Published online Jul 9, 2022. doi: 10.5492/wjccm.v11.i4.236
Peer-review started: February 3, 2022
First decision: April 13, 2022
Revised: April 16, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: July 9, 2022
Processing time: 154 Days and 2.8 Hours
Mortality is a well-established patient-important outcome in critical care studies. In contrast, morbidity is less uniformly reported (given the myriad of critical care illnesses and complications of each) but may have a common end-impact on a patient’s functional capacity and health-related quality-of-life (HRQoL). Survival with a poor quality-of-life may not be acceptable depending on individual patient values and preferences. Hence, as mortality decreases within critical care, it becomes increasingly important to measure intensive care unit (ICU) survivor HRQoL. HRQoL measurements with a preference-based scoring algorithm can be converted into health utilities on a scale anchored at 0 (representing death) and 1 (representing full health). They can be combined with survival to calculate quality-adjusted life-years (QALY), which are one of the most widely used methods of combining morbidity and mortality into a composite outcome. Although QALYs have been use for health-technology assessment decision-making, an emerging and novel role would be to inform clinical decision-making for patients, families and healthcare providers about what expected HRQoL may be during and after ICU care. Critical care randomized control trials (RCTs) have not routinely measured or reported HRQoL (until more recently), likely due to incapacity of some patients to participate in patient-reported outcome measures. Further differences in HRQoL measurement tools can lead to non-comparable values. To this end, we propose the validation of a gold-standard HRQoL tool in critical care, specifically the EQ-5D-5L. Both combined health-utility and mortality (disaggregated) and QALYs (aggregated) can be reported, with disaggregation allowing for determination of which components are the main drivers of the QALY outcome. Increased use of HRQoL, health-utility, and QALYs in critical care RCTs has the potential to: (1) Increase the likelihood of finding important effects if they exist; (2) improve research efficiency; and (3) help inform optimal management of critically ill patients allowing for decision-making about their HRQoL, in additional to traditional health-technology assessments.
Core Tip: Health-related quality-of-life and health-utility are patient-important outcome measures that rival even mortality. The purpose of the paper is to outline the steps required for wider adoption of health-related quality-of-life measures in critical care, and what benefits this measurement will yield.
- Citation: Lau VI, Johnson JA, Bagshaw SM, Rewa OG, Basmaji J, Lewis KA, Wilcox ME, Barrett K, Lamontagne F, Lauzier F, Ferguson ND, Oczkowski SJW, Fiest KM, Niven DJ, Stelfox HT, Alhazzani W, Herridge M, Fowler R, Cook DJ, Rochwerg B, Xie F. Health-related quality-of-life and health-utility reporting in critical care. World J Crit Care Med 2022; 11(4): 236-245
- URL: https://www.wjgnet.com/2220-3141/full/v11/i4/236.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i4.236
Mortality is a well-established, patient-important outcome used in critical care trials[1,2], which has many attractive features for use in clinical research. Mortality is a commonly occurring, unambiguous, dichotomous event, whose adjudication is less susceptible to bias. Unfortunately, most randomized controlled trials (RCTs) in critical care have failed to demonstrate consistent effects or improvements on mortality across a host of intensive care unit (ICU) interventions[3] potentially due to: (1) Under
Morbidity may be an intuitive alternative to mortality, but has unique challenges for research. Despite certain benefits of measuring morbidity (e.g. describes patient’s complications and potential suffering from those illnesses), it is less uniformly reported. With large variations in outcomes and complications, this results in a myriad of reported morbidity outcomes[1,2]. There is often a lack of common outcomes and standardization between studies[7], especially for different disease states and illnesses.
With carefully developed, defined, patient-centered outcomes like HRQoL and functional status, morbidity can better represent diverse illnesses and outcomes across critical care populations. Initiatives are being developed for critical care core outcome sets, which could include HRQoL[8]. It is important that we listen to our patients and their health proxies by capturing patient-centered values and self-reported HRQoL, whenever possible[9]. With a growing populace of ICU survivors, HRQoL and morbidity outcomes become increasingly important to measure and optimize in order to characterize the health states in which ICU patients survive[1]. However, we must address specific barriers and challenges to measuring HRQoL in the critical care population.
To this end, we present an overview of HRQoL, health-utility and QALYs, their specific applications, and unique challenges of its use in the critical care population. Furthermore, we present unique opportunities for HRQoL and health-utility research in the critical care population, which may include: (1) end-of-life decision-making and low-utility states, which may only be realized in critically ill patients; and (2) increased use of proxy measurements (e.g. substitute decision-makers) given that some patients may lack the capacity to participate in their reported outcomes. We present these issues not merely as responses to the technical challenges of measurement and application in critical care, but as a research imperative to paradigm shift in how we report and measure HRQoL and other patient-important outcomes in critically ill patients.
Health-related quality-of-life (HRQoL) is “an individual’s or a group’s perceived physical and mental health over time”[10]. Another definition states that HRQoL is a “multi-dimensional concept that includes domains related to physical, mental, emotional, and social functioning…[which] goes beyond direct measures of population health, life expectancy, and causes of death, and focuses on the impact health status has on quality-of-life.”
A health state can be used to describe HRQoL. Health states can be assigned preference weights and described as a health-utility value. In contrast to HRQoL, which describes one’s overall health qualitatively, a health-utility value seeks quantify HRQoL as a number, anchored to zero (representing death) to one (representing perfect health) [11]. However, health states less than zero can also be reported (e.g. “states worse than death”).
Various tools can be used ascertain HRQoL and health-utility values. These include direct methods (e.g. standard gamble, time-trade off) or indirect methods using HRQoL population-derived preference based utility scales (e.g. Health Utility Index Mark 3, Short Form-6D, EQ-5D). These health-utility scores can be leveraged to calculate quality-adjusted life-years.
The quality-adjusted life-year (QALY) is measured as a function of length of life (mortality) and time spent in a health-related quality-of-life state (morbidity), and combines the value of these attributes into a single index number[12]. Essentially, the QALY represents “time alive, scaled to reflect health state desirability…and individual values and preferences[9],” where a year in the hypothetical state of “perfect health” is worth one QALY. The QALY can be useful as a standard measure of health states across diverse treatments and settings, as it transforms different illnesses and their severity into a common physical and mental description of their health state. This allows comparisons to be made with a common denominator of QALYs[13]. For these reasons, the QALY is recommended as a measure of health outcomes for economic evaluations[2,12-16].
Despite criticisms (e.g. bias against elderly, against those with physical/mental disabilities)[13,17], QALYs remain widely used and are well-validated composite outcome measures for chronic health conditions (e.g. chronic obstructive pulmonary disease, congestive heart failure)[1,2]. QALYs can be estimated with an indirect generic preference-based health utility measure, making it patient-centered, with values and preferences for health states incorporated into its calculation[1].
For these reasons, we propose that HRQoL, health-utility and QALYs, rather than mortality alone, should be measured as an important secondary outcome in critical care research. For this incorporation to take place, critical care trialists must first measure HRQoL, which not currently routinely performed.
Critical care studies have not routinely measured HRQoL compared to mortality, likely due to: (1) The incapacitated status of patients; and (2) the time-consuming nature of certain pre-existing measurement tools.
There are two main methods of utility- or preference-based HRQoL measurement. The first are direct HRQoL measurement methods such as the standard gamble, time trade-off, visual analog scale (VAS), and discrete choice experiments[18]. Unfortunately, some of these methods are time-consuming, complex, and thus not always feasible in all studies[18].
The second group of methods are indirect HRQoL measurement tools, which utilize population-based preferences onto a health-utility scale indirectly via a generic utility-based HRQoL questionnaire[18]. These tools are derived from the general population, representing that society’s values. Commonly used generic instruments include the Short Form [SF]-36 or SF-6D[19], Health Utility Index mark 3 [HUI3][20], and the EQ-5D (Table 1)[21,22], and have been used prior in critical care studies[2].
| Utility measurement | Questionnaire description | Levels and health states | Tariff weighting acquisition | Information | Range of health-utility scores |
| European quality of life five dimensions (EQ-5D) | Five dimensions (mobility; self-care; usual activities; pain/discomfort; anxiety/depression) | 5 levels; 3125 health states | Sample of European general population (n = 3395); time trade off valuation; hypothetical scenarios | Mostly used in continental Europe and the United Kingdom | -0.59 to 1.00 |
| Short Form-36 (SF-36) | Ten physical (physical function, physical role limitations, bodily pain, general health perceptions, energy/vitality) and mental health (social functioning, emotional role limitations and mental health) dimensions | 4-6 levels; approximately 18000 health states | Sample of United Kingdom general population (n = 611); standard gamble valuation; hypothetical scenarios | Shorter versions available and applicable to SF-12 and SF-6D | 0.30 to 1.00 |
| Health utilities index mark 3 (HUI-3) | Eight dimensions (vision; hearing; speech; ambulation; dexterity; emotion; cognition; pain) | 5-6 levels; approximately 972000 health states | Representative sample of adults in Ontario, Canada (n = 504); visual analogue scale transformed into standard gamble; hypothetical scenarios | Closely related adaptation of HUI-2, with a more detailed descriptive system; mostly used in Canada | −0.36 to 1.00 |
The Short Form-36 is a proprietary, 36-item, 5-page questionnaire evaluating 10 comprehensive domains: physical functioning, physical role limitations, bodily pain, general health perceptions, energy/vitality, social functioning, emotion role limitations and mental health[19]. The SF-36 is time-consuming to complete, and some patients may have difficulty completing the entire questionnaire[23]. Although Chrispin et al[24] observed there was acceptability and reliability of the SF-36 when used in the ICU, they did not assess or formally validate the SF-36 against any other ICU-based HRQoL tools or illness severity scores. The SF-36 was used to derive the SF-6D (a utility-based instrument), using a subset of items/dimensions from the SF-36, which are occasionally used in critical care populations[25-27].
The HUI3, is an 8-item, 3-page questionnaire, which evaluates 8 domains: vision, hearing, speech, ambulation, dexterity, emotion, cognition. The HUI3 has not been widely used given the higher cost and proprietary licensing[20]. Although less cumbersome than the SF-36, both instruments require specific training to administer and complete.
The most commonly used indirect method in critical care cost-utility analyses is the EQ-5D[2,18,21]. The instrument is a 5-item, 3 or 5-level Likert scale with a built-in global health VAS for self-reporting health-utility built in its design. The 5 domains evaluated include: Mobility, self-care, usual activities, pain/discomfort, anxiety/depression[14,21,28-31]. Many of these domains are similar to Activities of Daily Living (ADLs)[1,2,28,29] and instrumental activities of daily living (IADLs)[32], which assess function within the patient-important context of how individuals live and work. These ADLs and IADLs are commonly assessed in critically ill patients[33,34], demonstrating the relevance and feasibility of using the EQ-5D in this setting. EQ-5D is shorter and easier to use than the SF-36 and HUI3, with only 5 fundamental patient-important HRQoL outcomes. In response, other HRQoL scales, such as the SF-36 have created shorter versions (e.g. SF-6D, etc.). The EQ-5D also has advantages over other HRQoL tools, including: (1) Coverage to low health-utilities, including less than zero (1); (2) no licensing fee for non-commercial use; (3) a built-in VAS for self-rating a patient’s health status; (4) a large number of versions and language translations; and (5) many country-specific population preference scoring systems to support cost-utility analyses[1,2,18].
Differences between HRQoL tools (e.g. EQ-5D vs SF) can also lead to scoring of different health-utility values for the same health state in the same patient, with each tool giving a slightly different result. The EQ-5D has been shown to have greater coverage at low health-utility states[1,2,29], which makes it a potentially useful HRQoL tool for use in critical care, as low health-utilities may be expected in this patient population as some are close to end-of-life. Despite being used in the ICU[25,26,35], there is no gold standard HRQoL measurement tool for use in the critical care setting, and none have been rigorously validated in the critically ill population[2]. At present, the EQ-5D is the most promising tool for HRQoL measurement, which merits focused evaluation in critical care.
Once measured, EQ-5D HRQoL measurements can be used in variety of ways. First, clinicians and researchers can use the EQ-5D-5L’s Likert-scale scores at face value, to determine what a patient’s health state is for the five domains[21,29-31]. This may inform the management plan for individual patients, such as referral to consulting services, such as physiotherapy or occupational therapy for physical domains, or psychiatry for mental health domains. Second, the EQ-5D HRQoL measurements can be converted into health-utility index score using a jurisdictional-specific algorithms, such as the validated time-trade off based scoring from the general Canadian population[36]. The Canadian scoring algorithm for the EQ-5D index utilizes population-based health-utility preferences which go from -0.59 to 1.0[18,36], whereby it can describe health states which patients consider to be “states worse than death”[2,18]. The index score can then be used to calculate the QALY, which is an aggregate measure of global health rating (health-utility) multiplied by the duration of time spent in that health state. The EQ-5D has become the most widely used and validated methods of combining morbidity and mortality into QALYs in medicine for a composite outcome[1,2].
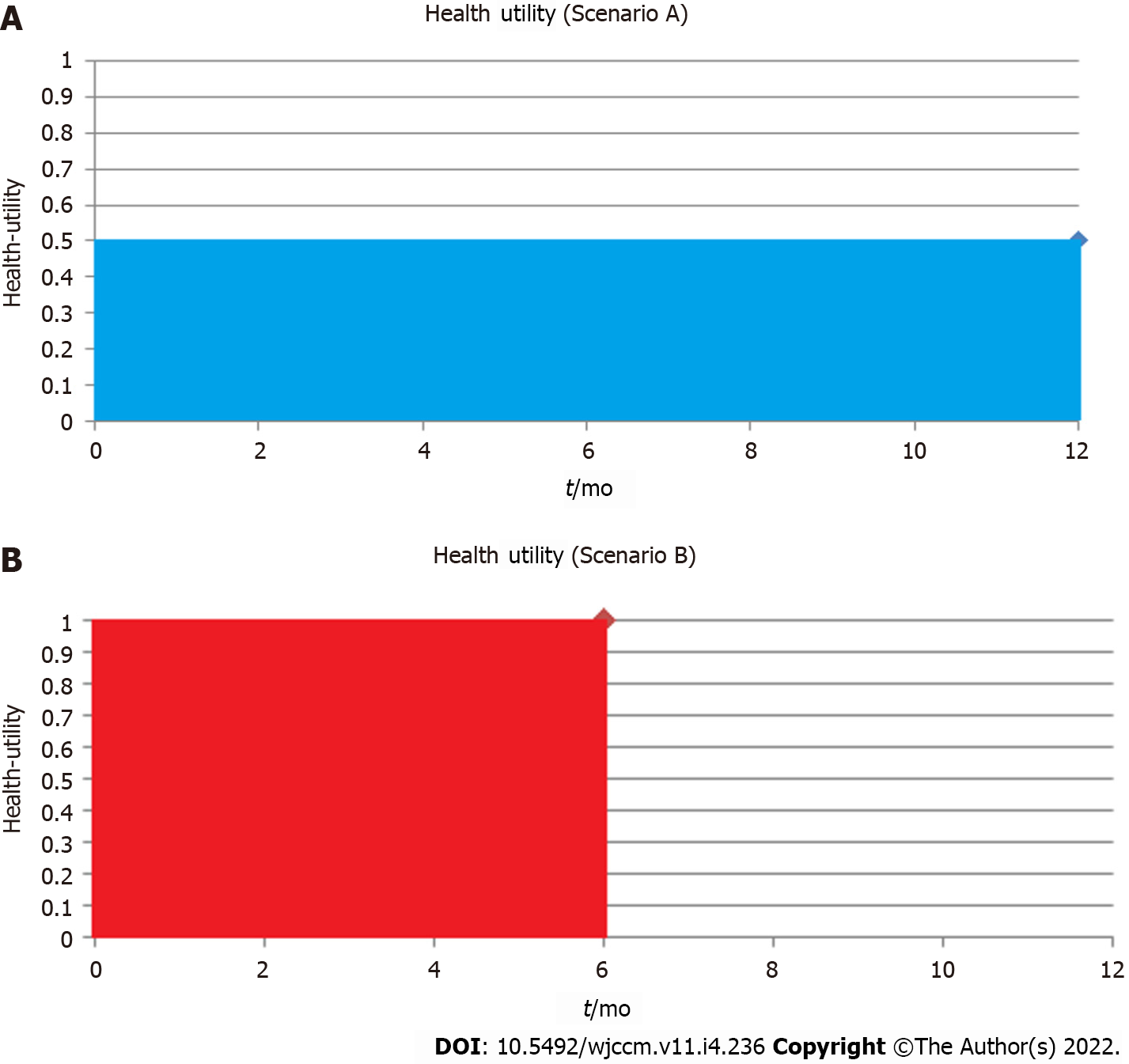
Like other composite outcomes, it is important to understand the individual component contributions of QALY including both the health-utility (morbidity) and time spent in that state (survival/mortality). For example, a study with 10 patients reported cumulative total of 5 QALYs at 1-year, this could be due to a myriad of combinations of health-utility and life-years. The effects are different if 10 patients survived to 1-year each at a health-utility of 0.5 (Figure 1A) vs a scenario where 10 patients survive only until 6-mo, but have full health (health-utility of 1) for the 6-mo prior to their deaths (Figure 1B). Both scenarios would yield a total 5 QALYs; however, each scenario may have different clinical implications to patients involved. Patients and clinical decision-makers may make different treatment choices in each scenario, in accordance with their values and preferences for quality-of-life vs duration of life.
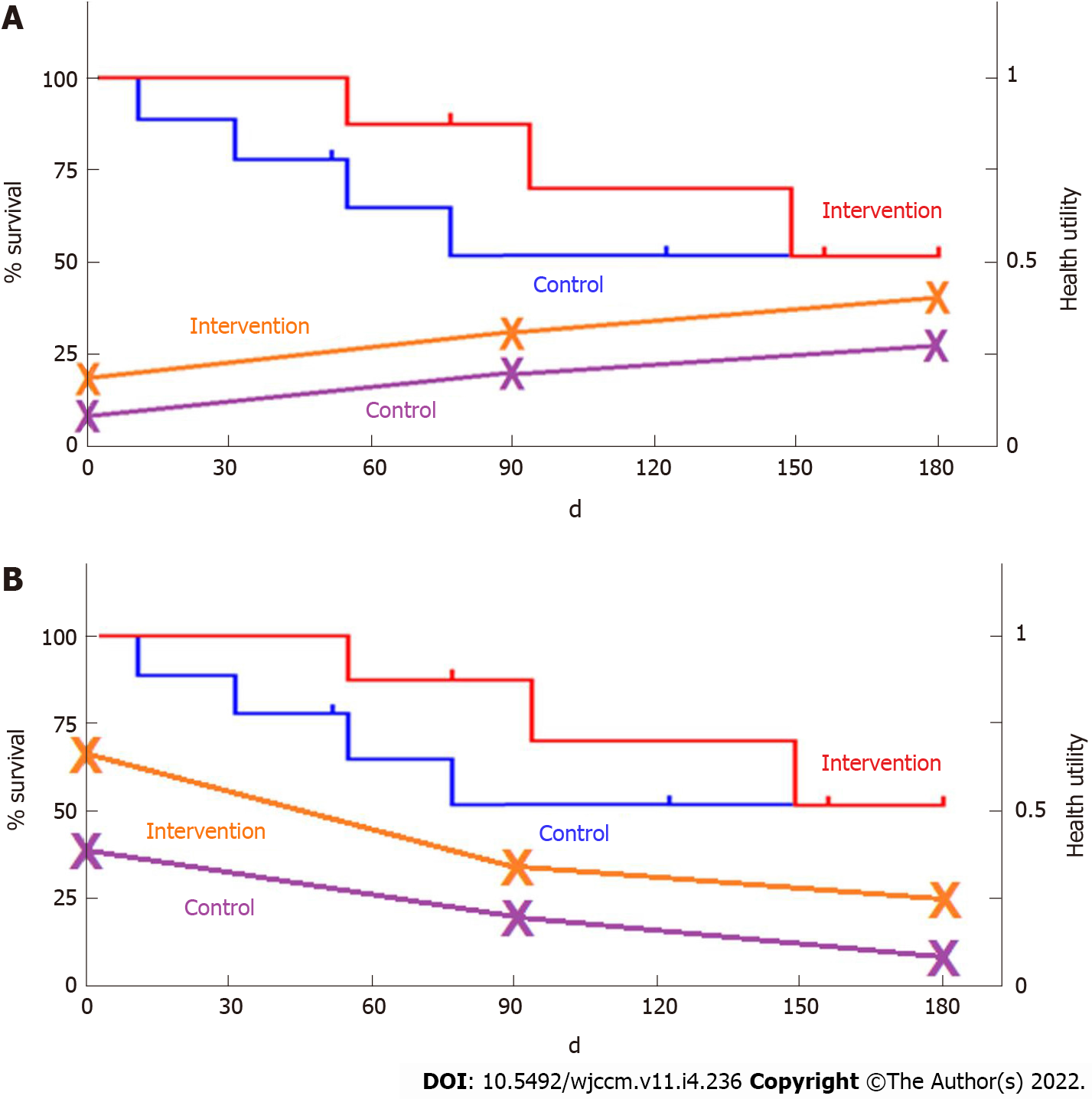
Disaggregation of QALYs into component parts of mortality and health-utility using graphical representation (can be shown on the same graph as a Kaplan-Meier curve) may be an important way to describe the specific drivers of QALYs changes (Figure 2). This novel methodology where health-utility and mortality are reported both separately and aggregated as QALYs, may further the acceptance of HRQoL, health-utility and QALYs in critical care. If healthcare providers, patients, and families are aware of what drives a particular QALY outcome difference, this may also help to inform future management plans for critically ill patients, better inform clinicians and families about the trajectory of HRQoL, and potentially impact upfront goals-of-care discussions and clinical decision-making.
Healthcare providers, patients, families, and healthcare policy-makers have demonstrated interest in survival and HRQoL before, during, and following discharge from critical care. With advances in ICU technology, our ability to sustain physiologic function of the body may minimize the effects of critical illness and treatment upon ICU survivors’ HRQoL[2,37], which could include their suffering alongside their illness. This is a very real concern, as many patients and families may choose to withdraw or defer life-sustaining ICU therapy based upon their individual values and preferences for HRQoL[2,37]. These concerns lend credence to the expression “alive and well” as a desired outcome following critical illness, as patient’s wishes and preferences for or against aggressive treatments are usually stable over time, including at end-of-life[38]. HRQoL is key to describe as a patient-important outcome. Furthermore, HRQoL can give a voice to patients as well as their families and friends as proxies.
HRQoL measurement and implementation in critical could mean: (1) An increase in the likelihood of finding important clinical effects for interventions, if they exist; (2) improve research efficiency by powering studies to QALYs rather than mortality; and (3) help inform optimal management of critically ill patients allowing for decision-making about their HRQoL, in additional to traditional health-technology assessments.
There are certain limitations to the measurement of HRQoL in the critical care population. First, there are incapacitated patients that would not be able to report their own HRQoL, emphasizing the need to validate a proxy tool (e.g. EQ-5D proxy versions) alongside the patient-reported tool. Second, proxies and patients may differ in rating or HRQoL[39]. Third, subjective vs objective HRQoL may differ (e.g. EQ-VAS score compared to EQ-5D-5L algorithm score), and could potentially be biased by a patient’s own preferences and values[39]. As compared to functional recovery scales, even though health-utility may be more patient-centric, it may also be less generalizable as they are mapped out to general population instead of just critically ill patients. Fourth, different components of HRQoL can move in different directions, making it difficult to assess the composite outcome, as different patients will value mortality and morbidity differently based on their preferences. Finally, most HRQoL measures are usually time-specific when the patient completes the questionnaire. Therefore, baseline measurements may either not be available (due to patient incapacity), or may be subject to recall bias from patients or proxies recalling past HRQoL.
There are also certain challenges associated with QALYs acceptance in general. First, QALYs in the critical care population can be skewed by mortality, presenting difficulties with analytic assumptions (e.g. parametric testing and reporting, although this can be addressed by non-parametric testing). Second, we are uncertain of the correct time-horizon to extend QALY measurements to for various critically illnesses, with longer time-horizons being affected by further lost-to-follow up and incomplete datasets. More routine HRQoL assessments at any health-related encounter could mitigate this issue (e.g. outpatient family practice, at any hospital admission). The relevant time-horizons will vary between illnesses and various patient populations, but perhaps at least a standardized set of time-horizons (e.g. in-hospital, 3 mo and 12 mo post-discharge) could be explored in critically ill patient populations. Finally, how should we measure and account for baseline imbalances in health-utility outside of a randomized control trial, and how should changes in responsiveness to treatment be anchored and reported?
Despite these challenges, there are substantial benefits to measuring HRQoL in critically ill patients. Therefore, we encourage researchers and clinicians to consider measuring HRQoL, with input from patients and proxies (e.g. surrogate decision-makers or caregivers), as some patients may never regain capacity to participate, but knowing what their values and preferences are is key to providing patient-centered care. We hope to provide the best available information (e.g. HRQoL measures, health-utility, QALYs) to decision-makers regarding HRQoL outcomes to aid both clinical decision-making alongside traditional health technology assessments.
We propose establishing a rapid, easy-to-use, broad metric, and well-validated HRQoL tool (both patient and proxy versions, which are available from EQ-5D) for use in critical care research as patient-important secondary outcome, which can be standardized across all studies allowing for comparability. We also propose reporting health-utility alongside mortality on Kaplan-Meier curves, to present a disaggregation of morbidity and mortality in addition to the aggregated quality-adjusted life-year.
Future work in this area should include: (1) Pilot validation of HRQoL patient and proxy tools in the critical care population during a cross-sectional study (approximately 50-100 patient recruitment) measuring: Pre-hospital baseline; admission; during ICU stay; and at discharge. We believe the EQ-5D could be validated in critical care (against Short-Form and correlated with other established illness severity scores), as it is the most promising tool at present; and (2) Future HRQoL validation studies for post-ICU follow-up (e.g. 3, 6, 12 mo) are required to determine long-term HRQoL outcomes. These steps will lay the foundation for feasible, reproducible, and interpretable patient-important outcome measures in critical care.
We also wish to acknowledge the support and contributions from the University of Alberta Department of Critical Care Medicine Research Office (Nadia Baig, Teresa Lawrence, Dawn Opgenorth, Lily Guan) and McMaster University’s Guidelines in Intensive Care, Development and Evaluation (GUIDE) group members (Sarah Culgin, Kate Nelson).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Gendy HA, Egypt; Gupta L, Indonesia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ferguson ND, Scales DC, Pinto R, Wilcox ME, Cook DJ, Guyatt GH, Schünemann HJ, Marshall JC, Herridge MS, Meade MO; Canadian Critical Care Trials Group. Integrating mortality and morbidity outcomes: using quality-adjusted life years in critical care trials. Am J Respir Crit Care Med. 2013;187:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Lau VI, Xie F, Basmaji J, Cook DJ, Fowler R, Kiflen M, Sirotich E, Iansavichene A, Bagshaw SM, Wilcox ME, Lamontagne F, Ferguson N, Rochwerg B. Health-Related Quality-of-Life and Cost Utility Analyses in Critical Care: A Systematic Review. Crit Care Med. 2021;49:575-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Santacruz CA, Pereira AJ, Celis E, Vincent JL. Which Multicenter Randomized Controlled Trials in Critical Care Medicine Have Shown Reduced Mortality? Crit Care Med. 2019;47:1680-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Van Cleave J, Kemper AR, Davis MM. Interpreting negative results from an underpowered clinical trial: warts and all. Arch Pediatr Adolesc Med. 2006;160:1126-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 6. | Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, Goligher EC, Jaber S, Ricard JD, Rittayamai N, Roca O, Antonelli M, Maggiore SM, Demoule A, Hodgson CL, Mercat A, Wilcox ME, Granton D, Wang D, Azoulay E, Ouanes-Besbes L, Cinnella G, Rauseo M, Carvalho C, Dessap-Mekontso A, Fraser J, Frat JP, Gomersall C, Grasselli G, Hernandez G, Jog S, Pesenti A, Riviello ED, Slutsky AS, Stapleton RD, Talmor D, Thille AW, Brochard L, Burns KEA. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 7. | Gaudry S, Messika J, Ricard JD, Guillo S, Pasquet B, Dubief E, Boukertouta T, Dreyfuss D, Tubach F. Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review. Ann Intensive Care. 2017;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Blackwood B, Marshall J, Rose L. Progress on core outcome sets for critical care research. Curr Opin Crit Care. 2015;21:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press. Available from: https://pure.york.ac.uk/portal/en/publications/methods-for-the-economic-evaluation-of-health-care-programmes(8f69bcee-cdac-44fa-871c-f821470df60a)/export.html. |
| 10. | Health-Related Quality of Life (HRQOL) | CDC. 2021. Available from: https://www.cdc.gov/hrqol/index.htm. |
| 11. | Payakachat N, Murawski MM, Summers KH. Health utility and economic analysis: theoretical and practical issues. Expert Rev Pharmacoecon Outcomes Res. 2009;9:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Neumann PJ, Cohen JT. QALYs in 2018-Advantages and Concerns. JAMA. 2018;319:2473-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Garrison LP Jr, Mansley EC, Abbott TA 3rd, Bresnahan BW, Hay JW, Smeeding J. Good research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report--Part II. Value Health. 2010;13:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Foreword | Guide to the methods of technology appraisal 2013 | Guidance | NICE. Available from: https://www.nice.org.uk/process/PMG9/chapter/Foreword. |
| 16. | CADTH. Guidelines for the Economic Evaluation of Health Technologies: Canada (4th Edition). 76.. [DOI] [Full Text] |
| 17. | Perfetto EM. ISPOR's Initiative on US Value Assessment Frameworks: A Missed Opportunity for ISPOR and Patients. Value Health. 2018;21:169-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339:b2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 20. | Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 1026] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 21. | Wailoo AJ, Hernandez-Alava M, Manca A, Mejia A, Ray J, Crawford B, Botteman M, Busschbach J. Mapping to Estimate Health-State Utility from Non-Preference-Based Outcome Measures: An ISPOR Good Practices for Outcomes Research Task Force Report. Value Health. 2017;20:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 22. | Brooks R, Boye KS, Slaap B. EQ-5D: a plea for accurate nomenclature. J Patient Rep Outcomes. 2020;4:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Andresen EM, Gravitt GW, Aydelotte ME, Podgorski CA. Limitations of the SF-36 in a sample of nursing home residents. Age Ageing. 1999;28:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Chrispin PS, Scotton H, Rogers J, Lloyd D, Ridley SA. Short Form 36 in the intensive care unit: assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia. 1997;52:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1580] [Cited by in RCA: 1625] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 26. | Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1968] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 27. | Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 28. | Stolk E, Ludwig K, Rand K, van Hout B, Ramos-Goñi JM. Overview, Update, and Lessons Learned From the International EQ-5D-5L Valuation Work: Version 2 of the EQ-5D-5L Valuation Protocol. Value Health. 2019;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 29. | McDonough CM, Grove MR, Tosteson TD, Lurie JD, Hilibrand AS, Tosteson AN. Comparison of EQ-5D, HUI, and SF-36-derived societal health state values among spine patient outcomes research trial (SPORT) participants. Qual Life Res. 2005;14:1321-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Dolan P, Roberts J. Modelling valuations for Eq-5d health states: an alternative model using differences in valuations. Med Care. 2002;40:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1091] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 32. | Guo HJ, Sapra A. Instrumental Activity of Daily Living. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2021 Available from: http://www.ncbi.nlm.nih.gov/books/NBK553126/. |
| 33. | Vest MT, Murphy TE, Araujo KL, Pisani MA. Disability in activities of daily living, depression, and quality of life among older medical ICU survivors: a prospective cohort study. Health Qual Life Outcomes. 2011;9:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Hopkins RO, Suchyta MR, Kamdar BB, Darowski E, Jackson JC, Needham DM. Instrumental Activities of Daily Living after Critical Illness: A Systematic Review. Ann Am Thorac Soc. 2017;14:1332-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 36. | Xie F, Pullenayegum E, Gaebel K, Bansback N, Bryan S, Ohinmaa A, Poissant L, Johnson JA; Canadian EQ-5D-5L Valuation Study Group. A Time Trade-off-derived Value Set of the EQ-5D-5L for Canada. Med Care. 2016;54:98-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 37. | Kyeremanteng K, Wan C, D'Egidio G, Neilipovitz D. Approach to economic analysis in critical care. J Crit Care. 2016;36:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Auriemma CL, Nguyen CA, Bronheim R, Kent S, Nadiger S, Pardo D, Halpern SD. Stability of end-of-life preferences: a systematic review of the evidence. JAMA Intern Med. 2014;174:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 39. | Flokstra-de Blok BM, Oude Elberink JN, Vlieg-Boerstra BJ, Duiverman EJ, Dubois AE. Measuring health-related quality of life: fundamental methodological issues. Clin Exp Allergy. 2009;39:1774; author reply 1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |