed children at high risk of asthma later[87]. In this study, the definitions of asthma were prioritized in order of decreasing priority: doctor-diagnosed asthma vs parent-diagnosed asthma and current asthma vs asthma during the previous year vs asthma at any time.
In a review published by Edmond et al[90] in 2012, no association was observed between childhood pneumonia and the development of subsequent asthma. Most studies on the association between viral LRTIs and the subsequent development of asthma have focused primarily on bronchiolitis such as LRTI. Early studies show that HRSV infections were associated with increased risk of asthma[44,86,88,89]. In this systematic review, regardless of the virus responsible for bronchiolitis in childhood, the association remained with asthma later. The risk was higher in non-HRSV viruses and more specifically in human metapneumovirus and RV, suggesting that the development of asthma after bronchiolitis in childhood is not different depending on the type of virus detected in the LRTI. This result is consistent with the meta-analysis of Liu et al[87], who had shown that childhood RV infections predisposed to the risk of developing asthma later. The systematic review by Fauroux et al[86] found that infections with non-HRSV respiratory viruses (influenza A, human bocavirus, human parainfluenza virus-3, human adenovirus, human metapneumovirus, and unknown etiology) were associated with a higher risk of subsequent asthma than HRSV.
The attribution of the causal role of preschool or adult asthma to bronchiolitis remains a subject of debate[91]. Several other factors such as female sex, passive smoking, overweight, low weight at birth, premature birth, or family history of atopy have been proposed as factors associated with asthma at school age[24,92-97]. Breastfeeding was also reported as a protective factor against asthma as a result of bronchiolitis in childhood[58,98]. These multiple other risk factors could interact additively with bronchiolitis to promote the development of asthma[45]. This meta-analysis appropriately assessed for the first time the confounders of the relationship between bronchiolitis in childhood and asthma later. This meta-analysis revealed that bronchiolitis is independently associated with subsequent asthma.
In this systematic review, we followed a rigorous methodology according to the PRISMA guidelines and applied a very sensitive research strategy accompanied by a very intensive manual search. We carefully collected and shared the individual data from the included studies and gave the individual reasons for exclusion of all articles examined entirely. We have explored and explained almost all sources of heterogeneity. The multiple sensitivity analyses gave consistent results with the overall results.
However, some methodological weaknesses must be considered in interpreting the results of this study and in future research on the subject. First, some subgroup analyses were probably limited by the small number of studies, particularly the non-bronchiolitis and non-HRSV studies. Apart from these areas eligible for improvement, future work should focus on assessing the sequelae of non-bronchiolitis LRTI with non-HRSV etiology, particularly in low income countries (Africa and Southeast Asia) where the data suggested that asthma could be associated with a significant burden[99]. Another potential limitation of this review would be the absence of data in the included studies concerning the type of asthma observed, which could be allergic asthma or not.
In conclusion, the current meta-analysis has shown that viral LRTI at ≤ 2 years, independently of the detected virus, is a predictive factor of asthma sequelae up to the age of 20. Health care workers and parents should be aware of these findings when managing viral LRTI in childhood.
We performed a literature search in PubMed and Global Index Medicus in December 2019 using keywords covering low respiratory tract infections AND common respiratory viruses AND asthma. The results of our research depicted in original articles, narrative reviews, and systematic reviews suggesting that human respiratory syncytial virus (HRSV) and rhinovirus (RV) bronchiolitis in childhood are associated with an increased risk of asthma later. This research also identified conflicting data on the influence of confounding factors on the high risk of developing asthma after bronchiolitis in childhood. It has also emerged from this research that the involvement of lower respiratory tract infections (LRTI) other than bronchiolitis and respiratory viruses other than HRSV and RV in the subsequent risk of asthma remains hypothetical to date.
Taking into account confounding factors, the influence of respiratory infections other than bronchiolitis in childhood and respiratory viruses other than HRSV and RV should be weighed against the risk of developing subsequent asthma.
This study was conducted to assess the influence of viral LRTI at < 2 years on the risk of subsequent asthma development.
This meta-analysis included cohort studies with viral LRTI at < 2 years as exposure and asthma as outcome. R software version 4.1.0 was used to calculate the odds ratios and their 95%CI using a random-effects model.
This study included 15 articles and demonstrated the implications of childhood viral LRTI in the risk of subsequent asthma development up to the age of 20 (odds ratio = 5.0, 95%CI: 3.3-7.5). This risk of developing asthma was not influenced in sensitivity analyses including only confounding factors with similar proportions between exposed and unexposed. The estimates were not affected by publication bias, but there was significant heterogeneity.
Childhood viral LRTIs, primarily HRSV bronchiolitis, are significantly associated with a risk of developing asthma later in life.
To curb the heavy burden of asthma in patients of all ages, we hope that the results of this review will encourage the implementation of a sensitization program for this association of viral LRTI in childhood and the subsequent asthma risk. Interventional studies are needed to involve the causality relationship between neonatal viral LRTI and the subsequent risk of asthma.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: Cameroon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alberca RW, Brazil S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
| 1. | GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1626] [Cited by in RCA: 1628] [Article Influence: 203.5] [Reference Citation Analysis (0)] |
| 2. | Domínguez-Ortega J, Phillips-Anglés E, Barranco P, Quirce S. Cost-effectiveness of asthma therapy: a comprehensive review. J Asthma. 2015;52:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Bartlett NW, McLean GR, Chang YS, Johnston SL. Genetics and epidemiology: asthma and infection. Curr Opin Allergy Clin Immunol. 2009;9:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Brunetti L, Colazzo D, Francavilla R, Tesse R, De Sario V, Lorè M, Armenio L. The role of pulmonary infection in pediatric asthma. Allergy Asthma Proc. 2007;28:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Busse WW, Lemanske RF Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 561] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 6. | Emuzyte R, Firantiene R, Petraityte R, Sasnauskas K. Human rhinoviruses, allergy, and asthma: a clinical approach. Medicina (Kaunas). 2009;45:839-847. [PubMed] |
| 7. | Gavala ML, Bertics PJ, Gern JE. Rhinoviruses, allergic inflammation, and asthma. Immunol Rev. 2011;242:69-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009;9:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Heymann PW, Platts-Mills TA, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J. 2005;24:S217-S222, discussion S220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010;10:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Kalina WV, Gershwin LJ. Progress in defining the role of RSV in allergy and asthma: from clinical observations to animal models. Clin Dev Immunol. 2004;11:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Kumar A, Grayson MH. The role of viruses in the development and exacerbation of atopic disease. Ann Allergy Asthma Immunol. 2009;103:181-6; quiz 186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Garcia-Garcia ML, Calvo Rey C, Del Rosal Rabes T. Pediatric Asthma and Viral Infection. Arch Bronconeumol. 2016;52:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Message SD, Johnston SL. Viruses in asthma. Br Med Bull. 2002;61:29-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Miller EK, Mackay IM. From sneeze to wheeze: what we know about rhinovirus Cs. J Clin Virol. 2013;57:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Papadopoulos NG, Kalobatsou A. Respiratory viruses in childhood asthma. Curr Opin Allergy Clin Immunol. 2007;7:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Piedimonte G. Respiratory syncytial virus and asthma: speed-dating or long-term relationship? Curr Opin Pediatr. 2013;25:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Proud D. Role of rhinovirus infections in asthma. Asian Pac J Allergy Immunol. 2011;29:201-208. [PubMed] |
| 19. | Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J. 2015;45:774-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Singh AM, Moore PE, Gern JE, Lemanske RF Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Song DJ. Rhinovirus and childhood asthma: an update. Korean J Pediatr. 2016;59:432-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Arruda LK, Solé D, Baena-Cagnani CE, Naspitz CK. Risk factors for asthma and atopy. Curr Opin Allergy Clin Immunol. 2005;5:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Goksör E, Amark M, Alm B, Gustafsson PM, Wennergren G. Asthma symptoms in early childhood--what happens then? Acta Paediatr. 2006;95:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Henry RL, Hodges IG, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child. 1983;58:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Hyvärinen M, Piippo-Savolainen E, Korhonen K, Korppi M. Teenage asthma after severe infantile bronchiolitis or pneumonia. Acta Paediatr. 2005;94:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Hyvärinen MK, Kotaniemi-Syrjänen A, Reijonen TM, Korhonen K, Korppi MO. Teenage asthma after severe early childhood wheezing: an 11-year prospective follow-up. Pediatr Pulmonol. 2005;40:316-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Jartti T, Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol. 2011;22:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Koponen P, Helminen M, Paassilta M, Luukkaala T, Korppi M. Preschool asthma after bronchiolitis in infancy. Eur Respir J. 2012;39:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Korppi M, Reijonen T, Pöysä L, Juntunen-Backman K. A 2- to 3-year outcome after bronchiolitis. Am J Dis Child. 1993;147:628-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Kotaniemi-Syrjänen A, Reijonen TM, Korhonen K, Korppi M. Wheezing requiring hospitalization in early childhood: predictive factors for asthma in a six-year follow-up. Pediatr Allergy Immunol. 2002;13:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Lauhkonen E, Koponen P, Nuolivirta K, Paassilta M, Toikka J, Korppi M. Lung function by impulse oscillometry at age 5-7 years after bronchiolitis at age 0-6 months. Pediatr Pulmonol. 2015;50:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Lemanske RF Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Piippo-Savolainen E, Korppi M. Wheezy babies--wheezy adults? Acta Paediatr. 2008;97:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Reijonen TM, Korppi M, Kuikka L, Savolainen K, Kleemola M, Mononen I, Remes K. Serum eosinophil cationic protein as a predictor of wheezing after bronchiolitis. Pediatr Pulmonol. 1997;23:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 37. | Törmänen S, Lauhkonen E, Saari A, Koponen P, Korppi M, Nuolivirta K. Excess weight in preschool children with a history of severe bronchiolitis is associated with asthma. Pediatr Pulmonol. 2015;50:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Valkonen H, Waris M, Ruohola A, Ruuskanen O, Heikkinen T. Recurrent wheezing after respiratory syncytial virus or non-respiratory syncytial virus bronchiolitis in infancy: a 3-year follow-up. Allergy. 2009;64:1359-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Wennergren G, Kristjánsson S. Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases. Eur Respir J. 2001;18:1044-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Almqvist C, Worm M, Leynaert B; working group of GA2LEN WP 2. 5 Gender. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 41. | Bont L, Aalderen WM, Kimpen JL. Long-term consequences of respiratory syncytial virus (RSV) bronchiolitis. Paediatr Respir Rev. 2000;1:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Everard ML. The relationship between respiratory syncytial virus infections and the development of wheezing and asthma in children. Curr Opin Allergy Clin Immunol. 2006;6:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Holt PG, Strickland DH, Sly PD. Virus infection and allergy in the development of asthma: what is the connection? Curr Opin Allergy Clin Immunol. 2012;12:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Kneyber MCJ, Steyerberg EW, de Groot R, Moll HA. Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatr. 2000;89:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 556] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 46. | Le Souëf PN. Gene-environmental interaction in the development of atopic asthma: new developments. Curr Opin Allergy Clin Immunol. 2009;9:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Lemanske RF. Viral infections and asthma inception. J Allergy Clin Immunol. 2004;114: 1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Lemanske RF Jr. Issues in understanding pediatric asthma: epidemiology and genetics. J Allergy Clin Immunol. 2002;109:S521-S524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109:362-367. [PubMed] |
| 50. | Puddu M, Fanos V. Respiratory syncytial virus infection and recurrent wheezing: what next? J Chemother. 2007;19 Suppl 2:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Saglani S. Viral infections and the development of asthma in children. Ther Adv Infect Dis. 2013;1:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, Murphy DD, Odio S, James HR, Patrie JT, Hunt W, O'Rourke AK, Davis MD, Steinke JW, Lu X, Kennedy J, Heymann PW. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499-1505.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 54. | Godfrey S. Bronchiolitis and asthma in infancy and early childhood. Thorax. 1996;51 Suppl 2:S60-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Mikalsen IB, Halvorsen T, Eide GE, Øymar K. Severe bronchiolitis in infancy: can asthma in adolescence be predicted? Pediatr Pulmonol. 2013;48:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Rylander E, Eriksson M, Freyschuss U. Risk factors for occasional and recurrent wheezing after RSV infection in infancy. Acta Paediatr Scand. 1988;77:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Wennergren G, Amark M, Amark K, Oskarsdóttir S, Sten G, Redfors S. Wheezing bronchitis reinvestigated at the age of 10 years. Acta Paediatr. 1997;86:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Walton RP, Johnston SL. Role of respiratory viral infections in the development of atopic conditions. Curr Opin Allergy Clin Immunol. 2008;8:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL; Palivizumab Long-Term Respiratory Outcomes Study Group. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34-42, 42.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 62. | Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões EA; C-CREW Investigators. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 63. | Edell D, Khoshoo V, Ross G, Salter K. Early ribavarin treatment of bronchiolitis: effect on long-term respiratory morbidity. Chest. 2002;122:935-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Centers for Reviews and Dissemination. CRD’s guidance for undertaking reviews in 301 healthcare: centers for reviews and dissemination. England: York Associates. |
| 65. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46994] [Article Influence: 2937.1] [Reference Citation Analysis (0)] |
| 66. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 11787] [Article Influence: 1309.7] [Reference Citation Analysis (1)] |
| 67. | Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1821] [Article Influence: 140.1] [Reference Citation Analysis (0)] |
| 68. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25685] [Article Influence: 1116.7] [Reference Citation Analysis (0)] |
| 69. | Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3226] [Cited by in RCA: 3276] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 70. | Kenmoe S, Bowo-Ngandji A, Kengne-Nde C, Ebogo-Belobo JT, Mbaga DS, Mahamat G, Demeni Emoh CP, Njouom R. Association between early viral LRTI and subsequent wheezing development, a meta-analysis and sensitivity analyses for studies comparable for confounding factors. PLoS One. 2021;16:e0249831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Backman K, Ollikainen H, Piippo-Savolainen E, Nuolivirta K, Korppi M. Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clin Exp Allergy. 2018;48:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Bertrand P, Lay MK, Piedimonte G, Brockmann PE, Palavecino CE, Hernández J, León MA, Kalergis AM, Bueno SM. Elevated IL-3 and IL-12p40 levels in the lower airway of infants with RSV-induced bronchiolitis correlate with recurrent wheezing. Cytokine. 2015;76:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Fjaerli HO, Farstad T, Rød G, Ufert GK, Gulbrandsen P, Nakstad B. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr. 2005;5:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | García-García ML, Calvo C, Casas I, Bracamonte T, Rellán A, Gozalo F, Tenorio T, Pérez-Breña P. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr Pulmonol. 2007;42:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Poorisrisak P, Halkjaer LB, Thomsen SF, Stensballe LG, Kyvik KO, Skytthe A, Schioetz PO, Bisgaard H. Causal direction between respiratory syncytial virus bronchiolitis and asthma studied in monozygotic twins. Chest. 2010;138:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed). 1982;284:1665-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 395] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 79. | Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500-505. [PubMed] |
| 80. | Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 776] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 81. | Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 82. | Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 682] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 83. | Singleton RJ, Redding GJ, Lewis TC, Martinez P, Bulkow L, Morray B, Peters H, Gove J, Jones C, Stamey D, Talkington DF, DeMain J, Bernert JT, Butler JC. Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska Native children. Pediatrics. 2003;112:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Strannegård O, Cello J, Bjarnason R, Sigurbergsson F, Sigurs N. Association between pronounced IgA response in RSV bronchiolitis and development of allergic sensitization. Pediatr Allergy Immunol. 1997;8:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Zomer-Kooijker K, van der Ent CK, Ermers MJ, Uiterwaal CS, Rovers MM, Bont LJ; RSV Corticosteroid Study Group. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9:e87162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Fauroux B, Simões EAF, Checchia PA, Paes B, Figueras-Aloy J, Manzoni P, Bont L, Carbonell-Estrany X. The Burden and Long-term Respiratory Morbidity Associated with Respiratory Syncytial Virus Infection in Early Childhood. Infect Dis Ther. 2017;6:173-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 87. | Liu L, Pan Y, Zhu Y, Song Y, Su X, Yang L, Li M. Association between rhinovirus wheezing illness and the development of childhood asthma: a meta-analysis. BMJ Open. 2017;7:e013034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Pérez-Yarza EG, Moreno A, Lázaro P, Mejías A, Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J. 2007;26:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 90. | Edmond K, Scott S, Korczak V, Ward C, Sanderson C, Theodoratou E, Clark A, Griffiths U, Rudan I, Campbell H. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS One. 2012;7:e31239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Kuehni CE, Spycher BD, Silverman M. Causal links between RSV infection and asthma: no clear answers to an old question. Am J Respir Crit Care Med. 2009;179:1079-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 93. | Goksör E, Amark M, Alm B, Gustafsson PM, Wennergren G. The impact of pre- and post-natal smoke exposure on future asthma and bronchial hyper-responsiveness. Acta Paediatr. 2007;96:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Lin HW, Lin SC. Environmental factors association between asthma and acute bronchiolitis in young children--a perspective cohort study. Eur J Pediatr. 2012;171:1645-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 900] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 96. | Sears MR, Holdaway MD, Flannery EM, Herbison GP, Silva PA. Parental and neonatal risk factors for atopy, airway hyper-responsiveness, and asthma. Arch Dis Child. 1996;75:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, Basterrechea M, Bisgaard H, Chatzi L, Corpeleijn E, Correia S, Craig LC, Devereux G, Dogaru C, Dostal M, Duchen K, Eggesbø M, van der Ent CK, Fantini MP, Forastiere F, Frey U, Gehring U, Gori D, van der Gugten AC, Hanke W, Henderson AJ, Heude B, Iñiguez C, Inskip HM, Keil T, Kelleher CC, Kogevinas M, Kreiner-Møller E, Kuehni CE, Küpers LK, Lancz K, Larsen PS, Lau S, Ludvigsson J, Mommers M, Nybo Andersen AM, Palkovicova L, Pike KC, Pizzi C, Polanska K, Porta D, Richiardi L, Roberts G, Schmidt A, Sram RJ, Sunyer J, Thijs C, Torrent M, Viljoen K, Wijga AH, Vrijheid M, Jaddoe VW, Duijts L. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 98. | Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 99. | Østergaard MS, Nantanda R, Tumwine JK, Aabenhus R. Childhood asthma in low income countries: an invisible killer? Prim Care Respir J. 2012;21:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |