Published online Jul 9, 2022. doi: 10.5492/wjccm.v11.i4.228
Peer-review started: November 14, 2021
First decision: April 7, 2022
Revised: April 23, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 9, 2022
Processing time: 234 Days and 14.8 Hours
The cholinergic anti-inflammatory pathway (CAP) refers to the anti-inflammatory effects mediated by the parasympathetic nervous system. Existence of this path
Core Tip: Cholinergic anti-inflammatory pathway is novel pathway of the inflammatory reflex. Activation of this pathway can suppress maladaptive inflammatory response seen in coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS). Nicotine is a potent activator of this pathway and may offer benefits in the management of COVID-19 ARDS, via immune suppressive effects similar to dexamethasone.
- Citation: Ahmad F. Medicinal nicotine in COVID-19 acute respiratory distress syndrome, the new corticosteroid. World J Crit Care Med 2022; 11(4): 228-235
- URL: https://www.wjgnet.com/2220-3141/full/v11/i4/228.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i4.228
A dramatic inflammatory response is a common manifestation of severe coronavirus disease 2019 (COVID-19) infection[1]. The purpose of such an inflammatory surge, under normal conditions, is to allow the body to attack, constrain, and kill invading organisms. However, that same inflammatory cascade has negative downstream consequences which can cause direct damage to the host.
Sepsis is the consequence of this hyperactive immune state, most commonly due to a poorly controlled infection or significant tissue injury[2]. The unbalanced immune reaction perpetuates further injury. Neutrophils are recruited and infiltrate the lungs where they undergo apoptosis, further causing tissue damage leading to the development of shock and acute respiratory distress syndrome (ARDS)[3]. These cells and the molecules they release are a potent force designed to neutralise pathogens, but cause significant collateral damage in the process. Another casualty of this inflammatory dysregulation is vasodilatation and microvascular thrombi that lead to poor tissue perfusion, further perpetuating the cycle of destruction. This self-perpetuating cycle of tissue damage and release of pro-inflammatory cytokines[4,5] causes further dysregulation of the immune system.
Cytokine is a term given to molecules that carry out inflammatory responses of the immune system, each having their respective receptors distributed across the body. They orchestrate most, if not all, of the consequences of sepsis. This phenomenon is now dubbed a ‘cytokine storm’[6] and has been particularly devastating in the current pandemic of COVID-19 infection[7,8].
In recent years many immune modulators have been administered to mitigate sepsis and shock but with limited success in changing the disease course, morbidity, and mortality outcomes. Tocilizumab was used widely during the initial phase of the COVID-19 pandemic in ICUs across the world. But it failed to demonstrate mortality benefits[9]. The reason could partly be explained by the fact that it has a narrow scope of action, only blocking the interleukin (IL)-6 receptor. Upregulation of alternate pathways of inflammation likely are at play. A mechanism to reduce the global immune response is required to suppress collectively the molecules perpetuating inflammation. Corticosteroids are touted as one of the strongest tools in our arsenal to achieve such a goal. Dexamethasone is the only drug we have at our disposal that has shown mortality benefits during the COVID-19 pandemic[10]. Although corticosteroids are considered to globally suppress inflammation, patients are still succumbing to this coronavirus infection despite high doses administered over several days. Other medications for global suppression of inflammation are needed.
One potential pathway that may hold promise in achieving global suppression of the immune system is the cholinergic anti-inflammatory pathway (CAP). CAP is a component of the inflammatory reflex, mediated by the cholinergic nervous system and augmenting its tone has been shown to decrease inflammation in both human and animal models. The first evidence of the cholinergic system having immunomodulatory properties dates back to 1987. Zabrodskiĭ[11] showed that Armin, an irreversible acetylcholinesterase inhibitor reduces mortality in animal models of sepsis. It was first recognized in humans when patients with Rheumatoid Arthritis and drug-resistant epilepsy underwent Vagal Nerve stimulation to ameliorate their recurrent seizures. After initiation of Vagal Nerve stimulation, patients incidentally reported improvement in joint pains[12].
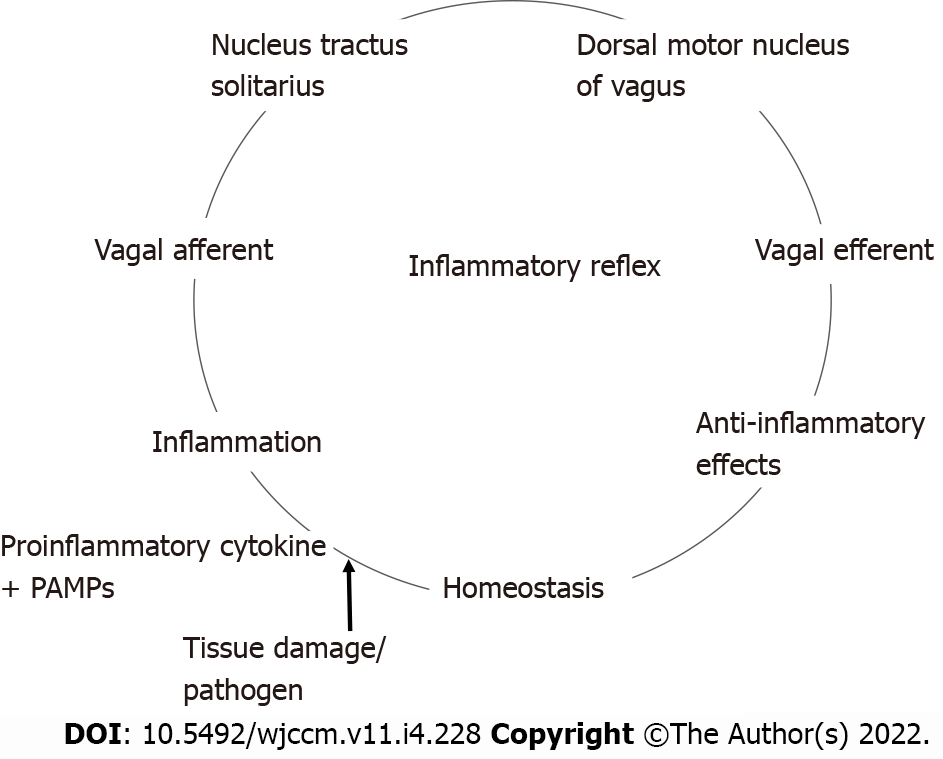
The inflammatory reflex[13] is a central nervous system mediated reflex arc that modulates the immune system. Like other prototypical reflexes, it has an incoming and outgoing arm. Instead of a sensory input that begets a motor response, this circuit senses inflammation and responds with appropriate inflammatory inhibition to reestablish homeostasis. The afferent arm is activated by the products of sterile or infectious inflammation.
The efferent arm is termed the CAP which, through diverse mechanisms, suppresses inflammation[14]. Both the afferent and efferent limbs of the reflex are transmitted predominantly by the vagus nerves. Tracey KJ team[15,16] has conducted extensive research in the potential therapeutic application of vagal stimulation in modulating the immune system, thereby providing initial major contributions to mapping this pathway (Figure 1)[17,18].
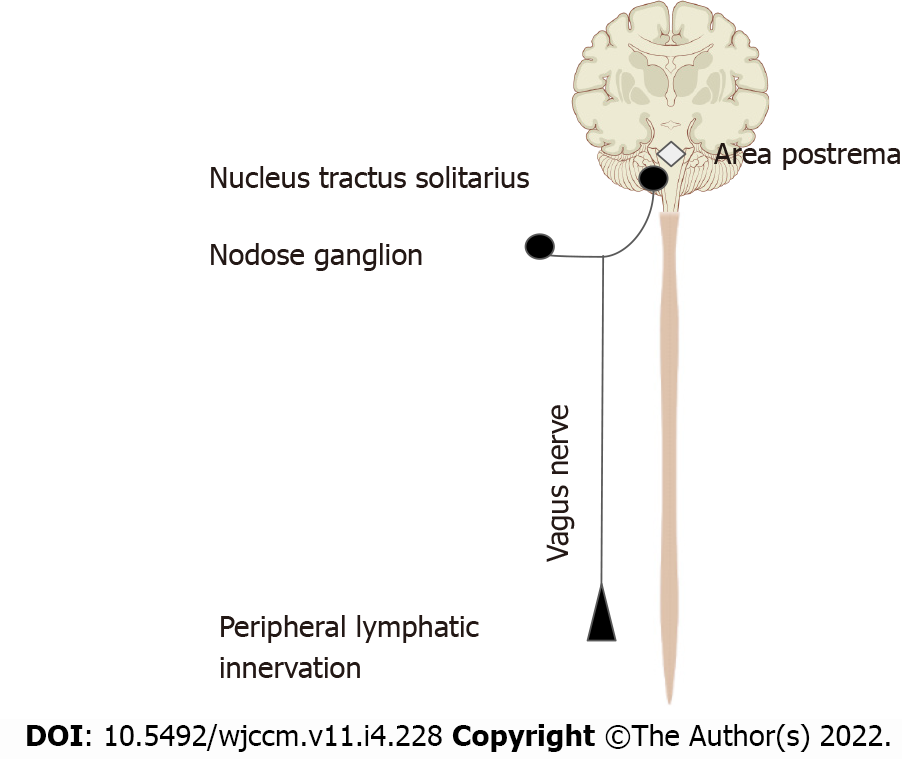
We are more familiar with the afferent limb of this pathway[19], which plays a role in triggering the mammalian febrile response. Disrupting the afferent arm, for example with a subdiaphragmatic vagotomy, prevented IL-1β induced fever in mice[20]. The afferent limb is activated by pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α) and IL-1β, neuropeptide Y and prostaglandins. Vagal fibers innervating visceral organs like the lungs and gastrointestinal tract demonstrate sensitivity to IL-1β. Furthermore, the nodose ganglion expresses Toll-like receptors[18] which are directly stimulated by pathogen associated molecular patterns such as those found on bacterial cell walls[21]. Area postrema directly expresses proinflammatory cytokine receptors[22]. The afferent limb converges on the nucleus tractus solitarius (NTS), the primary central vagal afferent nucleus. Interneurons connect the NTS to the dorsal motor nucleus of vagus (DMV), which are the primary efferent nuclei of the vagus nerve (Figure 2).
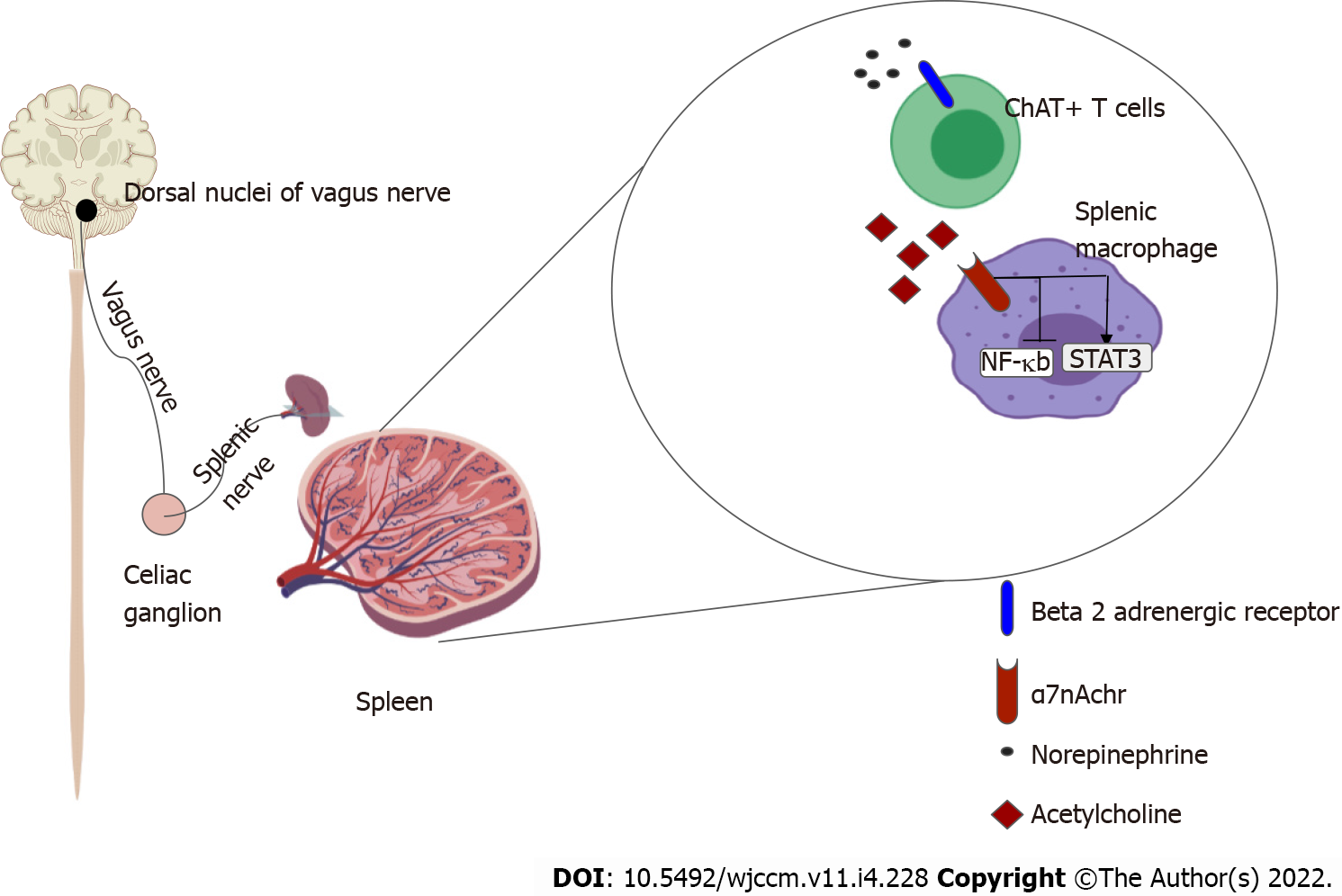
The systemic anti-inflammatory effects of CAP are thought to exert its effects via the spleen[23,24]. The efferent limb originates at the DMV, the motor nuclei of the vagus nerve. Motor signals are transmitted via cholinergic fibers down the vagus nerve to mount an anti-inflammatory response, reestablishing homeostasis. The vagus nerve does not directly innervate the spleen like it does with other visceral organs such as the heart, intestines and liver. So to realize a response from splenic lymphocytes and macrophages, the splenic nerve functions as an intermediary. The efferent pathway is as follows: Cholinergic fibers from the vagus nerve innervate the celiac ganglion; Noradrenergic neurons from the celiac ganglion, via the splenic nerve, innervate the spleen, and by releasing norepinephrine stimulate β-2 adrenergic receptors on choline-acetyltransferase positive T cells that reside in the spleen; Activation of the β-2 adrenergic receptors with norepinephrine induces the release of acetylcholine (ACh) from these splenic T cells; ACh then activates α-7 nicotinic acetylcholinergic receptor (α7nAChr) on the splenic macrophages; Activation of α7nAChr causes downstream inhibition of the NF-Kappa β pathway and subsequent suppression of pro-inflammatory cytokines. It also induces the release of anti-inflammatory molecules by activating the JAK2-STAT3 pathway[13,14].
Iatrogenic activation of the efferent limb of the inflammatory reflex, irrespective of the modality, has demonstrated anti-inflammatory effects in diverse pathological conditions[15] (Figure 3).
Augmenting the CAP offers an effective tool in controlling maladaptive inflammatory responses[25,26]. Modulating the cholinergic tone, irrespective of the modality used, has been shown to suppress inflammation[27]. Direct electrical stimulation of the vagus nerve aims to trigger an action potential that consequently activate this pathway downstream. Vagal nerve stimulation has been shown to suppress inflammation and decrease serum levels of TNF, IL-1β and IL-6[28-32]. Pharmacological modalities to increase the activity of CAP have also yielded similar results. Direct agonists of α7nAChr like the pharmacological agent nicotine have demonstrated anti-inflammatory properties[33-39]. Ongoing trials using GTS-1, a specific α7nAChr agonist, are being conducted in human models of sepsis[40,41]. Another feasible pharmacological strategy is to use inhibitors of acetylcholinesterase to delay degra
Practical modalities for bedside manipulation of CAP is limited. Vagal nerve stimulation has limited feasibility for critically ill septic patients. GTS-1, an α7nAChr agonist, is in an experimental phase acetylcholinesterase inhibitors like physostigmine increase cholinergic tone systemically and cause undesirable muscarinic side effects. That currently leaves nicotine as the only feasible and medically available potentiator of CAP as an agonist of α7nAChr. As such, it has demonstrated anti-inflammatory properties in ulcerative colitis and models of human sepsis[33,34].
Humans have been using nicotine since prehistoric times[49], mostly in the form of tobacco. Even though it is widely acknowledged that smoking or chewing tobacco is unequivocally injurious to health, nicotine by itself has not been shown to be harmful. Medicinal nicotine has demonstrated potent anti-inflammatory properties while being safe and possessing a low side-effect profile in short term administration. Nicotine administration in animal models of ARDS and sepsis have shown improved survival with lower serum inflammatory markers and reduced migration of neutrophils[36-38]. Human models of lipopolysaccharide (LPS) induced sepsis show faster resolution of sepsis[33]. Nicotine has also shown anti-inflammatory effects in patients with ulcerative colitis[34,35].
Nicotine patches are well suited as a modality for increasing nicotinic cholinergic receptor activity, and possess the following advantages: Nicotine does not have any underlying muscarinic effects and, therefore, lack concerns of increasing airway secretions that occur with acetylcholinesterase inhibitors like galantamine or physostigmine; Using a nicotine patch achieves therapeutic levels of nicotine in the blood within 4-6 h, offering a rapid drug onset profile[50]; The active drug nicotine has a short half-life of 2 h. Its metabolite, cotinine, has minimal biological activity[51]. This allows for rapid withdrawal of treatment if necessary. Most acetylcholinesterase inhibitors have a much longer half-life; The depot mechanism of drug delivery for the nicotine patch allows for a rapid onset, prolonged drug delivery during the duration of application, with a quick withdrawal time; The 24-h depot administration avoids repeated administrations and minimized nursing exposure for delivery of the medication; Ease of administration; Nicotine transdermal patches are widely used as clinical medication for nicotine replacement therapy in both the hospital and outpatient settings; There are minimal drug-drug interactions[52].
The data on the safety of nicotine on non-smoking patients in an inpatient setting is limited.
Safety data on current or former smokers receiving nicotine replacement therapy in ICU settings and hospital settings fail to demonstrate an increase in adverse events[53-58]. Potential side effects of medicinal nicotine administration are few. They may include hypertension and tachyarrhythmias. Rash at the site of the nicotine patch application has been described. Patients with end stage renal disease have a decreased rate of nicotine metabolism so the safety profile for patients on dialysis is uncertain[59,60].
The current ongoing pandemic of severe acute respiratory syndrome coronavirus 2 proves a new challenge for the medical community. Owing to the tremendous ingenuity and grit demonstrated by teams across the globe, we now have several promising vaccines which demonstrate remarkable efficacy. However, we are yet to develop a similarly promising tool for management of severe infection which is still very prevalent. Consequently, patients continue to succumb in ICUs across the world to the COVID-19 acute hypoxic respiratory failure and septic shock. Several touted treatment modalities during this pandemic have emerged only to quickly fall out of favour due to lack of documented benefit, including Hydroxychloroquine, Tocilizumab, and transfusion of convalescent plasma. Management for COVID-19 pneumonia, at present, comprises two parallel approaches. Remdesivir or other upcoming potential antivirals, to control viral replication and immunomodulators like dexamethasone to control the maladaptive immune response. Dexamethasone has shown utility in reducing mortality in patients with COVID-19 induced acute hypoxic respiratory failure. However, despite its use early in the course of the disease, many still deteriorate, requiring increased levels of oxygen support or even mechanical ventilation. Patients continue to die even with dexamethasone as part of their pharmacological regimen. Better modalities are needed to further improve patient outcomes. The hope is bringing to the attention of the medical community a fairly well studied, yet paradoxically unknown pathway of global immune modulation.
CAP is a part of a neural reflex termed the inflammatory reflex. It plays a central role in the neural control of inflammation. Inflammatory reflex has an afferent limb that senses systemic inflammation via the vagus nerve. This signal is relayed to the NTS, the sensory vagal nucleus in the central nervous system. Interneurons then communicate to the DMV, which is the primary motor nucleus of the vagus nerve. The efferent limb of the inflammatory reflex originates from the DMV via motor vagal fibers and trigger various anti-inflammatory mechanisms, reestablishing homeostasis. The systemic anti-inflammatory effects of CAP is thought to be due to suppression of pro-inflammatory cytokines from splenic macrophages. Nicotinic ACh receptors on these splenic macrophages are the point of convergence of this pathway’s systemic anti-inflammatory effect. This translates to survival benefits with lower levels of serum TNF-α, and IL-6, along with reduced migration of neutrophils in models of sepsis. The potential of augmenting this pathway to mitigate inflammation has been demonstrated in several animal and human studies.
Nicotine is a commonly used molecule that is a potent activator of α7nAChr, with demonstrated anti-inflammatory effects. Animal models of sepsis show improved survival with nicotine administration. Nicotine patch has been studied in the human model of LPS induced sepsis and demonstrated faster resolution of inflammation compared to controls. Nicotine transdermal patch has been used for decades as a means of nicotine delivery for nicotine replacement therapy in active tobacco users and has demonstrated a favorable safety profile. Thus, nicotine transdermal patch may offer a readily available tool with significant benefit-to-risk ratio in the setting of COVID-19 induced acute hypoxic respiratory failure.
With patients suffering daily across the globe with COVID ARDS, there is little downside to the administration of this relatively inexpensive, widely available medication with a high safety. There is presently a lack of literature regarding the use of nicotine in COVID-19 ARDS patients and it must be further studied first before being applied routinely.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Juneja D, India S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 434] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 2. | Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 549] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 3. | Yang SC, Tsai YF, Pan YL, Hwang TL. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed J. 2021;44:439-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, Nagarkatti M. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669-684. [PubMed] |
| 5. | Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm. 2013;2013:165974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 502] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 6. | Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1322] [Article Influence: 101.7] [Reference Citation Analysis (1)] |
| 7. | Caricchio R, Gallucci M, Dass C, Zhang X, Gallucci S, Fleece D, Bromberg M, Criner GJ; Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis. 2021;80:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 8. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2913] [Article Influence: 582.6] [Reference Citation Analysis (0)] |
| 9. | Huang E, Jordan SC. Tocilizumab for Covid-19 - The Ongoing Search for Effective Therapies. N Engl J Med. 2020;383:2387-2388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8:1170-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Zabrodskiĭ PF. [Effect of armin on nonspecific resistance factors of the body and on the primary humoral immune response]. Farmakol Toksikol. 1987;50:57-60. [PubMed] |
| 12. | Bonaz B, Picq C, Sinniger V, Mayol JF, Clarençon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | Tracey KJ. The inflammatory reflex. Nature. 2002;420:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2484] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 14. | Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 785] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 15. | Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125-134. [PubMed] |
| 16. | Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 17. | Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008-11013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 18. | Satoh T, Akira S. Toll-Like Receptor Signaling and Its Inducible Proteins. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 19. | Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 371] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Székely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am J Physiol. 1998;275:R63-R68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci. 2005;120:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140:1415-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 408] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 24. | Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623-1628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 540] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 25. | Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther. 2017;179:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 26. | Ren C, Tong YL, Li JC, Lu ZQ, Yao YM. The Protective Effect of Alpha 7 Nicotinic Acetylcholine Receptor Activation on Critical Illness and Its Mechanism. Int J Biol Sci. 2017;13:46-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Kanashiro A, Sônego F, Ferreira RG, Castanheira FV, Leite CA, Borges VF, Nascimento DC, Cólon DF, Alves-Filho JC, Ulloa L, Cunha FQ. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol Res. 2017;117:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113:8284-8289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 697] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 29. | Genovese MC, Gaylis NB, Sikes D, KivitzA, Horowitz DL, Peterfy C, Glass EV, Levine YA, Chernoff D. Safety and efficacy of neurostimulation with a miniaturised Vagus Nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: A two-stage multicentre, randomised pilot study. Lancet Rheumatol. 2020;2: E527-E538. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 2966] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 31. | Kohoutova M, Horak J, Jarkovska D, Martinkova V, Tegl V, Nalos L, Vistejnova L, Benes J, Sviglerova J, Kuncova J, Matejovic M, Stengl M. Vagus Nerve Stimulation Attenuates Multiple Organ Dysfunction in Resuscitated Porcine Progressive Sepsis. Crit Care Med. 2019;47:e461-e469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Hilderman M, Bruchfeld A. The cholinergic anti-inflammatory pathway in chronic kidney disease-review and vagus nerve stimulation clinical pilot study. Nephrol Dial Transplant. 2020;35:1840-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Guslandi M. Nicotine treatment for ulcerative colitis. Br J Clin Pharmacol. 1999;48:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Guslandi M, Tittobello A. Outcome of ulcerative colitis after treatment with transdermal nicotine. Eur J Gastroenterol Hepatol. 1998;10:513-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Özdemir-Kumral ZN, Özbeyli D, Özdemir AF, Karaaslan BM, Kaytaz K, Kara MF, Tok OE, Ercan F, Yegen BÇ. Protective Effect of Nicotine on Sepsis-Induced Oxidative Multiorgan Damage: Role of Neutrophils. Nicotine Tob Res. 2017;19:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Mabley J, Gordon S, Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 2011;34:231-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Kloc M, Ghobrial RM, Kubiak JZ. How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of COVID-19 infection? Immunol Lett. 2020;224:28-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Gonzalez-Rubio J, Navarro-Lopez C, Lopez-Najera E, Lopez-Najera A, Jimenez-Diaz L, Navarro-Lopez JD, Najera A. Cytokine Release Syndrome (CRS) and Nicotine in COVID-19 Patients: Trying to Calm the Storm. Front Immunol. 2020;11:1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Effects of the α7 nicotinic acetylcholine receptor agonist GTS-21 on the innate immune response in humans. Shock. 2011;36:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Garg BK, Loring RH. GTS-21 has cell-specific anti-inflammatory effects independent of α7 nicotinic acetylcholine receptors. PLoS One. 2019;14:e0214942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Ahmad F. COVID-19 induced ARDS, and the use of galantamine to activate the cholinergic anti-inflammatory pathway. Med Hypotheses. 2020;145:110331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Pinder N, Bruckner T, Lehmann M, Motsch J, Brenner T, Larmann J, Knebel P, Hoppe-Tichy T, Swoboda S, Weigand MA, Hofer S, Zimmermann JB. Effect of physostigmine on recovery from septic shock following intra-abdominal infection - Results from a randomized, double-blind, placebo-controlled, monocentric pilot trial (Anticholium® per Se). J Crit Care. 2019;52:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Li G, Zhou CL, Zhou QS, Zou HD. Galantamine protects against lipopolysaccharide-induced acute lung injury in rats. Braz J Med Biol Res. 2016;49:e5008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Liu ZH, Ma YF, Wu JS, Gan JX, Xu SW, Jiang GY. Effect of cholinesterase inhibitor galanthamine on circulating tumor necrosis factor alpha in rats with lipopolysaccharide-induced peritonitis. Chin Med J (Engl). 2010;123:1727-1730. [PubMed] |
| 46. | Yoshiyama Y, Kojima A, Ishikawa C, Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J Alzheimers Dis. 2010;22:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Consolim-Colombo FM, Sangaleti CT, Costa FO, Morais TL, Lopes HF, Motta JM, Irigoyen MC, Bortoloto LA, Rochitte CE, Harris YT, Satapathy SK, Olofsson PS, Akerman M, Chavan SS, MacKay M, Barnaby DP, Lesser ML, Roth J, Tracey KJ, Pavlov VA. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 49. | Musk AW, de Klerk NH. History of tobacco and health. Respirology. 2003;8:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | DeVeaugh-Geiss AM, Chen LH, Kotler ML, Ramsay LR, Durcan MJ. Pharmacokinetic comparison of two nicotine transdermal systems, a 21-mg/24-hour patch and a 25-mg/16-hour patch: a randomized, open-label, single-dose, two-way crossover study in adult smokers. Clin Ther. 2010;32:1140-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Moran VE. Cotinine: Beyond that Expected, More than a Biomarker of Tobacco Consumption. Front Pharmacol. 2012;3:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Hukkanen J, Jacob P 3rd, Peng M, Dempsey D, Benowitz NL. Effect of nicotine on cytochrome P450 1A2 activity. Br J Clin Pharmacol. 2011;72:836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Stefan MS, Pack Q, Shieh MS, Pekow PS, Bernstein SL, Raghunathan K, Nason KS, Lindenauer PK. The Association of Nicotine Replacement Therapy With Outcomes Among Smokers Hospitalized for a Major Surgical Procedure. Chest. 2020;157:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | de Jong B, Schuppers AS, Kruisdijk-Gerritsen A, Arbouw MEL, van den Oever HLA, van Zanten ARH. The safety and efficacy of nicotine replacement therapy in the intensive care unit: a randomised controlled pilot study. Ann Intensive Care. 2018;8:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Pathak V, Rendon IS, Lupu R, Tactuk N, Olutade T, Durham C, Stumacher R. Outcome of nicotine replacement therapy in patients admitted to ICU: a randomized controlled double-blind prospective pilot study. Respir Care. 2013;58:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Cartin-Ceba R, Warner DO, Hays JT, Afessa B. Nicotine replacement therapy in critically ill patients: a prospective observational cohort study. Crit Care Med. 2011;39:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Pack QR, Priya A, Lagu TC, Pekow PS, Atreya A, Rigotti NA, Lindenauer PK. Short-Term Safety of Nicotine Replacement in Smokers Hospitalized With Coronary Heart Disease. J Am Heart Assoc. 2018;7:e009424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Ng KT, Gillies M, Griffith DM. Effect of nicotine replacement therapy on mortality, delirium, and duration of therapy in critically ill smokers: a systematic review and meta-analysis. Anaesth Intensive Care. 2017;45:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Molander L, Hansson A, Lunell E, Alainentalo L, Hoffmann M, Larsson R. Pharmacokinetics of nicotine in kidney failure. Clin Pharmacol Ther. 2000;68:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Perry RJ, Griffiths W, Dextraze P, Solomon RJ, Trebbin WM. Elevated nicotine levels in patients undergoing hemodialysis. A role in cardiovascular mortality and morbidity? Am J Med. 1984;76:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |