Published online Jul 9, 2022. doi: 10.5492/wjccm.v11.i4.246
Peer-review started: November 6, 2021
First decision: January 12, 2022
Revised: January 17, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 9, 2022
Processing time: 242 Days and 17.4 Hours
Coronavirus disease 2019 (COVID-19) can be associated with life-threatening organ dysfunction due to septic shock, frequently requiring intensive care unit (ICU) admission, respi
To identify the proportion of severe COVID-19 patients with vasopressor support requirements, with and without hyperlactatemia, and describe their clinical outcomes and mortality.
We performed a single-center prospective cohort study. All adult patients admitted to the ICU with COVID-19 were included in the analysis and were further divided into three groups: Sepsis group, without both criteria; Vasoplegic Shock group, with persistent hypotension and vasopressor support without hyperlactatemia; and Septic Shock 3.0 group, with both criteria. COVID-19 was diagnosed using clinical and radiologic criteria with a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive RT-PCR test.
118 patients (mean age 63 years, 87% males) were included in the analysis (n = 51 Sepsis group, n = 26 Vasoplegic Shock group, and n = 41 Septic Shock 3.0 group). SOFA score at ICU admission and ICU length of stay were different between the groups (P < 0.001). Mortality was significantly higher in the Vasoplegic Shock and Septic Shock 3.0 groups when compared with the Sepsis group (P < 0.001) without a significant difference between the former two groups (P = 0.713). The log rank tests of Kaplan-Meier survival curves were also different (P = 0.007). Ventilator-free days and vasopressor-free days were different between the Sepsis vs Vasoplegic Shock and Septic Shock 3.0 groups (both P < 0.001), and similar in the last two groups (P = 0.128 and P = 0.133, respectively). Logistic regression identified the maximum dose of vasopressor therapy used (AOR 1.046; 95%CI: 1.012-1.082, P = 0.008) and serum lactate level (AOR 1.542; 95%CI: 1.055-2.255, P = 0.02) as the major explanatory variables of mortality rates (R2 0.79).
In severe COVID-19 patients, the Sepsis 3.0 criteria of septic shock may exclude approximately one third of patients with a similarly high risk of a poor outcome and mortality rate, which should be equally addressed.
Core Tip: Coronavirus disease 2019 (COVID-19) can be associated with life-threatening organ dysfunction due to septic shock, frequently requiring intensive care unit admission, respiratory and vasopressor support. Although most patients with severe COVID-19 meet the Sepsis-3.0 criteria for septic shock, it has been increasingly recognized that hyperlactatemia is frequently absent. Our data clearly show that one third of patients with Sepsis by the Sepsis 3.0 criteria present a risk of poor outcomes and a mortality rate similar to those with Septic Shock, which should be equally addressed.
- Citation: Cidade JP, Coelho L, Costa V, Morais R, Moniz P, Morais L, Fidalgo P, Tralhão A, Paulino C, Nora D, Valério B, Mendes V, Tapadinhas C, Povoa P. Septic shock 3.0 criteria application in severe COVID-19 patients: An unattended sepsis population with high mortality risk. World J Crit Care Med 2022; 11(4): 246-254
- URL: https://www.wjgnet.com/2220-3141/full/v11/i4/246.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i4.246
Coronavirus disease 2019 (COVID-19) can be associated with life-threatening organ dysfunction due to septic shock, frequently requiring intensive care unit (ICU) admission, respiratory and vasopressor support[1]. Surviving Sepsis Campaign guidelines for the management of critically ill adults with COVID-19 document a highly variable prevalence of septic shock in these patients ranging from 1 to 35%[2,3].
Clear clinical criteria of septic shock in this population are, therefore, pivotal for early recognition of patients more likely to have poor outcomes and high mortality.
Since its publication in 2016, the Sepsis 3.0 criteria for septic shock have been validated in several studies, as a superior predictor of in-hospital mortality, with an association of a greater than 40% hospital mortality rate[3-5]. Vasopressor requirement in the absence of hypovolemia and serum lactate level greater than 2 mmol/L (> 18 mg/dL) have been recommended for use as a clinical marker combination for risk stratification in patients with infection[3-6].
Although patients with severe COVID-19 frequently meet the Sepsis 3.0 criteria for septic shock, it has been increasingly recognized that, in this population, hyperlactatemia is frequently absent, even in markedly hypotensive patients requiring high doses of vasopressors. This potentially underrecognized population might still have a high illness severity and mortality risk, indicating the need for similar close clinical surveillance and prompt organ support as COVID-19 septic shock patients defined by Sepsis 3.0 criteria.
This study aimed to identify the proportion of patients with severe COVID-19 and hypotension despite adequate volume resuscitation, requiring vasopressor support to achieve a mean arterial pressure (MAP) > 65 mmHg, with and without hyperlactatemia, in the ICU, and describe their clinical outcomes and mortality rate.
A single-center prospective observational cohort study was conducted over a 9-month period between March 2020 and January 2021. Data were collected from consecutive adult patients, admitted to the ICU, using the patient’s electronic medical records, in Centro Hospitalar Lisboa Ocidental, in Lisbon, Portugal. The study was approved by the National Ethics Committee for Clinical Research (reference REC: 2020_EO_02).
Eligibility criteria included age equal to or above 18 years old and admission to an ICU with multi
Patients included in the analysis were further divided according to the presence of hyperlactatemia (lactate > 2 mmol/L) and persistent hypotension with vasopressor support, and 3 groups were identified: Sepsis group, without both criteria; Vasoplegic Shock group, with persistent hypotension with vasopressor support without hyperlactatemia; and Septic Shock 3.0 group, with both criteria.
Demographic characteristics were recorded at baseline for all patients including comorbidities, days of symptoms of SARS-CoV-2 infection and SOFA score at admission. Daily measurements of vital signs (including minimum MAP and maximum respiratory rate), ventilation variables (including minimum ratio partial pressure arterial oxygen and the fraction of inspired oxygen, time of ventilation in the prone position and duration of neuromuscular blockade), hemodynamic support (including the use of vasopressor therapy and maximum dosage of vasopressor support), renal function (including rate of replacement therapy and maximum creatinine level registered), laboratory variables (including hemoglobin, troponin I, lactate, C-reactive protein, and procalcitonin), prescribed therapies (remdesivir and dexamethasone) and outcomes (discharged alive or death in the ICU) were also collected for every admitted patient for statistical analysis.
The number of secondary infections per patient was also collected in the three groups. The association of (1) clinical suspicion of new onset infection, (2) with persistent or increased inflammatory serum biomarkers, (3) requiring antibiotic therapy, (4) in a patient with a length of ICU stay of at least 48 h were the criteria used for the definition of secondary infection. Positive microbiological cultures or microbial identification were not used as exclusion criteria for this definition.
Primary outcomes included 28-day mortality rate. As secondary outcomes, in-hospital mortality rate, ventilator-free days and vasopressor-free days at day 28 were determined.
All Gaussian distributed variables were expressed as mean and SD, and non-normally distributed variables as median [interquartile range (IQR)]. Categorical variables were expressed as numbers and percentages.
The chi-square test was used for categorical variables, and the t-test and Kruskal-Wallis test were used on continuous variables for statistical assessment of outcomes between groups. Kaplan-Meier survival curves and log-rank tests were also obtained to ascertain and compare survival between the groups.
Multiple logistic regression modeling for in-hospital mortality rate was carried out considering mini
To assess the ability of the “serum lactate level” and “maximum vasopressor therapy used” variables in predicting the primary endpoints, diagnostic performances were calculated and receiver operating characteristic (ROC) curves were constructed in order to ascertain the corresponding area under the ROC curve (AUROC).
In all the hypothesis tests, a P value less than 0.05 was considered statistically significant and the usual confidence intervals of 95% were chosen.
In total, 118 patients were included during the study period, 51 (43.2%) in the Sepsis group, 26 (22%) in the Vasoplegic Shock group, and 41 (34.8%) in the Septic Shock 3.0 group. No patient with hyperlactatemia and normal arterial blood pressure was identified. Patients’ baseline characteristics are summarized in Table 1.
| IQR | Sepsis | Vasoplegic shock | Septic shock 3.0 | Total | P |
| (n = 51) | (n = 26) | (n = 41) | (n = 118) | ||
| Age, years (mean ± SD) | 59.51 ± 13.7 | 61.9 ± 12.9 | 68.7 ± 10.6 | 63.3 ± 13.1 | 0.005 |
| Gender, males (n) | 38 | 19 | 30 | 87 | 0.986 |
| Body mass index (mean ± SD) | 27.56 ± 4.44 | 29.67 ± 6.7 | 27.9 ± 4.1 | 28.2 ± 4.9 | 0.591 |
| SOFA at admission [mean (IQR)] | 3.04 (2; 4) | 5.88 (3; 8) | 7.14 (4; 9) | 5.13 (2; 7.8) | < 0.001 |
| Mechanical ventilation (n) | 5 | 26 | 37 | 68 | < 0.001 |
| Length of mechanical ventilation, d [mean (IQR)] | 1.06 (0; 2) | 12.5 (4.75;17) | 19.3 (7.5; 28) | 9.9 (0; 17.3) | < 0.001 |
| Minimum paO2/FiO2 registered (mean ± SD) | 181.9 ± 82.1 | 104.9 ± 69.2 | 92 ± 64.5 | 133.7 ± 84.4 | < 0.001 |
| Ventilation in prone position, h [mean (IQR)] | 4.55 (3; 5.1) | 70.2 (0; 134.8) | 129.1 (0; 187.5) | 62.3 (0; 96) | < 0.001 |
| Length of neuromuscular blockade, d [mean (IQR)] | 0 (0; 0) | 6.5 (2; 9.3) | 8.3 (3; 16.5) | 4.9 (0; 8.3) | < 0.001 |
| Vasopressor support (n) | 0 | 26 | 41 | 67 | < 0.001 |
| Minimum blood pressure registered, mmHg (mean ± SD) | 60.1 ± 11.3 | 52.8 ± 8.1 | 48.7 ± 9.5 | 54.5 ± 11.2 | < 0.001 |
| Maximum dose of vasopressor therapy, µg/kg (mean ± SD) | - | 22.5 ± 18.8 | 30.5 ± 16.3 | 15.6 ± 18.9 | < 0.001 |
| Maximum serum lactate level, mg/dL (mean ± SD) | 1.64 ± 0.56 | 1.39 ± 0.35 | 3.88 ± 2.8 | 2.36 ± 2 | < 0.001 |
| Maximum serum troponin level, ng/mL [mean (IQR)] | 22.04 (6; 25) | 103.4 (17.75; 124.8) | 129.7 (40; 166.5) | 77.4 (13; 93) | < 0.001 |
| Minimum serum hemoglobin level, g/dL (mean ± SD) | 11.4 ± 2.1 | 9.3 ± 2 | 8.1 ± 1.8 | 9.8 ± 2.5 | < 0.001 |
| Maximum serum C-reactive protein, mg/dL (mean ± SD) | 18.2 ± 9.45 | 30.2 ± 9.9 | 31.9 ± 8.8 | 25.6 ± 11.3 | < 0.001 |
| Maximum serum Procalcitonin, ng/mL [mean (IQR)] | 2.29 (0.1; 0.8) | 6.65 (0.4; 5.9) | 10.4 (1.1; 12.4) | 6.23 (0.3; 5.9) | < 0.001 |
| Maximum creatinine level registered, mg/dL [mean (IQR)] | 1.68 (0.82; 1.2) | 2.66 (0.83; 2.54) | 3 (1.3; 3.8) | 2.36 (0.9; 2.8) | < 0.001 |
| Renal support therapy (n) | 4 (8%) | 7 (3%) | 20 (49%) | 31 (26%) | < 0.001 |
| Secondary infections, per patient [mean (IQR)] | 0.16 (0; 0) | 0.63 (0; 1) | 1.1 (0; 1.5) | 0.55 (0; 1) | < 0.001 |
| Remdesivir (n, %) | 22 (43%) | 13 (50%) | 20 (49%) | 55 (47%) | 0.8 |
| Corticosteroid therapy (n) | 14 (27%) | 4 (15%) | 20 (49%) | 38 (32%) | 0.01 |
The mean age was 63 (± 13.1) years and a statistically significant difference was observed between the three groups with an older subset of patients in the Septic Shock 3.0 group. There was no difference in gender or in patient body mass index distribution.
SOFA score at admission, respiratory support, hemodynamic support, maximum creatinine, C-reactive protein and maximum procalcitonin levels, shown in Table 1, were different between the 3 groups, but without statistical significance between the Vasoplegic Shock and Septic Shock 3.0 groups. In addition, maximum serum lactate level was not different between the Sepsis and Vasoplegic Shock groups (1.64 ± 0.56 mg/dL vs 1.39 ± 0.35 mg/dL, respectively, P = 0.134). Similarly, secondary infection rates per patient, were different between the three groups (P < 0.0001) without statistical significance between the Vasoplegic Shock and Septic Shock 3.0 groups (P = 0.041).
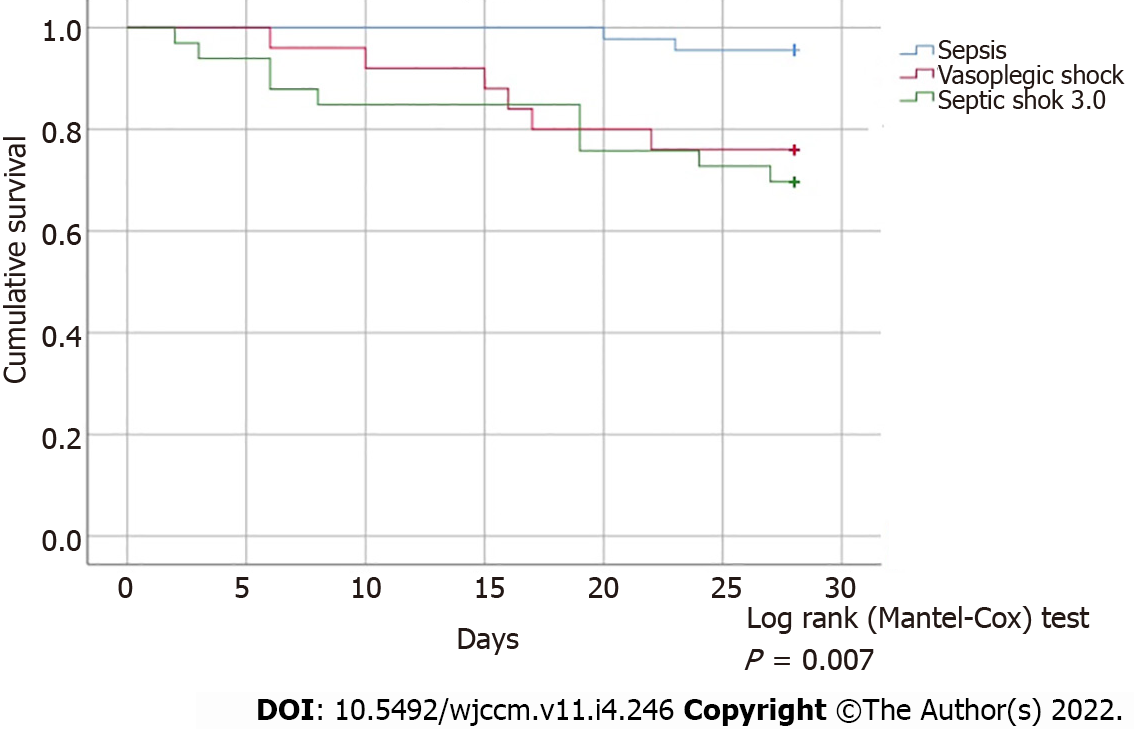
The analysis of primary outcomes revealed an overall in-hospital mortality of 23.7%. The mortality rate was significantly higher in the Vasoplegic Shock (26,9%) and Septic Shock 3.0 groups (46%) when compared to the Sepsis group (3.9%) (P = 0.026 and P = 0.0003, respectively) without statistical significance between the former two groups (P = 0.713). 28-day mortality rate was also not statistically different between the Vasoplegic Shock and Septic Shock 3.0 groups (P = 0.619) (Figure 1).
Secondary outcomes are presented in Table 2. Ventilator free-days and vasopressor free-days at day 28 were statistically different between the Sepsis group and Vasoplegic Shock (P < 0.001, in both tests) and Septic Shock 3.0 groups (P < 0.001, in both tests), without statistical differences between the last two groups (P = 0.128 and P = 0.133, respectively).
| Sepsis | Vasoplegic shock | Septic shock 3.0 | Total | P | |
| (n = 51) | (n = 26) | (n = 41) | (n = 118) | ||
| Ventilator free-days at day 28 (mean ± SD) | 25.8 ± 6.4 | 11.4 ± 9.1 | 5.17 ± 8.9 | 15.4 ± 12.3 | < 0.001 |
| Vasopressor free-days at day 28 (mean ± SD) | 26.9 ± 5.5 | 15.7 ± 10.4 | 7.76 ± 10.2 | 17.8 ± 12 | < 0.001 |
| ICU length of stay, days (mean ± SD) | 6.86 ± 5.1 | 15.9 ± 8.2 | 24.3 ± 15.1 | 14.9 ± 12.8 | < 0.001 |
| In-hospital death rate (n) | 2 | 7 | 19 | 28 | < 0.001 |
Multivariable logistic regression analysis adjusted for gender, age, and SOFA score at admission, identified the maximum dose of vasopressor therapy used (AOR 1.046; 95%CI: 1.012-1.082, P = 0.008) and serum lactate level (AOR 1.542; CI 95%: 1.055-2.255, P = 0.02) as the major explanatory variables of mortality rates (R20.79).
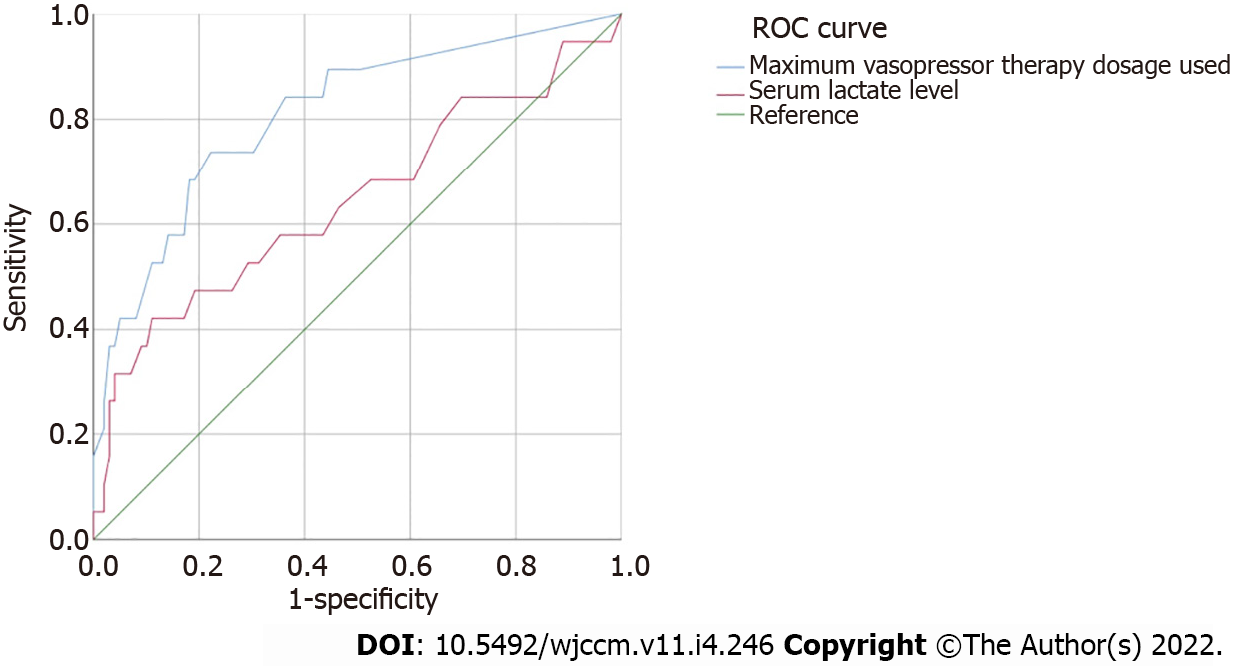
The AUROC curves for prediction of 28-day mortality rate, by serum lactate level and maximum vasopressor therapy dosage used, were constructed and are presented in Figure 2. The highest AUROC was for the maximum vasopressor therapy dosage used (0.81; 95%CI: 0.696-0.922) when compared to serum lactate level (0.645; 95%CI: 0.491-0.799).
Despite the general acceptance of the Sepsis-3 Task Force update of the defining criteria for septic shock, several lines of investigation have questioned its clinical sensitivity to reliably perform clinical decision-making and identification of patients with a high risk of complications and mortality[7-12]. This was further questioned when its criteria were preferably indicated for a coding and epidemiological application, and not intended as a clinical screening tool.
Our study clearly shows that using the Sepsis 3.0 criteria there was a proportion of hypotensive patients with vasopressor support without hyperlactatemia (n = 26; 22%), that, despite being classified as “Sepsis”, had outcomes that were clearly different to those found in that group and superimposable to those in the Septic Shock 3.0 group. This potential discriminative inaccuracy favors patients to be diagnosed with Sepsis, despite illness severity and mortality similar to Septic Shock 3.0 patients, and they should be treated equally.
Furthermore, COVID-19 patients’ mortality rates have been strongly and positively associated with ventilation and hemodynamic support, especially when critically ill and in need of ICU care[13,14], depending on reliable criteria to institute prompt and adequate organ support and improve outcomes.
Our data show that the use of hyperlactatemia as a criterion to clinically classify COVID-19 patients as having septic shock may undermine the sensitivity of our assessment of patients’ severity and prognosis in this population. This evidence is in accordance with previously published studies describing the existence of different ICU patients’ profiles, within the definition of Sepsis with concomitant different outcome and mortality rates[15,16].
The overlap in ventilator and vasopressor free-days and in-hospital mortality rate and 28-day mortality rates (Table 2), in the Vasoplegic Shock and Septic Shock 3.0 groups, provides evidence that further supports the premise of a similar illness severity between these two groups. These data might indicate that occult hypoperfusion may still be present in COVID-19 patients[17], even with normal serum lactate levels, accounting for its systemic dysfunction and compromising patients’ survivability. This was reinforced by the fact that the maximum dose of vasopressor therapy used was one of the major explanatory variables of mortality rates across the three groups when adjusted to lactate levels.
Moreover, COVID-19 patients belonging to the Septic shock 3.0 group presented with higher values of SOFA on ICU admission, a higher need for mechanical ventilation, poorer respiratory severity indices, and higher dosages of vasopressor support, when compared to patients in the Vasoplegic Shock group. However, no statistically significant differences were found between these two groups regarding these indices. These results are similar to those previously obtained by Verboom et al[18] in 2019, which demonstrated a high percentage of agreement in mortality between patients with and without hyperlactatemia, under septic shock conditions.
Our study provides evidence that the use of Sepsis 3.0 criteria can undervalue severely ill COVID-19 patients. According to their clinical requirements and prognosis, a group of patients, equally severe to Septic Shock 3.0, are being classified as having Sepsis. It is clear that it would be safer for these patients (those with persistent hypotension with vasopressor support without hyperlactatemia) to have a different classification, to account for their increased mortality risk and poor prognosis, in addition to their subsequent need for close clinical monitoring, prompt diagnosis, and adequate resuscitation. This is in concordance with significantly better accuracy of hypotension with vasopressor support when compared to hyperlactatemia, to predict the mortality rate of COVID-19 patients.
These study results are strengthened by the robust structure and data prospectively collected. Furthermore, the homogeneity of supportive care across the compared groups limits some potential biases on the analyzed outcomes. However, it is not without some limitations. Although COVID-19 pneumonia was necessary for statistical analysis eligibility, it lacked information on potential confounders of co-infections or other causes of shock, before ICU admission. On the other hand, the potential complications during ICU stay that could justify hyperlactatemia, not directly related to COVID-19 infection, were also not registered.
In severe COVID-19 patients, the Sepsis 3.0 criteria for septic shock may exclude approximately one-third of patients with a similarly high risk of poor outcomes and mortality rate, which should be equally addressed. Considering the importance of early recognition of septic shock in COVID-19 patients to improve their survival, the presence of hypotension with vasopressor support, even without hyperlactatemia, demonstrated strong prognostic accuracy for mortality.
The Sepsis 3.0 criteria for sepsis and septic shock have been extensively used in the definition of severe patients, admitted to hospital care and intensive care, in order to adequately define a subset of patients with poor prognosis and higher mortality rates.
Since its publication in 2016, its use has been presented as a good diagnostic tool to define these patients and to promptly initiate organic support. Coronavirus disease 2019 (COVID-19) patients present a strong association with life-threatening organ dysfunction due to septic shock and frequently require intensive care unit (ICU) admission and organ support.
COVID-19 patients frequently lack hyperlactatemia, a necessary clinical criteria to define septic shock using the Septic Shock 3.0 criteria. Therefore, this could potentially lead to an unrecognized subset of these patients who have a high illness severity and mortality risk, and are inaccurately classified as having sepsis.
This study aimed to identify the proportion of patients with severe COVID-19 with vasopressor requirements without hyperlactatemia and describe their clinical outcomes and mortality rate.
A single-center prospective observational cohort study was conducted in a tertiary hospital in Portugal, analyzing adult patients, admitted to the ICU, with COVID-19 pneumonia. Data collection was extensive, providing data on comorbidities, clinical status, severity indices, respiratory, hemodynamic, and renal dysfunction and the outcome of these COVID-19 patients.
Twenty-two percent of the analyzed COVID-19 patients were found to have persistent hypotension despite adequate volume resuscitation, requiring vasopressor support, and without hyperlactatemia. This "Vasoplegic Shock" group was found to have high 28-day and hospital mortality rates, and few vasopressor-free days and ventilator-free days, without significant differences to those in the "Septic Shock" group, but significantly different to those in the Sepsis group. Multivariable logistic regression identified the maximum dose of vasopressor therapy used and serum lactate level as the major explanatory variables of mortality rates. However, the highest AUROC was for the maximum vasopressor therapy dosage used when compared to serum lactate level.
The Sepsis 3.0 criteria for septic shock may exclude approximately one-third of patients with similar clinical severity, poor outcomes, and mortality rate, which should be equally addressed.
Further studies are needed to identify a subset of COVID-19 patients, who were not initially admitted to the ICU, despite persistent hypotension with vasopressor requirements, and describe their clinical course and outcomes, further demonstrating a potential need to redefine the septic shock criteria in COVID-19 patients in order to maximize early recognition and prompt adequate surveillance and support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Deshwal H, United States; Solanki SL; India S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Del Sole F, Farcomeni A, Loffredo L, Carnevale R, Menichelli D, Vicario T, Pignatelli P, Pastori D. Features of severe COVID-19: A systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 2. | Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854-887. [PubMed] |
| 3. | Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, Arabi YM, Loeb M, Ng Gong M, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Machado F, Wunsch H, Crowther M, Cecconi M, Koh Y, Burry L, Chertow DS, Szczeklik W, Belley-Cote E, Greco M, Bala M, Zarychanski R, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Nainan Myatra S, Arrington A, Kleinpell R, Citerio G, Lewis K, Bridges E, Memish ZA, Hammond N, Hayden FG, Alshahrani M, Al Duhailib Z, Martin GS, Kaplan LJ, Coopersmith CM, Antonelli M, Rhodes A. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med. 2021;49:e219-e234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 265] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 4. | Sinha S, Ray B. Sepsis-3: How useful is the new definition? J Anaesthesiol Clin Pharmacol. 2018;34:542-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Chen QH, Shao J, Liu WL, Wang HL, Liu L, Gu XH, Zheng RQ. Predictive accuracy of Sepsis-3 definitions for mortality among adult critically ill patients with suspected infection. Chin Med J (Engl). 2019;132:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Freund Y, Lemachatti N, Krastinova E, Van Laer M, Claessens YE, Avondo A, Occelli C, Feral-Pierssens AL, Truchot J, Ortega M, Carneiro B, Pernet J, Claret PG, Dami F, Bloom B, Riou B, Beaune S; French Society of Emergency Medicine Collaborators Group. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA. 2017;317:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 7. | Giamarellos-Bourboulis EJ, Tsaganos T, Tsangaris I, Lada M, Routsi C, Sinapidis D, Koupetori M, Bristianou M, Adamis G, Mandragos K, Dalekos GN, Kritselis I, Giannikopoulos G, Koutelidakis I, Pavlaki M, Antoniadou E, Vlachogiannis G, Koulouras V, Prekates A, Dimopoulos G, Koutsoukou A, Pnevmatikos I, Ioakeimidou A, Kotanidou A, Orfanos SE, Armaganidis A, Gogos C; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement in early risk identification. Clin Microbiol Infect. 2017;23:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Tusgul S, Carron PN, Yersin B, Calandra T, Dami F. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand J Trauma Resusc Emerg Med. 2017;25:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2508] [Article Influence: 278.7] [Reference Citation Analysis (0)] |
| 10. | Ranzani OT, Prina E, Menéndez R, Ceccato A, Cilloniz C, Méndez R, Gabarrus A, Barbeta E, Bassi GL, Ferrer M, Torres A. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality. A Validation and Clinical Decision-Making Study. Am J Respir Crit Care Med. 2017;196:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Sterling SA, Puskarich MA, Glass AF, Guirgis F, Jones AE. The Impact of the Sepsis-3 Septic Shock Definition on Previously Defined Septic Shock Patients. Crit Care Med. 2017;45:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Carneiro AH, Póvoa P, Gomes JA. Dear Sepsis-3, we are sorry to say that we don't like you. Rev Bras Ter Intensiva. 2017;29:4-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V, Tassara A, Cid C, Catalano HN, Agarwal A, Foroutan F, Rada G. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020;15:e0241955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 14. | Andrés M, Leon-Ramirez JM, Moreno-Perez O, Sánchez-Payá J, Gayá I, Esteban V, Ribes I, Torrus-Tendero D, González-de-la-Aleja P, Llorens P, Boix V, Gil J, Merino E; COVID19-ALC research group. Fatality and risk features for prognosis in COVID-19 according to the care approach - a retrospective cohort study. PLoS One. 2021;16:e0248869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ranzani OT, Monteiro MB, Ferreira EM, Santos SR, Machado FR, Noritomi DT; Grupo de Cuidados Críticos Amil. Reclassifying the spectrum of septic patients using lactate: severe sepsis, cryptic shock, vasoplegic shock and dysoxic shock. Rev Bras Ter Intensiva. 2013;25:270-278. [PubMed] |
| 16. | Sterling SA, Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Summers RL, Jones AE; Emergency Medicine Shock Research Network (EMSHOCKNET). Characteristics and outcomes of patients with vasoplegic versus tissue dysoxic septic shock. Shock. 2013;40:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Verboom DM, Frencken JF, Ong DSY, Horn J, van der Poll T, Bonten MJM, Cremer OL, Klein Klouwenberg PMC. Robustness of sepsis-3 criteria in critically ill patients. J Intensive Care. 2019;7:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |